Abstract
Individuals with Type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) are at increased risk for fragility fractures. Bone mineral density (BMD) is decreased in T1DM but often normal or even elevated in T2DM when compared with age-matched non-DM populations. However, bone turnover is decreased in both T1DM and T2DM. The pathophysiologic mechanisms leading to bone fragility is multifactorial, and potentially leads to reduced bone formation, altered bone microstructure and decreased bone strength. Interestingly, different antidiabetic treatments may influence fracture risk due to effects on glycemic control, triggering of hypoglycemic events or osteoblastogenesis.
Keywords: bone metabolism, diabetes mellitus, bone remodeling, biomarkers
INTRODUCTION
Diabetes mellitus (DM) is a chronic metabolic non-communicable disease with increasing global prevalence. By 2015, there were over 415 million adults living with DM, and this number is expected to increase to 642 million by 2040.1 Apart from the major DM-related complications such as cardiovascular diseases, osteoporotic fracture is increasingly recognized as an important complication of type 1 DM (T1DM) and type 2 DM (T2DM) in both men and women.2 Worldwide, over 9 million osteoporotic fractures occur annually, and the effect of reduced bone mineral density (BMD), including osteoporosis, is predicted to result in over 5 million disability adjusted life years (DALY) and 188,000 deaths each year. The incidence of hip fractures in individuals with T1DM was 383 per 100,000, six-fold higher than the overall incidence of hip fracture in the age-matched, nondiabetic population.3 The odds ratio of vertebral fracture in T2DM was 1,86 and 4,73 in women and men,4 respectively, with a relative risk of 1,83 (95% CI: 1,25-1,53).5 These studies were largely done using the cross-sectional design and showed only associations rather than causality of DM and the incidence of fracture. However, taken together, these data indeed show the increased fracture risk in individuals with DM. The presence of microvascular complications in DM have also been associated with reduction of BMD in T1DM5 and with bone micro-architectural abnormalities in T2DM.6-9
Increasing evidence shows the interaction between plasma glucose levels and bone metabolism, revealing mechanisms through which bone fragility may develop in DM. Whether this interaction translates into increased risk for fragility fractures and decreased BMD in all DM populations remains unclear. Studies reported conflicting findings of changes in BMD. Whereas BMD is decreased in T1DM,10-15 it is either increased or unchanged in T2DM.16-21 Intriguingly, a meta-analysis found that both DM types are associated with increased risk of hip fracture.2 In this review, we discuss bone metabolism and remodeling, the pathophysiologic mechanisms by which bone fragility may occur in DM, and the effects of glucose-lowering drugs on bone health.
Bone Metabolism and Remodeling
The structural components of bone consist of a largely mineralized extracellular matrix, collagen, and cells. Bone is a living organ that is continuously being remodeled, in a process that involves a balance in the tearing down of bone structure (bone resorption) and its rebuilding (bone formation). This resorption and formation allows for the repair of micro-fractures and the modification of structure in response to stress.22 Bone resorption is initiated by osteoclasts, which attach to bone surface and secrete acid and hydrolytic enzymes that resorb bone, releasing minerals and collagen fragments.23 After osteoclastic resorption is completed, a reversal phase takes place in which mononuclear cells prepare the bone surface for new osteoblasts to begin bone formation by laying down a layer of glycoprotein-rich material to which the osteoblasts can adhere.24 Bone formation is subsequently initiated by osteoblasts, which produced type I collagen and other proteins, such as osteocalcin, which then form osteoid, a substrate for which mineralization can occur. The newly formed osteoid then begins to accumulate matrix molecules and mineralize.22 In healthy adults, bone resorption and formation is a tightly balanced process. Both high or low rates of remodeling with an imbalanced bone resorption and formation can be associated with decreased or increased bone mass.
The synthesis of type I collagen during the bone formation phase involves the intertwining of one alpha-2 and two alpha-1 polypeptide chains to form a helical structure known as procollagen, followed by cleavage of their amino-terminal and carboxy-terminal peptides to form tropocollagen. The N-telopeptide (NTX) is the pyridinoline crosslink in the N-telopeptide region that joins alpha-1 chains to alpha-2 chains,25 whereas the Ctelopeptide (CTX) is a fragment of the alpha-1 peptide with an isomerized bond between the aspartate and the glycine from the carboxytelopeptide region.26 NTX and CTX, together with the bone-specific alkaline phosphatase and amino terminal propeptide of type 1 procollagen (P1NP) are the most clinically useful markers of bone turnover.27,28 Osteoblasts produce osteocalcin, which is also used as a marker of bone formation.29 Furthermore, bone resorption results in the release of bone mineral and the collagen-rich osteoid, whereas osteoid formation involves the production of the byproducts of collagen and other proteins. These substances may be released in the circulation, and can be measured in serum and urine to provide information on the rate of bone resorption and formation, and are collectively termed in the clinic as "bone turnover markers" (BTM)23 (Table 1).
Table 1.
Bone turnover markers
| Markers | Full name | Origin | Comment | Source of Variability |
||
|---|---|---|---|---|---|---|
| Renal | Liver | Circadian rhythm | ||||
| Resorption | ||||||
| u-CTX | Urinary carboxy-terminal cross-linking telopeptide of type I collagen | Osteoclastic hydrolysis of collagen, generated by cathepsin K | Requires adjustment to levels of urinary creatinine Specificity: collagen type I, with highest contribution probably from bone Changes in levels of u-CTX were reported in both T1DM and T2DM Pioglitazone is associated with increased levels of u-CTX110 |
X | ||
| s-CTX | Serum carboxy-terminal cross-linking telopeptide of type I collagen |
Osteoclastic hydrolysis of collagen, generated by cathepsin K | Source of variability: food consumed (so must be collected after an overnight fast) Changes in levels of s-CTX were reported in both T1DM and T2DM Pioglitazone is associated with increased levels of s-CTX110 |
X | X | X |
| u-NTX | Urinary amino-terminal cross-linking telopeptide of type I collagen |
Osteoclastic hydrolysis of collagen type I | Requires adjustment to levels of urinary creatinine Specificity: collagen type I, with highest contribution probably from bone Changes in levels of u-NTX were reported in both T1DM and T2DM Pioglitazone is associated with increased levels of u-NTX110 |
X | ||
| s-NTX | Serum amino-terminal cross-linking telopeptide of type I collagen |
Osteoclastic hydrolysis of collagen type I, generated by cathepsin K | Specificity: collagen type I, with highest contribution probably from bone Changes in levels of u-NTX were reported in both T1DM and T2DM Pioglitazone is associated with increased levels of s-NTX110 |
X | X | |
| s-ICTP or CTX-MMP | Carboxy-terminal crosslinking telopeptide of type I collagen |
Osteoclastic hydrolysis of collagen generated by matrix metalloproteinases | Specificity: collagen type I, with highest contribution probably from bone Marker is not responsive to usual treatments for osteoporosis Lower s-PICP to s-ICTP ratio were reported in T2DM1 Troglitazone use in T2DM individuals is associated with a decrease in s-ICTP111 |
X | X | X |
| u-DPD | Urinary deoxypyridinoline |
Proteolytic hydrolysis of collagen, found in bone |
Requires adjustment to levels of urinary creatinine Specificity: highest contribution from bone Sources of variability: UV radiation Changes in levels of u-DPD were reported in both T1DM and T2DM112 Troglitazone use in T2DM individuals is associated with a decrease in u-DPD111 |
X | ||
| u-PYD | Urinary pyridinoline , | Found in bone cartilage, tendon, blood vessels | Requires adjustment to urinary creatinine Specificity: highest contribution from bone and cartilage Sources of variability: active arthritis and UV radiation |
X | X | |
| s-TRAP | Serum tartrate- | Includes two isoforms: | Sources of variability: influenced by haemolysis and blood clotting | X | ||
| resistant acid | type 5a (platelets, | Changes in levels of s-OC were reported in both T1DM and | ||||
| phosphatase | erythrocytes and other sources) and type 5b (osteoclasts) | T2DM112 Levels of s-TRAP is affected by long-term use of insulin in T1DM36 |
||||
| Formation | ||||||
| s-OC | Serum osteocalcin | Hydroxyapatite-binding protein exclusively synthesised by osteoblasts and odontoblasts | Specificity: specific marker of osteoblast function Rapid degradation in serum may lead to heterogeneity of OC fragments measured Sources of variability: large inter-laboratory variation Changes in levels of s-OC were reported in both T1DM and T2DM |
X | X | |
| u-OC | Urinary osteocalcin | Hydroxyapatite-binding protein exclusively synthesised by osteoblasts and odontoblasts |
Adjusted to levels of urinary creatinine (/Cr) Specificity: specific marker of osteoblast function Changes in levels of u-OC were reported in non-insulin dependent DM112 |
X | X | |
| s-ALP | Serum alkaline phosphatase (total) | Ubiquitous, membrane bound tetrameric enzyme located on the outer cell surface of various tissues: liver, bone, intestine, spleen, kidney and placenta |
Specificity: non-specific for bone (about 50% is liver isoform in healthy individuals) Changes in levels of s-ALP were reported in both T1DM and T2DM Troglitazone use in T2DM individuals is associated with a decrease in s-ALP111 |
X | ||
| s-BALP | Serum bone-specific alkaline phosphatase | Ubiquitous, membrane bound tetrameric enzyme located on the outer cell surface of osteoblasts |
Specificity: specific for bone, but with some cross-reactivity with liver isoform (up to 20%) Changes in levels of s-BALP were reported in T2DM112 Troglitazone use in T2DM individuals is associated with a decrease in s-BALP111 |
X | ||
| s-PICP | Procollagen type I C propeptide | Precursor molecules of collagen type I synthesised by osteoblasts | Specificity: mostly derived from bone collagen type I (around 90%). Short serum half-life. Regulated by hormones (thyroid, IGF-1) Lower s-PICP to s-ICTP ratio were reported in T2DM12 |
X | ||
| s-PINP | Procollagen type I N propeptide | Precursor molecules of collagen type I synthesised by osteoblasts | Specificity: mostly derived from bone collagen type I A ssay: may recognise trimer alone (intact) or trimer and monomer (total PINP) Changes in levels of s-P1NP were reported in both T1DM and T2DM |
X | ||
Adapted from Vasikaran et al.109
Fracture Risk and Diabetes Mellitus
Fracture risk is significantly higher in both T1DM and T2DM populations when compared to the general population.2 The incidence of hip fracture in individuals with T1DM were reported to be six times higher than in the population (mean age 65 years) and 2,5-fold higher than in the T2DM population.3
T1DM
A meta-analysis of 5 studies reported that T1DM is associated with an overall relative risk (RR) of 8,9 (95% CI 7,1–11,2) for hip fractures when compared with an age-matched nondiabetic population.2 Most studies in young and older, male and female individuals with T1DM reported a decrease in BMD at the radius and femur.30-38 This decrease ranges from 22 to 37%.5 Individuals with T1DM showed decreased trabecular and/or cortical volumetric BMD at the distal radius or tibia compared with non-diabetic controls,30,39-43 and some studies reported the associations of these alterations with poor glycemic control.40, 41
T2DM
The risk of hip fracture is particularly increased in individuals with T2DM.21,44,45 The risk is even higher in those treated with insulin3,46 and poor glycemic control,47 as reflected by high HbA1c levels, which may indicate the more advanced disease state. Studies have also reported increased fracture risk in individuals with more hypoglycemic episodes.48 A meta-analysis of four cohorts showed that the RR of hip fractures reached 2,7 (95% CI, 1,7-4,4).2 The risks for other fractures appear to also increase in T2DM compared to healthy individuals, such as fractures of the wrist 49 and foot,21,50 as well as of the vertebrae.4
Although earlier studies reported lower or unchanged BMD, recent large studies found that in T2DM, in contrast with T1DM, BMD is increased when compared to controls.20,49, 51-60 Furthermore, this increase in BMD remained after adjustment for body weight and composition,55, 60 and ranges between 5 to 10% above age-matched, non-diabetic controls.50 Bone fragility depends not only on the reduction in bone mineral mass, as reflected by BMD, but also from changes to the bone microstructure and the components of the bone material. This is likely to account for the increased risk of fracture despite the increased BMD seen in individuals with T2DM. Indeed, MRI studies revealed greater cortical porosity in individuals with T2DM compared with non-diabetic controls,61,62 a finding repeated by a study using quantitative CT (Xtreme-CT), especially in those with fractures and/or microvascular complications.6-9 Recent diagnostic advances enable the measurement of in vivo bone material strength (BMS) by the minimally invasive, bone microindentation testing.7,63 Postmenopausal women with T2DM demonstrated lower BMS and greater radial cortical porosity. Poor BMS was correlated with poor long-term glycemic control over the past 10 years.7 A study in a similar population with fragility fractures suggests that severe deficits in cortical bone quality, as depicted by an increase in porosity, is a likely cause of fragility fractures.8 Regardless of the difference in BMD alterations between T1DM and T2DM, DM alone has been shown to be predictive of increased post-fracture mortality risk during hospitalization64 and up to one year after discharge65,66 in individuals with hip fracture.
Mechanisms of DM-induced Bone Fragility
The mechanisms of DM-induced bone fragility in T1DM and T2DM are complex and only partially overlap.67 Individuals with T1DM are mainly experiencing β‑cell failure and low levels of IGF1which disrupt the function of osteoblasts during growth. As a result, low peak bone mass can occur at a young age.68 In contrast, individuals with T2DM developed bone fragility at a later stage of the disease, and consequently, at a later age due to the lack of insulin, glucose toxicity, advanced glycation end products (AGEs), cytokines and adipokines that are affecting osteocyte, bone turnover and collagen.69
Low Bone Turnover
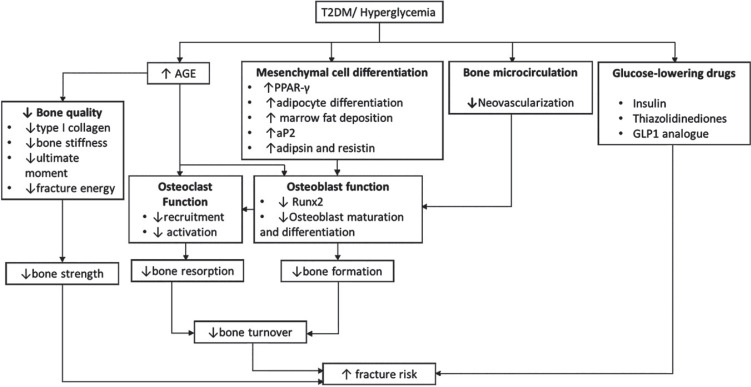
Most published studies in individuals with DM have reported low bone turnover (Table 2). Osteocalcin level, a marker of bone formation, is decreased in both T1DM and T2DM,70-72 and is negatively correlated with HBA1c level.70 The negative correlation with HBA1c was also reported for CTX, a marker of bone resorption.70 When looking separately at T1DM and T2DM, osteocalcin levels have been reported to be decreased in T1DM and only borderline significantly decreased in T2DM.73 Similarly, P1NP and NTX also tended to be lower in individuals with DM.5 Consistently, histological study of DM found decreased number of osteoblasts and osteoid.74 In general, the processes involved in the decreased bone formation in T2DM include a decrease in bone quality, alterations of the mesenchymal cell differentiation and bone microcirculation, as well as changes in osteoblasts and osteoclasts (Figure 1).
Table 2.
Studies reporting on bone turnover in individuals with DM
| Study author | Participants | BTM measured | Comments |
|---|---|---|---|
| Reyes-Garcia et al.; 2013110 | 78 T2D (43 men, 35 women), 55 controls | OC (ns)- RIA) (DiaSorin, Stillwater, Minnesota USA; normal range 1.8–6.6 ng/ml, CTX ↓ EIA (Elecsys ft CrossLaps, Roche Diagnostics SL, |
Vertebral fractures in 27.7% of T2D and 21.7% of controls Cross-sectional |
| Yamamoto et al.; 2012111 | 255 T2D (postmenopausal women and men), 240 controls |
Barcelona, Spain; normal range 0.01-6 ng/ml) OC↓, CTX↓ (electrochemiluminescence immunoassay on an automated analyzer; Roche Diagnostics GmbH, Mannheim, Germany), PTH↓ |
Excluded if serum creatinine was higher than normal range |
| Manavalan et al.; 2012112 | 18 T2D PM, 27 controls PM | OC↓, ELISA (IDS), CTX ↓ ELISA | At least 1 year use of antiglycemic medication eGFR b 60 ml/min excluded |
| Bhattoa et al.; 2013113 | 68 male T2D, 68 male controls | OC↓, CTX↓ electrochemiluminescence immunoassay (Roche Diagnostics GmbH, Mannheim, Germany). | Renal disease excluded Case-control |
| Ardawi et al.; 2013114 | 482 T2D PM women, 482 controls PM | LIASON autoanalyzer (DiaSorin Inc., Stillwater, MN, USA) | VF in 24.5% of T2D and none in controls |
| Hamilton et al.; 2012115 | 26 T1D, 27 T2D | CTX ↑, OC (ns), PTH (ns) | |
| Akin et al.; 2003116 | 57 T2D PM, 20 controls PM | OC↓, NTX↓ | BMI significantly lower in controls, fasting, chronic disease excluded |
| Reyes-Garcia et al.; 2013110 | 78 T2D, 55 controls | CTX↓j, PTH↓, enzyme immunoassay (EIA) and ELISA | Vertebral fractures in 27.7% of T2D and 21.7% of controls |
| Jiajue et al.; 2014117 | 236 T2D PM, 1055 controls PM | CTX↓, PINP↓ | Renal disease excluded |
| Farr et al.; 20147 | 30 T2D PM, 30 controls PM | CTX↓, PINP↓ | Stage 4 and 5 chronic kidney diseases excluded MI significantly lower in controls. Performs microindentation |
| Manavalan et al.; 2012112 | 18 T2D PM, 27 controls PM | Circulating OC(+) cells ↓ | At least 1 year use of antiglycemic medication eGFR b 60 ml/min excluded |
| Bhattoa et al.; 2013118 | 68 male T2D, 68 male controls | OCj, CTX↓ | Renal disease excluded |
| Gaudio et al.; 2012119 | 40 T2D PM, 40 controls PM | CTX↓ | Renal bone disease excluded |
| Ardawi et al.; 2013114 | 482 T2D PM, 482 controls PM | IGF-1 ↓, sclerostin T, OC↓, CTX↓, PINP↓, NTX↓ | VF in 24.5% of T2D and none in controls |
| Hernandez et al.; 2013120 | 2431 subjects of these 45 T2D | CTX and P1NP↓ in T2DM individuals who use statins | PM females and older men , Coexisting medical disorder that might affect bone metabolism was excluded. |
| Sarkar and Choudhury; 2013121 | 108 T2D, 50 controls | OC↓ | T2D was newly diagnosed. |
| Movahed et. al.; 2012122 | 382 PM of these 102 T2D | OC↓, CTX↓ | The diabetes group is a subgroup of the total population. |
| Sosa et al.; 1996123 | 47 female NIDDM, 252 female controls | OC (ns), ALP (ns) | No renal disorders |
| Chen et al.; 2013124 | 55 T2D, 27 controls | Plasma ALP↑, OC↓ | No history of metabolic bone disease |
Alkaline phosphatase (ALP), C-terminal cross-link of collagen (CTX), estimated glomerular filtration rate (eGFR), insulin-like growth factor-1 (IGF-1), myocardial infarction (MI), Non-insulin dependent diabetes mellitus (NIDDM), not significant (ns), tosteocalcin (OC), procollagen type 1 N-terminal propeptide (P1NP), postmenopausal (PM), parathyroid hormone (PTH), type 1 diabetes (T1D), type 2 diabetes (T2D), vertebral fracture (VF),
Figure 1.
Process involved in the decrease of bone turnover and increase of fracture risk.
Adipokines
Adiponectin, a protein hormone secreted by adipose tissue, was found to be decreased in T2DM.75 Adiponectin was reported to have an anabolic effect on osteoblasts and inhibits osteoclastic activity in vitro.76 However, clinical studies reported conflicting findings on whether there were negative correlations between adiponectin levels and BMD in individuals with T2DM. Leptin, another adipokine which is secreted by white adipose, bone marrow adipocytes and osteoblastic cells, was found to be lower in individuals with DM compared with controls. A negative correlation between leptin and NTX was found in individuals with T2DM, whereas a positive correlation was found with leptin and Z-scores at the distal radius, but not at the femoral neck or lumbar spine.77 Interestingly, in vitro and animal studies showed that high glucose level increases the expression of adipogenic markers such as the peroxisome proliferator-activated receptor (PPAR)-γ, adipocyte fatty acid binding protein (aP2), resistin and adipsin, whereas it suppresses cell growth, mineralization, and expression of osteogenic markers including Runx2, collagen I, osteocalcin, osteonectin.78,79 Further studies are needed to precisely explain the role of adiponectins in affecting bone fragility.
Advanced Glycation End Products (AGEs)
Individuals with DM have increased levels of AGEs due to hyperglycemia and increased levels of oxidative stress.80 The main mechanisms by which AGEs contribute to damaging the bone tissue are: 1) by forming cross-links with target protein, permanently altering cellular structure, and 2) by interacting with specific receptors to increase oxidative stress and inflammation.81 The receptor for AGEs (RAGE) initiates the intracellular signaling through the binding of AGEs.82 The soluble isoform of RAGE (known as soluble RAGE, sRAGE) is thought to be produced by proteolytic cleavage of disintegrin and metalloproteinase domain-containing proteins (ADAMs). Activation of the RAGE signaling pathway leads to a positive feedback loop by enhancing the NF-kB expression. Subsequently, important inflammatory mediators, including tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), IL-6, and C-reactive protein (CRP) are upregulated through both AGE- and NF-kB-mediated pathways.82 Increased AGE concentration is negatively associated with bone density and mineralization,83 and the cross-linking of AGE with collagen alters the mechanical properties of bone, disrupting its remodeling, increasing its stiffness and fragility.84-86 Pentosidine, a well-known AGE, was also shown to disrupt osteoblast differentiation.87 Studies found that poor glycemic control was associated with increased risk of fractures in individuals with DM, and suggest that HbA1c level of <8% could reduce fracture risk in individuals with DM.
Insulin and IGF1
Insulin exerts an anabolic effect on bones by promoting osteoblast proliferation and differentiation.88 Animal studies have shown that diabetic rodents have impaired bone formation following bone injury whereas insulin injection normalized it.89 Insulin deficiency, as in T1DM, is characterized by low levels or activity of insulin-like growth factor 1 (IGF1). The stimulating activity of IGF1 on osteoblasts is inhibited by high concentration of AGEs or glucose.90,91 In contrast with T1DM, T2DM is a disease that mainly shows insulin resistance. It remains unclear how in T2DM insulin resistance and insulin deficiency at its later stage may affect bone metabolism and fragility.
Pro-inflammatory cytokines
Pro-inflammatory cytokines have been implicated in both T1DM and T2DM and in the development of complications of both diseases. Elevated pro-inflammatory cytokine levels, such as TNF and IL-6, can activate osteoclastogenesis and inhibit osteoblast differentiation.92,93 Indirectly, the reactive oxygen species generated due to the exposure of tissue to IL-1, IL-6 and TNF can affect the differentiation and survival of osteoclasts, osteoblasts, and osteocytes.
Glucose-lowering Drugs and Bone Metabolism
Antidiabetic treatment is aimed at achieving good glucose control to reduce the risk of complications. Data showed that 1% reduction in HBA1c levels led to 37% reduction in microvascular complication endpoints.94 As HBA1c, microvascular complications and bone fragility have been shown to be interrelated, it is reasonable to consider that optimal glucose control may reduce fracture risk. Individuals with poor glycemic control have increased risk for fractures.47,95,96 In individuals with T2DM, HBA1c levels ≥7,5% were reported to have 62% higher risk for fractures compared to those with HBA1c levels <7,5%. The ACCORD trial reported that there was no substantial benefit for fracture prevention or BMD changes in lowering HBA1c below 7,5%.
Insulin was shown to increase the risk of falls in insulin-treated individuals if their HBA1c levels were ≤6%. It appeared that more aggressive glycemic control in elderly individuals with long term disease might increase hypoglycemic events and thus the risk for falls and fractures.97 Metformin, the first line drug for DM, was found from most clinical studies to have positive or
neutral effect on BMD and fracture risk in large cohorts.46,98,99 Sulfonylureas show neutral effect on BTM levels, and studies on its clinical effect has not been established.46 However, sulfonylureas should be avoided in individuals at risk for bone fragility due to its risk for inducing hypoglycemic events.67,100 Thiazolidinediones, which includes rosiglitazone and pioglitazone, activate peroxisome proliferator-activated receptors (PPARs), particularly PPAR-γ. In vitro and in vivo studies show increased adipogenesis and impaired osteoblastogenesis. Meta-analyses confirmed an increased risk for fractures (OR 2.23, 95% CI 1.65–3.01101 and OR=1.94; 95%CI: 1.60-2.35102) in women treated with pioglitazone or rosiglitazone, but not in men. The evidence on the incretin-based treatments, GLP1 analogues and DPP4 inhibitors, are less conclusive.67 A meta-analysis found that two different GLP1 analogues, liraglutide and exenatide, had protective and negative effects, respectively, on fracture risk. However, these studies were not designed for bone outcomes and differ in their design and power.103 Studies on DPP4 inhibitors also did not find consistent effects on fracture outcomes.104,105 Sodium/glucose co‑transporter 2 (SGLT2) inhibitors are new generation antidiabetics which exert effects by inhibiting glucose reabsorption in the proximal tubule of the kidney.106 Data has also not been consistent in this group of drugs. While dapagliflozin and empagliflozin seem to have a neutral effect on bone turnover and BMD parameters, canagliflozin was reported to cause bone loss at the hips107,108 and increase the risk for hip fractures.
CONCLUSIONS
Fracture risk is known to be increased in both T1DM and T2DM. Levels of BTM were also lower in individuals with DM compared to non-DM controls. Despite increasing data on the association between BMD, BTM and fracture in individuals with DM, there are still challenges in identifying those with high fracture risk. Oxidative stress, inflammation and the production of AGEs increase the risk of complications. Additionally, disturbances in bone collagen metabolism and bone mineralization also reduce bone strength, while altered fat metabolism also affects bone health. A population of individuals are treated with insulin, but its use has been associated with increased fracture risk.46 It remains unclear whether insulin use is merely a marker for the severity or duration of disease, or induces more hypoglycemic events that lead to falls. Furthermore, it is unknown whether in DM, changes in bone metabolism occurs earlier in the disease course. It is therefore important to consider the treatment approach and education of fall prevention in these individuals who are already at increased risk for fractures. Medications with favorable effect on bone metabolism such as metformin or incretin-based treatments may be the preferred treatment while thiazolidinediones should be used with careful evaluation and patient education. Evaluation by use of BTM may be of benefit, but needs further studies in particular populations of individuals with DM such as premenopausal women or the Indonesian population.
Statement of Authorship
All authors certified fulfillment of ICMJE authorship criteria.
Author Disclosure
The authors declared no conflict of interest.
Funding Source
The authors received research grant from Hibah Penelitian Unggulan Perguruan Tinggi (PUPT) from the Directorate of Research and Public Services, Ministry of Research, Technology and Higher Education.
Authors are required to accomplish, sign and submit scanned copies of the JAFES Author Form consisting of: (1) Authorship Certification, that all the requirements for authorship have been met by each author, and that the final version of the manuscript has been read and approved by all authors; (2) the Author Declaration, that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere; (3) the Statement of Copyright Transfer [accepted manuscripts become the permanent property of the JAFES and are licensed with an Attribution-Share Alike-Non-Commercial Creative Commons License. Articles may be shared and adapted for non-commercial purposes as long as they are properly cited]; and the ICMJE form for Disclosure of Potential Conflicts of Interest. For original articles, authors are required to submit a scanned copy of the Ethics Review Approval of their research as well as registration in trial registries as appropriate. For manuscripts reporting data from studies involving animals, authors are required to submit a scanned copy of the Institutional Animal Care and Use Committee approval. For Case Reports or Series, and Images in Endocrinology, consent forms, are required for the publication of information about patients; otherwise, authors declared that all means have been exhausted for securing such consent. Articles and any other material published in the JAFES represent the work of the author(s) and should not be construed to reflect the opinions of the Editors or the Publisher.
References
- 1.International Diabetes Federation IDF Diabetes Atlas, 7th ed, 2015. [Google Scholar]
- 2.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166(5):495-505. PMID: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- 3.Janghorbani M, Feskanich D, Willett WC, Hu F. Prospective study of diabetes and risk of hip fracture: The nurses’ health study. Diabetes Care. 2006;29(7):1573-8. PMID: 10.2337/dc06-0440. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto M, Yamaguchi T, Yamauchi M, Kaji H, Sugimoto T. Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Miner Res. 2009;24(4):702-9. PMID: 10.1359/jbmr.081207. [DOI] [PubMed] [Google Scholar]
- 5.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes -- a meta-analysis. Osteoporos Int. 2007;18(4):427-44. PMID: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 6.Burghardt AJ, Issever AS, Schwartz AV, et al. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95(11):5045-55. PMID: PMCID: 10.1210/jc.2010-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farr JN, Drake MT, Amin S, Melton LJ 3rd, McCready LK, Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res. 2014;29(4):787-95. PMID: PMCID: 10.1002/jbmr.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patsch JM, Burghardt AJ, Yap SP, et al. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res. 2013;28(2):313-24. PMID: PMCID: 10.1002/jbmr.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shanbhogue VV, Hansen S, Frost M, et al. Compromised cortical bone compartment in type 2 diabetes mellitus patients with microvascular disease. Eur J Endocrinol. 2016;174(2):115-24. PMID: 10.1530/EJE-15-0860. [DOI] [PubMed] [Google Scholar]
- 10.Muñoz-Torres M, Jódar E, Escobar-Jiménez F, López-Ibarra PJ, Luna JD. Bone mineral density measured by dual X-ray absorptiometry in Spanish patients with insulin-dependent diabetes mellitus. Calcif Tissue Int. 1996;58(5):316-9. PMID: . [DOI] [PubMed] [Google Scholar]
- 11.Miazgowski T, Czekalski S. A 2-year follow-up study on bone mineral density and markers of bone turnover in patients with long-standing insulin-dependent diabetes mellitus. Osteoporos Int. 1998;8(5):399-403. PMID: 10.1007/s001980050082. [DOI] [PubMed] [Google Scholar]
- 12.Jehle PM, Jehle DR, Mohan S, Böhm BO. Serum levels of insulin-like growth factor system components and relationship to bone metabolism in type 1 and type 2 diabetes mellitus patients. J Endocrinol. 1998;159(2):297-306. PMID: . [DOI] [PubMed] [Google Scholar]
- 13.Tuominen JT, Impivaara O, Puukka P, Rönnemaa T. Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care. 1999;22(7):1196-200. PMID: . [DOI] [PubMed] [Google Scholar]
- 14.Gallacher SJ, Fenner JA, Fisher BM, et al. An evaluation of bone density and turnover in premenopausal women with type 1 diabetes mellitus. Diabet Med. 1993;10(2):129-33. PMID: . [DOI] [PubMed] [Google Scholar]
- 15.Weber G, Beccaria L, de'Angelis M, et al. Bone mass in young patients with type I diabetes. Bone Miner. 1990;8(1):23-30. PMID: . [DOI] [PubMed] [Google Scholar]
- 16.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332(12):767-73. PMID: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 17.Ivers RQ, Cumming RG, Mitchell P, Peduto AJ; Blue Mountains Eye Study Diabetes and risk of fracture: The Blue Mountains Eye Study. Diabetes Care. 2001;24(7):1198-203. PMID: . [DOI] [PubMed] [Google Scholar]
- 18.Nicodemus KK, Folsom AR; Iowa Women's Health Study Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care. 2001;24(7):1192-7. PMID: . [DOI] [PubMed] [Google Scholar]
- 19.Wakasugi M, Wakao R, Tawata M, Gan N, Koizumi K, Onaya T. Bone mineral density measured by dual energy x-ray absorptiometry in patients with non-insulin-dependent diabetes mellitus. Bone. 1993;14(1):29-33. PMID: . [DOI] [PubMed] [Google Scholar]
- 20.van Daele PL, Stolk RP, Burger H, et al. Bone density in non-insulin-dependent diabetes mellitus. The Rotterdam Study. Ann Intern Med. 1995;122(6):409-14. PMID: . [DOI] [PubMed] [Google Scholar]
- 21.Schwartz AV, Sellmeyer DE, Ensrud KE, et al. Older women with diabetes have an increased risk of fracture: A prospective study. J Clin Endocrinol Metab. 2001;86(1):32-8. PMID: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 22.Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci. 2006;1092:385-96. PMID: 10.1196/annals.1365.035. [DOI] [PubMed] [Google Scholar]
- 23.Taylor AK, Lueken SA, Libanati C, Baylink DJ. Biochemical markers of bone turnover for the clinical assessment of bone metabolism. Rheum Dis Clin North Am. 1994;20(3):589-607. PMID: . [PubMed] [Google Scholar]
- 24.McKee MD, Nanci A. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: Ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc Res Tech. 1996;33(2):141-64. PMID: . [DOI] [PubMed] [Google Scholar]
- 25.Hanson DA, Weis MA, Bollen AM, Maslan SL, Singer FR, Eyre DR. A specific immunoassay for monitoring human bone resorption: Quantitation of type I collagen cross‑linked N‑telopeptides in urine. J Bone Miner Res. 1992;7(11):1251-8. PMID: . [DOI] [PubMed] [Google Scholar]
- 26.Christgau S, Rosenquist C, Alexandersen P, et al. Clinical evaluation of the Serum CrossLaps One Step ELISA, a new assay measuring the serum concentration of bone-derived degradation products of type I collagen C-telopeptides. Clin Chem. 1998;44(11):2290-300. PMID: . [PubMed] [Google Scholar]
- 27.Fink E, Cormier C, Steinmetz P, Kindermans C, Le Bouc Y, Souberbielle JC. Differences in the capacity of several biochemical bone markers to assess high bone turnover in early menopause and response to alendronate therapy. Osteoporos Int. 2000;11(4):295-303. PMID: 10.1007/PL00004183. [DOI] [PubMed] [Google Scholar]
- 28.Hannon R, Blumsohn A, Naylor K, Eastell R. Response of biochemical markers of bone turnover to hormone replacement therapy: Impact of biological variability. J Bone Miner Res. 1998;13(7):1124-33. PMID: 10.1359/jbmr.1998.13.7.1124. [DOI] [PubMed] [Google Scholar]
- 29.Seibel MJ. Biochemical markers of bone turnover part I: Biochemistry and variability. Clin Biochem Rev. 2005;26(4):97-122. PMID: PMCID: . [PMC free article] [PubMed] [Google Scholar]
- 30.Forst T, Beyer J, Pfützner A, et al. Peripheral osteopenia in adult patients with insulin‑dependent diabetes mellitus. Diabet Med. 1995;12(10):874-9. PMID: . [DOI] [PubMed] [Google Scholar]
- 31.Kemink SA, Hermus AR, Swinkels LM, Lutterman JA, Smals AG. Osteopenia in insulin-dependent diabetes mellitus; prevalence and aspects of pathophysiology. J Endocrinol Invest. 2000;23(5):295-303. PMID: 10.1007/BF03343726. [DOI] [PubMed] [Google Scholar]
- 32.Hadjidakis DJ, Raptis AE, Sfakianakis M, Mylonakis A, Raptis SA. Bone mineral density of both genders in type 1 diabetes according to bone composition. J Diabetes Complications. 2006;20(5):302-7. PMID: 10.1016/j.jdiacomp.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Strotmeyer ES, Cauley JA, Orchard TJ, Steenkiste AR, Dorman JS. Middle-aged premenopausal women with type 1 diabetes have lower bone mineral density and calcaneal quantitative ultrasound than nondiabetic women. Diabetes Care. 2006;29(2):306-11. PMID: . [DOI] [PubMed] [Google Scholar]
- 34.Mastrandrea LD, Wactawski-Wende J, Donahue RP, Hovey KM, Clark A, Quattrin T. Young women with type 1 diabetes have lower bone mineral density that persists over time. Diabetes Care. 2008;31(9):1729-35. PMID: PMCID: 10.2337/dc07-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campos Pastor MM, López-Ibarra PJ, Escobar-Jiménez F, Serrano Pardo M, García-Cervigón AG. Intensive insulin therapy and bone mineral density in type 1 diabetes mellitus: A prospective study. Osteoporos Int. 2000;11(5):455-9. PMID: . [DOI] [PubMed] [Google Scholar]
- 36.Eller-Vainicher C, Zhukouskaya VV, Tolkachev YV, et al. Low bone mineral density and its predictors in type 1 diabetic patients evaluated by the classic statistics and artificial neural network analysis. Diabetes Care. 2011;34(10):2186-91. PMID: PMCID: 10.2337/dc11-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joshi A, Varthakavi P, Chadha M, Bhagwat N. A study of bone mineral density and its determinants in type 1 diabetes mellitus. J Osteoporos. 2013;2013:397814. PMID: PMCID: 10.1155/2013/397814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soto N, Pruzzo R, Eyzaguirre F, et al. Bone mass and sex steroids in postmenarcheal adolescents and adult women with type 1 diabetes mellitus. J Diabetes Complications. 2011;25(1):19-24. PMID: 10.1016/j.jdiacomp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Bechtold S, Dirlenbach I, Raile K, Noelle V, Bonfig W, Schwarz HP. Early manifestation of type 1 diabetes in children is a risk factor for changed bone geometry: Data using peripheral quantitative computed tomography. Pediatrics. 2006;118(3):e627-34. PMID: 10.1542/peds.2005-2193. [DOI] [PubMed] [Google Scholar]
- 40.Danielson KK, Elliott ME, LeCaire T, Binkley N, Palta M. Poor glycemic control is associated with low BMD detected in premenopausal women with type 1 diabetes. Osteoporos Int. 2009;20(6):923-33. PMID: PMCID: 10.1007/s00198-008-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heap J, Murray MA, Miller SC, Jalili T, Moyer-Mileur LJ. Alterations in bone characteristics associated with glycemic control in adolescents with type 1 diabetes mellitus. J Pediatr. 2004;144(1):56-62. PMID: 10.1016/j.jpeds.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 42.Lettgen B, Hauffa B, Möhlmann C, Jeken C, Reiners C. Bone mineral density in children and adolescents with juvenile diabetes: Selective measurement of bone mineral density of trabecular and cortical bone using peripheral quantitative computed tomography. Horm Res. 1995;43(5):173-5. PMID: . [DOI] [PubMed] [Google Scholar]
- 43.Saha MT, Sievänen H, Salo MK, Tulokas S, Saha HH. Bone mass and structure in adolescents with type 1 diabetes compared to healthy peers. Osteoporos Int. 2009;20(8):1401-6. PMID: 10.1007/s00198-008-0810-0. [DOI] [PubMed] [Google Scholar]
- 44.Forsén L, Meyer H, Midthjell K, Edna TH. Diabetes mellitus and the incidence of hip fracture: Results from the Nord-Trøndelag Health Survey. Diabetologia. 1999;42(8):920-5. PMID: 10491750 10.1007/s001250051248. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed LA, Joakimsen RM, Berntsen GK, Fønnebø V, Schirmer H. Diabetes mellitus and the risk of non-vertebral fractures: The Tromsø study. Osteoporos Int. 2006;17(4):495-500. PMID: 16283065 10.1007/s00198-005-0013-x. [DOI] [PubMed] [Google Scholar]
- 46.Napoli N, Strotmeyer ES, Ensrud KE, et al. Fracture risk in diabetic elderly men: The MrOS study. Diabetologia. 2014;57(10):2057-65. PMID: PMCID: 10.1007/s00125-014-3289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li CI, Liu CS, Lin WY, et al. Glycated hemoglobin level and risk of hip fracture in older people with type 2 diabetes: A competing risk analysis of Taiwan Diabetes Cohort Study. J Bone Miner Res. 2015; 30(7):1338-46. PMID: 10.1002/jbmr.2462. [DOI] [PubMed] [Google Scholar]
- 48.Johnston S, Conner C, Aagren M, Ruiz K, Bouchard J. Association between hypoglycaemic events and fall‑related fractures in Medicare‑covered patients with type 2 diabetes. Diabetes Obes Metab. 2012;14(7):634-43. PMID: 10.1111/j.1463-1326.2012.01583.x. [DOI] [PubMed] [Google Scholar]
- 49.de Liefde II, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA. Bone mineral density and fracture risk in type-2 diabetes mellitus: The Rotterdam Study. Osteoporos Int. 2005;16(12):1713-20. PMID: 10.1007/s00198-005-1909-1. [DOI] [PubMed] [Google Scholar]
- 50.Bonds DE, Larson JC, Schwartz AV, Strotmeyer ES, et al. Risk of fracture in women with type 2 diabetes: The Women’s Health Initiat.ive Observational Study. J Clin Endocrinol Metab. 2006;91(9):3404-10. PMID: 10.1210/jc.2006-0614. [DOI] [PubMed] [Google Scholar]
- 51.Kwon DJ, Kim JH, Chung KW, et al. Bone mineral density of the spine using dual energy x‑ray absorptiometry in patients with non‑insulin‑dependent diabetes mellitus. J Obstet Gynaecol Res. 1996;22(2):157-62. PMID: . [DOI] [PubMed] [Google Scholar]
- 52.Pérez-Castrillón JL, De Luis D, Martín-Escudero JC, Asensio T, del Amo R, Izaola O. Non-insulin-dependent diabetes, bone mineral density, and cardiovascular risk factors. J Diabetes Complications. 2004;18(6):317-21. PMID: 10.1016/S1056-8727(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 53.Lunt M, Masaryk P, Scheidt-Nave C, et al. The effects of lifestyle, dietary dairy intake and diabetes on bone density and vertebral deformity prevalence: The EVOS study. Osteoporos Int. 2001;12(8):688-98. PMID: . [DOI] [PubMed] [Google Scholar]
- 54.Hanley D, Brown J, Tenenhouse A, et al. Associations among disease conditions, bone mineral density, and prevalent vertebral deformities in men and women 50 years of age and older: Cross‑sectional results from the Canadian Multicentre Osteoporosis Study. J Bone Miner Res. 2003;18(4):784-90. PMID: 10.1359/jbmr.2003.18.4.784. [DOI] [PubMed] [Google Scholar]
- 55.Dennison E, Syddall H, Sayer AA, Craighead S, Phillips D, Cooper C. Type 2 diabetes mellitus is associated with increased axial bone density in men and women from the Hertfordshire Cohort Study: Evidence for an indirect effect of insulin resistance? Diabetologia. 2004;47(11):1963-8. PMID: 10.1007/s00125-004-1560-y. [DOI] [PubMed] [Google Scholar]
- 56.Barrett-Connor E, Holbrook TL. Sex differences in osteoporosis in older adults with non—insulin-dependent diabetes mellitus. JAMA. 1992;268(23):3333-7. PMID: . [PubMed] [Google Scholar]
- 57.Gerdhem P, Isaksson A, Akesson K, Obrant KJ. Increased bone density and decreased bone turnover, but no evident alteration of fracture susceptibility in elderly women with diabetes mellitus. Osteoporos Int. 2005;16(12):1506-12. PMID: 10.1007/s00198-005-1877-5. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz AV, Sellmeyer DE, Strotmeyer ES, et al. Diabetes and bone loss at the hip in older black and white adults. J Bone Miner Res. 2005; 20(4):596-603. PMID: 10.1359/JBMR.041219. [DOI] [PubMed] [Google Scholar]
- 59.Dobnig H, Piswanger-Solkner JC, Roth M, et al. Type 2 diabetes mellitus in nursing home patients: effects on bone turnover, bone mass, and fracture risk. J Clin Endocrinol Metab. 2006;91(9):3355-63. PMID: 10.1210/jc.2006-0460. [DOI] [PubMed] [Google Scholar]
- 60.Strotmeyer ES, Cauley JA, Schwartz AV, et al. Diabetes is associated independently of body composition with BMD and bone volume in older white and black men and women: The Health, Aging, and Body Composition Study. J Bone Miner Res. 2004;19(7):1084-91. PMID: 10.1359/JBMR.040311. [DOI] [PubMed] [Google Scholar]
- 61.Pritchard JM, Giangregorio LM, Atkinson SA, et al. Changes in trabecular bone microarchitecture in postmenopausal women with and without type 2 diabetes: A two year longitudinal study. BMC Musculoskelet Disord. 2013;14:114. PMID: PMCID: 10.1186/1471-2474-14-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pritchard JM, Giangregorio LM, Atkinson SA, et al. Association of larger holes in the trabecular bone at the distal radius in postmenopausal women with type 2 diabetes mellitus compared to controls. Arthritis Care Res (Hoboken). 2012;64(1):83-91. PMID: PMCID: 10.1002/acr.20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Randall C, Bridges D, Guerri R, et al. Applications of a new handheld reference point indentation instrument measuring bone material strength. J Med Device. 2013;7(4):0410051-6. PMID: PMCID: 10.1115/1.4024829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dubey A, Aharonoff GB, Zuckerman JD, Koval KJ. The effects of diabetes on outcome after hip fracture. Bull Hosp Jt Dis. 2000;59(2):94-8. PMID: . [PubMed] [Google Scholar]
- 65.Huang YF, Shyu YI, Liang J, Chen MC, Cheng HS, Wu CC. Diabetes and health outcomes among older Taiwanese with hip fracture. Rejuvenation Res. 2012;15(5):476-82. PMID: 10.1089/rej.2011.1308. [DOI] [PubMed] [Google Scholar]
- 66.Muraki S, Yamamoto S, Ishibashi H, Nakamura K. Factors associated with mortality following hip fracture in Japan. J Bone Miner Metab. 2006;24(2):100-4. PMID: 10.1007/s00774-005-0654-z. [DOI] [PubMed] [Google Scholar]
- 67.Napoli N, Chandran M, Pierroz DD, et al. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13(4):208-19. PMID: 10.1038/nrendo.2016.153. [DOI] [PubMed] [Google Scholar]
- 68.Hough FS, Pierroz DD, Cooper C, Ferrari SL; IOF CSA Bone and Diabetes Working Group . Mechanisms in endocrinology: Mechanisms and evaluation of bone fragility in type 1 diabetes mellitus. Eur J Endocrinol. 2016;174(4):R127-38. PMID: 10.1530/EJE-15-0820. [DOI] [PubMed] [Google Scholar]
- 69.Napoli N, Strollo R, Paladini A, Briganti SI, Pozzilli P, Epstein S. The alliance of mesenchymal stem cells, bone, and diabetes. Int J Endocrinol. 2014;2014:690783. PMID: PMCID: 10.1155/2014/690783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maggio AB, Ferrari S, Kraenzlin M, et al. Decreased bone turnover in children and adolescents with well controlled type 1 diabetes. J Pediatr Endocrinol Metab. 2010;23(7):697-707. PMID: . [DOI] [PubMed] [Google Scholar]
- 71.Adami S. Bone health in diabetes: Considerations for clinical management. Curr Med Res Opin. 2009;25(5):1057-72. PMID: 10.1185/03007990902801147. [DOI] [PubMed] [Google Scholar]
- 72.Díaz-López A, Bulló M, Juanola-Falgarona M, et al. Reduced serum concentrations of carboxylated and undercarboxylated osteocalcin are associated with risk of developing type 2 diabetes mellitus in a high cardiovascular risk population: A nested case-control study. J Clin Endocrinol Metab. 2013;98(11):4524-31. PMID: 10.1210/jc.2013-2472. [DOI] [PubMed] [Google Scholar]
- 73.Starup-Linde J, Eriksen SA, Lykkeboe S, Handberg A, Vestergaard P. Biochemical markers of bone turnover in diabetes patients—a metaanalysis, and a methodological study on the effects of glucose on bone markers. Osteoporos Int. 2014;25(6):1697-708. PMID: 10.1007/s00198-014-2676-7. [DOI] [PubMed] [Google Scholar]
- 74.Leite Duarte ME, da Silva RD. Histomorphometric analysis of the bone tissue in patients with non-insulin-dependent diabetes (DMNID). Rev Hosp Clin Fac Med Sao Paulo. 1995;51(1):7-11. PMID: . [PubMed] [Google Scholar]
- 75.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86(5):1930-5. PMID: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 76.Williams GA, Wang Y, Callon KE, et al. In vitro and in vivo effects of adiponectin on bone. Endocrinology. 2009;150(8):3603-10. PMID: 10.1210/en.2008-1639. [DOI] [PubMed] [Google Scholar]
- 77.Tamura T, Yoneda M, Yamane K, et al. Serum leptin and adiponectin are positively associated with bone mineral density at the distal radius in patients with type 2 diabetes mellitus. Metabolism. 2007;56(5):623-8. PMID: 10.1016/j.metabol.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 78.Wang W, Zhang X, Zheng J, Yang J. High glucose stimulates adipogenic and inhibits osteogenic differentiation in MG-63 cells through cAMP/protein kinase A/extracellular signal-regulated kinase pathway. Mol Cell Biochem. 2010;338(1-2):115-22. PMID: 10.1007/s11010-009-0344-6. [DOI] [PubMed] [Google Scholar]
- 79.Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, McCabe LR. Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology. 2005;146(8):3622-31. PMID: PMCID: 10.1210/en.2004-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bucala R, Vlassara H. Advanced glycosylation end products in diabetic renal and vascular disease. Am J Kidney Dis. 1995;26(6):875-88. PMID: . [DOI] [PubMed] [Google Scholar]
- 81.Gefter JV, Shaufl AL, Fink MP, Delude RL. Comparison of distinct protein isoforms of the receptor for advanced glycation end-products expressed in murine tissues and cell lines. Cell Tissue Res. 2009;337(1):79-89. PMID: 10.1007/s00441-009-0791-0. [DOI] [PubMed] [Google Scholar]
- 82.Ramasamy R, Yan SF, Schmidt AM. Advanced glycation endproducts: From precursors to RAGE: Round and round we go. Amino Acids. 2012;42(4):1151-61. PMID: PMCID: 10.1007/s00726-010-0773-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Odetti P, Rossi S, Monacelli F, et al. Advanced glycation end products and bone loss during aging. Ann N Y Acad Sci. 2005;1043:710-7. PMID: 10.1196/annals.1333.082. [DOI] [PubMed] [Google Scholar]
- 84.Hein GE. Glycation endproducts in osteoporosis—is there a pathophysiologic importance? Clin Chim Acta. 2006;371(1-2):32-6. PMID: 10.1016/j.cca.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 85.Dong XN, Qin A, Xu J, Wang X. In situ accumulation of advanced glycation endproducts (AGEs) in bone matrix and its correlation with osteoclastic bone resorption. Bone. 2011;49(2):174-83. PMID: PMCID: 10.1016/j.bone.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang S, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007;40(4):1144-51. PMID: PMCID: 10.1016/j.bone.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanguineti R, Storace D, Monacelli F, Federici A, Odetti P. Pentosidine effects on human osteoblastsin vitro. Ann N Y Acad Sci. 2008;1126:166-72. PMID: 10.1196/annals.1433.044. [DOI] [PubMed] [Google Scholar]
- 88.Yang J, Zhang X, Wang W, Liu J. Insulin stimulates osteoblast proliferation and differentiation through ERK and PI3K in MG‑63 cells. Cell Biochem Funct. 2010;28(4):334-41. PMID: 10.1002/cbf.1668. [DOI] [PubMed] [Google Scholar]
- 89.Gandhi A, Beam HA, O'Connor JP, Parsons JR, Lin SS. The effects of local insulin delivery on diabetic fracture healing. Bone. 2005;37(4):482-90. PMID: 10.1016/j.bone.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 90.McCarthy AD, Etcheverry SB, Cortizo AM. Effect of advanced glycation endproducts on the secretion of insulin-like growth factor-I and its binding proteins: Role in osteoblast development. Acta Diabetol. 2001;38(3):113-22. PMID: . [DOI] [PubMed] [Google Scholar]
- 91.Terada M, Inaba M, Yano Y, et al. Growth-inhibitory effect of a high glucose concentration on osteoblast-like cells. Bone. 1998;22(1):17-23. PMID: 9437509. [DOI] [PubMed] [Google Scholar]
- 92.Gilbert L, He X, Farmer P, et al. Inhibition of osteoblast differentiation by tumor necrosis factor-α 1. Endocrinology. 2000;141(11):3956-64. PMID: 10.1210/endo.141.11.7739. [DOI] [PubMed] [Google Scholar]
- 93.Glantschnig H, Fisher JE, Wesolowski G, Rodan GA, Reszka AA. MCSF, TNFα and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003;10(10): 1165-77. PMID: 10.1038/sj.cdd.4401285. [DOI] [PubMed] [Google Scholar]
- 94.Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ. 2000;321 (7258):405-12. PMID: PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oei L, Zillikens MC, Dehghan A, Buitendijk GH, et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: The Rotterdam Study. Diabetes Care. 2013;36(6):1619-28. PMID: PMCID: 10.2337/dc12-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schneider AL, Williams EK, Brancati FL, Blecker S, Coresh J, Selvin E. Diabetes and risk of fracture-related hospitalization: The atherosclerosis risk in communities study. Diabetes Care. 2013;36(5):1153-8. PMID: PMCID: 10.2337/dc12-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schwartz AV, Vittinghoff E, Sellmeyer DE, et al. Diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care. 2008;31(3):391-6. PMID: PMCID: 10.2337/dc07-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005;48(7):1292-9. PMID: 10.1007/s00125-005-1786-3. [DOI] [PubMed] [Google Scholar]
- 99.Solomon DH, Cadarette SM, Choudhry NK, Canning C, Levin R, Stürmer T. A cohort study of thiazolidinediones and fractures in older adults with diabetes. J Clin Endocrinol Metab. 2009;94(8):2792-8. PMID: PMCID: 10.1210/jc.2008-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Del Prato S, Camisasca R, Wilson C, Fleck P. Durability of the efficacy and safety of alogliptin compared with glipizide in type 2 diabetes mellitus: A 2‑year study. Diabetes Obes Metab. 2014;16(12):1239-46. PMID: 10.1111/dom.12377. [DOI] [PubMed] [Google Scholar]
- 101.Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. Can Med Assoc J. 2009;180(1):32-9. PMID: PMCID: 10.1503/cmaj.080486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu ZN, Jiang YF, Ding T. Risk of fracture with thiazolidinediones: An updated meta-analysis of randomized clinical trials. Bone. 2014;68:115-23. PMID: 10.1016/j.bone.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 103.Su B, Sheng H, Zhang M, et al. Risk of bone fractures associated with glucagon-like peptide-1 receptor agonists’ treatment: A meta-analysis of randomized controlled trials. Endocrine. 2015;48(1):107-15. PMID: 10.1007/s12020-014-0361-4. [DOI] [PubMed] [Google Scholar]
- 104.Monami M, Dicembrini I, Antenore A, Mannucci E. Dipeptidyl peptidase-4 inhibitors and bone fractures: A Meta-analysis of randomized clinical trials. Diabetes Care 2011. 34(11): 2474–6. PMID: PMCID: 10.2337/dc11-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mosenzon O, Wei C, Davidson J, et al. Incidence of fractures in patients with type 2 diabetes in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38(11):2142-50. PMID: 10.2337/dc15-1068. [DOI] [PubMed] [Google Scholar]
- 106.DeFronzo R, Davidson J, Del Prato S. The role of the kidneys in glucose homeostasis: A new path towards normalizing glycaemia. Diabetes Obes Metab. 2012;14(1):5-14. PMID: 10.1111/j.1463-1326.2011.01511.x. [DOI] [PubMed] [Google Scholar]
- 107.Bilezikian JP, Watts NB, Usiskin K, et al. Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with canagliflozin. J Clin Endocrinol Metab. 2015;101(1):44-51. PMID: PMCID: 10.1210/jc.2015-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Watts NB, Bilezikian JP, Usiskin K, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2015;101(1):157-66. PMID: PMCID: 10.1210/jc.2015-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vasikaran S, Eastell R, Bruyère O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: A need for international reference standards. Osteoporos Int. 2011;22(2):391-420. PMID: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 110.Reyes-García R, Rozas-Moreno P, López-Gallardo G, et al. Serum levels of bone resorption markers are decreased in patients with type 2 diabetes. Acta Diabetol. 2013;50(1):47-52. PMID: 10.1007/s00592-011-0347-0. [DOI] [PubMed] [Google Scholar]
- 111.Yamamoto M, Yamaguchi T, Nawata K, Yamauchi M, Sugimoto T. Decreased PTH levels accompanied by low bone formation are associated with vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2012;97(4):1277-84. PMID: 10.1210/jc.2011-2537. [DOI] [PubMed] [Google Scholar]
- 112.Manavalan JS, Cremers S, Dempster DW, et al. Circulating osteogenic precursor cells in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97(9):3240-50. PMID: PMCID: 10.1210/jc.2012-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bhattoa HP, Wamwaki J, Kalina E, Foldesi R, Balogh A, Antal-Szalmas P. Serum sclerostin levels in healthy men over 50 years of age. J Bone Miner Metab. 2013;31(5):579-84. PMID: 10.1007/s00774-013-0451-z. [DOI] [PubMed] [Google Scholar]
- 114.Ardawi MS, Akhbar DH, Alshaikh A, et al. Increased serum sclerostin and decreased serum IGF-1 are associated with vertebral fractures among postmenopausal women with type-2 diabetes. Bone. 2013;56(2):355-62. PMID: 10.1016/j.bone.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 115.Hamilton EJ, Rakic V, Davis WA, et al. A five-year prospective study of bone mineral density in men and women with diabetes: The Fremantle Diabetes Study. Acta Diabetol. 2012;49(2):153-8. PMID: 10.1007/s00592-011-0324-7. [DOI] [PubMed] [Google Scholar]
- 116.Akin O, Göl K, Aktürk M, Erkaya S. Evaluation of bone turnover in postmenopausal patients with type 2 diabetes mellitus using biochemical markers and bone mineral density measurements. Gynecol Endocrinol. 2003;17(1):19-29. PMID: . [PubMed] [Google Scholar]
- 117.Jiajue R, Jiang Y, Wang O, et al. Suppressed bone turnover was associated with increased osteoporotic fracture risks in non-obese postmenopausal Chinese women with type 2 diabetes mellitus. Osteoporos Int. 2014;25(8):1999-2005. PMID: 10.1007/s00198-014-2714-5. [DOI] [PubMed] [Google Scholar]
- 118.Bhattoa HP, Onyeka U, Kalina E, et al. Bone metabolism and the 10-year probability of hip fracture and a major osteoporotic fracture using the country-specific FRAX algorithm in men over 50 years of age with type 2 diabetes mellitus: A case-control study. Clin Rheumatol. 2013;32(8):1161-7. PMID: 10.1007/s10067-013-2254-y. [DOI] [PubMed] [Google Scholar]
- 119.Gaudio A, Privitera F, Battaglia K, et al. Sclerostin levels associated with inhibition of the Wnt/β-catenin signaling and reduced bone turnover in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97(10):3744-50. PMID: 10.1210/jc.2012-1901. [DOI] [PubMed] [Google Scholar]
- 120.Hernández JL, Olmos JM, Romaña G, et al. Bone turnover markers in statin users: A population-based analysis from the Camargo Cohort Study. Maturitas. 2013;75(1):67-73. PMID: 10.1016/j.maturitas.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 121.Sarkar P, Choudhury A. Relationships between serum osteocalcin levels versus blood glucose, insulin resistance and markers of systemic inflammation in central Indian type 2 diabetic patients. Eur Rev Med Pharmacol Sci. 2013;17(12):1631-5. PMID: . [PubMed] [Google Scholar]
- 122.Movahed A, Larijani B, Nabipour I, et al. Reduced serum osteocalcin concentrations are associated with type 2 diabetes mellitus and the metabolic syndrome components in postmenopausal women: The crosstalk between bone and energy metabolism. J Bone Miner Metab. 2012;30(6):683-91. PMID: 10.1007/s00774-012-0367-z. [DOI] [PubMed] [Google Scholar]
- 123.Sosa M, Dominguez M, Navarro MC, et al. Bone mineral metabolism is normal in non-insulin-dependent diabetes mellitus. J Diabetes Complications. 1996;10(4):201-5. PMID: . [DOI] [PubMed] [Google Scholar]
- 124.Chen H, Li X, Yue R, Ren X, Zhang X, Ni A. The effects of diabetes mellitus and diabetic nephropathy on bone and mineral metabolism in T2DM patients. Diabetes Res Clin Pract. 2013;100(2):272-6. PMID: 10.1016/j.diabres.2013.03.007. [DOI] [PubMed] [Google Scholar]