Abstract
The development of programmed cell death-1 inhibitor (PD-1) has shed light on the treatment of tumors with deficiencies in DNA mismatch repair system or microsatellite instability (dMMR/MSI). However, predicting the subset in this group that will benefit from PD-1 blockade remains a challenge. In this study, we aimed to investigate the relationship between the degree of microsatellite instability and the responses to anti-PD-1 immunotherapy. 33 patients with colorectal adenocarcinoma who had a known MSI status and received anti-PD-1 immunotherapy were included. PCR results for MSI of the whole cohort were collected and treatment response was evaluated. Our data indicated that objective response rate (ORR) in instability-high group (instability loci ≥ 3) was significantly higher than ORR in instability-intermediate group (13/16 versus 6/17, P = 0.008). Besides, patients in instability-high group had significant longer progression-free survival (log-rank test, P = 0.004), and a significant increase in T lymphocyte infiltration and cytolytic activity in tumors. Future study might implement the intensity of microsatellite instability for more delicate selection for anti-PD-1 therapy in patient with dMMR/MSI-H tumors.
Keywords: dMMR/MSI-H, Anti-PD-1 immunotherapy, Colorectal cancer
To the Editor
Defects in DNA mismatch repair (dMMR) promote a frequent insertion and/or deletion hypermutable state in nucleotide repeats regions termed microsatellite instability-high (MSI-H) [1]. Colorectal cancers (CRCs) with dMMR/MSI-H have favorable response to the programmed cell death-1 (PD-1) blockade immunotherapy [2]. However, there are still 45–70% of such tumors which do not respond to immune checkpoint blockade, so predicting the subset that will benefit from PD-1 blockade remains a challenge [3–5]. In the current study, we aimed to evaluate whether the degree of microsatellite instability can predict the diversity of responses to anti-PD-1 immunotherapy in dMMR/MSI-H colorectal cancers.
Methods
Patients’ data were collected from a prospectively maintained database in Sun Yat-sen University Cancer Center, Guangzhou, China. Inclusion criteria were as follows: (1) pathologically confirmed colorectal adenocarcinoma; (2) a known MSI status; (3) received at least one dose of anti-PD-1 therapy. Patients with tumors demonstrating no instability loci (microsatellite stable, MSS) were excluded, except for those whose tumor was proved to be dMMR. MSI or dMMR status was prospectively determined using the American National Cancer Institute-recommended Polymerase Chain Reaction (PCR) for MSI or immunohistochemistry (IHC) for dMMR. IHC analyses of CD3 and CD8 were performed using the standard methods on pretreatment specimens. The ethical committees in our center approved this study procedure and waive the necessity of informed consult.
Treatment response was evaluated every two cycles of treatment according to RECIST v1.1. Objective response rate (ORR) was defined as the portion of patients with complete response (CR) or partial response (PR). Progression-free survival (PFS) was calculated from the date of initial anti-PD-1 treatment to either the date of the first progression or death due to CRC. ORRs between groups were compared by χ2 test. PFS was estimated using the Kaplan–Meier method and compared between groups with the log-rank test. Multivariate analyses were performed using the Cox proportional hazards model.
Results
Thirty-three patients were included in the study, with eighteen men and a median age of 45 years (range, 19 to 67). Baseline characteristics were shown in Table 1. Two patients were diagnosed with stage II disease (one local recurrent), seven patients were with stage III disease, while 24 patients were with stage IV disease. Among them, nineteen patients had received ≥ 2 lines of prior systemic therapies before anti-PD-1 immunotherapy. Thirteen patients were treated with a single-agent anti-PD-1 antibody, and the median cycles of therapy given were 8 (range 1–31).
Table 1.
Baseline characteristics of patients according to the degrees of MSI
| Variables | Instability loci < 3 | Instability loci ≥ 3 | P value |
|---|---|---|---|
| n | 17 | 16 | |
| Age-median (range), year | 45.0 (19.0–64.0) | 45.5 (30.0–67.0) | 0.783 |
| Sex-n | 0.112 | ||
| Male | 7 | 11 | |
| Female | 10 | 5 | |
| Tumor stage-n | 0.619 | ||
| II/III | 4 | 5 | |
| IV | 13 | 11 | |
| Lines of therapy-n | 0.119 | ||
| First line | 5 | 9 | |
| Second or late line | 12 | 7 | |
| Combined with chemotherapy-n | 0.055 | ||
| Yes | 13 | 7 | |
| No | 4 | 9 |
As shown in Table 2, we found that three patients identified as d-MMR but with microsatellite stability (MSS) disease had no response to anti-PD-1 treatment. By contrast, all patients with five instability loci achieved PR or CR (Additional file 1: Figure S1). To investigate the relationship between the degree of MSI and responses to anti-PD-1 therapy, we classified the patients into instability-intermediate (instability loci < 3) and instability-high subgroups (instability loci ≥ 3) according to the degrees of MSI. Baseline characteristics were comparable between the two groups (Table 1). However, ORR in instability-high group was significantly higher than ORR in instability-intermediate group (13/16 versus 6/17, P = 0.008).
Table 2.
Treatment response and ORR of patients stratified by the number of instability loci
| Number of instability loci | Number of cases | CR* | PR | SD | PD | ORR |
|---|---|---|---|---|---|---|
| 0 | 3 | 0 | 0 | 2 | 1 | 0 |
| 1 | 5 | 0 | 0 | 2 | 3 | 0 |
| 2 | 9 | 2 | 4 | 1 | 2 | 0.67 |
| 3 | 9 | 5 | 2 | 1 | 1 | 0.78 |
| 4 | 4 | 2 | 1 | 1 | 0 | 0.75 |
| 5 | 3 | 1 | 2 | 0 | 0 | 1 |
CR complete response, PR partial response, SD stable disease, PD progressive disease, ORR objective response rate
*For patients who underwent surgical resection and had a pathological confirmed complete response, treatment response was recorded as CR
During a median follow-up of 11.2 months (range, 2.0–36.3), twelve patients had the disease progression. All patients remained alive at the wrighting of this article. Univariable analysis showed that the degree of microsatellite instability was associated with PFS after anti-PD-1 immunotherapy (Additional file 1: Table S1). Patients in instability-high subgroup have significant longer PFS (log-rank test, P = 0.004, Fig. 1). After excluding the confounding effects of sex, age and combined treatment by multivariate Cox proportional hazard model, instability-high was demonstrated to be an independent predictor for the longer PFS (HR = 0.136 [0.024–0.781], P = 0.025; Additional file 1: Table S2). Further univariable analysis of the location of the MSI loci showed that mutation in the loci of BAT25 and BAT26 were associated with longer PFS (Additional file 1: Table S3). To exclude the confounding effect of the number of instability loci, we did the multivariate cox regression for PFS, and BAT25 retains its predictive capability (HR = 0.037 [0.002–0.571], P = 0.018; Additional file 1: Table S4).
Fig. 1.
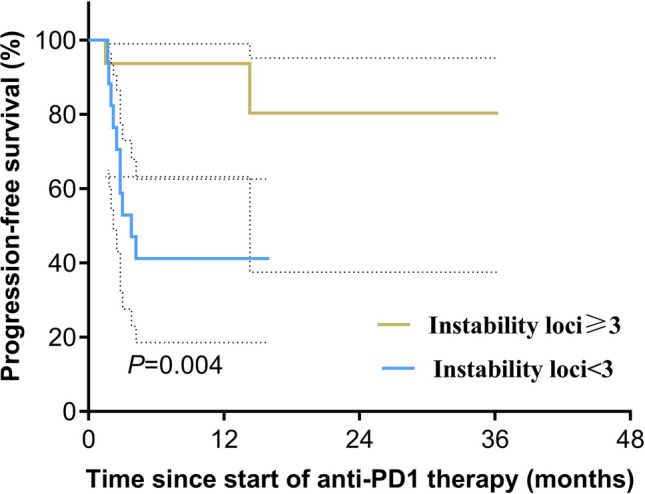
Progression-free survival of the two groups according to the degree of MSI. Survival was analyzed using the log-rank test. Dashed lines are 95% confidence intervals
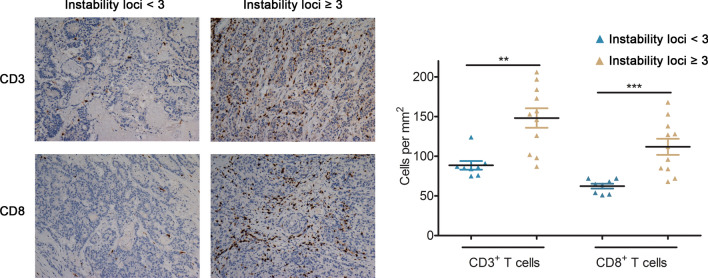
IHC analyses of CD3 and CD8 were performed and the number of CD3+ and CD8+ T cell infiltration per mm2 was counted in each group. A significant increase in CD3+ and CD8+ T lymphocyte infiltration and cytolytic activity were also found in tumors in instability-high subgroup (Fig. 2).
Fig. 2.
The degrees of microsatellite instability in relation to T cell infiltration in dMMR/MSI-H CRC. Representative immunohistochemical images of pretreatment tumor specimens stained with CD3 and CD8. Images are shown at ×20 with a 100-μM scale bar in each image
Discussion
Our study indicated that the degree of microsatellite instability could predict a patient’s response to anti-PD-1 immunotherapy, and was an independent predictor for PFS in dMMR/MSI-H CRCs. This is in line with the recent finding in mouse models of microsatellite instability [6]. Underling mechanisms might involve the increased tumors immunogenicity and lymphocytic infiltration, as MSI-H reflects a genome-wide instability which eventually results in high mutational burden.
The main limitation of this study is the small sample size. But our data suggest that responds to anti-PD-1 are substantially diverse within dMMR tumors and that it highlights the possibility of more delicate selection for anti-PD-1 therapy in patients with dMMR/MSI-H.
Supplementary Information
Additional file 1: Figure S1. The intensity of instability loci and the patient’s response to anti-PD-1 treatment. A. The representative PCR result of the colorectal cancer patient (case 6) with five microsatellite instability loci. B. The representative MRI images of the patient (case 6) showing a sigmoid colon mass before and after anti-PD-1 immunotherapy. Table S1. Univariate analyses of the prognostic factors for progression-free survival of the cohort (N = 33). Table S2. Multivariate Cox regression analyses of the prognostic factors for progression-free survival of the whole cohort (N = 33). Table S3. Univariate analyses of the location of the MSI loci for progression-free survival of the cohort (N = 33). Table S4. Multivariate Cox regression analyses for progression-free survival of the whole cohort (N = 33).
Acknowledgements
None.
Abbreviations
- dMMR
Defects in DNA mismatch repair
- MSI-H
Microsatellite instability-high
- CRCs
Colorectal cancers
- PD-1
Programmed cell death-1
- PCR
Polymerase Chain Reaction
- IHC
Immunohistochemistry
- ORR
Objective response rate
- CR
Complete response
- PR
Partial response
- PFS
Progression-free survival
Authors’ contributions
DX and MYC designed the study. YHG, PRD, JPY, DX and MYC provided the material and technical support. QXW, CHQ and MYC analysed the data and drafted the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the Grants from the Nature Science Foundation of China (Nos. 81672407 and 81872001) and the Program for physician-scientist in Sun Yat-sen University Cancer Center (No. 09010101).
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The ethical committees in Sun Yat-sen University Center approved this study procedure and waive the necessity of informed consult for the retrospective design of this study.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dan Xie, Email: xiedan@sysucc.org.cn.
Mu-Yan Cai, Email: caimy@sysucc.org.cn.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40164-020-00193-z.
References
- 1.Kim TM, Laird PW, Park PJ. The landscape of microsatellite instability in colorectal and endometrial cancer genomes. Cell. 2013;155(4):858–868. doi: 10.1016/j.cell.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 4.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le DT, Kim TW, Van Cutsem E, Geva R, Jäger D, Hara H, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 2020;38(1):11–19. doi: 10.1200/JCO.19.02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandal R, Samstein RM, Lee KW, Havel JJ, Wang H, Krishna C, et al. Genetic diversity of tumors with mismatch repair deficiency influences anti-PD-1 immunotherapy response. Science. 2019;364(6439):485–491. doi: 10.1126/science.aau0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. The intensity of instability loci and the patient’s response to anti-PD-1 treatment. A. The representative PCR result of the colorectal cancer patient (case 6) with five microsatellite instability loci. B. The representative MRI images of the patient (case 6) showing a sigmoid colon mass before and after anti-PD-1 immunotherapy. Table S1. Univariate analyses of the prognostic factors for progression-free survival of the cohort (N = 33). Table S2. Multivariate Cox regression analyses of the prognostic factors for progression-free survival of the whole cohort (N = 33). Table S3. Univariate analyses of the location of the MSI loci for progression-free survival of the cohort (N = 33). Table S4. Multivariate Cox regression analyses for progression-free survival of the whole cohort (N = 33).
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.