In the title pyridazinone derivative, the chlorophenyl and pyridazinone rings being almost perpendicular, while the phenyl ring of the styryl group is coplanar with the pyridazinone ring. In the crystal, N—H⋯O hydrogen bonds form inversion dimers with an  (8) ring motif and C—H⋯Cl hydrogen bonds also occur.
(8) ring motif and C—H⋯Cl hydrogen bonds also occur.
Keywords: crystal structure, Hirshfeld surface analysis, pyridazine derivative, pyridazinone
Abstract
The title pyridazinone derivative, C19H14Cl2N2O, an important pharmacophore with a wide variety of biological applications is not planar, the chlorophenyl and pyridazinone rings being almost perpendicular, subtending a dihedral angle of 85.73 (11)°. The phenyl ring of the styryl group is coplanar with the pyridazinone ring [1.47 (12)°]. In the crystal, N—H⋯O hydrogen bonds form inversion dimers with an R 2 2(8) ring motif and C—H⋯Cl hydrogen bonds also occur. The roles of the intermolecular interactions in the crystal packing were clarified using Hirshfeld surface analysis, and two-dimensional fingerprint plots indicate that the most important contributions to the crystal packing are from H⋯H (37.9%), C⋯H/H⋯C (18.7%), Cl⋯H/ H⋯Cl (16.4%) and Cl⋯C/C⋯Cl (6.7%) contacts.
Chemical context
Pyridazines are an important family of six-membered aromatic heterocycles containing two nitrogen atoms. Pyridazinone is an important pharmacophore possessing a wide range of biological activities including antitumor (Bouchmaa et al., 2018 ▸, 2019 ▸), anti-inflammatory (Boukharsa et al., 2018 ▸), antihypertensive (Siddiqui et al., 2011 ▸), antidepressant (Boukharsa et al., 2016 ▸), anti-HIV (Livermore et al., 1993 ▸), antihistaminic (Tao et al. 2012 ▸), analgesic (Gökçe et al., 2009 ▸) and anticonvulsant (Partap et al., 2018 ▸) and is used in glucan synthase inhibitors (Zhou et al., 2011 ▸) and herbicidal agents (Asif et al., 2013 ▸). The chemistry of pyridazinones has been an interesting field of study for decades and this nitrogen heterocycle has become a scaffold of choice for the development of potential drug candidates (Dubey et al., 2015 ▸; Thakur et al., 2010 ▸).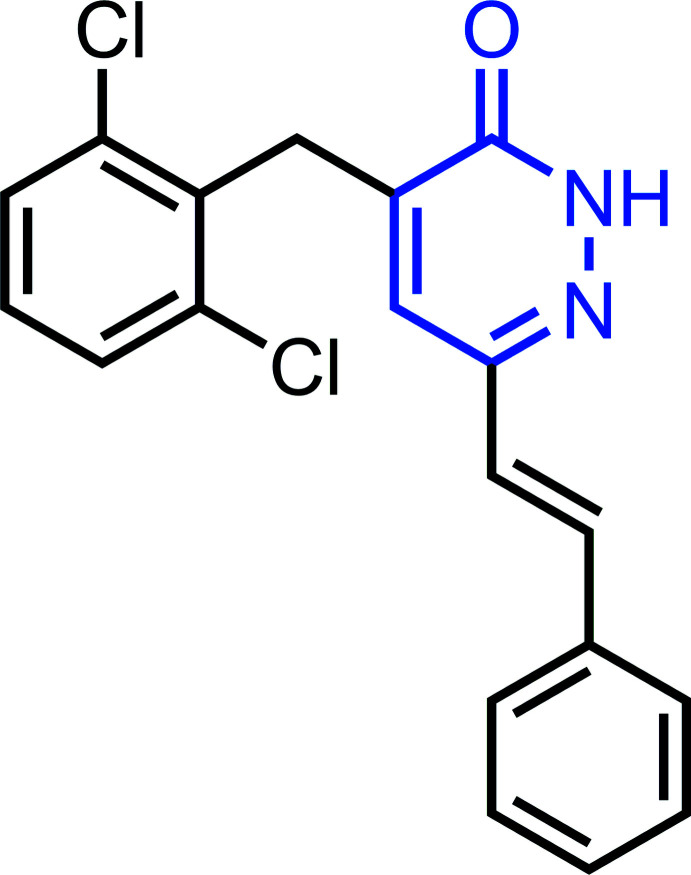 In a continuation of our studies towards the synthesis, molecular structures, Hirshfeld surfaces analysis and DFT studies of new pyridazin-3(2H)-one derivatives (Daoui et al., 2020 ▸, 2021 ▸; El Kalai et al., 2021 ▸), we report herein the crystal structure and Hirshfeld surface analysis of 4-(2,6-dichlorobenzyl)-6-[(E)-2-phenylethenyl]pyridazin-3(2H)-one.
In a continuation of our studies towards the synthesis, molecular structures, Hirshfeld surfaces analysis and DFT studies of new pyridazin-3(2H)-one derivatives (Daoui et al., 2020 ▸, 2021 ▸; El Kalai et al., 2021 ▸), we report herein the crystal structure and Hirshfeld surface analysis of 4-(2,6-dichlorobenzyl)-6-[(E)-2-phenylethenyl]pyridazin-3(2H)-one.
Structural commentary
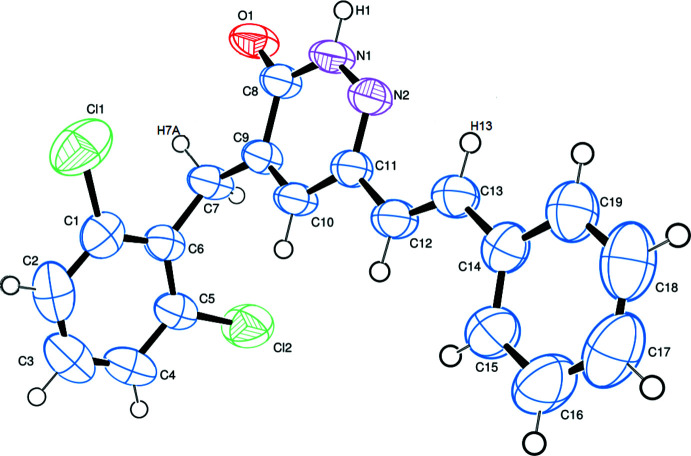
The molecular structure of the title compound is shown in Fig. 1 ▸. The C1–C6 phenyl ring and the pyridazinone ring (N1/N2/C8–C11) are almost perpendicular, subtending a dihedral angle of 85.73 (11)°. The C14–C19 phenyl ring of the styryl group is coplanar with the pyridazinone ring [1.47 (12)°]. The carbonyl group has a C8=O1 bond length of 1.236 (2) Å, and the C8—N1 and C11—N2 bond lengths in the pyridazine ring are 1.357 (3) and 1.305 (2) Å, respectively. The N1—N2 bond length is 1.344 (2) Å.
Figure 1.
The molecular structure of the title compound, with the atom labelling. Displacement ellipsoids are drawn at the 50% probability level.
Supramolecular features
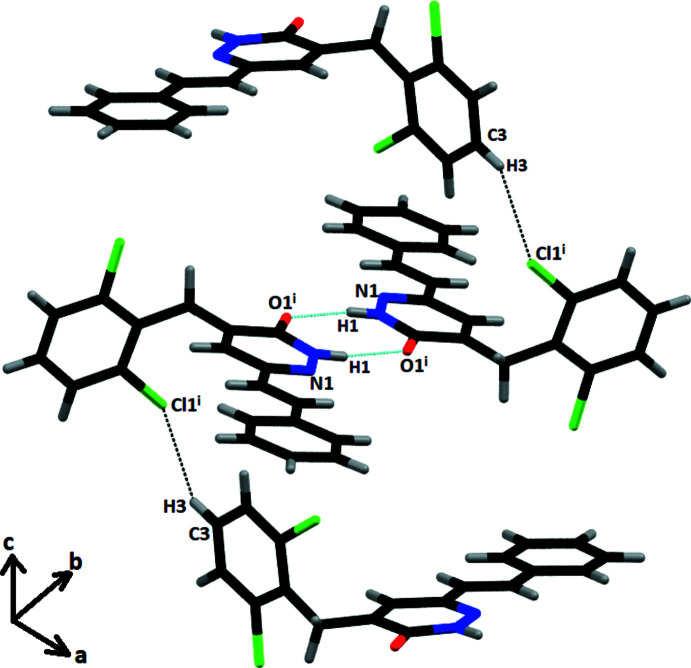
In the crystal, pairs of N—H⋯O hydrogen bonds form inversion dimers with an 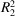 (8) ring motif (Table 1 ▸, Fig. 2 ▸). C3—H3⋯Cl1 hydrogen bonds are also observed. C—H⋯π interactions between the
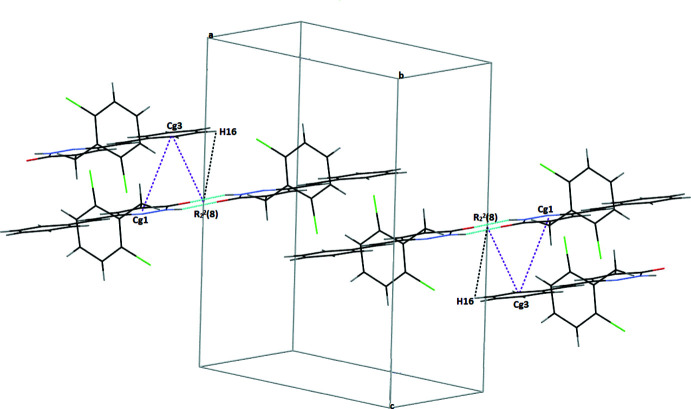
(8) ring motif (Table 1 ▸, Fig. 2 ▸). C3—H3⋯Cl1 hydrogen bonds are also observed. C—H⋯π interactions between the 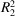 (8) dimer rings and H16 atoms [centroid-to-centroid distance of 3.501 (9) Å; length between dimer ring and C14–C19 ring = 3.569 (12) Å] also occur (Fig. 3 ▸). π–π interactions also occur with a centroid–centroid distance Cg1⋯Cg3(−x + 1, −y + 2, −z + 1) of 3.9107 (15) Å where Cg1 and Cg3 are the centroids of the N1/N2/C8–C11 and C14–C19 rings, respectively (Fig. 3 ▸).
(8) dimer rings and H16 atoms [centroid-to-centroid distance of 3.501 (9) Å; length between dimer ring and C14–C19 ring = 3.569 (12) Å] also occur (Fig. 3 ▸). π–π interactions also occur with a centroid–centroid distance Cg1⋯Cg3(−x + 1, −y + 2, −z + 1) of 3.9107 (15) Å where Cg1 and Cg3 are the centroids of the N1/N2/C8–C11 and C14–C19 rings, respectively (Fig. 3 ▸).
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1⋯O1i | 0.86 | 1.92 | 2.772 (2) | 171 |
| C3—H3⋯Cl1ii | 0.93 | 2.97 | 3.824 (3) | 153 |
| C7—H7A⋯O1 | 0.97 | 2.42 | 2.803 (2) | 103 |
| C13—H13⋯N2 | 0.93 | 2.51 | 2.845 (3) | 101 |
Symmetry codes: (i) 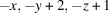 ; (ii)
; (ii) 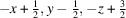 .
.
Figure 2.
View of the crystal structure of the title compound. N—H⋯O hydrogen bonds are represented by red dashed lines and C—H⋯N and C—H⋯O interactions are shown as blue dashed lines.
Figure 3.
Packing diagram showing the intermolecular interactions in the title compound (C—H⋯π interactions shown as black dashed lines and π–π interactions as purple dashed lines).
Database survey
A survey of the Cambridge Structural Database (CSD version 5.41, update of March 2020; Groom et al., 2016 ▸) reveals six comparable pyridazine derivatives, 1-(6-benzoyl-2-phenyl-2,3-dihydropyridazin-4-yl)ethanone 1-(4-benzoyl-2-phenyl-2,3-dihydropyridazin-6-yl)ethanone (AQIKOB; Al-Awadi et al., 2011 ▸), 4-(2′-chloro-6′-fluorophenyl)-2,5-dioxo-8-phenyl-1,2,3,4,5,6-hexahydropyrido(2,3-d)pyridazine (BARQOA; Pita et al., 2000 ▸), 4-[(2,6-dichlorophenyl)methyl]-6-phenylpyridazin-3(2H)-one (BOBXEY; El Kali, Kansiz et al., 2019 ▸), ethyl {5-[(3-chlorophenyl)methyl]-6-oxo-3-phenylpyridazin-1(6H)-yl}acetate (FODQUN; El Kalai, Baydere et al., 2019 ▸), 4-benzyl-2-[2-(4-fluorophenyl)-2-oxoethyl]-6-phenylpyridazin-3(2H)-one (NOLDUQ; Daoui et al., 2019 ▸) and 4-benzyl-6-p-tolylpyridazin-3(2H)-one (YOTVIN; Oubair et al., 2009 ▸). Of these, BOBXEY, (II), is very similar to the title compound. The phenyl ring and the pyridazine ring are twisted with respect to each other, making a dihedral angle of 21.76 (18)° and the phenyl ring (C1–C6) of the benzyl group is inclined to the pyridazine ring by 79.61 (19)°. Relevant bond lengths in (II) are C17=O1 = 1.229 (5), C17—N2 = 1.388 (5) Å and C10—N1 =1.299 (4) Å. The N1—N2 bond lengths in (I) and (II) are virtually the same, with values of 1.348 (2) and 1.353 (4) Å, respectively. In the structure of YOTVIN, N—H⋯O bonds are also observed.
Hirshfeld surface analysis
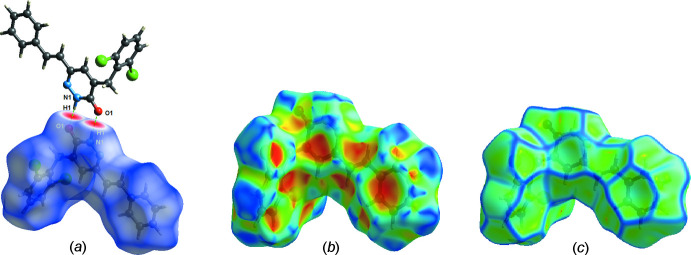
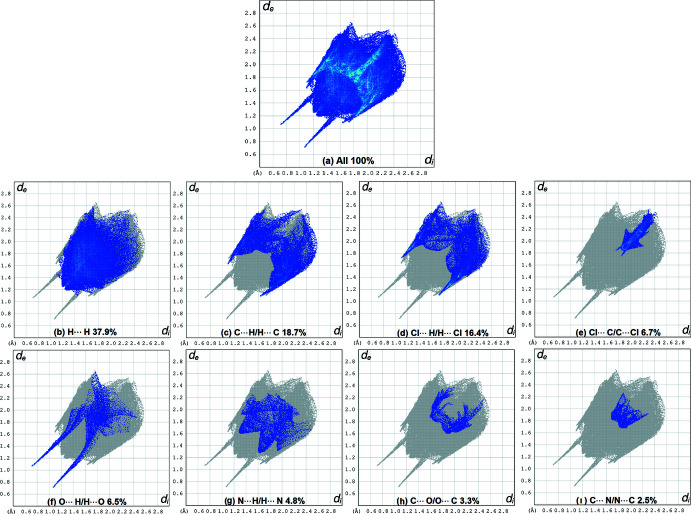
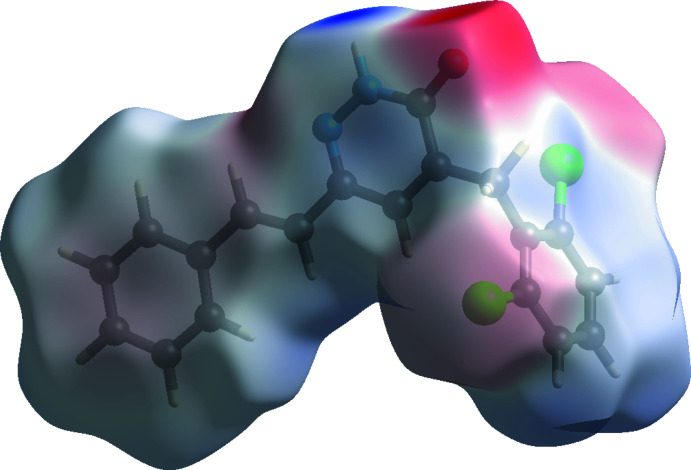
A Hirshfeld surface (HS) study of the title compound was undertaken using CrystalExplorer17.5 (Turner et al., 2017 ▸) to visualize and study the intermolecular contacts. The d norm surface of the title compound is illustrated in Fig. 4 ▸ a. The shape-index, a tool for visualizing π–π stacking interactions by the presence of adjacent red and blue triangles is given in Fig. 4 ▸ b while Fig. 4 ▸ c shows the curvedness map of the title compound. The absence of prominent red and blue triangles in the shape-index map, as well as the absence of large green regions in the curvedness map, confirms that π–π and C—H⋯π interactions are weak. Fig. 5 ▸ shows fingerprint plots that quantitatively summarize the nature and type of intermolecular contacts. The highest contribution to the Hirshfeld surface is from H⋯H contacts (Fig. 5 ▸ b). Other interactions and their respective contributions are C⋯H/H⋯C (18.7%), Cl⋯H/H⋯Cl (16.4%), Cl⋯C/C⋯Cl (6.7%), O⋯H/H⋯O (6.5%), N⋯H/H⋯N (4.8%), C⋯O/O⋯C (3.3%) and C⋯N/N⋯C (2.5%). The acceptor and donor atoms participating in the hydrogen bond appear as blue (donors) and red regions (acceptors) corresponding to positive and negative potential, respectively, in the HS mapped over the electrostatic potential, in the range −0.099–0.165 a.u., as shown in Fig. 6 ▸.
Figure 4.
(a) Hirshfeld surface mapped over d norm for visualizing the intermolecular interactions of the title compound, (b) shape-index map and (c) curvedness map of the title molecule.
Figure 5.
Two-dimensional fingerprint plots for the title compound showing the relative contributions of the atom pairs to the Hirshfeld surface.
Figure 6.
A view of the three-dimensional Hirshfeld surface of the title compound plotted over electrostatic potential.
Synthesis and crystallization
To a solution of (E)-6-styryl-4,5-dihydropyridazin-3(2H)-one (0.2 g, 1 mmol) and 2,6-dichlorobenzaldehyde (0.175 g, 1 mmol) in 30 ml of ethanol, sodium ethanoate (0.23 g, 2.8 mmol) was added. The mixture was refluxed for 3 h. The reaction mixture was cooled, diluted with cold water and acidified with concentrated hydrochloric acid. The precipitate was filtered, washed with water, dried and recrystallized from ethanol. Colourless single-crystals were obtained by slow evaporation at room temperature.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. C-bound H atoms were positioned geometrically with C—H distances of 0.93–0.97 Å and refined as riding, with U iso(H) = 1.2U eq(C). The N-bound H atom was located in a difference-Fourier map and refined with N—H = 0.86 Å.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C19H14Cl2N2O |
| M r | 357.22 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 296 |
| a, b, c (Å) | 10.1306 (5), 10.7019 (6), 15.7749 (7) |
| β (°) | 97.715 (4) |
| V (Å3) | 1694.78 (15) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.39 |
| Crystal size (mm) | 0.72 × 0.47 × 0.13 |
| Data collection | |
| Diffractometer | Stoe IPDS 2 |
| Absorption correction | Integration (X-RED32; Stoe & Cie, 2002 ▸) |
| T min, T max | 0.796, 0.937 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 20123, 5828, 2944 |
| R int | 0.047 |
| (sin θ/λ)max (Å−1) | 0.746 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.064, 0.170, 1.01 |
| No. of reflections | 5828 |
| No. of parameters | 217 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.33, −0.21 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S205698902001573X/dx2034sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S205698902001573X/dx2034Isup2.hkl
Supporting information file. DOI: 10.1107/S205698902001573X/dx2034Isup3.cml
CCDC reference: 2047452
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Crystal data
| C19H14Cl2N2O | F(000) = 736 |
| Mr = 357.22 | Dx = 1.400 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 10.1306 (5) Å | Cell parameters from 16480 reflections |
| b = 10.7019 (6) Å | θ = 1.9–32.4° |
| c = 15.7749 (7) Å | µ = 0.39 mm−1 |
| β = 97.715 (4)° | T = 296 K |
| V = 1694.78 (15) Å3 | Plate, colorless |
| Z = 4 | 0.72 × 0.47 × 0.13 mm |
Data collection
| Stoe IPDS 2 diffractometer | 5828 independent reflections |
| Radiation source: sealed X-ray tube, 12 x 0.4 mm long-fine focus | 2944 reflections with I > 2σ(I) |
| Plane graphite monochromator | Rint = 0.047 |
| Detector resolution: 6.67 pixels mm-1 | θmax = 32.0°, θmin = 2.3° |
| rotation method scans | h = −12→15 |
| Absorption correction: integration (X-RED32; Stoe & Cie, 2002) | k = −15→15 |
| Tmin = 0.796, Tmax = 0.937 | l = −23→23 |
| 20123 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.064 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.170 | H-atom parameters constrained |
| S = 1.01 | w = 1/[σ2(Fo2) + (0.0652P)2 + 0.3156P] where P = (Fo2 + 2Fc2)/3 |
| 5828 reflections | (Δ/σ)max < 0.001 |
| 217 parameters | Δρmax = 0.33 e Å−3 |
| 0 restraints | Δρmin = −0.21 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl2 | 0.32888 (9) | 0.44720 (8) | 0.41784 (5) | 0.1022 (3) | |
| Cl1 | 0.06171 (9) | 0.57454 (9) | 0.67843 (6) | 0.1101 (3) | |
| O1 | −0.00667 (14) | 0.83981 (14) | 0.49196 (12) | 0.0698 (4) | |
| N2 | 0.30239 (16) | 0.97613 (15) | 0.57214 (12) | 0.0561 (4) | |
| N1 | 0.17344 (16) | 0.95588 (15) | 0.54273 (13) | 0.0582 (4) | |
| H1 | 0.1229 | 1.0208 | 0.5381 | 0.070* | |
| C6 | 0.19680 (18) | 0.50436 (17) | 0.55109 (14) | 0.0520 (5) | |
| C11 | 0.37910 (19) | 0.87772 (18) | 0.58104 (13) | 0.0508 (4) | |
| C9 | 0.2000 (2) | 0.73781 (17) | 0.53008 (13) | 0.0508 (4) | |
| C8 | 0.11329 (19) | 0.84552 (18) | 0.51939 (14) | 0.0536 (5) | |
| C10 | 0.32911 (19) | 0.75621 (18) | 0.56121 (14) | 0.0533 (5) | |
| H10 | 0.3862 | 0.6880 | 0.5698 | 0.064* | |
| C12 | 0.5217 (2) | 0.8968 (2) | 0.61209 (14) | 0.0568 (5) | |
| H12 | 0.5752 | 0.8261 | 0.6204 | 0.068* | |
| C7 | 0.1371 (2) | 0.61526 (18) | 0.50303 (16) | 0.0609 (6) | |
| H7A | 0.0433 | 0.6192 | 0.5095 | 0.073* | |
| H7B | 0.1431 | 0.6031 | 0.4427 | 0.073* | |
| C14 | 0.7197 (2) | 1.0298 (2) | 0.66024 (15) | 0.0615 (5) | |
| C13 | 0.5792 (2) | 1.0060 (2) | 0.62890 (15) | 0.0607 (5) | |
| H13 | 0.5245 | 1.0759 | 0.6199 | 0.073* | |
| C1 | 0.1675 (2) | 0.4752 (2) | 0.63198 (16) | 0.0650 (6) | |
| C5 | 0.2807 (2) | 0.4200 (2) | 0.51757 (16) | 0.0631 (6) | |
| C19 | 0.7618 (3) | 1.1510 (3) | 0.67726 (17) | 0.0761 (7) | |
| H19 | 0.7004 | 1.2159 | 0.6691 | 0.091* | |
| C2 | 0.2149 (3) | 0.3694 (3) | 0.67623 (18) | 0.0840 (8) | |
| H2 | 0.1934 | 0.3533 | 0.7307 | 0.101* | |
| C15 | 0.8136 (2) | 0.9359 (3) | 0.67285 (19) | 0.0776 (7) | |
| H15 | 0.7882 | 0.8536 | 0.6611 | 0.093* | |
| C4 | 0.3270 (3) | 0.3132 (2) | 0.5602 (2) | 0.0831 (8) | |
| H4 | 0.3810 | 0.2579 | 0.5351 | 0.100* | |
| C3 | 0.2937 (3) | 0.2891 (3) | 0.6385 (2) | 0.0897 (9) | |
| H3 | 0.3249 | 0.2168 | 0.6672 | 0.108* | |
| C18 | 0.8940 (3) | 1.1772 (3) | 0.7063 (2) | 0.0951 (9) | |
| H18 | 0.9209 | 1.2595 | 0.7168 | 0.114* | |
| C16 | 0.9453 (3) | 0.9629 (4) | 0.7027 (2) | 0.0969 (9) | |
| H16 | 1.0072 | 0.8984 | 0.7115 | 0.116* | |
| C17 | 0.9850 (3) | 1.0827 (4) | 0.7194 (2) | 0.1007 (10) | |
| H17 | 1.0736 | 1.1002 | 0.7397 | 0.121* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl2 | 0.1168 (6) | 0.1083 (6) | 0.0881 (5) | 0.0366 (5) | 0.0379 (5) | 0.0082 (4) |
| Cl1 | 0.1042 (6) | 0.1263 (7) | 0.1069 (6) | −0.0057 (5) | 0.0406 (5) | −0.0446 (5) |
| O1 | 0.0467 (8) | 0.0506 (8) | 0.1070 (13) | 0.0102 (6) | −0.0080 (8) | −0.0061 (8) |
| N2 | 0.0479 (9) | 0.0427 (9) | 0.0767 (12) | 0.0047 (7) | 0.0040 (8) | −0.0020 (8) |
| N1 | 0.0459 (9) | 0.0409 (8) | 0.0860 (13) | 0.0104 (7) | 0.0017 (8) | −0.0034 (8) |
| C6 | 0.0467 (9) | 0.0412 (9) | 0.0653 (12) | −0.0024 (8) | −0.0024 (9) | −0.0062 (9) |
| C11 | 0.0469 (10) | 0.0460 (10) | 0.0587 (12) | 0.0051 (8) | 0.0047 (9) | 0.0004 (9) |
| C9 | 0.0506 (10) | 0.0406 (9) | 0.0597 (12) | 0.0066 (8) | 0.0019 (9) | −0.0011 (8) |
| C8 | 0.0486 (10) | 0.0434 (10) | 0.0671 (13) | 0.0070 (8) | 0.0014 (9) | −0.0014 (9) |
| C10 | 0.0488 (11) | 0.0415 (10) | 0.0682 (13) | 0.0114 (8) | 0.0029 (9) | −0.0003 (9) |
| C12 | 0.0488 (10) | 0.0493 (11) | 0.0708 (14) | 0.0067 (8) | 0.0027 (9) | −0.0009 (9) |
| C7 | 0.0519 (11) | 0.0451 (10) | 0.0813 (15) | 0.0073 (9) | −0.0074 (10) | −0.0068 (10) |
| C14 | 0.0553 (11) | 0.0673 (14) | 0.0618 (13) | −0.0049 (10) | 0.0073 (10) | −0.0002 (11) |
| C13 | 0.0526 (11) | 0.0545 (12) | 0.0740 (14) | 0.0054 (10) | 0.0049 (10) | 0.0029 (10) |
| C1 | 0.0605 (12) | 0.0645 (13) | 0.0692 (14) | −0.0135 (10) | 0.0054 (11) | −0.0135 (11) |
| C5 | 0.0652 (13) | 0.0512 (11) | 0.0716 (14) | 0.0087 (10) | 0.0042 (11) | −0.0006 (10) |
| C19 | 0.0754 (16) | 0.0741 (16) | 0.0785 (16) | −0.0148 (13) | 0.0092 (13) | −0.0052 (13) |
| C2 | 0.095 (2) | 0.0852 (19) | 0.0684 (16) | −0.0303 (16) | −0.0025 (15) | 0.0149 (14) |
| C15 | 0.0567 (13) | 0.0771 (16) | 0.0962 (19) | −0.0020 (12) | −0.0002 (12) | 0.0039 (14) |
| C4 | 0.0901 (18) | 0.0569 (14) | 0.099 (2) | 0.0237 (13) | 0.0024 (16) | 0.0022 (14) |
| C3 | 0.105 (2) | 0.0610 (16) | 0.097 (2) | 0.0033 (15) | −0.0089 (18) | 0.0160 (15) |
| C18 | 0.098 (2) | 0.103 (2) | 0.0835 (19) | −0.043 (2) | 0.0090 (16) | −0.0134 (17) |
| C16 | 0.0554 (14) | 0.125 (3) | 0.106 (2) | 0.0002 (16) | −0.0032 (14) | 0.010 (2) |
| C17 | 0.0643 (17) | 0.141 (3) | 0.093 (2) | −0.031 (2) | −0.0051 (15) | −0.002 (2) |
Geometric parameters (Å, º)
| Cl2—C5 | 1.733 (3) | C14—C15 | 1.379 (3) |
| Cl1—C1 | 1.740 (3) | C14—C19 | 1.380 (3) |
| O1—C8 | 1.236 (2) | C14—C13 | 1.465 (3) |
| N2—C11 | 1.305 (2) | C13—H13 | 0.9300 |
| N2—N1 | 1.344 (2) | C1—C2 | 1.382 (4) |
| N1—C8 | 1.357 (3) | C5—C4 | 1.376 (3) |
| N1—H1 | 0.8600 | C19—C18 | 1.385 (4) |
| C6—C1 | 1.384 (3) | C19—H19 | 0.9300 |
| C6—C5 | 1.392 (3) | C2—C3 | 1.362 (4) |
| C6—C7 | 1.492 (3) | C2—H2 | 0.9300 |
| C11—C10 | 1.415 (3) | C15—C16 | 1.384 (4) |
| C11—C12 | 1.476 (3) | C15—H15 | 0.9300 |
| C9—C10 | 1.349 (3) | C4—C3 | 1.350 (4) |
| C9—C8 | 1.445 (3) | C4—H4 | 0.9300 |
| C9—C7 | 1.495 (3) | C3—H3 | 0.9300 |
| C10—H10 | 0.9300 | C18—C17 | 1.366 (5) |
| C12—C13 | 1.317 (3) | C18—H18 | 0.9300 |
| C12—H12 | 0.9300 | C16—C17 | 1.358 (5) |
| C7—H7A | 0.9700 | C16—H16 | 0.9300 |
| C7—H7B | 0.9700 | C17—H17 | 0.9300 |
| C11—N2—N1 | 116.38 (17) | C12—C13—H13 | 116.4 |
| N2—N1—C8 | 127.90 (16) | C14—C13—H13 | 116.4 |
| N2—N1—H1 | 116.1 | C2—C1—C6 | 123.2 (2) |
| C8—N1—H1 | 116.1 | C2—C1—Cl1 | 118.7 (2) |
| C1—C6—C5 | 114.9 (2) | C6—C1—Cl1 | 118.08 (19) |
| C1—C6—C7 | 121.7 (2) | C4—C5—C6 | 122.6 (2) |
| C5—C6—C7 | 123.3 (2) | C4—C5—Cl2 | 117.6 (2) |
| N2—C11—C10 | 121.83 (18) | C6—C5—Cl2 | 119.75 (17) |
| N2—C11—C12 | 117.79 (18) | C14—C19—C18 | 121.0 (3) |
| C10—C11—C12 | 120.38 (17) | C14—C19—H19 | 119.5 |
| C10—C9—C8 | 118.07 (18) | C18—C19—H19 | 119.5 |
| C10—C9—C7 | 125.98 (17) | C3—C2—C1 | 118.7 (3) |
| C8—C9—C7 | 115.93 (17) | C3—C2—H2 | 120.6 |
| O1—C8—N1 | 121.52 (17) | C1—C2—H2 | 120.6 |
| O1—C8—C9 | 123.72 (18) | C14—C15—C16 | 120.8 (3) |
| N1—C8—C9 | 114.76 (17) | C14—C15—H15 | 119.6 |
| C9—C10—C11 | 121.03 (17) | C16—C15—H15 | 119.6 |
| C9—C10—H10 | 119.5 | C3—C4—C5 | 119.7 (3) |
| C11—C10—H10 | 119.5 | C3—C4—H4 | 120.2 |
| C13—C12—C11 | 125.18 (19) | C5—C4—H4 | 120.2 |
| C13—C12—H12 | 117.4 | C4—C3—C2 | 120.9 (3) |
| C11—C12—H12 | 117.4 | C4—C3—H3 | 119.6 |
| C6—C7—C9 | 115.12 (17) | C2—C3—H3 | 119.6 |
| C6—C7—H7A | 108.5 | C17—C18—C19 | 120.2 (3) |
| C9—C7—H7A | 108.5 | C17—C18—H18 | 119.9 |
| C6—C7—H7B | 108.5 | C19—C18—H18 | 119.9 |
| C9—C7—H7B | 108.5 | C17—C16—C15 | 120.6 (3) |
| H7A—C7—H7B | 107.5 | C17—C16—H16 | 119.7 |
| C15—C14—C19 | 117.8 (2) | C15—C16—H16 | 119.7 |
| C15—C14—C13 | 122.9 (2) | C16—C17—C18 | 119.5 (3) |
| C19—C14—C13 | 119.3 (2) | C16—C17—H17 | 120.2 |
| C12—C13—C14 | 127.2 (2) | C18—C17—H17 | 120.2 |
| C11—N2—N1—C8 | −1.5 (3) | C5—C6—C1—C2 | −1.3 (3) |
| N1—N2—C11—C10 | −0.3 (3) | C7—C6—C1—C2 | 176.1 (2) |
| N1—N2—C11—C12 | 179.18 (19) | C5—C6—C1—Cl1 | −179.26 (16) |
| N2—N1—C8—O1 | −178.9 (2) | C7—C6—C1—Cl1 | −1.8 (3) |
| N2—N1—C8—C9 | 1.7 (3) | C1—C6—C5—C4 | 2.4 (3) |
| C10—C9—C8—O1 | −179.6 (2) | C7—C6—C5—C4 | −175.0 (2) |
| C7—C9—C8—O1 | 1.9 (3) | C1—C6—C5—Cl2 | −178.40 (17) |
| C10—C9—C8—N1 | −0.1 (3) | C7—C6—C5—Cl2 | 4.2 (3) |
| C7—C9—C8—N1 | −178.7 (2) | C15—C14—C19—C18 | −0.2 (4) |
| C8—C9—C10—C11 | −1.4 (3) | C13—C14—C19—C18 | −179.5 (2) |
| C7—C9—C10—C11 | 177.0 (2) | C6—C1—C2—C3 | −0.4 (4) |
| N2—C11—C10—C9 | 1.7 (3) | Cl1—C1—C2—C3 | 177.5 (2) |
| C12—C11—C10—C9 | −177.8 (2) | C19—C14—C15—C16 | 0.9 (4) |
| N2—C11—C12—C13 | −2.5 (3) | C13—C14—C15—C16 | −179.9 (3) |
| C10—C11—C12—C13 | 177.0 (2) | C6—C5—C4—C3 | −1.7 (4) |
| C1—C6—C7—C9 | 78.6 (3) | Cl2—C5—C4—C3 | 179.0 (2) |
| C5—C6—C7—C9 | −104.2 (2) | C5—C4—C3—C2 | −0.2 (5) |
| C10—C9—C7—C6 | 31.4 (3) | C1—C2—C3—C4 | 1.2 (4) |
| C8—C9—C7—C6 | −150.2 (2) | C14—C19—C18—C17 | −0.7 (4) |
| C11—C12—C13—C14 | 179.6 (2) | C14—C15—C16—C17 | −0.7 (5) |
| C15—C14—C13—C12 | 3.0 (4) | C15—C16—C17—C18 | −0.2 (5) |
| C19—C14—C13—C12 | −177.8 (2) | C19—C18—C17—C16 | 0.9 (5) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1···O1i | 0.86 | 1.92 | 2.772 (2) | 171 |
| C3—H3···Cl1ii | 0.93 | 2.97 | 3.824 (3) | 153 |
Symmetry codes: (i) −x, −y+2, −z+1; (ii) −x+1/2, y−1/2, −z+3/2.
Funding Statement
This work was funded by Ondokuz Mayis Üniversitesi grant PYOFEN.1906.19.001.
References
- Al-Awadi, N. A., Ibrahim, M. R., Al-Etaibi, A. M. & Elnagdia, M. H. (2011). Arkivoc (ii) pp. 310–321, https://doi.org/10.3998/ark.5550190.0012.225
- Asif, M. (2013). Mini-Rev. Org. Chem. 10, 113–122.
- Bouchmaa, N., Mrid, R. B., Boukharsa, Y., Bouargalne, Y., Nhiri, M., Idir, A., Taoufik, J., Ansar, M. & Zyad, A. (2019). Drug Res (Stuttg), 69, 528–536. [DOI] [PubMed]
- Bouchmaa, N., Tilaoui, M., Boukharsa, Y., Jaâfari, A., Mouse, H. A., Ali Oukerrou, M., Taoufik, J., Ansar, M. & Zyad, A. (2018). Pharm. Chem. J. 51, 893–901.
- Boukharsa, Y., Lakhlili, W., El harti, J., Meddah, B., Tiendrebeogo, R. Y., Taoufik, J., El Abbes Faouzi, M., Ibrahimi, A. & Ansar, M. (2018). J. Mol. Struct. 1153, 119–127.
- Boukharsa, Y., Meddah, B., Tiendrebeogo, R. Y., Ibrahimi, A., Taoufik, J., Cherrah, Y., Benomar, A., Faouzi, M. E. A. & Ansar, M. (2016). Med. Chem. Res. 25, 494–500.
- Daoui, S., Baydere, C., Akman, F., El Kalai, F., Mahi, L., Dege, N., Topcu, Y., Karrouchi, K. & Benchat, N. (2021). J. Mol. Struct. 1225, 129–180.
- Daoui, S., Baydere, C., Chelfi, T., El Kalai, F., Dege, N., Karrouchi, K. & Benchat, N. (2020). Acta Cryst. E76, 432–437. [DOI] [PMC free article] [PubMed]
- Daoui, S., Faizi, M. S. H., Kalai, F. E., Saddik, R., Dege, N., Karrouchi, K. & Benchat, N. (2019). Acta Cryst. E75, 1030–1034. [DOI] [PMC free article] [PubMed]
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Dubey, S. & Bhosle, P. A. (2015). Med. Chem. Res. 24, 3579–3598.
- El Kalai, F., Baydere, C., Daoui, S., Saddik, R., Dege, N., Karrouchi, K. & Benchat, N. (2019). Acta Cryst. E75, 892–895. [DOI] [PMC free article] [PubMed]
- El Kali, F., Kansiz, S., Daoui, S., Saddik, R., Dege, N., Karrouchi, K. & Benchat, N. (2019). Acta Cryst. E75, 650–654. [DOI] [PMC free article] [PubMed]
- El Kalai, F., Karrouchi, K., Baydere, C., Daoui, S., Allali, M., Dege, N., Benchat, N. & Brandán, S. A. (2021). J. Mol. Struct. 1223, 129–213.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Gökçe, M., Utku, S. & Küpeli, E. (2009). Eur. J. Med. Chem. 44, 3760–3764. [DOI] [PubMed]
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Livermore, D., Bethell, R. C., Cammack, N., Hancock, A. P., Hann, M. M. & Green, D. (1993). J. Med. Chem. 36, 3784–3794. [DOI] [PubMed]
- Macrae, C. F., Sovago, I., Cottrell, S. J., Galek, P. T. A., McCabe, P., Pidcock, E., Platings, M., Shields, G. P., Stevens, J. S., Towler, M. & Wood, P. A. (2020). J. Appl. Cryst. 53, 226–235. [DOI] [PMC free article] [PubMed]
- Oubair, A., Daran, J.-C., Fihi, R., Majidi, L. & Azrour, M. (2009). Acta Cryst. E65, o1350–o1351. [DOI] [PMC free article] [PubMed]
- Partap, S., Akhtar, M. J., Yar, M. S., Hassan, M. Z. & Siddiqui, A. A. (2018). Bioorg. Chem. 77, 74–83. [DOI] [PubMed]
- Pita, B., Sotelo, E., Suárez, M., Raviña, E., Ochoa, E., Verdecia, Y., Novoa, H., Blaton, N., de Ranter, C. & Peeters, O. M. (2000). Tetrahedron, 56, 2473–2479.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Siddiqui, A. A., Mishra, R., Shaharyar, M., Husain, A., Rashid, M. & Pal, P. (2011). Bioorg. Med. Chem. Lett. 21, 1023–1026. [DOI] [PubMed]
- Spek, A. L. (2020). Acta Cryst. E76, 1–11. [DOI] [PMC free article] [PubMed]
- Stoe & Cie (2002). X-AREA and X-RED32. Stoe & Cie, Darmstadt, Germany.
- Tao, M., Aimone, L. D., Gruner, J. A., Mathiasen, J. R., Huang, Z., Lyons, J., Raddatz, R. & Hudkins, R. L. (2012). Bioorg. Med. Chem. Lett. 22, 1073–1077. [DOI] [PubMed]
- Thakur, A. S., Verma, P. & Chandy, A. (2010). Asian. J. Res. Chem. 3, 265–271.
- Turner, M. J., MacKinnon, J. J., Wolff, S. K., Grimwood, D. J., Spackman, P. R., Jayatilaka, D. & Spackman, M. A. (2017). CrystalExplorer17.5. University of Western Australia.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Zhou, G., Ting, P. C., Aslanian, R., Cao, J., Kim, D. W., Kuang, R., Lee, J. F., Schwerdt, J., Wu, H., Jason Herr, R., Zych, A. J., Yang, J., Lam, S., Wainhaus, S., Black, T. A., McNicholas, P. M., Xu, Y. & Walker, S. S. (2011). Bioorg. Med. Chem. Lett. 21, 2890–2893. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S205698902001573X/dx2034sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S205698902001573X/dx2034Isup2.hkl
Supporting information file. DOI: 10.1107/S205698902001573X/dx2034Isup3.cml
CCDC reference: 2047452
Additional supporting information: crystallographic information; 3D view; checkCIF report