TO THE EDITOR: Coronavirus disease 2019 (COVID-19) commonly presents with cough, fever, dyspnea, and myalgia [1]. Many patients develop severe complications, including thrombosis. The initial presentation of pulmonary embolism (PE) is similar to that of COVID-19 infection. An early study of patients with COVID-19 in the intensive care unit (ICU) demonstrated a 31% rate of venous thrombosis, 85% of which was PE [2]. A meta-analysis by Henrina et al. [3] analyzed 1,237 pooled patients from eight studies and found that venous thromboembolism was associated with higher mortality, need for ICU admission, and mechanical ventilation. To better understand the incidence of PE in a more general population of patients with upper respiratory symptoms suspicious of COVID-19 infection or PE, we studied the incidence of pulmonary embolism in patients presenting to our institution with upper respiratory symptoms during the height of the pandemic. We correlated this with COVID-19 infection status and other clinical characteristics to better understand the association with pulmonary embolism.
Under our Internal Review Board (IRB)-approved protocol (protocol number AAAS9652 approved 3/16/2020 to study patients between 2/28/2020–04/08/2020), we retrospectively queried our radiology record system, Catalyst, to identify reports of patients with a computed tomography angiogram (CTA) for the evaluation of pulmonary embolism during this time period. One hundred and thirty-four patients were identified; however, upon chart review, eight of these patients did not have all of the clinical data available; therefore, 126 patients were included in the study. Demographics and clinical information were obtained via chart review, including if patients were confirmed to have COVID-19 by a reverse transcription polymerase chain reaction test, information on comorbid conditions, highest D-dimer level during hospitalization (reported in mg/mL fibrinogen equivalent units), and clinical course of their disease, including outcomes and treatments. Chest CT scans were reviewed to assess for PE and for the presence or absence of consolidation. Consolidation was defined as a dense opacity in the pulmonary parenchyma that obscured blood vessels in the region. Clinical information regarding DVT, active cancer, and recent surgery was obtained for patients with PE.
Student’s t-tests and chi-squared tests were used for continuous and categorical variables, respectively, to evaluate differences between the COVID-19-positive and -negative groups and differences in patients with or without pulmonary embolism. Multivariable logistic regression analysis was employed to evaluate the characteristics associated with pulmonary embolism.
Of the 126 patients who met the inclusion criteria for this study, 51 tested positive for SARS-CoV-2 infection and 75 tested negative. Table 1 shows the demographic and clinical information for patients who were COVID-19-positive and COVID-19-negative. Twenty-four of the 126 patients had a PE, a rate of 19%, comprised of 10 COVID-19-positive patients (20%), and 14 COVID-19-negative patients (19%). There was no significant difference in the rate of pulmonary embolism between patients who were COVID-19-positive versus those who were COVID-19-negative. There was also no difference in the average age, rate of comorbid hypertension (HTN), diabetes mellitus (DM), or highest D-dimer between groups.
Table 1.
Demographics and clinical characteristics of COVID-19-positive and COVID-19-negative patients.
| COVID-19-Positive (%) | COVID-19-Negative (%) | P | |
|---|---|---|---|
| N of patients | 51 | 75 | |
| Average age (range) | 52.5 (23–86) | 55.6 (24–84) | 0.30 |
| Male sex | 29 (57) | 24 (32) | <0.01 |
| Oxygen saturation, % (range) | 94.1 (66–100) | 96.7 (88–100) | <0.01 |
| Consolidation | 34 (67) | 21 (28) | <0.01 |
| DM | 7 (14) | 12 (16) | 0.92 |
| HTN | 16 (31) | 29 (39) | 0.52 |
| Highest D-dimer (range) | 5.3 (0.2–20.0) | 3.3 (0.3–20.0) | 0.20 |
| Positive PE | 10 (20) | 14 (19) | 0.92 |
| PE and DVT | 1 (10) | 4 (29) | 0.27 |
| PE and recent surgery | 1 (10) | 1 (7) | 0.80 |
| PE and cancer | 0 (10) | 1 (7) | ∞ |
Abbreviations: DM, diabetes mellitus; DVT, deep vein thrombosis; HTN, hypertension; PE, pulmonary embolism.
There was a slight preponderance of male patients who tested positive for COVID-19 infection, which may have been related to sample size. There was a significantly lower average oxygen saturation on presentation in COVID-19- positive patients, likely due to several cases of severely low oxygen saturation in this population. There was a significantly increased rate of consolidation on chest CT in COVID-19-positive patients.
Certain clinical conditions predispose to pulmonary embolism. In patients with PE, we evaluated active cancer, DVT, and recent surgery to assess whether PE may have been provoked by a comorbid condition and whether these associations differed according to COVID-19 status. Table 1 also demonstrates comorbid conditions in patients found to have PE; 1/10 COVID-19-positive and 4/14 COVID-19-negative patients had concomitant DVT, 1/10 COVID-19-positive and 1/14 COVID-19-negative patients had recent surgery, and 0/10 COVID-19-positive and 1/14 COVID-19-negative patients had active cancer. There was no significant difference in any of these comorbid conditions between patients who were COVID-19-positive and those who were COVID-19-negative.
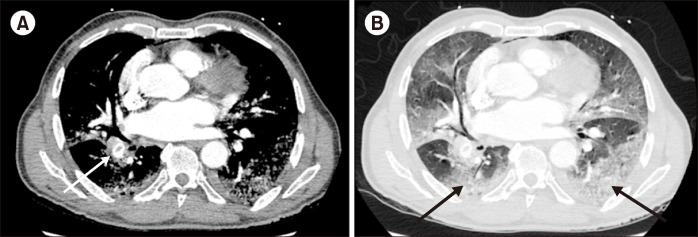
Table 2 shows the multivariable logistic regression analysis of patients with and without pulmonary embolism, demonstrating no difference in the rate of COVID-19 infection. Age, sex, presenting oxygen saturation, and comorbid HTN and DM were not associated with an increased rate of pulmonary embolism. The presence of consolidation on chest CT was associated with a higher risk of PE (OR 34.7, P=0.02). Patients with a higher D-dimer also had a higher risk of PE (OR 1.2, P=0.01). Fig. 1 demonstrates a patient with COVID-19 infection with bilateral consolidations and a pulmonary embolus.
Table 2.
Multivariable analysis with outcome of PE. Patients with consolidation had a higher risk of PE [OR 34.69, 95% CI (1.8, 664.31), P=0.02]; patients with higher d-dimer also had a higher risk of PE [OR 1.15, 95% CI (1.03, 1.29), P=0.01].
| (-) PE | (+) PE | OR | 95% CI | P | |
|---|---|---|---|---|---|
| N of patients | 102 | 24 | |||
| Average age | 54.8 | 52.7 | 0.98 | 0.92–1.1 | 0.73 |
| Male sex | 44 (43%) | 9 (38%) | 0.80 | 0.13–5.0 | 0.82 |
| Oxygen saturation, % | 95.6 | 95.7 | 1.00 | 0.9–1.1 | 0.96 |
| Consolidation | 40 (40%) | 14 (58%) | 34.70 | 1.8–664.3 | 0.02 |
| DM | 13 (13%) | 6 (25%) | 2.60 | 0.4–18.0 | 0.34 |
| HTN | 38 (37%) | 7 (29%) | 2.10 | 0.3–16.0 | 0.48 |
| COVID-19 | 41 (40%) | 10 (42%) | 0.25 | 0.0–2.0 | 0.19 |
| Highest D-dimer (average) | 2.9 | 10.8 | 1.20 | 1.0–1.3 | 0.01 |
Abbreviations: COVID-19, coronavirus-2019; DM, diabetes mellitus; HTN, hypertension.
Fig. 1.
This figure demonstrates an acute pulmonary embolism (white arrow) in a COVID-19 infected patient in soft tissue (A) and lung (B) windows. Pulmonary disease is characterized by peripheral and lower lobe predominant ground glass opacities (black arrows), typical of COVID-19 infection.
Research has shown that D-dimer levels correlate with worse outcomes in COVID-19 (Pernia et al., manuscript under review). We found that elevated D-dimer levels correlated with the incidence of PE, yet there was no increased incidence of PE on chest CT in patients who were COVID- 19-positive compared to those who were COVID-19-negative. The role of microthrombi in COVID-19 infection has been controversial but is increasingly recognized as being important in disease pathophysiology. The first reported cases of COVID-19 infection did not identify pulmonary microthrombi [4], which was corroborated by subsequent autopsy [5] and biopsy studies [6]. However, using ultrasound-based minimally invasive autopsies, a study from Brazil demonstrated that the majority of patients had pulmonary microthrombi in arterioles in both damaged and preserved regions of the lung. This group also found secondary signs of coagulation cascade activation, including endothelial tumefaction and large numbers of megakaryocytes [7]. The disparity in pathologic findings may have been due to the limited number of patients and the method of pulmonary parenchymal sampling in studies lacking pulmonary microthrombi. Together, these studies suggest that microthrombi may be the cause of elevated D-dimer levels and perfusion abnormalities in these patients. Given the lack of randomized trials assessing anticoagulation in COVID-19 infection, widespread use of general venous thromboembolism prophylaxis guidelines for hospitalized patients has also been employed [8].
Studies have demonstrated an increased incidence of thrombotic events in patients with COVID-19 [2, 9], and the low rate of PE demonstrated in our study was an unexpected finding. In thrombosis, as well as in diabetes research, distinction is made between microvascular and macrovascular thrombosis. This distinction is also applicable in COVID-19 infection. Clinical imaging of microthrombi is challenging given the limits of resolution in diagnostic radiology [10]. Dual-energy CT is a technique that uses differences in beam attenuation at different energies to assess material densities within tissue. It has been used to demonstrate differences in pulmonary perfusion secondary to pulmonary embolism. A recent study published in Lancet Infectious Diseases using dual-energy CT to assess patients with COVID-19 infection demonstrated striking perfusion abnormalities in the absence of visualized pulmonary embolism [11]. Widespread use of dual-energy CT to assess pulmonary perfusion in patients with COVID-19 may provide important clinical information in the absence of pulmonary embolism on chest CT.
The limitations of our study include the fact that it was a single center study. Given the widespread nature of the pandemic and numerous early reports of increased thrombotic events, clinicians may have changed their ordering practices during this time period. The data available for this retrospective study allowed us to assess the rate of pulmonary embolism in patients with COVID-19 versus those with similar symptoms without COVID-19. A prospective study may have provided additional information as to whether these patients went on to develop pulmonary emboli at a later time point. However, given that these limitations equally affected the COVID-19-negative and -positive populations, it is unlikely that they would have introduced significant differences in the conclusions drawn from these data.
Our study demonstrated no difference in the rate of pulmonary embolism in patients with COVID-19 compared to patients presenting with similar symptoms without COVID-negative. Mounting evidence suggests that patients with severe COVID-19 have diffuse microthrombotic disease resulting in pulmonary parenchymal infarcts. Further studies testing this hypothesis will provide evidence to strengthen the guidelines for treating microthrombotic disease in patients with COVID-19 infection.
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest
Mary M. Salvatore- Speaker and Consultant: Genentech, Boehringer Ingelheim. Grant funding: Genentech, Boehringer Ingelheim. The remaining authors have no conflicts to disclose.
REFERENCES
- 1.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–7. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henrina J, Putra ICS, Cahyadi I, Gunawan HFH, Cahyadi A, Suciadi LP. Clinical characteristics and outcomes of venous thromboembolism in patients hospitalized for COVID-19: systematic review and meta-analysis. medRxiv. 2020:20130922. doi: 10.1101/2020.06.14.20130922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–4. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–33. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian S, Xiong Y, Liu H, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Modern Pathol. 2020;33:1007–14. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost. 2020;18:1517–9. doi: 10.1111/jth.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198–225. doi: 10.1182/bloodadvances.2018022954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clerkin KJ, Fried JA, Raikhelkar J, et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–55. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 10.Allen J, Howell K. Microvascular imaging: techniques and opportunities for clinical physiological measurements. Physiol Meas. 2014;35:R91–141. doi: 10.1088/0967-3334/35/7/R91. [DOI] [PubMed] [Google Scholar]
- 11.Lang M, Som A, Mendoza DP, et al. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30367-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]