Abstract
Objective
This study determined the correlation between erythrocyte acetylcholinesterase (AChE) activity, serum malondialdehyde (MDA) and insulin sensitivity in agricultural workers and non-agricultural workers.
Methodology
The cross-sectional comparative study was undertaken in 45 agricultural and 45 non-agricultural workers from Nat-Kan Village, Magway Township. Erythrocyte acetylcholinesterase activity and serum malondialdehyde were measured by spectrophotometric method. Insulin sensitivity was calculated by homeostasis model assessment (HOMA-IR).
Results
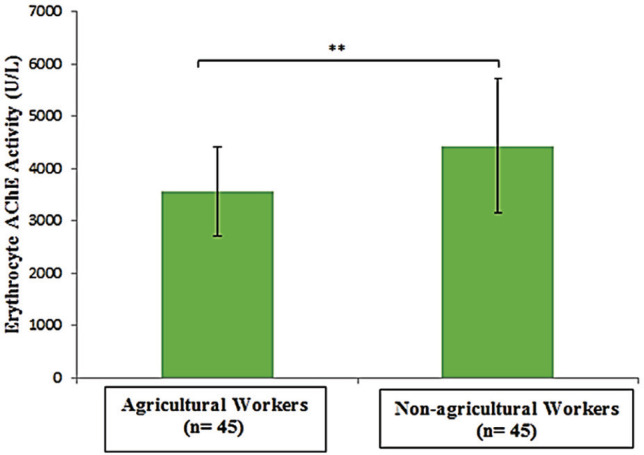
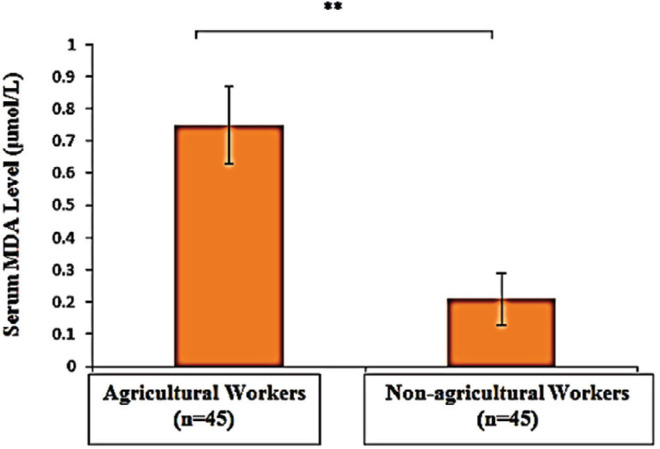
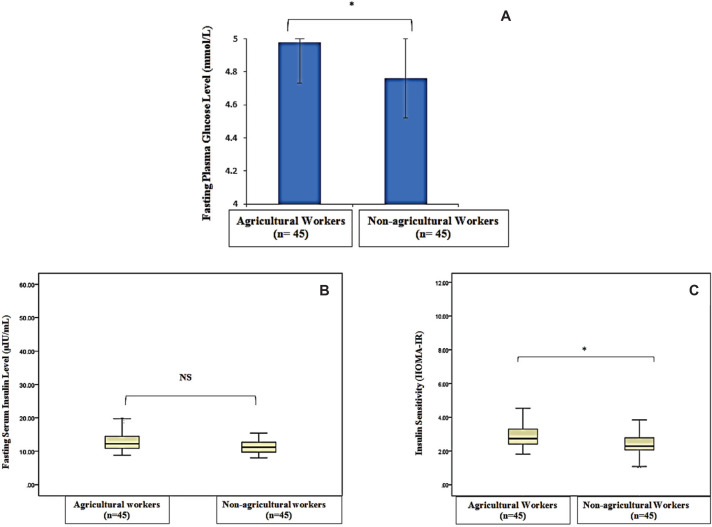
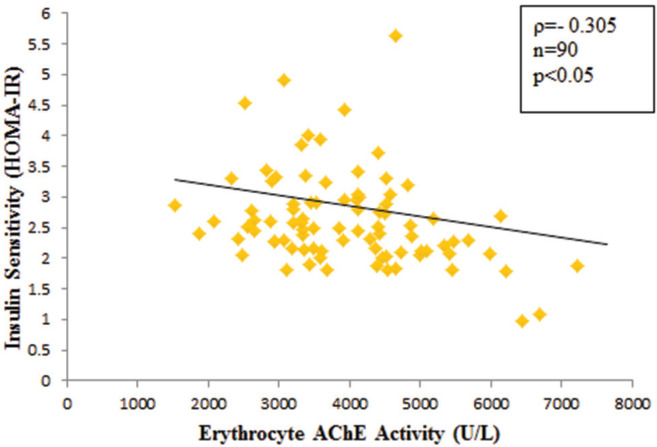
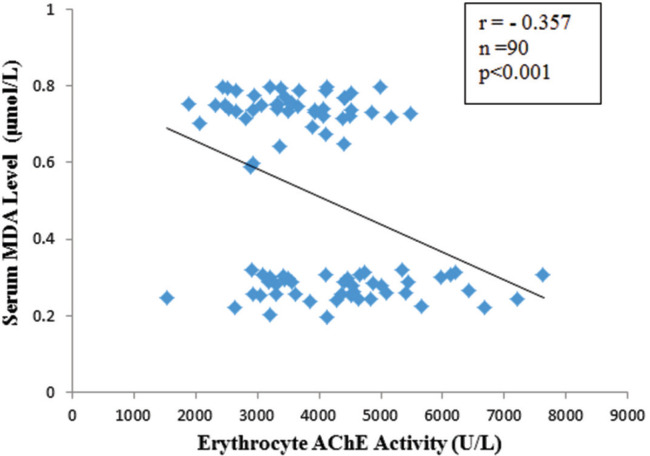
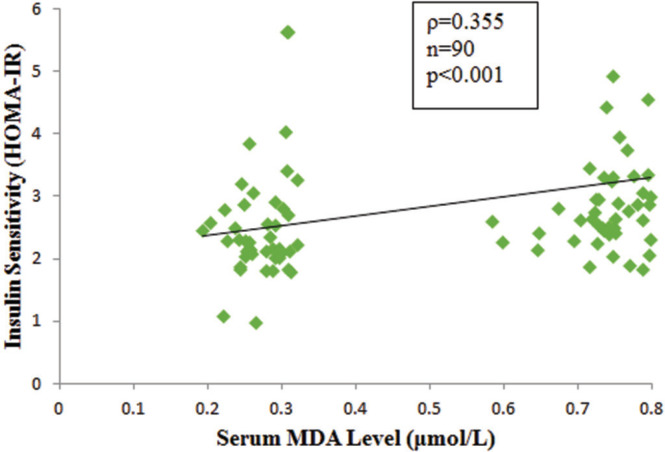
Mean erythrocyte AChE activity was significantly lower in agricultural (3553.99 IU/L) compared with nonagricultural workers (4432.68 IU/L) (p<0.001). A significant high level of mean serum MDA was observed in agricultural workers (0.74 versus 0.28 μmol/L, p<0.001). Median HOMA-IR value was significantly higher in agricultural (2.74) than that of non-agricultural workers (2.28) (p<0.05). The risk of insulin resistance was 2.8 times greater in agricultural workers than non-agricultural workers (OR 2.8, 95% CI, 1.18 to 6.72). Erythrocyte AChE activity had weak negative correlation with serum MDA level (r=-0.357, p<0.001) and HOMA-IR (ρ= -0.305, p<0.05). There was a significant but weak positive correlation between serum MDA level and HOMA-IR (ρ=0.355, p<0.001).
Conclusion
Organophosphate pesticide exposure lowered erythrocyte AChE activity and increased oxidative stress. Oxidative stress is partly attributed to the development of insulin resistance.
Keywords: erythrocyte acetylcholinesterase activity, serum malondialdehyde level, HOMA-IR, agricultural workers
INTRODUCTION
With agriculture as the main industry in Myanmar, many workers have become reliant on toxic pesticides to increase farm productivity. Agricultural workers are inevitably exposed to pesticides during preparation and application of the pesticide spray solution. As a consequence, agricultural workers face a greater risk of exposure to pesticides than non-agricultural workers.
Organophosphate (OP) pesticides are liquid at room temperature and can be absorbed through intact skin and through the gut after ingestion of contaminated food. They also penetrate the skin, respiratory tract epithelium and cornea, as they easily vaporize.1 As inhibitors of the enzyme acetylcholinesterase (AChE), OP pesticides allow accumulation of acetylcholine (ACh) and continuous stimulation of ACh receptors at the neuromuscular junction. This is the suggested mechanism of toxicity of OP pesticides.2
Chronic exposure to OP pesticides affect different organs and body systems such as the skeletal muscles, gastrointestinal tract, urinary bladder, secretory glands, central nervous system and respiratory system. These are manifested as weakness, glandular secretion, fasciculation, acute pancreatitis, convulsion and respiratory failure.3 Hyperglycemia has often been reported to be one of the most common manifestations of chronic OP pesticide toxicity in experimental animal models as well as in cases of human exposure.4-8
The cholinergic action of OP pesticides in inhibiting AChE and increasing ACh activity induces hyperesthesia, intermittent spasm, muscular tremors, sustained seizures and muscle fasciculation. These involuntary energydemanding activities trigger the release of glucose by glycogenolysis and gluconeogenesis. There is an ensuing increase in glucose-6-phosphatase enzymes leading to hyperglycemia. These also stimulate glycolysis in the liver and muscle, and the subsequent release of adenosine triphosphate (ATP) to meet the body’s energy requirements. Thus, OP pesticides may influence the pathways involved in enzymatic glucose homeostasis.6,9,10 In addition to hyperglycemia, inhibition of AChE by OP pesticides can induce significant increases in hepatic lipid peroxidation, as indicated by the biomarker malondialdehyde.10-12 This is the proposed mechanism underlying the causal relation between oxidative stress and insulin resistance.13,14 However, only one study reported the association between serum concentration of malathion and elevated HOMA-IR among 98 farmers without diabetes.14
While research activities on the toxic effects of OP pesticides in developed countries have increased public awareness, we have limited studies of our own. The data may be used to institute programs to promote awareness of the health problems of pesticide usage in our country. Thus, this study aims to investigate erythrocyte AChE activity and insulin sensitivity in agricultural and non-agricultural workers in Nat-Kan Village, Magway Township. This study will determine the effects and mechanisms involved in OP pesticide toxicity on glucose homeostasis. The findings will be used to help farmers become aware of the potential dangers of pesticide handling and use in daily farming activities, and to prevent accidental poisoning.
METHODOLOGY
This cross-sectional comparative study was undertaken in villagers 18 to 45 years of age living in Nat-Kan Village. History taking and physical examination were carried out according to the pro forma. After explaining the procedure for data collection, written informed consent was obtained. Weight measurement was done by using a calibrated bathroom scale. Standing height was measured with the use of a measuring tape. After body mass index (BMI) calculation, subjects with BMI between 18.5 to 24.9 kg/m2 were included.
The sample size was calculated by the following formula:
r = n2/n1, q1=1-p1, q2=1-p2 p = p1+p2r/1+r, q = 1-p
P₁ =Proportion in group 1= 27.4% (derived from Panda et al32) P₂ =Proportion in group 2 = 57.6%
Z1- α/2 =reliability coefficient = 1.96 (95% Confidence Interval)
∆ = 0.05
Z1- β = 0.84 (80% power of the test)
n1= n2= 39
Drop out = 10%
Required n1= n2 = 39+4 = 43
Therefore, required sample size for each group (exposed and non-exposed group) was 43.
Forty-five agricultural workers who have been using OP pesticides (Acephate and Chlorpyrifos) in the processes of formulating, mixing, loading and spraying for a period of at least one year were recruited in this study. However, different types of pesticides have also been used in orchards to control various insect pests. The agricultural workers recruited in the present study were also exposed to other types of pesticides such as Imidacloprid, Cypermethrin, Acetamiprid and Azoxystrobin (Table 1). Another control group of 45 subjects whose work did not involve pesticide application (sellers, carpenters and bricklayers) and lived in an area at least 100 meters away from processes of pesticide use were selected as control subjects. Control subjects were matched to study group by age, sex and BMI. This study was approved by the Ethics and Research Committee of the University of Medicine, Magway.
Table 1.
Active compounds in pesticides commonly used in Magway Region
| Brand Name | Item | Active compound |
|---|---|---|
| Min Ma Haw | Cupro Star 85 | Copper oxychloride 85% |
| No Two Super | Acetamiprid 15% + Lambdacyhalothrin 10% | |
| Aw Ba | Revive 25 SC | Azoxystrobin 25% w/v |
| Phosdrin 40EC | Chlorpyrifos 40% | |
| Carbofuran 3G | Carbofuran 3% | |
| AZPHATE 75SP | Acephate 75% | |
| Better 25 WP | Acetamiprid 20% + Lambdacyhalothrin 5% | |
| Demon 1.8 EC | Abamectin 1.8% | |
| Unity 32.5 SC | Difenoconazole 12.5% + Azoxystrobin 20% | |
| Cyclone 505 EC | Chlorpyrifos 45.9% + Cypermethrin 4.59% | |
| THUNDER 250 EC | Cypermethrin 25% | |
| DOZER 20 WP | Imidacloprid 20% | |
| DOZER 70 WP | Imidacloprid 70% | |
| ALARM 15 WP | Emamectin benzoate 5 % + Lambda-cyhalothrin 10% | |
| Min Mida | 70 WDG | Imidacloprid 70% |
| Syntech | Carbine 3 G | Carbofuran 3% |
| Shwe Chin Thae | COBRA 40 EC | Chlorpyrifos 40% |
All 90 subjects were assessed for their eligibility to the study by inclusion and exclusion criteria. Subjects with a history of heavy smoking, heavy alcohol drinking, hypertension, heart disease, diabetes mellitus, chronic renal disease, signs and symptoms of heavy metal poisoning (e.g. diarrhea, nausea, vomiting, abdominal pain and shortness of breath) and intake of antioxidant drugs (e.g. zinc tablets) were excluded from the study. Heavy smoking was defined as consumption of at least 20 cigarettes daily, and heavy alcohol drinking as more than 30 mL per day.
Visits were made from June to August during pesticide spraying season. The investigator visited agricultural workers every two weeks for three times: the first visit was performed for observation of pesticide application processes, the second for observation of continuation of the process, and the third for preparation of data collection. During visits, safe handling procedures for pesticide storage and application were explained to agricultural workers. Signs and symptoms of acute or severe pesticide poisoning, such as fever, dizziness, confusion, restlessness, muscle twitching, staggering gait, slurred speech, fits and unconsciousness, were also noted. Moreover, first aid and immediate assistance were provided to any person found to have mild or severe pesticide poisoning. Subjects with severe poisoning were excluded from the study.
Data collection was also done during pesticide spraying season. On the day of blood extraction, all subjects were instructed to be present at the office of the local administrator at 0600H after fasting for 10 hours (from 2000H to 0600H). Upon arrival, a 7 mL blood sample taken by venipuncture was collected from each subject in 3 separate blood collecting tubes: 1 mL in a tube containing sodium fluoride 10 mg for determination of blood sugar, 3 mL in a plain tube for serum separation, and 3 mL in an ethylenediaminetetraacetic acid (EDTA) tube for packed cell separation. All blood samples were transported to the Common Research Laboratory, University of Medicine, Magway using an effective cold chain system.
Upon arrival, the blood sample in the plain tube was allowed to clot for 30 minutes at room temperature. Once clotted, centrifugation at 3000 rpm for 15 minutes was done. Serum was collected in two separate sample tubes and stored at -4°C until blood sample analysis: one for determination of serum MDA and another for serum insulin. The sample in the EDTA tube was centrifuged at 1000 rpm for 15 minutes. The supernatant plasma and buffy coat were removed. Packed cells were washed three times with isotonic saline. These were then stored at 2ºC before analysis. AChE activity was determined within 3 days. Blood glucose level was determined on the same day of blood collection. Those with fasting plasma glucose (FPG) more than 100 mg/dL were excluded from the study. Erythrocyte AChE activity and serum MDA were measured by spectrophotometric method.
Ellman’s spectrophotometrical method for cholinesterase activity determines the rate of production of thiocholine as acetylthiocholine is hydrolyzed. The enzyme activity is measured by following the increase in yellow color produced from thiocholine when it reacts with dithiobisnitrobenzoate ion. This method has the sensitivity to determine human erythrocyte AChE activity. The Ellman assay for AChE is well known in the detection of OP pesticide exposure. We utilized Ellman’s spectrophotometrical method to investigate the erythrocyte AChE activity in our study.15
Fasting plasma glucose was measured by glucose oxidase method. Fasting serum insulin was determined by enzymelinked immunosorbent assay (ELISA). Insulin sensitivity was calculated by HOMA-IR as shown in the equation:16
Data entry and analysis were done by SPSS Statistics version 16.0. Normally distributed variables were expressed in mean (SD). Comparison of the data between study group and control group was done by unpaired Student’s t test. Skewed data were expressed as median (interquartile range, IQR) and they were computed by non-parametric tests using the Mann-Whitney U test. Correlation studies were done by Pearson’s correlation. Another non-parametric test, Spearman’s rank, was used when the data did not follow a normal distribution. Chisquared test was used to determine the risk of insulin resistance in agricultural workers. Differences were considered significant when p<0.05.
RESUlTS
A total of 100 subjects, composed of 50 agricultural workers exposed to OP pesticides and 50 non-agricultural workers, were initially recruited to participate in this study. Of these, three agricultural workers and two control subjects were dropped due to incomplete data. Another two agricultural workers with hypoglycemia were excluded from this study. Three control subjects who were unable to follow instructions were also excluded. The final group included 45 agricultural and 45 non-agricultural workers. The general characteristics of the study participants are shown in Table 2. There were no significant differences in age, sex distribution and BMI.
Table 2.
General characteristics of study participants
| Parameter | Agricultural workers (n=45) | Non-agricultural workers (n=45) |
|---|---|---|
| Mean age, year (SD) | 28.67 (4.12) | 27.53 (4.00) |
| Sex (Male:Female) | 1:1 | 1:1 |
| Mean body weight, kg (SD) | 62.56 (4.12) | 59.47 (3.33) |
| Mean height, m (SD) | 1.65 (0.57) | 1.61 (0.47) |
| Mean body mass index, kg/m2 (SD) | 22.91 (1.13) | 22.92 (1.35) |
The comparison of erythrocyte AChE and MDA parameters are shown in Figures 1 and 2. Mean erythrocyte AChE activity of agricultural workers (3,553.99 ± 855.6 IU/L) was significantly lower compared to the nonagricultural workers (4,432.68 ± 1,287.86 IU/L) (p<0.001). However, mean serum MDA was significantly higher in agricultural workers (0.74 ± 0.05 μmol/L) compared with non-agricultural workers (0.28 ± 0.06 μmol/L) (p<0.001). Agricultural workers had a higher mean FPG, whereas serum insulin levels were not significantly different between study groups (Figure 3A and B). A higher HOMAIR value in agricultural workers [median IQR, 2.74 (2.373.3)] compared to non-agricultural workers [median IQR, 2.28 (2.03-2.78)] indicated that insulin sensitivity was significantly reduced in agricultural workers (p<0.05) (Figure 3C). Erythrocyte AChE activity was weakly and negatively correlated with serum MDA level (r=0.357, p<0.001) as well as HOMA-IR (ρ=-0.305, p<0.05) (Figures 4 and 5). A significant weak positive correlation between serum MDA level and HOMA-IR was also demonstrated (ρ=0.355, p<0.001).
Figure 1.
Comparison of erythrocyte acetylcholinesterase (AChE) activity in agricultural and non-agricultural workers. **, significant difference (p<0.001).
Figure 2.
Comparison of serum malondialdehyde (MDA) levels in agricultural and non-agricultural workers. **, significant difference (p<0.001).
Figure 3.
Comparison of fasting plasma glucose (A), fasting serum insulin (B) and insulin sensitivity by homeostasis model assessment (HOMA -IR) (C) in agricultural (n=45) and non-agricultural workers (n=45). *, significant difference (p<0.05).
Figure 4.
Correlation between erythrocyte acetylcholinesterase (AChE) activity and serum malondialdehyde (MDA) level in agricultural and non-agricultural workers. r, Pearson’s correlation coefficient; n, total number of subjects.
Figure 5.
Correlation between erythrocyte acetylcholinesterase (AChE) activity and insulin sensitivity by homeostasis model assessment (HOMA-IR) in agricultural and non-agricultural workers. ρ, Spearman’s correlation coefficient; n, total number of subjects.
DISCUSSION
Nowadays, the living population is continually exposed to numerous environmental contaminants such as industrial waste, polluted air and pesticides. In Myanmar, where agriculture is the main industry, pesticides are used countrywide for pest control and crop protection. Pesticides are among the leading causes of environmental pollution, with harmful effects on human health. Organophosphate pesticides are a diverse group of chemicals that have been commonly used since 1975. The chemicals mostly used in these compounds are acephate, diazinon, dimethoate, parathion, phosmet, malathion, fenthion, dichlorvos, chlorpyrifos, ethion and azamethiphos. In Myanmar, many pesticides are commercially distributed under the names like Min-Ma-Haw, Aw-Ba, Shwe-ChinThae, Shwe-Nagar and Ar-Mo. These are composed of organophosphate compounds and other pesticides such as copper oxychloride, imidacloprid and carbendazim. Table 1 lists the pesticides that are utilized in Magway Region, Myanmar. Among them, Aw-Ba was the brand most commonly used in the region at the time of sample collection. Other brand alternatives were also reported to be in use. The agricultural workers who participated in the present study were also exposed OP and other pesticides such as imidacloprid, cypermethrin, acetamiprid and azoxystrobin. These other pesticides are considered to have low acute and chronic toxicity to humans.17 All agricultural workers who participated in study had been working as pesticide spraying service professionals for many years, with a mean duration of OP pesticide exposure of 4 ± 1.5 years. The mean frequency of pesticide spraying process by pesticide applicators was 58.2 ± 1.4 per year.
Many animal and human studies have reported the association of OP pesticide toxicity with hyperglycemia, suggesting an underlying influence on liver enzymes involved in glucose homeostasis pathways.5,6,18,19 Moreover, some studies postulated that pancreatic toxicity and oxidative stress induced by OP pesticides may impair glucose homeostasis.6,9,10 There is increasing interest on the effect of environmental pesticide exposure on glucose homeostasis. The present study investigated insulin resistance in agricultural workers exposed to these pesticides. (Figure 6)
Figure 6.
Correlation between serum malondialdehyde (MDA) level and insulin sensitivity by homeostasis model assessment (HOMA-IR) in agricultural and nonagricultural workers. ρ, Spearman’s correlation coefficient; n, total number of subjects.
Measurement of erythrocyte AChE activity has been recognized as a human biological marker of OP pesticide exposure. Inhibition of acetylcholinesterase activity in erythrocyte membranes has been reported as a useful indicator of chronic exposure to OP.3,20,21 Upon entering the body, OP pesticides avidly form covalent bonds with oxygen in serine, located at the active site of AChE. These transform into irreversible phosphorylated inactive AChE.21,22 While erythrocyte AChE activity is also reduced in aging RBC, chronic renal failure and heavy metal exposure, these conditions were part of the exclusion criteria in the present study based on history taking and physical examination.24,25 Based on a study on Kenyan agricultural workers, cutoffs for AChE activity were defined as low (≤3.95 IU/mL, high inhibition), medium (3.95 to ≥5.95 IU/mL, mild inhibition) and normal (>5.95 IU/mL, no inhibition).20 The mean value of erythrocyte AChE activity of agricultural workers in the present study was 3,553.99 ± 855.6 IU/L (range 1,867 to 5,470 IU/L), significantly lower compared to the results of other studies.3,20,21 Thirty out of 45 agricultural workers in the present study had high inhibition of erythrocyte AChE activity, while the. remaining had mild inhibition Erythrocyte AChE activity of agricultural workers was significantly lower compared to non-agricultural workers in the present study (p<0.001). This was in agreement with the findings of previous studies.3,18,20 The low level of erythrocyte AChE activity in agricultural compared to non-agricultural workers showed that there was an inhibitory effect on erythrocyte AChE activity by OP pesticides.22,23
In the present study, the mean erythrocyte AChE activity in non-agricultural workers were 4,432.68 ± 1,287.86 IU/L (range, 3,129 IU/L to 7,632 IU/L). Accordingly, normal erythrocyte AChE activity (more than 5.95 IU/mL) was seen only in seven control subjects in the present study. Most of control subjects (n=38) in the present study had mild inhibition of erythrocyte AChE activity due to indirect exposure to OP pesticides. The findings also suggested that non-agricultural control subjects who lived at least 100 meters away from pesticide application process were not spared from OP pesticide exposure. Farms that use pesticides were located around the villages where they were residing. Non-agricultural workers may be exposed to OP pesticides indirectly by means of inhalation and ingestion of contaminated food and water. One limitation of our study was that investigation of the status of anemia and heavy metal contamination of food and water, which possess an inhibitory effect on AChE activity, were not performed.
The present study also investigated the serum MDA level to detect oxidative stress.The mean serum MDA was significantly higher in agricultural (0.74 ± 0.05 μmol/L) compared to non-agricultural workers (0.28 ± 0.06 μmol/L) (p<0.001). This finding was similar to other studies.10,11,12 Lipids are susceptible to oxidation, and lipid peroxidation products are potential biomarkers for oxidative stress status.10,11,12 MDA is the most relevant and widely used in determining free radical reactions.26,27 Accordingly, the significantly higher levels of serum MDA in agricultural workers was interpreted as reliable evidence of oxidative stress. The present study used thiobarbituric acid reactive substances to measure lipid peroxidation.28 However, determination of MDA with thiobarbituric acid is considered nonspecific, as it is only considered as an indirect measure of reactive oxygen species (ROS), and it can react with peroxidation products of non-lipid origin. It may lead to substantial controversy in the quantification of serum MDA level.
Organophosphate pesticides inhibit the AChE enzyme and consequently increase the activity of the nicotinic type of ACh receptor, resulting in uncoordinated nerve and muscle stimulation. This manifests as sustained seizures and muscle fasciculation.28 Thus, oxygen and glucose demand as well as ATP requirements are greatly increased in the muscle. Thereafter, the glycolytic pathway is activated to meet the increase in energy requirement. Nicotinamide adenine dinucleotide + H or NADH, a side product of the pathway, is oxidized by using atmospheric oxygen, leading to increased production of free radicals in the form of ROS. These ROS alter the cellular macromolecules of lipids, proteins and deoxyribonucleic acid. Lipids are the most susceptible to oxidative stress, causing release of MDA by the oxidation of the lipid layer of the cellular membrane.30,31
The present study also showed a significant but weak negative correlation between serum MDA level and AChE activity (r=-0.357, n=90, p<0.001). This finding was similar to a previous study, where serum MDA level was found to be significantly increased with the severity of OP pesticide poisoning, suggestive of increased oxidative stress.32 It may be inferred that higher serum MDA levels of agricultural workers in the present study might be partly due to exposure to OP pesticides through the mechanism of oxidative stress.
Fasting plasma glucose level was significantly higher in agricultural (4.98 ± 0.40 mmol/L) compared to nonagricultural workers (4.76 ± 0.32 mmol/L) (p<0.05) in our study, despite falling within normal range (3.5 to 6.1 mmol/L). Previous animal studies reported the hyperglycemic effect of malathion, acephate and dimethorate compounds of OP pesticides after intraperitoneal injection in rats.4,5 It has also been shown that acephate and malathion OP pesticide compounds enhance the breakdown of hepatic glycogen contents, with acephate causing an increase in hepatic glucose-6-phosphate activity.4,6 Additionally, the development of hyperglycemia in OP pesticides-exposed rats was found to be due to stimulation of hepatic gluconeogenesis and glycogenolysis.
With advanced modern farming technique, pesticides quickly became widely used by farm workers. Consequently, an increasing awareness of the association between hyperglycemia and organophosphate pesticides in humans was borne out of many studies.18,33,34 A longitudinal study done observed the increased risk of diabetes in pesticide applicators who had used OP compounds.7 Another study noted that 28 out of 102 patients with a history of OP pesticide poisoning had random blood sugar levels >140 mg/dL.32 Similarly, a study of acute OP pesticide poisoning cases reported hyperglycemia in 21%.19 Because these previous studies examined patients with OP pesticide poisoning and our study examined agricultural workers who were exposed to OP in pesticide application process, the results cannot be compared. It can be assumed that normal FPG levels of agricultural workers in the present study were attributable to low doses of pesticide exposure. However, because of the stimulation of the enzymatic glucose homeostasis and subsequent release of glucose to meet involuntary energy demanding activities by OP exposure, significant increases in fasting plasma glucose level in agricultural workers in the present study may be due to direct exposure, or a certain amount of exposure through pollution compared to non-agricultural workers.6,9
The gold standard method for measuring insulin sensitivity is the euglycemic hyperinsulinemic clamp (EHC) method developed by DeFronzo et al.35 The homeostasis model assessment is recommended for large scale studies because of its simplicity and validity, with an excellent correlation with insulin sensitivity measured using EHC.16 HOMA requires the measurement of plasma insulin level in the basal level (i.e. less than 2.1 mIU/L) and the plasma glucose level at or below 3.5 mmol/L. For normal subjects, a one unit increase in plasma glucose level from 3.5 mmol/L (i.e. 4.5 mmol/L) increases plasma insulin secretion by 5 mIU/L from its basal value. Therefore, for a given level of blood glucose, prevailing insulin level reflects insulin resistance. HOMA uses fasting plasma glucose and fasting insulin concentrations related by the feedback of glucose on pancreatic beta cells to increase insulin secretion. Insulin resistance (HOMA-IR) may then be mathematically computed using a derived equation.
In the present study, HOMA-IR value was significantly higher in agricultural than in non-agricultural workers [median IQR, 2.74 (2.37-3.3) versus 2.28 (2.03-2.78), p<0.05]. This finding of decreased insulin sensitivity in agricultural workers was in line with another study in farmers who were exposed to malathion pesticides.14 A HOMA-IR value of more than 2.6 was regarded as insulin resistance.36 Accordingly, insulin resistance was observed in 13 (28.8%) control subjects as well as 24 (53.3%) agricultural workers in this study. The risk of insulin resistance was 2.8 times increased in agricultural workers compared to controls [odds ratio (OR), 2.8; 95% confidence interval (CI), 1.18-6.72). This study shows that agricultural workers who were exposed to OP pesticides have a greater risk for insulin resistance.
A previous longitudinal study revealed that incident diabetes was 51%, 63%, and 94% in applicators who had used the organochlorine insecticides aldrin, chlordane, and heptachlor, respectively.7 In the present study, pesticide applicators mostly used chlopyrifos and acephate. However, a recent study reported that the occurrence of diabetes mellitus in Thai farmers was associated with all types of pesticide (OR, 1.35; 95% CI, 1.04-1.76), especially with organophosphates (OR, 2.22; 95% CI, 1.17-4.19).8
The present study also showed that HOMA-IR had a positive correlation with oxidative stress, as measured by MDA (ρ=0.355, p<0.001), and a negative correlation with erythrocyte AChE activity (ρ=-0.305, p<0.05) in all study groups. There are many relevant underlying mechanisms that the oxidative pathway is linked to erythrocyte AChE activity and insulin sensitivity (HOMA-IR).
Accordingly, oxidative stress may lead to the development of insulin resistance, based on the proposed the mechanisms on their correlation.13,37 In the presence of oxidative stress, ROS is produced in increased amounts by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4 (NOX4), a powerful oxidizing enzyme. As a consequence, there is a shift in the phosphatidylinositol-3-kinase (PI3K) insulin signaling pathway to phosphorylate RacGTPase as an alternative to phosphotidylinositol biphosphate (PIP2). Subsequently phosphorylated RacGTPase amplifies the activity of NOX4 resulting in increased ROS, which in turn activate casein kinase-2 (CK2) and continue to activate the retromer. It causes the trans-Golgi network downstream. Glucose transporter type 4 (GLUT4) is transported to lysosomes for degradation instead of the plasma membrane. Thus, intravascular glucose levels remain elevated and insulin resistance ensues.5,38
Another possible mechanism is endoplasmic reticulum (ER) dysfunction. The endoplasmic reticulum is important for cellular functions, such as intracellular calcium storage.39,40 Cellular stress conditions increase endoplasmic reticulum demand, a condition called ER stress. Unfolding protein response (UPR) and sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) pump activity are activated in the organelle itself, a condition also called ER homeostasis.40 When ER homeostasis is impaired, as in prolonged exposure to oxidative stress, SERCA activities are reduced, decreasing endoplasmic reticulum calcium concentration and subsequently increasing intracellular calcium concentration, contributing to insulin resistance.39
CONClUSION
We found that agricultural workers had significantly lower erythrocyte AChE activity than non-agricultural workers. Insulin sensitivity was decreased in agricultural workers based on the observed increase in HOMA-IR scores. Serum MDA level was significantly higher in agricultural compared to non-agricultural workers.
We also found a significant negative correlation between erythrocyte AChE activity and serum MDA level as well as HOMA-IR score. A positive correlation was also observed between serum MDA and HOMA-IR score. Insulin sensitivity was decreased significantly with increased oxidative stress and increased inhibition of erythrocyte AChE activity, the latter indicative of the severity of OP pesticide exposure. OP pesticides inhibit the erythrocyte AChE activity and consequently increase the activity of nicotinic type ACh receptor, leading to oxidative stress. These findings support the hypothesis that OP pesticide exposure increases oxidative stress and contribute to the development of insulin resistance.
Acknowledgments
This research was approved by the University of Medicine, Magway. The authors wish to acknowledge with deep respect, Professor Dr. Aye Aye Oo, Professor and Head of Preventive and Social Medicine, University of Medicine, Magway for sample size calculation and data analysis. The authors deeply thank all participating volunteers who willingly gave consent for their great cooperation in this study.
Limitations of the study
The present study does not explore other conditions which may also affect AChE activity, such as anemia and heavy metal contamination of food and water. Determination of MDA by thiobarbituric acid reactive substances test is nonspecific and may affect adequate quantification of serum MDA level. As a preliminary study, ours is the first to assess erythrocyte AChE activity in agricultural workers exposed to organophosphate pesticides, and its association with oxidative stress and insulin sensitivity in agricultural workers in our country. As a cross-sectional study, it cannot clearly establish causality between exposures and outcomes. The study is not powered for a multivariate analysis where the interaction of various environmental and subject -related factors can be analyzed to look at the effects on the outcome of insulin sensitivity.
Statement of Authorship
All authors certified fulfillment of ICMJE authorship criteria.
Author Disclosure
The authors declared no conflict of interest.
Funding Source
None.
Authors are required to accomplish, sign and submit scanned copies of the JAFES Author Form consisting of: (1) Authorship Certification, that authors contributed substantially to the work, that the manuscript has been read and approved by all authors, and that the requirements for authorship have been met by each author; (2) the Author Declaration, that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere, that the article does not infringe or violate any copyrights or intellectual property rights, and that no references have been made to predatory/ suspected predatory journals; (3) the Author Contribution Disclosure, which lists the specific contributions of authors; and (4) the Author Publishing Agreement which retains author copyright, grants publishing and distribution rights to JAFES, and allows JAFES to apply and enforce an Attribution-Non-Commercial Creative Commons user license. Authors are also required to accomplish, sign, and submit the signed ICMJE form for Disclosure of Potential Conflicts of Interest. For original articles, authors are required to submit a scanned copy of the Ethics Review Approval of their research as well as registration in trial registries as appropriate. For manuscripts reporting data from studies involving animals, authors are required to submit a scanned copy of the Institutional Animal Care and Use Committee approval. For Case Reports or Series, and Images in Endocrinology, consent forms, are required for the publication of information about patients; otherwise, appropriate ethical clearance has been obtained from the institutional review board. Articles and any other material published in the JAFES represent the work of the author(s) and should not be construed to reflect the opinions of the Editors or the Publisher.
References
- 1.Evison D, Hinsley D and Rice P. Chemical weapons. BMJ.2002;324(7333): 332-5. PMID: PMCID: 10.1136/bmj.324.7333.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdollahi M, Balali-Mood M, Akhgari M, Jannat B, Kebriaeezadeh A, Nikfar S. A survey of cholinesterase activity in healthy and organophosphate-exposed populations. Irn J Med Sci. 1996;21(1&2): 63-66. [Google Scholar]
- 3.Shadnia S, Azizi E, Hosseini R, et al. Evaluation of oxidative stress and genotoxicity in organophosphorus insecticide formulators. Hum Exp Toxicol. 2005;24(9):439-45. PMID: 10.1191/0960327105ht549oa. [DOI] [PubMed] [Google Scholar]
- 4.Deotare ST, Chakrabarti CH. Effect of acephate (orthene) on tissue levels of thiamine, pyruvic acid, lactic acid, glycogen and blood sugar. Indian J Physiol Pharmacol. 1981;25(3):259-64. PMID: . [PubMed] [Google Scholar]
- 5.Reena K, Ajay K, Sharma CB. Haematological changes induced by dimethoate in rat. Arh Hig Rada Toksikol. 1989;40(1):23-7. PMID: . [PubMed] [Google Scholar]
- 6.Abdollahi M, Donyavi M, Pournourmohammadi S, Saadat M. Hyperglycemia associated with increased hepatic glycogen phosphorylase and phosphoenolpyruvate carboxykinase in rats following subchronic exposure to malathion. Comp Biochem Physiol C Toxicol Pharmacol. 2004;137(4):343-7. PMID: 10.1016/j.cca.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Montgomery MP, Kamel F, Saldana TM, Alavanja MCR, Sandler DP. Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural Health Study, 1993-2003. Am J Epidemiol. 2008;167:(10):1235-46. PMID: PMCID: 10.1093/aje/kwn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juntarawijit C, Juntarawijit Y. Association between diabetes and pesticides: A case-control study among Thai farmers. Environ Health Prev Med. 2018;23(1):3. PMID: PMCID: 10.1186/s12199-018-0692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hers HG. Mechanisms of blood glucose homeostasis. J Inherit Metab Dis. 1990;13(4):395-410. PMID: 10.1007/bf01799497. [DOI] [PubMed] [Google Scholar]
- 10.Ogut S, Gultekin F, Kisioglu AN, Kucukoner E. Oxidative stress in the blood of farm workers following intensive pesticide exposure. Toxicol Ind Health. 2011;27(9):820-5. PMID: 10.1177/0748233711399311. [DOI] [PubMed] [Google Scholar]
- 11.Rastogi SK, Satyanarayan PVV, Ravishankar D, Tripathi S. A study on oxidative stress and antioxidant status of agricultural workers exposed to organophosphorus insecticides during spraying. Indian J Occup Environ Med. 2009;13(3):131-4. PMID: PMCID: https://dx.doi.org/10.4103%2F0019-5278.58916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muniz JF, McCauley L, Scherer J, et al. Biomarkers of oxidative stress and DNA damage in agricultural workers: A pilot study. Toxic Appl Pharmacol. 2008;227(1):97-107. PMID: 10.1016/j.taap.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Guerrero-Hernández A, Leon-Aparicio D, Chavez-Reyes J, OlivaresReyes JA and DeJesus S. Endoplasmic reticulum stress in insulin resistance and diabetes. Cell Calcium. 2014;56(5):311-22. PMID: 10.1016/j.ceca.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Raafat N, Abass MA, Salem HM. Malathion exposure and insulin resistance among a group of farmers in Al-Sharkia governorate. Clin Biochem. 2012;45(18): 1591-5. PMID: 10.1016/j.clinbiochem.2012.07.108. [DOI] [PubMed] [Google Scholar]
- 15.Ellman GL, Courtney KD, Andres V Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88-95. PMID: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-9. PMID: 10.1007/bf00280883. [DOI] [PubMed] [Google Scholar]
- 17.Office of Pesticide Programs Label Review Manual. United States: United States Environmental Protection Agency, 2018. https://www.epa.gov/sites/production/files/2018-04/documents/lrm-completemar-2018.pdf.
- 18.Meller D, Fraser I, Kryger M. Hyperglycemia in anticholinesterase poisoning. Can Med Assoc J. 1981;124(6):745-8. PMID: PMCID: . [PMC free article] [PubMed] [Google Scholar]
- 19.Kempegowda P. Glycemic changes in acute anticholinesterase insecticide poisoning. West Lond Med J. 2013;5(1):27-33. [Google Scholar]
- 20.Ohayo-Mitoko GJA, Kromhout H, Simwa JM, Boleij JSM, Heederik D. Self reported symptoms and inhibition of acetylcholinesterase activity among Kenyan agricultural workers. Occup Environ Med. 2000;57(3):195-200. 10.1136/oem.57.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranjbar A, Pasalar P, Abdollahi M. Induction of oxidative stress and acetylcholinesterase inhibition in organophosphorous pesticide manufacturing workers. Hum Exp Toxicol. 2002;21(4):179-82. PMID: 10.1191/0960327102ht238oa. [DOI] [PubMed] [Google Scholar]
- 22.Namba T, Nolte CT, Jackrel J, Grob D. Poisoning due to organophosphate insecticides: Acute and chronic manifestations. Am J Med. 1971;50(4):475-92. PMID: 10.1016/0002-9343(71)90337-8. [DOI] [PubMed] [Google Scholar]
- 23.Haddad LM, Winchester JF. Clinical management of poisoning and drug overdose. Philadelphia: WB Saunders Company, 1983. [Google Scholar]
- 24.Tsakiris S, Schulpis KH, Tjamouranis J, Michelakakis H, Karikas GA. Reduced acetylcholinesterase activity in erythrocyte membranes from patients with phenylketonuria. Clin Biochem. 2002;35(8):615-9. PMID: 10.1016/s0009-9120(02)00381-8. [DOI] [PubMed] [Google Scholar]
- 25.Saldanha C. Human erythrocyte acetylcholinesterase in health and disease. Molecules. 2017;22(9):E1499. PMID: PMCID: 10.3390/molecules22091499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raoofi R, Jahromi HK, Jahromi ZK, Abedi HA, Sameni H, Pourahmad M. Antioxidant effects of green-tea on biochemical and histopathological changes of liver in male rats poisoned by malathion insecticide. Int J Med Res Health Sci, 2016;5(5):361-70. [Google Scholar]
- 27.Hashmi MA, Ahsan B, Shah SIA, Khan MIU. Antioxidant capacity and lipid peroxidation product in pulmonary tuberculosis. Al Ameen J Med Sci. 2012;5(3):313-9. [Google Scholar]
- 28.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407-21. PMID: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 29.Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366(1-2):1-13. PMID: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Verma RS, Mehta A, Srivastava N. In vivo chlorpyrifos induced oxidative stress: Attenuation by antioxidant vitamins. Pestic Biochem Phys. 2007;88(2):191-6. 10.1016/j.pestbp.2006.11.002. [DOI] [Google Scholar]
- 31.Aly N, El-Gendy K, Mahmoud F, El-Sebae AK. Protective effect of vitamin C against chlorpyrifos oxidative stress in male mice. Pestic Biochem Physiol. 2010;97(1):7-12. 10.1016/j.pestbp.2009.11.007. [DOI] [Google Scholar]
- 32.Panda S, Nanda R, Mangaraj M, Rathod PK, Mishra PK. Glycemic status in organophosphorus poisoning. J Nepal Health Res Counc. 2015;13(31):214-9. PMID: . [PubMed] [Google Scholar]
- 33.Elinav E, Shapira Y, Ofran Y, Hassin T, Ben-Dov IZ. Nearfatal amitraz intoxication: The overlooked pesticide. Basic Clin Pharmacol Toxicol. 2005;97(3):185-7. PMID: 10.1111/j.1742-7843.2005.pto_97399.x. [DOI] [PubMed] [Google Scholar]
- 34.Badrane N, Askour M, Berechid K, Abidi K, Dendane T, Zeggwagh AA. Severe oral and intravenous insecticide mixture poisoning with diabetic ketoacidosis: A case report. BMC Res Notes. 2014;7(1):485. PMID: PMCID: 10.1186/1756-0500-7-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214-23. PMID: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 36.Singh Y, Garg MK, Tandon N, Marwaha RK. A study of insulin resistance by HOMA-IR and its cut-off value to identify metabolic syndrome in urban Indian adolescents. J Clin Res Pediatr Endocrinol. 2013;5(4):245-51. PMID: PMCID: 10.4274/Jcrpe.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2007;29(1):42-61. PMID: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 38.Fu S, Watkins SM, Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15(5):623-34. PMID: 10.1016/j.cmet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Yalcin A, Hotamisligil GS. Impact of ER protein homeostasis on metabolism. Diabetes. 2013;62(3):691-3. PMID: PMCID: 10.2337/db12-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walter P, Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science. 2011;334(6059):1081-6. PMID: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]