Abstract
With the creation of the Somatic Symptom and Related Disorders category of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition in 2013, the functional neurological (symptom) disorder diagnostic criteria underwent transformative changes. These included an emphasis on ‘rule-in’ physical examination signs/semiological features guiding diagnosis and the removal of a required proximal psychological stressor to be linked to symptoms. In addition, the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition somatization disorder, somatoform pain disorder and undifferentiated somatoform disorder conditions were eliminated and collapsed into the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition somatic symptom disorder diagnosis. With somatic symptom disorder, emphasis was placed on a cognitive-behavioural (psychological) formulation as the basis for diagnosis in individuals reporting distressing bodily symptoms such as pain and/or fatigue; the need for bodily symptoms to be ‘medically unexplained’ was removed, and the overall utility of this diagnostic criteria remains debated. A consequence of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition restructuring is that the diagnosis of somatization disorder that encompassed individuals with functional neurological (sensorimotor) symptoms and prominent other bodily symptoms, including pain, was eliminated. This change negatively impacts clinical and research efforts because many patients with functional neurological disorder experience pain, supporting that the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition would benefit from an integrated diagnosis at this intersection. We seek to revisit this with modifications, particularly since pain (and a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition somatization disorder comorbidity, more specifically) is associated with poor clinical prognosis in functional neurological disorder. As a first step, we systematically reviewed the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition somatization disorder literature to detail epidemiologic, healthcare utilization, demographic, diagnostic, medical and psychiatric comorbidity, psychosocial, neurobiological and treatment data. Thereafter, we propose a preliminary revision to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition allowing for the specifier functional neurological disorder ‘with prominent pain’. To meet this criterion, core functional neurological symptoms (e.g. limb weakness, gait difficulties, seizures, non-dermatomal sensory loss and/or blindness) would have ‘rule-in’ signs and pain (>6 months) impairing social and/or occupational functioning would also be present. Two optional secondary specifiers assist in characterizing individuals with cognitive-behavioural (psychological) features recognized to amplify or perpetuate pain and documenting if there is a pain-related comorbidity. The specifier of ‘with prominent pain’ is etiologically neutral, while secondary specifiers provide additional clarification. We advocate for a similar approach to contextualize fatigue and mixed somatic symptoms in functional neurological disorder. While this preliminary proposal requires prospective data and additional discussion, these revisions offer the potential benefit to readily identify important functional neurological disorder subgroups—resulting in diagnostic, treatment and pathophysiology implications.
Keywords: functional neurological disorder, somatization disorder, pain, psychogenic, conversion disorder
This article revisits the functional neurological disorder – pain intersection by systematically reviewing the somatization disorder literature. We propose a preliminary diagnostic criteria revision denoting the etiologically neutral specifier functional neurological disorder “with prominent pain”. Proposals are also suggested to characterize individuals “with prominent fatigue” and “with prominent mixed somatic symptoms”.
Graphical Abstract
Graphical Abstract.
Introduction
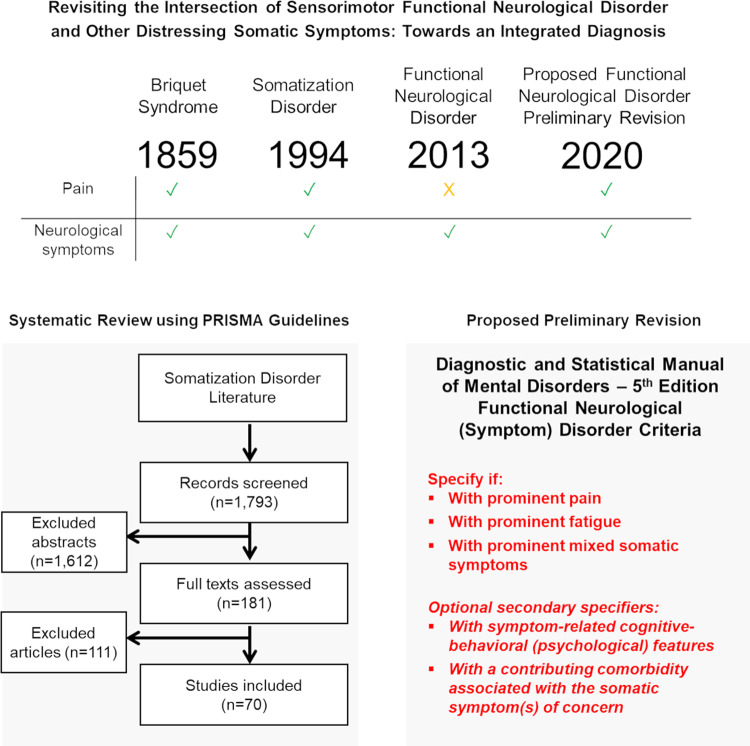
Pierre Briquet, the French physician and psychologist, published his Treatise on Hysteria in 1859 on 430 patients that provided the basis for the modern-day somatization disorder (SD) (Briquet syndrome) diagnosis (see Fig. 1) (Briquet, 1859; Mai and Merskey, 1980, 1981). Briquet wrote that hysteria was a ‘neurosis of the brain in which the observed phenomena consist chiefly of a perturbation of vital activities, which serve as the manifestation of affective feeling’. While sensorimotor functional neurological symptoms were part of the original symptom complex, pain was a core symptom. Briquet wrote, ‘there is not a single woman with this neurosis who does not have some muscle pain during the course of the illness’ (Briquet, 1859). In the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Somatization disorder (DSM-IV-SD) category, the presence of at least one functional neurological symptom occurring at some point during the illness course was required—along with four pain symptoms, two gastrointestinal symptoms and one sexual symptom(American Psychiatric Association, 2000). While the DSM-IV-SD criteria were criticized for its somewhat arbitrary symptom domain requirements(Mayou et al., 2005; Rief et al., 2011), a strength was having one diagnosis encompass the frequently encountered intersection of functional neurological disorder (FND) with prominent pain.
Figure 1.
Depicts Pierre Briquet (1796–1881; left panel) alongside his Treatise on Hysteria book published in 1859 (right panel). Left panel image reproduced with permission from Fontoura P. The ‘Ajuda Paralyses’: history of a neuropsychiatric debate in mid-19th-century Portugal. Brain 2010; 133: 3141–52. Right panel image is in the public domain. Source: Bibliothèque nationale de France, département Sciences et techniques.
With Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), the ‘Somatic Symptom and Related Disorders’ section underwent major changes (American Psychiatric Association, 2013; Dimsdale et al., 2013). The DSM-IV-SD, somatoform pain disorder and undifferentiated somatoform disorder diagnoses were re-conceptualized into one condition—somatic symptom disorder (SSD). The SSD diagnostic criteria removed the need for physical symptoms to be ‘medically unexplained’, and instead emphasized a cognitive-behavioural (psychological) formulation whereby individuals were deemed to engage with bodily symptoms (>6 months duration) using unhelpful thought patterns, behavioural strategies and/or emotional responses. Hypochondriasis was reframed as illness anxiety disorder, and conversion disorder was re-conceptualized as FND. Major changes to the FND diagnostic criteria included an emphasis on positive neurological examination signs ‘ruling-in’ diagnosis, as well as the removal of the need to relate a proximal stressor to symptom onset; the requirement to exclude feigning, a diagnostic challenge relevant only to a minority of cases, was also eliminated(Stone et al., 2010a). Notably, the DSM-5 FND diagnostic category focuses on motor symptoms (e.g. limb weakness, abnormal movements, seizures) and sensory deficits (e.g. non-dermatomal sensory loss, blindness). As such, a single diagnosis encompassing patients with FND and prominent pain is now no longer present in the DSM-5 framework, requiring clinicians to consider dual FND and SSD diagnoses. This is problematic given that the psychological diagnostic criteria for SSD and its potential application to individuals with known symptom-related medical problems have been met with mixed reviews (van der Feltz-Cornelis et al., 2018; Lehmann et al., 2019; Burton et al., 2020; Scamvougeras and Howard, 2020), resulting in variable use of the SSD diagnosis amongst FND experts (Aybek et al., 2020).
In FND, pain is common and clinically relevant (Glass et al., 2018). For example, in a large cohort (n = 107) of patients with functional limb weakness, pain beyond the affected limb (64%), headache (40%) and back pain (38%) were frequently present and differentiated FND from neurological controls (Stone et al., 2010b). In 160 functional movement disorder patients, one-fourth reported pain with functional motor symptom onset (Gelauff et al., 2020). Robust associations have also been described between functional dystonia and complex regional pain syndrome (Popkirov et al., 2019). Notably, the presence of chronic pain differentiates individuals with functional (psychogenic non-epileptic/dissociative) seizures from those with epilepsy (Gazzola et al., 2012). In pediatric FND, pain is especially common, with one study in 194 children reporting that 56% had concurrent pain (Kozlowska et al., 2007). Reduced quality of life and poor clinical outcomes have been linked to pain in FND (Ibrahim et al., 2009; Myers et al., 2012). In addition, FND patients with predominantly pain-related medical disability have been excluded from physiotherapy clinical trials, highlighting that this subgroup is being identified indirectly as a distinct entity (Nielsen et al., 2017). Relatedly, pathophysiological models of FND emphasizing altered predictive processing, multimodal integration and emotion processing fit well with a close intersection between sensorimotor FND and pain (Edwards et al., 2012; Diez et al., 2019; Pick et al., 2019), further supporting the need to better characterize the FND—pain intersection.
In this article, we first revisit the explicit intersection of FND and pain in the DSM-IV-SD diagnosis by performing a systematic review of the DSM-IV-SD literature to detail relevant epidemiologic, healthcare utilization, demographic, diagnostic, medical and psychiatric comorbidity, psychosocial, neurobiological and treatment data. Thereafter, we subsequently propose a preliminary revision to the DSM-5 FND diagnostic criteria that allows for the etiologically neutral specifier of FND ‘with prominent pain’ (akin to a FND plus syndrome). This important distinction will greatly aid cohort characterization across diagnostic, treatment and pathophysiology studies, including providing increased clarity regarding the types of patients enrolled in clinical and translational research studies.
Materials and methods
Search strategy
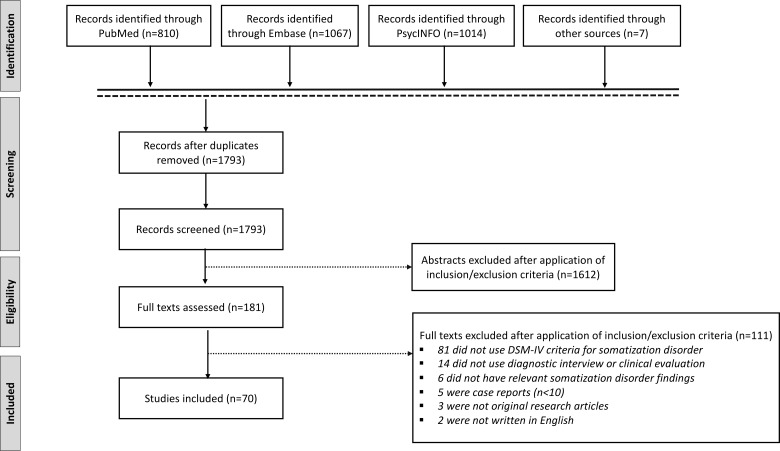
This study was registered in PROSPERO (CRD42020159179). We searched PubMed, PsycINFO and Embase with the terms ‘somatization disorder’ OR ‘Briquet’ from inception to 30 June 2019 in accord with PRISMA guidelines. Reference lists from identified original research articles and reviews were also scrutinized to identify articles meeting eligibility criteria.
Eligibility criteria
Original research studies with patients meeting DSM-IV criteria for SD were included. Only articles in which the DSM-IV-SD diagnosis was obtained by interview (structured or unstructured), as opposed to symptom checklist or self-report questionnaire, were included (Carson et al., 2015). We identified within and between-group studies in the following content areas: epidemiology, healthcare utilization, diagnosis, medical and psychiatric/psychological comorbidities, predisposing vulnerabilities, neural mechanisms, treatment and prognosis. Case reports or series (n < 10), review articles, those not written in English, and content published only in abstract or dissertation form were excluded. Articles not using DSM-IV-SD diagnostic criteria [e.g. abridged SD, Diagnostic and Statistical Manual of Mental Disorders, Third Edition, International Classification of Diseases, Tenth Edition or Perley & Guze criteria (Perley and Guze, 1962)] were also excluded (see Table 1). The rationale for this exclusion criterion is that only DSM-IV-SD diagnostic criteria explicitly required at least one functional neurological symptom. Structural neuroimaging studies of DSM-IV-SD were also omitted given that they were previously reviewed elsewhere (Begue et al., 2019).
Table 1.
The historical evolution of Briquet syndrome diagnostic criteria
| Diagnostic criteria | Name(s) | Date first described | Number of symptoms required | Number of symptom groups required | Neurologic symptoms | Age of onset requirement |
|---|---|---|---|---|---|---|
| Briquet | Briquet Syndrome or Hysteria | 1859 | 25 | 9 of 10a | Not required | Onset of symptoms by 30 |
| Robins and O'Neal | Hysteria | 1953 | 18 | 7 of 10a | Not required | More than one major operation or two hospitalizations by 21 |
| Perley and Guze | Hysteria | 1962 | 15 | 9 of 10a | Not required | Significant medical history by 35 |
| Feighner | Hysteria | 1967 |
Definite diagnosis: 25 Probable diagnosis: 20–24 |
9 of 10a | Not required | Chronic or recurrent illness by 30 |
| ICD-9 | Hysteria unspecified | 1977 | Not specified | Not specified | Not specified | Not specified |
| DSM-III | SD | 1980 |
Women: 14 Men: 12 |
Not required | Not required | Onset of symptoms by 30 |
| DSM-III-R | SD | 1987 | 13 | Not required | Not required | Onset of symptoms by 30 |
| Abridged Escobar or Somatic Symptom Index | SD | 1987 |
Women: 6 Men: 4 |
Not required | Not required | Not specified |
| ICD-10 | SD | 1990 | Not specified | Not specified | Not required | 2-year duration of unexplained somatic symptoms needed |
| DSM-IV | SD | 1994 | 8 | 4b | Required | Onset of symptoms by 30 |
| DSM-5 | SSD | 2013 | 1 | Not specified | Not required | >6 months of symptoms duration |
Group 1: Feeling sickly for most of life, or headache; Group 2: blindness, paralysis, anaesthesia, aphonia, fits or convulsions, unconsciousness, amnesia, deafness, hallucinations or urinary retention; Group 3: fatigue, lump in the throat, fainting spells, visual blurring, weakness or dysuria; Group 4: breathing difficulty, palpitation, anxiety attacks, chest pain or dizziness; Group 5: anorexia, weight loss, marked fluctuations in weight, nausea, abdominal bloating, food intolerances, diarrhea or constipation; Group 6: abdominal pain or vomiting; Group 7: dysmenorrhea, menstrual irregularity, including amenorrhea for at least 2 months, or excessive menstrual bleeding; Group 8: sexual indifference, sexual frigidity, dyspareunia, other sexual difficulties or vomiting for all 9 months of pregnancy; Group 9: back pain, joint pain, extremity pain, burning pains of the sexual organs, mouth or rectum or other bodily pains; Group 10: nervousness, fears, depressed feelings, need to quit working or inability to carry on regular duties because of feeling sick, crying easily, feeling life was hopeless, thinking a good deal about dying, wanting to die, thinking of suicide or suicide attempts.
Group 1: pain symptoms; Group 2: gastrointestinal symptoms; Group 3: functional neurological symptoms; Group 4: sexual symptoms.
Data extraction
EndNote was used to compile abstracts from search results of all three databases. After removing duplicates, J.M. and P.R.A. independently applied inclusion/exclusion criteria to determine articles to be read. Discrepancies between the two reviewers were independently resolved by D.L.P. From the list of articles selected, J.M. and P.R.A. narrowed down studies based on inclusion/exclusion criteria. Included articles were evaluated for quality using the National Institutes of Health Study Quality Assessment Tools guidelines (National Institutes of Health: National Heart Lung and Blood Institute) (see Supplementary Table 1). See Fig. 2 for a PRISMA flow diagram of the systematic review (Moher et al., 2009).
Figure 2.
PRISMA flow diagram of the systematic review of somatization disorder, as defined using DSM-IV diagnostic criteria.
Results
Epidemiology
The prevalence of DSM-IV-SD was recorded in eight studies (Escobar et al., 1998; Lynch et al., 1999; Simon and Gureje, 1999; Fink et al., 2004, 2005; Smith et al., 2005; Prerana et al., 2017; Chander et al., 2019). The largest study (n = 5447) performed in 14 countries, determined that DSM-IV-SD had a prevalence of 1.4% among primary care outpatients (Simon and Gureje, 1999). Two studies performed in psychiatry clinics, found that 2.6–6.7% met criteria for DSM-IV-SD (Prerana et al., 2017; Chander et al., 2019). Among first time neurology referrals (n = 120), 7% in one cohort were diagnosed with DSM-IV-SD (Fink et al., 2005).
Healthcare utilization
Five studies investigated healthcare utilization in DSM-IV-SD patients, although none had an isolated DSM-IV-SD cohort (Lynch et al., 1999; Hiller et al., 2003; Smith et al., 2005; Frostholm et al., 2014; Weiss et al., 2017), and two studies had very limited inclusion of DSM-IV-SD patients and will not be further discussed (Lynch et al., 1999; Smith et al., 2005). Weiss et al examined 254 patients (55 with DSM-IV-SD) across 7 outpatient psychotherapy clinics, identifying that those with DSM-IV-SD had a higher number of outpatient doctor visits (an average of 36.5 outpatient doctor visits in the prior year) compared to those with undifferentiated somatoform disorder, somatoform pain disorder and severe DSM-5 SSD (x = 25.9, 24.7 and 32.5 outpatient doctor visits, respectively) (Weiss et al., 2017). The somatoform disorder cohort (including those with DSM-IV-SD) showed two times higher outpatient healthcare utilization compared to the general German population. Negative illness perceptions in 144 patients with somatoform disorders (26 with DSM-IV-SD) correlated with greater healthcare expenditures (Frostholm et al., 2014). In 172 subjects (54 with DSM-IV-SD) enrolled in an inpatient cognitive behavioural therapy (CBT) treatment programme for somatic symptoms, individuals with DSM-IV-SD had higher outpatient costs than those with abridged SD, and the entire cohort had 2.5% higher outpatient costs compared to average German healthcare system costs (Hiller et al., 2003).
Demographics
Seven studies investigated demographic characteristics in DSM-IV-SD cohorts (n > 50) (Guz et al., 2004; Allen et al., 2006; Garcia-Campayo et al., 2007; Kuwabara et al., 2007; Manchikanti et al., 2007; Fjorback et al., 2013; Prerana et al., 2017). DSM-IV-SD had a female predominance (75–89%), with an age range typically between 20 and 50 years old (Guz et al., 2004; Allen et al., 2006; Garcia-Campayo et al., 2007; Kuwabara et al., 2007; Manchikanti et al., 2007; Fjorback et al., 2013; Prerana et al., 2017). Educational background reflected regional differences; 22 ± 8.4 years of schooling were reported in a Japanese cohort (Kuwabara et al., 2007), while only 14% of participants in a Turkish DSM-IV-SD study completed college (Guz et al., 2004). Rates of unemployment were reported in 4 of 7 studies and ranged from 5% to 45% (Guz et al., 2004; Allen et al., 2006; Fjorback et al., 2013; Prerana et al., 2017). Patients receiving disability ranged from 19% to 53% (Allen et al., 2006; Garcia-Campayo et al., 2007; Fjorback et al., 2013). The majority of patients were married (Guz et al., 2004; Allen et al., 2006; Garcia-Campayo et al., 2007; Fjorback et al., 2013; Prerana et al., 2017).
Diagnostic criteria
Four studies evaluated the stability or specificity of the DSM-IV-SD diagnostic criteria (Lynch et al., 1999; Simon and Gureje, 1999; Fink et al., 2005; Smith et al., 2005). A random sample of 3196 primary care patients from the World Health Organization Psychological Problems in General Health Care study conducted in 15 sites across 14 countries were assessed regarding the stability of a lifetime DSM-IV-SD diagnosis (Simon and Gureje, 1999). Of the 74 (2.3% of total sample) patients initially meeting lifetime DSM-IV-SD criteria, only 21 (28%) of those same individuals in a subsequent interview 1 year later again endorsed a history of DSM-IV-SD. This suggests that the DSM-IV-SD diagnosis was subject to recall bias (an issue not necessarily specific to the DSM-IV-SD diagnosis). Compared to other multiple somatic symptom classification systems, the DSM-IV-SD diagnostic criteria were the most narrowly defined and restrictive (Lynch et al., 1999; Fink et al., 2005; Smith et al., 2005). For example, in 119 patients attending a primary care centre, only 1 (0.84%) met DSM-IV-SD criteria; 10 (8%) met SD when only one gastrointestinal symptom was required and sexual/reproductive symptoms were excluded (Lynch et al., 1999). Similarly, the DSM-IV-SD prevalence was 1% versus 7% by International Classification of Diseases, Tenth Edition in a sample of 198 patients with medically unexplained symptoms (Fink et al., 2005); in 206 primary care patients with medically unexplained symptoms, only 3 (1.5%) met DSM-IV-SD criteria while 39 (19%) met the abridged Escobar criteria (Smith et al., 2005).
Medical comorbidities—functional somatic disorders
Eight studies evaluated the intersection of DSM-IV-SD and medical comorbidities (Hiller et al., 2000; Miller et al., 2001; North et al., 2004; Schrag et al., 2004; Manchikanti et al., 2007, 2008; Padhy et al., 2016; Chander et al., 2019). The intersection of DSM-IV-SD and functional somatic disorders were primarily investigated in two distinct groups, patients with irritable bowel syndrome (IBS) and individuals with chronic pain disorders (Hiller et al., 2000; Miller et al., 2001; North et al., 2004; Manchikanti et al., 2007, 2008; Padhy et al., 2016). Three studies were conducted in IBS cohorts (n = 24–56), two from outpatient gastroenterology (Miller et al., 2001; North et al., 2004). The prevalence of DSM-IV-SD in those with IBS ranged from 16% to 25% (Miller et al., 2001; North et al., 2004; Padhy et al., 2016). In these studies, patients with IBS with comorbid DSM-IV-SD showed increased abnormal illness behaviours, greater psychiatric comorbidities and higher rates of other functional somatic disorders compared to individuals with IBS alone (Miller et al., 2001; North et al., 2004; Padhy et al., 2016). Three studies looked at the intersection of chronic pain and DSM-IV-SD (Hiller et al., 2000; North et al., 2004; Manchikanti et al., 2007). Ten of 60 individuals attending an inpatient chronic pain programme had DSM-IV-SD and were grouped together with 31 total patients framed as ‘other functional somatic disorders’ (Hiller et al., 2000). The chronic pain group with a comorbid other functional somatic disorder displayed a greater number of bodily symptoms and pain symptoms, but similar psychological pain distress profiles, depression and anxiety as the chronic pain only group. In a separate outpatient cohort of 500 patients taking opioids for chronic pain, 30% had comorbid DSM-IV-SD (Manchikanti et al., 2007). Higher rates of current illicit drug use were also observed in men with SD versus without (22% versus 9%) (Manchikanti et al., 2007). In 438 patients with chronic spinal pain, a DSM-IV-SD diagnosis (n = 162) did not significantly influence false positive (placebo) rates following a single anesthetic administration (Manchikanti et al., 2008). While not well studied, in addition to functional somatic disorders, patients with DSM-IV-SD were also reported to have cardiovascular and endocrine conditions, and peripheral injuries (including fractures) as common comorbid medical conditions (Schrag et al., 2004; Chander et al., 2019).
Psychiatric comorbidities
Eleven studies characterized psychiatric comorbidities in DSM-IV-SD (Battaglia et al., 1998; Rief et al., 2001; Carey et al., 2003; Mohlman et al., 2004; Brown et al., 2005; Garcia-Campayo et al., 2007; Ozturk and Sar, 2008; Sertoz et al., 2009; Spitzer et al., 2009; Taycan et al., 2014; Chander et al., 2019). Many studies reported high rates of mood disorders (10–90%) (Brown et al., 2005; Sertoz et al., 2009; Taycan et al., 2014; Chander et al., 2019); panic attacks (41%) (Brown et al., 2005); lifetime post-traumatic stress disorder (PTSD) or complex PTSD (14–55%) (Brown et al., 2005; Spitzer et al., 2009; Taycan et al., 2014); generalized anxiety disorder (45%) (Brown et al., 2005); any anxiety disorder (15%) (Chander et al., 2019); lifetime dissociative disorder (28–50%) (Brown et al., 2005; Taycan et al., 2014) and borderline personality disorder (15%) (Taycan et al., 2014) within DSM-IV-SD cohorts. Similarly, DSM-IV-SD was found to be highly comorbid within PTSD (35%), panic disorder (11%) and dissociative disorder (33%) populations (Carey et al., 2003; Mohlman et al., 2004; Ozturk and Sar, 2008).
The largest sample of DSM-IV-SD patients (n = 70) reported that 39% of patients did not have another DSM-IV Axis I comorbidity (Garcia-Campayo et al., 2007). The remaining DSM-IV-SD population had comorbid mood disorders [major depressive disorder (MDD) 13%; dysthymia 11%], anxiety disorders (panic disorder 13%; generalized anxiety disorder 10%; agoraphobia 4%), or another mood/anxiety disorder (6%). Sixty-four percent of individuals with DSM-IV-SD also met criteria for one or more personality disorders (Garcia-Campayo et al., 2007).
Several studies also examined relationships between psychiatric comorbidities and dimensional psychological characteristics in DSM-IV-SD. One study used the Brief Symptom Inventory to assess differences in psychopathology between DSM-IV-SD with complex PTSD (n = 10), DSM-IV-SD without complex PTSD (n = 18) and MDD without complex PTSD (n = 27) (Spitzer et al., 2009). Here, patients with DSM-IV-SD and complex PTSD exhibited higher psychoticism and interpersonal problems than both other groups, and higher obsessive compulsive and anger–hostility tendencies than the DSM-IV-SD without complex PTSD group. Both DSM-IV-SD groups showed greater obsessionality, anxiety, anger–hostility and global severity of psychopathology compared to the MDD group (Spitzer et al., 2009). Furthermore, another study reported that the co-occurrence of major depression and somatization (37.5% with full-criteria DSM-IV-SD) was linked to increased psychopathology on the Symptom Checklist-90 Revised (Rief et al., 2001). In another study, 18 DSM-IV-SD patients with comorbid panic disorder showed higher novelty seeking scores compared to 41 individuals with panic disorder and 22 healthy controls (HCs), and novelty seeking positively correlated with number of somatic symptoms (Battaglia et al., 1998).
DSM-IV-SD versus Other DSM-IV somatoform disorders
Five studies compared DSM-IV-SD to other somatoform disorders (Escobar et al., 1998; Sanyal et al., 1998; Guz et al., 2004; Espirito-Santo and Pio-Abreu, 2009; Kırpınar et al., 2016), including two examining similarities and differences between DSM-IV-SD and hypochondriasis (Escobar et al., 1998; Kırpınar et al., 2016) and three comparing DSM-IV-SD and DSM-IV conversion disorder (Sanyal et al., 1998; Guz et al., 2004; Espirito-Santo and Pio-Abreu, 2009). In a primary care setting of 1456 outpatients, 49 patients with hypochondriasis and 20 with DSM-IV-SD were identified (Escobar et al., 1998). One in five patients with DSM-IV-SD also had hypochondriasis; a higher rate of DSM-IV-SD amongst hypochondriasis patients was also observed with a prevalence of 9% versus 1% in those without hypochondriasis. Kırpınar et al. characterized a consecutive sample of 73 outpatients in a somatoform disorders unit ([DSM-IV-SD (n = 51) and hypochondriasis (n = 22)], identifying a higher female prevalence in the DSM-IV-SD group (71% versus 41%) and higher heath anxiety in the hypochondriasis group (Kırpınar et al., 2016). The two cohorts showed similar Beck Anxiety Inventory, Hamilton Depression Rating Scale, Somatosensory Amplification Scale, Somatoform Dissociation Questionnaire, Dissociative Experiences Scale and State-Trait Anxiety Inventory scores.
DSM-IV-SD (n = 40), conversion disorder (n = 26) and dissociative disorders (n = 38) were compared in one study (Espirito-Santo and Pio-Abreu, 2009), with more commonalities found amongst the dissociative and conversion disorder groups (e.g. greater dissociation) than between the DSM-IV-SD and conversion disorder groups. More depression and paranoia symptoms were seen in those with dissociative disorders, while more somatic and obsessive symptoms were appreciated in the DSM-IV-SD group. In a separate study, 71 DSM-IV-SD patients had lower paranoia and psychotic personality traits compared to 87 individuals with DSM-IV conversion disorder (Guz et al., 2004). Individuals with DSM-IV-SD also exhibited lower self-appraisal compared to both patients with conversion disorder and HCs (Sanyal et al., 1998).
Comorbid DSM-IV-SD in FND
Six studies reported on the co-occurrence of DSM-IV-SD and FND (Interian et al., 2004; Schrag et al., 2004; Marchetti et al., 2008; Stone et al., 2010b; Epstein et al., 2016; Gelauff et al., 2019). In one study, 8 (12.5%) of 36 patients with functional movement disorder met criteria for comorbid SD (Epstein et al., 2016); 12 (32%) of 38 patients with probable or diagnosed functional dystonia had comorbid DSM-IV-SD in another study (Schrag et al., 2004). Likewise, 19% of patients experiencing functional (non-epileptic/dissociative) seizures (n = 27) also had DSM-IV-SD (Marchetti et al., 2008). In a large sample of 107 patients with functional limb weakness, 27% had a comorbid DSM-IV-SD (Stone et al., 2010b). However, the presence of functional neurological symptoms in 120 patients with medically unexplained symptoms was not predictive of a DSM-IV-SD diagnosis (Interian et al., 2004). Importantly, the dual diagnosis of DSM-IV-SD and FND correlated with poor clinical outcomes in a 14-year follow-up study of 76 patients with functional limb weakness (Gelauff et al., 2019).
Dimensional psychopathology
Eleven studies measured dimensional psychological characteristics using self-report or clinician-rated scales in DSM-IV-SD samples (Sanyal et al., 1998; Brown et al., 2005; Ozturk and Sar, 2008; Sertoz et al., 2009; Spitzer et al., 2009; Landa et al., 2012; Taycan et al., 2014; Kırpınar et al., 2016; Prerana et al., 2017; Davoodi et al., 2018, 2019 ). Patients with DSM-IV-SD reported elevated depression scores compared to HCs in one study (Taycan et al., 2014). Another study compared DSM-IV-SD patients to those with MDD, finding more anxiety in the DSM-IV-SD cohort (Spitzer et al., 2009). The number of bodily complaints positively correlated with depression and anxiety scores in an DSM-IV-SD sample (Prerana et al., 2017). Higher rates of suicide attempts and self-mutilation were also identified in DSM-IV-SD compared to HCs (Ozturk and Sar, 2008; Taycan et al., 2014). Dissociation in DSM-IV-SD has also been characterized in multiple studies (Brown et al., 2005; Taycan et al., 2014; Kırpınar et al., 2016). Individuals with DSM-IV-SD exhibited increased dissociation and more trance-like states compared to HCs (Taycan et al., 2014), as well as higher dissociative amnesia rates compared to neurological controls (i.e. dystonia) (Brown et al., 2005).
Studies have characterized an external locus of control in DSM-IV-SD compared to HCs (Sanyal et al., 1998). Increased alexithymia, higher level of mistrust and an unmet need for interpersonal closeness were also identified in a mixed DSM-IV somatoform disorder cohort (n = 20) compared to HCs (n = 20) (Landa et al., 2012). Furthermore, worse body image and self-esteem were identified in a mixed somatoform disorders sample (38% with DSM-IV-SD) compared to both healthy and medical controls (patients with breast cancer status-post total mastectomy) (Sertoz et al., 2009). By contrast, when compared to patients with MDD, individuals with DSM-IV-SD exhibited less severe maladaptive schemas (Davoodi et al., 2018) and use of more adaptive emotional regulation strategies (Davoodi et al., 2019).
Traumatic life events
Three studies investigated childhood trauma burden in DSM-IV-SD cohorts (Brown et al., 2005; Spitzer et al., 2008; Taycan et al., 2014). Forty women with DSM-IV-SD reported a greater number of childhood trauma types and were more likely to have experienced physical abuse (20%), emotional abuse (25%) and emotional neglect (30%) compared to 40 healthy women (Taycan et al., 2014). In another study, 28 individuals with DSM-IV-SD reported more childhood sexual (43%) and physical abuse (54%) compared to 28 patients with MDD (Spitzer et al., 2008). Furthermore, the severity of sexual and physical abuse distinguished the DSM-IV-SD group from the MDD cohort (Spitzer et al., 2008). Similarly, more severe early-life maltreatment distinguished DSM-IV-SD from neurological populations, with one study showing that 22 DSM-IV-SD patients reported more severe childhood physical abuse, increased emotional abuse exposure, greater number of emotional abuse perpetrators, and a longer duration of emotional abuse compared to 19 dystonia controls (Brown et al., 2005). Those with DSM-IV-SD also reported more family conflict during childhood (Brown et al., 2005). In a qualitative, interview-based study, several patients with DSM-IV-SD articulated links between early-life maltreatment, somatic symptoms and healthcare use (Morse et al., 1997).
Regarding lifetime trauma, 40 women with DSM-IV-SD reported more traumatic events in adulthood (mean = 2.6) than 40 healthy women (mean = 1.2), and a higher number of adulthood traumatic experiences predicted the DSM-IV-SD diagnosis (Taycan et al., 2014). Three studies assessed lifetime trauma in mixed cohorts (Carey et al., 2003; Landa et al., 2012; Brown et al., 2014). Twenty individuals in a somatoform disorder cohort comprised of DSM-IV-SD, somatoform pain and undifferentiated somatoform disorders experienced more lifetime trauma than 20 HCs, and 76% of the traumatic experiences by the patient group were interpersonal in nature (e.g. assault, divorce and separation) (Landa et al., 2012). In primary care, 36 individuals with DSM-IV-SD experienced more traumatic lifetime events than 165 medical patients without DSM-IV-SD (Carey et al., 2003). In 898 twins discordant for lifetime trauma exposure, adverse experiences correlated with increased risk of developing DSM-IV-SD (Brown et al., 2014); this highlights the potential etiological relevance of adversity in DSM-IV-SD.
Pathophysiology
Ten inter-related neuroimaging articles reported resting-state functional MRI (fMRI) findings in a single cohort of 25 drug-naive patients with DSM-IV-SD compared to 28 HCs (Su et al., 2014; Song et al., 2015; Su et al., 2015, 2016; Wang et al., 2016; Wei et al., 2016; Guo et al., 2017; Li et al., 2018; Ou et al., 2018; Pan et al., 2019) (see Supplementary Table 2). Among the findings identified, patients with DSM-IV-SD showed increased functional connectivity within the right inferior temporal gyrus compared to HCs (Su et al., 2015). Measuring local and distant functional connectivity, patients with DSM-IV-SD exhibited increased short-range functional connectivity in the right superior frontal gyrus and left pallidum, and increased long-range functional connectivity in the left middle frontal gyrus and right inferior temporal gyrus (Guo et al., 2017).
In a single photon emission computed tomography study performed in 11 DSM-IV-SD patients, the most common findings were a normal single photon emission computed tomography image (n = 4) and right cerebellum hypoperfusion (n = 4) (Garcia-Campayo et al., 2001). Another study used magnetic resonance spectroscopy to measure brain metabolites in patients with DSM-IV-SD, fibromyalgia and HCs (10 subjects per group). The combined DSM-IV-SD and fibromyalgia cohort showed increased posterior cingulate glutamate levels, but this did not remain statistically significant when only comparing DSM-IV-SD patients and HCs (Fayed et al., 2012).
One electrophysiology study measured attention and working memory mechanisms in 25 DSM-IV-SD patients and HCs using auditory-evoked potentials (Garcia Campayo et al., 2007). Patients with DSM-IV-SD had greater latency in the time needed to perceive, identify and classify new information (Garcia Campayo et al., 2007). In an autonomic study, 10 DSM-IV-SD patients showed higher baseline electrodermal activity (a marker of increased sympathetic tone) compared to individuals with DSM-IV conversion disorder and HCs (Sanyal et al., 1998). Three articles investigated inflammatory markers in DSM-IV-SD, although no consistent pattern emerged across studies (Rief et al., 2001; Hossain et al., 2007a, b).
Treatment
Three treatment studies were specifically performed in DSM-IV-SD cohorts (Allen et al., 2001, 2006; Fjorback et al., 2013). In a small within-group study, 11 individuals assigned to 10 weekly outpatient CBT sessions reported less physical discomfort and improved physical functioning at the end of treatment (Allen et al., 2001). In a trial comparing outpatient CBT plus a psychiatric consultation intervention (CBT+PCI; n = 43) to a PCI alone (n = 41), the CBT+PCI treatment arm showed greater improvement in somatic symptoms and physical functioning (Allen et al., 2006). Fifty-nine subjects assigned to eight sessions (plus one follow-up) of mindfulness therapy showed improved general health, health anxiety, physical symptoms, anxiety and depression as compared to 60 patients receiving PCI (Fjorback et al., 2013).
In addition, several mixed-cohort treatment studies included individuals with DSM-IV-SD. In a bodily distress syndrome cohort (n = 120; 45% meeting DSM-IV-SD criteria), subjects who received nine sessions (over 4 months) of outpatient CBT-based treatment plus PCI showed greater improvement in physical functioning, bodily pain and vitality than those who received a PCI alone (Schröder et al., 2012). In an inpatient CBT study comparing a mixed-somatoform disorders cohort (n = 172, 31% meeting DSM-IV-SD criteria) to a mixed psychiatric cohort (n = 123, primarily mood and anxiety disorders), both groups comparably improved in physical and mental health measures (Hiller et al., 2003). In a somatization syndrome cohort (28% meeting DSM-IV-SD criteria), 107 subjects receiving eight sessions of inpatient CBT plus symptom management training and 84 subjects who underwent eight sessions of inpatient CBT plus relaxation training similarly improved in somatic symptoms, mental health and life satisfaction (Bleichhardt et al., 2004). Additional mixed-cohort studies with DSM-IV-SD making up <20% of the cohort have shown efficacy for intensive inpatient treatment (involving CBT) (Rief and Hiller, 2003), CBT-based outpatient treatment (Martin et al., 2007; Zonneveld et al., 2012; Schröder et al., 2013), outpatient mindfulness therapy (van Ravesteijn et al., 2013) and progressive muscle relaxation (Schröder et al., 2013). Other studies have shown efficacy using antidepressants (Voon and Lang, 2005), and multidisciplinary treatment (Schrag et al., 2004). See Supplementary Table 3 for additional treatment trial details in DSM-IV-SD.
Discussion
This systematic review of DSM-IV-SD identified a number of observations that parallel themes emerging in the DSM-5 FND literature (Ludwig et al., 2018; Baslet et al., 2020; Perez et al., 2020). Patients with DSM-IV-SD were predominantly (although not exclusively) female with increased medical and psychiatric comorbidities, including functional somatic disorders (e.g. IBS) and elevated rates of mood, anxiety, trauma-related and personality disorders. Dimensionally, individuals with DSM-IV-SD reported increased alexithymia, dissociation, an external locus of control and health anxiety. Of etiological relevance, patients with DSM-IV-SD reported increased childhood and lifetime adverse life experiences, including higher rates compared to healthy and neuropsychiatric populations. A large twin study discordant for lifetime trauma exposure showed positive associations between adverse life experiences and increased risk for DSM-IV-SD (Brown et al., 2014). Treatment studies also identified that skills-based psychotherapy was effective in reducing somatic symptoms in patients with DSM-IV-SD. Interestingly, neural mechanisms were particularly understudied in DSM-IV-SD, which may relate in part to the low 1-3% prevalence rates driven in the context of overly stringent diagnostic criteria.
The above overlapping characteristics between DSM-IV-SD and FND have relevant clinical and research implications in FND based on three additional observations: (i) pain is a common comorbid symptom in FND [including particularly high rates in pediatric FND (Kozlowska et al., 2007)] that can limit treatment engagement and incur a poor prognosis (Glass et al., 2018); (ii) a subset of FND patients meet criteria for comorbid DSM-IV-SD (Interian et al., 2004; Schrag et al., 2004; Marchetti et al., 2008; Stone et al., 2010b; Epstein et al., 2016); (iii) comorbid DSM-IV-SD in FND is associated with a poor prognosis (Gelauff et al., 2019). These interrelated themes demonstrate the importance of explicitly considering pain in the assessment, management and research of patients with FND, and underscore the need to better identify the co-occurrence of pain in FND populations. Furthermore, based on neurological examination alone, the assessment of pain requires a more nuanced approach that is not as straight forward as a ‘rule-in’ diagnosis of motor FND based on clinical features such as Hoover sign or tremor entrainment with high diagnostic specificity (Daum et al., 2014). Given concerns regarding SSD diagnostic criteria (based in part on its psychological framework) (van der Feltz-Cornelis et al., 2018; Burton et al., 2020), there is also evidence of heterogeneity in the willingness of FND clinical experts to diagnose SSD in patients with FND (Aybek et al., 2020); an alternative would be to view pain as a commonly present, yet non-specific symptom of FND. The concern with the latter approach is that pragmatically, the presence or absence of pain has high clinical and research relevance, including but not limited to that predominant pain symptoms have been an exclusion criteria for physiotherapy trials in FND (Nielsen et al., 2017). In a randomized feasibility study of physiotherapy for motor FND conducted by Nielsen and colleagues, 27% of the 210 patients screened were excluded based on predominant pain; notably, 47% of the 60 enrolled nonetheless reported ‘severe to extreme’ pain ratings (Nielsen et al., 2017). Regarding neural mechanisms, the central pain matrix implicated in chronic pain disorders overlaps with salience (cingulo-insular) network areas implicated in the pathophysiology of FND (Denk et al., 2014; Begue et al., 2019), underscoring that pain has neurobiological consequences that need to be considered in FND pathophysiology research. Thus, to further advance clinical and research efforts in FND, there is a critical need to provide a practical approach to identify patients that also have prominent pain.
As a preliminary proposal that we hope will catalyse considerable discussion in the field [including amongst the FND Society (www.fndsociety.org) leadership], we argue that a revision to the DSM-5 should be considered to explicitly identify the subset of patients with FND that also have prominent pain. In Fig. 3, we detail the proposed diagnostic criteria allowing for the diagnosis of FND ‘with prominent pain’ (a specifier). Here, patients would meet rule-in criteria for sensorimotor FND and concurrently endorse pain symptoms for at least 6 months that are also impairing to social and/or occupational functioning. In an effort to remain etiologically neutral while acknowledging the biopsychosocial complexity of predisposing and perpetuating factors, we suggest an optional secondary specifier of ‘with cognitive-behavioral (psychological) features’. We propose this optional secondary specifier for the following reasons: (i) an exploration of psychological factors early in the diagnostic assessment for patients with physical symptoms can be challenging for both patients and clinicians alike (and psychological factors related to pain may not be present in all patients); (ii) identification of psychological constructs either amplifying or perpetuating pain have translatable clinical utility (CBT treatment targets), suggesting that there is merit in identifying this subgroup; (iii) the use of ‘rule-in’ psychological criteria as proposed in SSD and related diagnostic formulations remain debated, with a recent European workgroup for functional somatic disorders taking a similar etiologically neutral stance to that outlined above (Burton et al., 2020).
Figure 3.
Preliminary proposal for a revision to the DSM-5 for FND. We suggest the addition of three new specifiers: ‘with prominent pain’; ‘with prominent fatigue’ and ‘with prominent mixed somatic symptoms’. Patients must first meet complete criteria for FND (criteria A–D). In addition, pain, fatigue and/or mixed somatic symptoms should themselves be impairing to social and/or occupational functioning and present for at least 6 months. The above three specifiers are etiologically neutral, which acknowledges the biopsychosocial heterogeneity present in the development and maintenance of these somatic symptoms. To provide additional clarification, we also propose two optional secondary specifiers: (i) with symptom-related cognitive-behavioural (psychological) features; and (ii) with a contributing comorbidity associated with the somatic symptom(s) of concern. The former optional specifier allows the identification of individuals displaying psychological constructs either amplifying or perpetuating pain that can have clinical utility (e.g. CBT treatment targets). The latter optional specifier encourages an appropriate (not necessarily exhaustive) medical workup for the identified somatic symptoms, as well as aids the characterization of relevant medical and neurological comorbidities (including functional somatic disorders) that has been a shortcoming of FND research to date. If a relevant comorbidity is present, this should be noted when using this optional specifier. Regardless of whether or not this latter optional specifier is used, clinicians should be mindful to evaluate pain, fatigue and other somatic symptoms without ‘rule-in’ physical examination features in FND patients as they would in other populations (to prevent premature diagnostic anchoring).
An additional consideration is whether an individual’s chronic pain symptoms are driven by another recognized medical/neurological/functional somatic disorder known to cause pain (e.g. small fibre neuropathy, fibromyalgia, severe lumbar stenosis) or is part of the intrinsic disease processes occurring in FND itself. Here, we recommend another optional secondary descriptive specifier: ‘with a contributing comorbidity associated with the somatic symptom(s) of concern’. If such a condition is present, this can be recorded. The rationale for this specifier is 2-fold: (i) encourages an appropriate (not necessarily exhaustive) medical workup for the individual’s pain symptoms; and (ii) makes transparent if an identified pain-related comorbidity is present, which can have both treatment and research implications. As a cautionary note, regardless of whether or not this optional specifier is used, clinicians should evaluate pain, fatigue and other somatic symptoms without ‘rule-in’ physical examination features in patients with FND as they would in other populations (to prevent premature diagnostic anchoring). While prospective studies are needed to test the reliability and utility of this suggested DSM-5 revision, we speculate that this diagnostic approach will identify the vast majority of patients with FND that are also endorsing prominent pain symptoms.
Operationalizing an FND with prominent pain subgroup has clear clinical and research advantages. By aiding the identification of this important (and likely prevalent) FND subgroup, this approach allows clinicians to consider guiding individuals towards interdisciplinary mind-body pain programmes that may be potentially more suitable for initial management in comparison to emerging motor FND care models (Jimenez et al., 2019). It also allows for the prioritization of treatment targets using a stepwise treatment approach in which a patient’s most prominent physical symptoms are addressed first. In our opinion, this could optimize the likelihood of success with physiotherapy. With an improved ability to operationalize inclusion criteria, our proposal will also catalyse clinical trial research in this potentially more treatment refractory and costly population. Furthermore, with the boom in using brain imaging approaches to elucidate the neurocircuitry of FND, and to identify prognostic biomarkers, there is growing need to more precisely characterize patients that may help explain the high-degree of variability found in FND studies to date (Begue et al., 2019).
A related question not yet addressed is how to also contextualize other prominent physical symptoms in patients with FND, most notably but not limited to fatigue (Aybek et al., 2020; Gelauff et al., 2020). Like pain, fatigue is a common symptom in FND that is also linked to reduced quality of life and reduced treatment engagement (Věchetová et al., 2018). We suggest that the same approach taken for pain can also be used to categorize FND patients with prominent fatigue symptoms. This would include using the specifier ‘with prominent fatigue’ for those individuals that endorse fatigue (>6 months) is limiting their occupational and/or social functioning. Optional secondary specifiers can denote the presence or absence of cognitive-behavioural (psychological) features and the co-occurrence with a contributing comorbidity known to be associated with fatigue (e.g. multiple sclerosis, chronic fatigue syndrome). Lastly, for patients with prominent pain and fatigue, or those exhibiting a combination of two or more non-sensorimotor symptoms (e.g. widespread body pain and gastrointestinal distress), the diagnostic specifier FND ‘with prominent mixed somatic symptoms’ can be recorded (see Fig. 3).
Our systematic review and proposed revised DSM-5 diagnostic criteria for FND have several limitations. Alternatives approaches such as systematically reviewing the frequency and relevance of pain, fatigue and other somatic symptoms in FND more broadly were not performed. Such efforts in the future could further inform the preliminary DSM-5 revisions proposed here (e.g. 6-month duration was chosen to be consistent with the symptom duration requirement for SSD, however, other durations could be considered). It is also important to note that many of the observations identified in DSM-IV-SD, such as increased rates of mood and anxiety disorders, alexithymia, dissociation and adverse life events, are non-specific features found in a range of other neuropsychiatric disorders. However, our proposed DSM-5 FND diagnostic criteria revisions will help catalyse new research in FND with prominent pain compared to a range of healthy, medical and neuropsychiatric populations, which will allow for rigorous investigations of the relevance and specificity of clinical and neurobiological associations in this subgroup. In addition, we want to explicitly note that we are not advocating for a return of the term ‘SD’, which is fraught with limitations including a dualistic mind-brain framing and an arbitrary selection of symptom clusters—must precarious of which is the sexual symptoms cluster. Another question is the potential benefit, or lack thereof, of revising the diagnostic criteria for FND within the DSM-5 framework. While we recognize that psychological factors may not universally play important predisposing and/or perpetuating roles in all patients, for many patients cognitive-behavioural (psychological) factors are relevant—particularly for developing patient-centred treatment plans. As such, it is important to continue to actively engage psychiatrists, psychologists and allied mental health professionals as equal partners alongside neurologists and allied rehabilitation specialists in FND-related clinical and research activities. It will be important, however, for the FND field to achieve consensus across neurologic and psychiatric perspectives, and we hope our etiologically neutral specifier ‘with prominent pain’ assists in these efforts. We also welcome our proposal being considered in future diagnostic criteria considerations by the FND Society. Lastly, we acknowledge that this article does not address the question of how to contextualize patients without sensorimotor FND that experience other distressing physical symptoms (with normal neurological examinations and negative medical evaluations). While there are a range of potential diagnostic classification systems being actively debated (each with their own strengths and weaknesses) (Burton et al., 2020), it will be important to bring together leaders (and patients) across the FND and functional somatic disorder fields to obtain consensus across the various stakeholders.
In conclusion, the intersection of FND with other prominent somatic symptoms, most notably pain, is clinically and prognostically relevant. The changes made from DSM-IV to DSM-5 eliminated DSM-IV-SD that encompassed individuals with functional neurological symptoms and other prominent somatic symptoms. We propose a preliminary revision to the DSM-5 diagnostic criteria for FND that adds an etiologically neutral specifier noting the presence of other prominent non-sensorimotor physical symptoms. While prospective research studies are needed to validate our proposal, we hope that this article catalyses discussion on how to optimally contextualize pain, fatigue and other physical symptoms in patients with FND across diagnostic, treatment and pathophysiology studies.
Supplementary Material
Acknowledgements
We thank the reviewers for their helpful feedback on an earlier version of this manuscript, including Professor Jon Stone who disclosed his identity.
Funding
D.L.P. and J.M. were funded by the Sidney R. Baer Jr Foundation. D.L.P. also received funding from the National Institute of Mental Health K23MH111983-04 grant and the Massachusetts General Hospital Physician-Scientist Career Development Program. S.A. was funded by the Swiss National Research Foundation (PP00P3_176985).
Supplementary material
Supplementary material is available at Brain Communications online.
Competing interests
D.L.P. has received honoraria for continuing medication education lectures in functional neurological disorder. All other authors do not report any disclosures or conflicts of interest.
Glossary
- CBT =
Cognitive behavioural therapy
- DSM-5 =
Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition
- DSM-IV =
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- DSM-IV-SD =
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Somatization disorder
- FND =
functional neurological disorder
- HC =
healthy control
- IBS =
irritable bowel syndrome
- ICD-10 =
International Classification of Diseases, Tenth Edition
- MDD =
major depressive disorder
- PCI =
psychiatric consultation intervention
- PTSD =
post-traumatic stress disorder
- SD =
somatization disorder
- SSD =
somatic symptom disorder
Contributor Information
Julie Maggio, Functional Neurology Research Group, Cognitive Behavioral Neurology Unit, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Department of Physical Therapy, Massachusetts General Hospital, Boston, MA, USA.
Priyanka R Alluri, Functional Neurology Research Group, Cognitive Behavioral Neurology Unit, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Sara Paredes-Echeverri, Functional Neurology Research Group, Cognitive Behavioral Neurology Unit, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Anna G Larson, Functional Neurology Research Group, Cognitive Behavioral Neurology Unit, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Petr Sojka, Department of Psychiatry, University Hospital Brno, Czech Republic.
Bruce H Price, Functional Neurology Research Group, Cognitive Behavioral Neurology Unit, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Department of Neurology, McLean Hospital, Belmont, MA, USA.
Selma Aybek, Department of Neurology, Inselspital University Hospital Bern and Clinical Neurosciences Bern, Bern University, Switzerland.
David L Perez, Functional Neurology Research Group, Cognitive Behavioral Neurology Unit, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Division of Neuropsychiatry, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
References
- Allen LA, Woolfolk RL, Escobar JI, Gara MA, Hamer RM. Cognitive-behavioral therapy for somatization disorder: a randomized controlled trial. Arch Intern Med 2006; 166: 1512–8. [DOI] [PubMed] [Google Scholar]
- Allen LA, Woolfolk RL, Lehrer PM, Gara MA, Escobar JI. Cognitive behavior therapy for somatization disorder: A preliminary investigation. J Behav Ther Exp Psychiatry 2001; 32: 53–62. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edn., Text Revision (DSM-IV-TR); 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th edn., 2013.
- Aybek S, Lidstone SC, Nielsen G, MacGillivray L, Bassetti CL, Lang AE, et al. What is the role of a specialist assessment clinic for FND? Lessons from three national referral centers. J Neuropsychiatry Clin Neurosci 2020; 32: 79–84. [DOI] [PubMed] [Google Scholar]
- Baslet G, Bajestan S, Aybek S, Modirrousta M, Prince J, Cavanna A, et al. Evidence-based practice of the clinical assessment of psychogenic nonepileptic seizures (PNES): a report from the American Neuropsychiatric Association Committee on Research. J Neuropsychiatry Clin Neurosci 2020. [DOI] [PubMed]
- Battaglia M, Bertella S, Bajo S, Politi E, Bellodi L. An investigation of the co-occurrence of panic and somatization disorders through temperamental variables. Psychosom Med 1998; 60: 726–9. [DOI] [PubMed] [Google Scholar]
- Begue I, Adams C, Stone J, Perez DL. Structural alterations in functional neurological disorder and related conditions: a software and hardware problem? NeuroImage Clin 2019; 22: 101798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleichhardt G, Timmer B, Rief W. Cognitive-behavioural therapy for patients with multiple somatoform symptoms—a randomised controlled trial in tertiary care. J Psychosom Res 2004; 56: 449–54. [DOI] [PubMed] [Google Scholar]
- Briquet P, Traité clinique et therapeutique de l'hystérie. Paris: J.B. Bailliere et fils; 1859. [Google Scholar]
- Brown RC, Berenz EC, Aggen SH, Gardner CO, Knudsen GP, Reichborn-Kjennerud T, et al. Trauma exposure and Axis I psychopathology: A cotwin control analysis in Norwegian young adults. Psychol Trauma 2014; 6: 652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RJ, Schrag A, Trimble MR. Dissociation, childhood interpersonal trauma, and family functioning in patients with somatization disorder. Am J Psychiatry 2005; 162: 899–905. [DOI] [PubMed] [Google Scholar]
- Burton C, Fink P, Henningsen P, Lowe B, Rief W, Group E-S. Functional somatic disorders: discussion paper for a new common classification for research and clinical use. BMC Med 2020; 18: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey PD, Stein DJ, Zungu-Dirwayi N, Seedat S. Trauma and posttraumatic stress disorder in an urban Xhosa primary care population: prevalence, comorbidity, and service use patterns. J Nerv Ment Dis 2003; 191: 230–6. [DOI] [PubMed] [Google Scholar]
- Carson AJ, Stone J, Hansen CH, Duncan R, Cavanagh J, Matthews K, et al. Somatic symptom count scores do not identify patients with symptoms unexplained by disease: a prospective cohort study of neurology outpatients. J Neurol Neurosurg Psychiatry 2015; 86: 295–301. [DOI] [PubMed] [Google Scholar]
- Chander KR, Manjunatha N, Binukumar B, Kumar CN, Math SB, Reddy YCJ. The prevalence and its correlates of somatization disorder at a quaternary mental health centre. Asian J Psychiatry 2019; 42: 24–7. [DOI] [PubMed] [Google Scholar]
- Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry 2014; 85: 180–90. [DOI] [PubMed] [Google Scholar]
- Davoodi E, Wen A, Dobson KS, Noorbala AA, Mohammadi A, Farahmand Z. Early maladaptive schemas in depression and somatization disorder. J Affect Disord 2018; 235: 82–9. [DOI] [PubMed] [Google Scholar]
- Davoodi E, Wen A, Dobson KS, Noorbala AA, Mohammadi A, Farahmand Z. Emotion regulation strategies in depression and somatization disorder. Psychol Rep 2019; 122: 2119–36. [DOI] [PubMed] [Google Scholar]
- Denk F, McMahon SB, Tracey I. Pain vulnerability: a neurobiological perspective. Nat Neurosci 2014; 17: 192–200. [DOI] [PubMed] [Google Scholar]
- Diez I, Ortiz-Teran L, Williams B, Jalilianhasanpour R, Ospina JP, Dickerson BC, et al. Corticolimbic fast-tracking: enhanced multimodal integration in functional neurological disorder. J Neurol Neurosurg Psychiatry 2019; 90: 929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimsdale JE, Creed F, Escobar J, Sharpe M, Wulsin L, Barsky A, et al. Somatic symptom disorder: an important change in DSM. J Psychosom Res 2013; 75: 223–8. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Adams RA, Brown H, Parees I, Friston KJ. A Bayesian account of ‘hysteria’. Brain 2012; 135: 3495–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein SA, Maurer CW, LaFaver K, Ameli R, Sinclair S, Hallett M. Insights into chronic functional movement disorders: the value of qualitative psychiatric interviews. Psychosomatics 2016; 57: 566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar JI, Gara M, Waitzkin H, Silver RC, Holman A, Compton W. DSM-IV hypochondriasis in primary care. Gen Hosp Psychiatry 1998; 20: 155–9. [DOI] [PubMed] [Google Scholar]
- Espirito-Santo H, Pio-Abreu JL. Psychiatric symptoms and dissociation in conversion, somatization and dissociative disorders. Aust N Z J Psychiatry 2009; 43: 270–6. [DOI] [PubMed] [Google Scholar]
- Fayed N, Andres E, Rojas G, Moreno S, Serrano-Blanco A, Roca M, et al. Brain dysfunction in fibromyalgia and somatization disorder using proton magnetic resonance spectroscopy: a controlled study. Acta Psychiatr Scand 2012; 126: 115–25. [DOI] [PubMed] [Google Scholar]
- Fink P, Hansen MS, Oxhøj ML. The prevalence of somatoform disorders among internal medical inpatients. J Psychosom Res 2004; 56: 413–8. [DOI] [PubMed] [Google Scholar]
- Fink P, Hansen MS, Søndergaard L. Somatoform disorders among first-time referrals to a neurology service. Psychosomatics 2005; 46: 540–8. [DOI] [PubMed] [Google Scholar]
- Fjorback LO, Arendt M, Ornbol E, Walach H, Rehfeld E, Schroder A, et al. Mindfulness therapy for somatization disorder and functional somatic syndromes: randomized trial with one-year follow-up. J Psychosom Res 2013; 74: 31–40. [DOI] [PubMed] [Google Scholar]
- Frostholm L, Petrie KJ, Ørnbøl E, Fink P. Are illness perceptions related to future healthcare expenditure in patients with somatoform disorders? Psychol Med 2014; 44: 2903–11. [DOI] [PubMed] [Google Scholar]
- Garcia-Campayo J, Alda M, Sobradiel N, Olivan B, Pascual A. Personality disorders in somatization disorder patients: a controlled study in Spain. J Psychosom Res 2007; 62: 675–80. [DOI] [PubMed] [Google Scholar]
- Garcia Campayo J, Pascual López A, Almárcegui Lafita C, Morales Bara I, Dolz Zaera I, De Vicente Álvarez-Manzaneda E. P300 endogen evoked potentials in somatization disorder: a controlled study. Actas Esp Psiquiatr 2007; 35: 52–8. [PubMed] [Google Scholar]
- Garcia-Campayo J, Sanz-Carrillo C, Baringo T, Ceballos C. SPECT scan in somatization disorder patients: an exploratory study of eleven cases. Aust N Z J Psychiatry 2001; 35: 359–63. [DOI] [PubMed] [Google Scholar]
- Gazzola DM, Carlson C, Rugino A, Hirsch S, Starner K, Devinsky O. Psychogenic nonepileptic seizures and chronic pain: a retrospective case-controlled study. Epilepsy Behav 2012; 25: 662–5. [DOI] [PubMed] [Google Scholar]
- Gelauff JM, Carson A, Ludwig L, Tijssen MAJ, Stone J. The prognosis of functional limb weakness: a 14-year case-control study. Brain 2019; 142: 2137–48. [DOI] [PubMed] [Google Scholar]
- Gelauff JM, Rosmalen JGM, Gardien J, Stone J, Tijssen MAJ. Shared demographics and comorbidities in different functional motor disorders. Parkinsonism Relat Disord 2020; 70: 1–6. [DOI] [PubMed] [Google Scholar]
- Glass SP, Matin N, Williams B, Mello J, Stephen CD, Young SS, et al. Neuropsychiatric factors linked to adherence and short-term outcome in a U.S. functional neurological disorders clinic: a retrospective cohort study. J Neuropsychiatry Clin Neurosci 2018; 30: 152–9. [DOI] [PubMed] [Google Scholar]
- Guo W, Liu F, Chen J, Wu R, Li L, Zhang Z, et al. Anatomical distance affects cortical–subcortical connectivity in first-episode, drug-naive somatization disorder. J Affect Disord 2017; 217: 153–8. [DOI] [PubMed] [Google Scholar]
- Guz H, Doganay Z, Ozkan A, Colak E, Tomac A, Sarisoy G. Conversion and somatization disorders: dissociative symptoms and other characteristics. J Psychosom Res 2004; 56: 287–91. [DOI] [PubMed] [Google Scholar]
- Hiller W, Fichter MM, Rief W. A controlled treatment study of somatoform disorders including analysis of healthcare utilization and cost-effectiveness. J Psychosom Res 2003; 54: 369–80. [DOI] [PubMed] [Google Scholar]
- Hiller W, Heuser J, Fichter MM. The DSM-IV nosology of chronic pain: a comparison of pain disorder and multiple somatization syndrome. Eur J Pain 2000; 4: 45–55. [DOI] [PubMed] [Google Scholar]
- Hossain I, Islam SN, Khan Md NI, Islam S, Hasnat A. Serum level of copper, zinc and manganese in somatization disorder patients. German J Psychiatry 2007. a; 10: 41–5. [Google Scholar]
- Hossain I, Sadat N, Hossain K, Qusar S, Islam SN, Islam S, et al. Serum immunoglobulin profile in somatization disorder patients. German J Psychiatry 2007. b; 10: 13–7. [Google Scholar]
- Ibrahim NM, Martino D, van de Warrenburg BP, Quinn NP, Bhatia KP, Brown RJ, et al. The prognosis of fixed dystonia: a follow-up study. Parkinsonism Relat Disord 2009; 15: 592–7. [DOI] [PubMed] [Google Scholar]
- Interian A, Gara MA, Diaz-Martinez AM, Warman MJ, Escobar JI, Allen LA, et al. The value of pseudoneurological symptoms for assessing psychopathology in primary care. Psychosom Med 2004; 66: 141–6. [DOI] [PubMed] [Google Scholar]
- Jimenez XF, Aboussouan A, Johnson J. Functional neurological disorder responds favorably to interdisciplinary rehabilitation models. Psychosomatics 2019; 60: 556–62. [DOI] [PubMed] [Google Scholar]
- Kırpınar I, Deveci E, Kılıç A, Camur D. Somatization disorder and hypochondriasis: as like as two peas? Anadolu Psikiyatri Derg 2016; 17: 165. [Google Scholar]
- Kozlowska K, Nunn KP, Rose D, Morris A, Ouvrier RA, Varghese J. Conversion disorder in Australian pediatric practice. J Am Acad Child Adolesc Psychiatry 2007; 46: 68–75. [DOI] [PubMed] [Google Scholar]
- Kuwabara H, Otsuka M, Shindo M, Ono S, Shioiri T, Someya T. Diagnostic classification and demographic features in 283 patients with somatoform disorder. Psychiatry Clin Neurosci 2007; 61: 283–9. [DOI] [PubMed] [Google Scholar]
- Landa A, Bossis AP, Boylan LS, Wong PS. Beyond the unexplainable pain: relational world of patients with somatization syndromes. J Nerv Ment Dis 2012; 200: 413–22. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Jonas C, Pohontsch NJ, Zimmermann T, Scherer M, Lowe B. General practitioners' views on the diagnostic innovations in DSM-5 somatic symptom disorder—a focus group study. J Psychosom Res 2019; 123: 109734. [DOI] [PubMed] [Google Scholar]
- Li R, Liu F, Su Q, Zhang Z, Zhao J, Wang Y, et al. Bidirectional causal connectivity in the cortico-limbic-cerebellar circuit related to structural alterations in first-episode, drug-naive somatization disorder. Front Psychiatry 2018; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig L, Pasman JA, Nicholson T, Aybek S, David AS, Tuck S, et al. Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta-analysis of case-control studies. Lancet Psychiatry 2018; 5: 307–20. [DOI] [PubMed] [Google Scholar]
- Lynch DJ, McGrady A, Nagel R, Zsembik C. Somatization in family practice: comparing 5 methods of classification. Prim Care Companion J Clin Psychiatry 1999; 1: 85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai FM, Merskey H. Briquet's Treatise on hysteria. A synopsis and commentary. Arch Gen Psychiatry 1980; 37: 1401–5. [DOI] [PubMed] [Google Scholar]
- Mai FM, Merskey H. Briquet's concept of hysteria: an historical perspective. Can J Psychiatry 1981; 26: 57–63. [DOI] [PubMed] [Google Scholar]
- Manchikanti L, Cash KA, Pampati V, Fellows B. Influence of psychological variables on the diagnosis of facet joint involvement in chronic spinal pain. Pain Physician 2008; 11: 145–60. [PubMed] [Google Scholar]
- Manchikanti L, Giordano J, Boswell MV, Fellows B, Manchukonda R, Pampati V. Psychological factors as predictors of opioid abuse and illicit drug use in chronic pain patients. J Opioid Manag 2007; 3: 89–100. [DOI] [PubMed] [Google Scholar]
- Marchetti RL, Kurcgant D, Neto JG, von Bismark MA, Marchetti LB, Fiore LA. Psychiatric diagnoses of patients with psychogenic non-epileptic seizures. Seizure 2008; 17: 247–53. [DOI] [PubMed] [Google Scholar]
- Martin A, Rauh E, Fichter M, Rief W. A one-session treatment for patients suffering from medically unexplained symptoms in primary care: a randomized clinical trial. Psychosomatics 2007; 48: 294–303. [DOI] [PubMed] [Google Scholar]
- Mayou R, Kirmayer LJ, Simon G, Kroenke K, Sharpe M. Somatoform disorders: time for a new approach in DSM-V. Am J Psychiatry 2005; 162: 847–55. [DOI] [PubMed] [Google Scholar]
- Miller AR, North CS, Clouse RE, Wetzel RD, Spitznagel EL, Alpers DH. The association of irritable bowel syndrome and somatization disorder. Ann of Clin Psychiatry 2001; 13: 25–30. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohlman J, de Jesus M, Gorenstein EE, Kleber M, Gorman JM, Papp LA. Distinguishing generalized anxiety disorder, panic disorder, and mixed anxiety states in older treatment-seeking adults. J Anxiety Disord 2004; 18: 275–90. [DOI] [PubMed] [Google Scholar]
- Morse DS, Suchman AL, Frankel RM. The meaning of symptoms in 10 women with somatization disorder and a history of childhood abuse. Arch Fam Med 1997; 6: 468–76. [DOI] [PubMed] [Google Scholar]
- Myers L, Lancman M, Laban-Grant O, Matzner B, Lancman M. Psychogenic non-epileptic seizures: predisposing factors to diminished quality of life. Epilepsy Behav 2012; 25: 358–62. [DOI] [PubMed] [Google Scholar]
- Nielsen G, Buszewicz M, Stevenson F, Hunter R, Holt K, Dudziec M, et al. Randomised feasibility study of physiotherapy for patients with functional motor symptoms. J Neurol Neurosurg Psychiatry 2017; 88: 484–90. [DOI] [PubMed] [Google Scholar]
- NIH: National Heart Lung and Blood Institute. Study quality assessment tools. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- North CS, Downs D, Clouse RE, Alrakawi A, Dokucu ME, Cox J, et al. The presentation of irritable bowel syndrome in the context of somatization disorder. Clin Gastroenterol Hepatol 2004; 2: 787–95. [DOI] [PubMed] [Google Scholar]
- Ou Y, Liu F, Chen J, Pan P, Wu R, Su Q, et al. Increased coherence-based regional homogeneity in resting-state patients with first-episode, drug-naive somatization disorder. J Affect Disord 2018; 235: 150–4. [DOI] [PubMed] [Google Scholar]
- Ozturk E, Sar V. Somatization as a predictor of suicidal ideation in dissociative disorders. Psychiatry Clin Neurosci 2008; 62: 662–8. [DOI] [PubMed] [Google Scholar]
- Padhy SK, Mishra S, Sarkar S, Bang LG, Panigrahi M. Comparison of psychiatric morbidity in patients with irritable bowel syndrome and non-ulcer dyspepsia. Ind Psychiatry J 2016; 25: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Ou Y, Su Q, Liu F, Chen J, Zhao J, et al. Voxel-based global-brain functional connectivity alterations in first-episode drug-naive patients with somatization disorder. J Affect Disord 2019; 254: 82–9. [DOI] [PubMed] [Google Scholar]
- Perez DL, Aybek S, Popkirov S, Kozlowska K, Stephen CD, Anderson J, et al. A review and expert opinion on the neuropsychiatric assessment of motor functional neurological disorders. J Neuropsychiatry Clin Neurosci 2020. [DOI] [PubMed]
- Perley MJ, Guze SB. Hysteria—the stability and usefulness of clinical criteria. A quantitative study based on a follow-up period of six to eight years in 39 patients. N Engl J Med 1962; 266: 421–6. [DOI] [PubMed] [Google Scholar]
- Pick S, Goldstein LH, Perez DL, Nicholson TR. Emotional processing in functional neurological disorder: a review, biopsychosocial model and research agenda. J Neurol Neurosurg Psychiatry 2019; 90: 704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkirov S, Hoeritzauer I, Colvin L, Carson AJ, Stone J. Complex regional pain syndrome and functional neurological disorders—time for reconciliation. J Neurol Neurosurg Psychiatry 2019; 90: 608–14. [DOI] [PubMed] [Google Scholar]
- Prerana G, Jigar H, Singh S, Shweta C, Singh S, Ibrahim A, et al. Somatization disorder: are we moving towards an over-generalized and overinclusive diagnosis in DSM-V? Acta Med Int 2017; 4: 110–19. [Google Scholar]
- Rief W, Hiller W. A new approach to the assessment of the treatment effects of somatoform disorders. Psychosomatics 2003; 44: 492–8. [DOI] [PubMed] [Google Scholar]
- Rief W, Mewes R, Martin A, Glaesmer H, Brähler E. Evaluating new proposals for the psychiatric classification of patients with multiple somatic symptoms. Psychosom Med 2011; 73: 760–8. [DOI] [PubMed] [Google Scholar]
- Rief W, Pilger F, Ihle D, Bosmans E, Egyed B, Maes M. Immunological differences between patients with major depression and somatization syndrome. Psychiatry Res 2001; 105: 165–74. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Chattopadhyay PK, Biswas D. Electro-dermal arousal and self-appraisal in patients with somatization disorder. Indian J Clin Psychology 1998; 25: 144–8. [Google Scholar]
- Scamvougeras A, Howard A. Somatic symptom disorder, medically unexplained symptoms, somatoform disorders, functional neurological disorder: how DSM-5 got it wrong. Can J Psychiatry 2020; 65: 301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag A, Trimble M, Quinn N, Bhatia K. The syndrome of fixed dystonia: an evaluation of 103 patients. Brain 2004; 127: 2360–72. [DOI] [PubMed] [Google Scholar]
- Schröder A, Heider J, Zaby A, Göllner R. Cognitive Behavioral therapy versus progressive muscle relaxation training for multiple somatoform symptoms: results of a randomized controlled trial. Cogn Ther Res 2013; 37: 296–306. [Google Scholar]
- Schröder A, Rehfeld E, Ornbøl E, Sharpe M, Licht RW, Fink P. Cognitive-behavioural group treatment for a range of functional somatic syndromes: randomised trial. Br J Psychiatry 2012; 200: 499–507. [DOI] [PubMed] [Google Scholar]
- Sertoz OO, Doganavsargil O, Elbi H. Body image and self-esteem in somatizing patients. Psychiatry Clin Neurosci 2009; 63: 508–15. [DOI] [PubMed] [Google Scholar]
- Simon GE, Gureje O. Stability of somatization disorder and somatization symptoms among primary care patients. Arch Gen Psychiatry 1999; 56: 90–5. [DOI] [PubMed] [Google Scholar]
- Smith RC, Gardiner JC, Lyles JS, Sirbu C, Dwamena FC, Hodges A, et al. Exploration of DSM-IV criteria in primary care patients with medically unexplained symptoms. Psychosom Med 2005; 67: 123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Su Q, Jiang M, Liu F, Yao D, Dai Y, et al. Abnormal regional homogeneity and its correlations with personality in first-episode, treatment-naive somatization disorder. Int J Psychophysiol 2015; 97: 108–12. [DOI] [PubMed] [Google Scholar]
- Spitzer C, Barnow S, Gau K, Freyberger HJ, Grabe HJ. Childhood maltreatment in patients with somatization disorder. Aust N Z J Psychiatry 2008; 42: 335–41. [DOI] [PubMed] [Google Scholar]
- Spitzer C, Barnow S, Wingenfeld K, Rose M, Lowe B, Grabe HJ. Complex post-traumatic stress disorder in patients with somatization disorder. Aust N Z J Psychiatry 2009; 43: 80–6. [DOI] [PubMed] [Google Scholar]
- Stone J, LaFrance WC, Jr, Levenson JL, Sharpe M. Issues for DSM-5: conversion disorder. Am J Psychiatry 2010. a; 167: 626–7. [DOI] [PubMed] [Google Scholar]
- Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain 2010. b; 133: 1537–51. [DOI] [PubMed] [Google Scholar]
- Su Q, Yao D, Jiang M, Liu F, Jiang J, Xu C, et al. Dissociation of regional activity in default mode network in medication-naive, first-episode somatization disorder. PLoS One 2014; 9: e99273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Q, Yao D, Jiang M, Liu F, Jiang J, Xu C, et al. Increased functional connectivity strength of right inferior temporal gyrus in first-episode, drug-naive somatization disorder. Aust N Z J Psychiatry 2015; 49: 74–81. [DOI] [PubMed] [Google Scholar]
- Su Q, Yao D, Jiang M, Liu F, Long L, Dai Y, et al. Decreased interhemispheric functional connectivity in insula and angular gyrus/supramarginal gyrus: significant findings in first-episode, drug-naive somatization disorder. Psychiatry Res 2016; 248: 48–54. [DOI] [PubMed] [Google Scholar]
- Taycan O, Sar V, Celik C, Erdogan-Taycan S. Trauma-related psychiatric comorbidity of somatization disorder among women in eastern Turkey. Compr Psychiatry 2014; 55: 1837–46. [DOI] [PubMed] [Google Scholar]
- van der Feltz-Cornelis CM, Elfeddali I, Werneke U, Malt UF, Van den Bergh O, Schaefert R, et al. A European research agenda for somatic symptom disorders, bodily distress disorders, and functional disorders: results of an estimate-talk-estimate Delphi expert study. Front Psychiatry 2018; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ravesteijn H, Lucassen P, Bor H, van Weel C, Speckens A. Mindfulness-based cognitive therapy for patients with medically unexplained symptoms: a randomized controlled trial. Psychother Psychosom 2013; 82: 299–310. [DOI] [PubMed] [Google Scholar]
- Věchetová G, Slovák M, Kemlink D, Hanzlíková Z, Dušek P, Nikolai T, et al. The impact of non-motor symptoms on the health-related quality of life in patients with functional movement disorders. J Psychosom Res 2018; 115: 32–7. [DOI] [PubMed] [Google Scholar]
- Voon V, Lang AE. Antidepressant treatment outcomes of psychogenic movement disorder. J Clin Psychiatry 2005; 66: 1529–34. [DOI] [PubMed] [Google Scholar]
- Wang H, Guo W, Liu F, Chen J, Wu R, Zhang Z, et al. Clinical significance of increased cerebellar default-mode network connectivity in resting-state patients with drug-naive somatization disorder. Medicine (Baltimore) 2016; 95: e4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Su Q, Jiang M, Liu F, Yao D, Dai Y, et al. Abnormal default-mode network homogeneity and its correlations with personality in drug-naive somatization disorder at rest. J Affect Disord 2016; 193: 81–8. [DOI] [PubMed] [Google Scholar]
- Weiss FD, Rief W, Kleinstauber M. Health care utilization in outpatients with somatoform disorders: descriptives, interdiagnostic differences, and potential mediating factors. Gen Hosp Psychiatry 2017; 44: 22–9. [DOI] [PubMed] [Google Scholar]
- Zonneveld LN, van Rood YR, Timman R, Kooiman CG, Van't Spijker A, Busschbach JJ. Effective group training for patients with unexplained physical symptoms: a randomized controlled trial with a non-randomized one-year follow-up. PLoS One 2012; 7: e42629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.