Abstract
Background
Tofogliflozin, an SGLT2 inhibitor, is associated with favorable metabolic effects, including improved glycemic control and serum lipid profile and decreased body weight, visceral adipose tissue, and blood pressure (BP). This study evaluated the effects of tofogliflozin on the brachial-ankle pulse wave velocity (baPWV) in patients with type 2 diabetes (T2DM) without a history of apparent cardiovascular disease.
Methods
The using tofogliflozin for possible better intervention against atherosclerosis for type 2 diabetes patients (UTOPIA) trial is a prospective, randomized, open-label, multicenter, parallel-group, comparative study. As one of the prespecified secondary outcomes, changes in baPWV over 104 weeks were evaluated in 154 individuals (80 in the tofogliflozin group and 74 in the conventional treatment group) who completed baPWV measurement at baseline.
Results
In a mixed-effects model, the progression in the right, left, and mean baPWV over 104 weeks was significantly attenuated with tofogliflozin compared to that with conventional treatment (– 109.3 [– 184.3, – 34.3] (mean change [95% CI] cm/s, p = 0.005; – 98.3 [– 172.6, – 24.1] cm/s, p = 0.010; – 104.7 [– 177.0, – 32.4] cm/s, p = 0.005, respectively). Similar findings were obtained even after adjusting the mixed-effects models for traditional cardiovascular risk factors, including body mass index (BMI), glycated hemoglobin (HbA1c), total cholesterol, high-density lipoprotein (HDL)-cholesterol, triglyceride, systolic blood pressure (SBP), hypertension, smoking, and/or administration of drugs, including hypoglycemic agents, antihypertensive agents, statins, and anti-platelets, at baseline. The findings of the analysis of covariance (ANCOVA) models, which included the treatment group, baseline baPWV, and traditional cardiovascular risk factors, resembled those generated by the mixed-effects models.
Conclusions
Tofogliflozin significantly inhibited the increased baPWV in patients with T2DM without a history of apparent cardiovascular disease, suggesting that tofogliflozin suppressed the progression of arterial stiffness.
Trial Registration UMIN000017607. Registered 18 May 2015. (https://www.umin.ac.jp/icdr/index.html)
Keywords: Arterial stiffness, Pulse wave velocity, Arteriosclerosis, Diabetes, SGLT2 inhibitor, Tofogliflozin
Background
Sodium-glucose cotransporter 2 (SGLT2) inhibitors, a class of antidiabetic agents, have a pleiotropic effect, and thus diminish several cardiovascular risk factors by reducing visceral adipose tissue, body weight, and blood pressure (BP); improving the blood lipid profile; and generating a renoprotective effect independent of glycemic effects [1, 2]. Previous clinical trials have shown that SGLT2 inhibitors significantly reduced major cardiovascular (CV) adverse events and/or hospitalization for heart failure in patients with type 2 diabetes mellitus (T2DM) at high risk of cardiovascular disease (CVD) [3–5]. In addition, a recent meta-analysis indicated that SGLT2 inhibitors have robust benefits in reducing hospitalization for heart failure and progression of renal disease, regardless of existing atherosclerotic CVD or a history of heart failure [6]. These results suggest that SGLT2 inhibitors exert a broad range of beneficial effects on the CV system through multiple mechanisms.
Reduction in the arterial wall elasticity increases the vascular wall stress, resulting in the progression of atherosclerosis, an increase in the left ventricular afterload, left ventricular diastolic dysfunction, and coronary perfusion disorder. Moreover, deterioration in arterial stiffness was associated with hospitalization for new-onset heart failure in asymptomatic patients with CV risk factors [7]. Thus, arterial stiffness may reflect not only atherosclerotic changes but also risks of an extensive range of CVDs including cardiac dysfunction.
Previous studies have evaluated the effect of SGLT2 inhibitors on arterial stiffness using several indices [8–19], some of which indicated the beneficial effect of SGLT inhibitors. Pulse wave velocity (PWV) is commonly used to measure the severity of arterial stiffness, showing the structural and functional properties of the arterial wall [20, 21]. However, evidence on the effect of SGLT2 inhibitors on the progression of PWV remains limited [8–11].
This study evaluated the effects of tofogliflozin, an SGLT2 inhibitor that has been clinically used in Japan, on the brachial-ankle pulse wave velocity (baPWV) as one of the prespecified secondary outcomes of the using tofogliflozin for possible better intervention against atherosclerosis for type 2 diabetes patients (UTOPIA) trial [22, 23].
Methods
Study design
The study design, study schedule, and outcomes of the original UTOPIA trial have been described in detail previously [22, 23]. In brief, the UTOPIA trial was a prospective, randomized, open-label, multicenter, parallel-group comparative study to evaluate the efficacy of tofogliflozin in preventing the progression of atherosclerosis over a 2-year intervention period in patients with T2DM. The study period was 104 weeks following patient registration (registration period: January to November 2016). All randomized patients were followed up until the scheduled end of the study, irrespective of adherence to or discontinuation of study medication for any reason. Clinical and biochemical data were collected at 0, 26, 52, 78, and 104 weeks after randomization. Primary study outcomes were changes in mean intima–media thickness (IMT) of the common carotid artery. As one of the prespecified secondary outcomes, changes in the right and left baPWV values over the 104-week observation period were evaluated. In addition, as a post-hoc analysis, the effects of tofogliflozin on changes in the mean baPWV values (mean derived from the left- and right-side baPWV values) were evaluated.
This study was registered at the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR), a nonprofit organization in Japan, and met the requirements of the International Committee of Medical Journal Editors (UMIN000017607).
Study population
The eligibility criteria of the original UTOPIA trial have been previously described in detail [22]. The inclusion criteria were: (1) Japanese with T2DM and inadequate glycemic control (HbA1c ≥ 6% but < 9%), along with the inability to achieve the blood glucose level stated in the Diabetes Treatment Guidelines of 2014–2015 despite being on drugs, except SGLT2 inhibitors—with diet and physical therapy, on diet and physical therapy without being on drugs for at least 12 weeks, or on SGLT2 inhibitors in the past but without them for at least 12 weeks before signing the consent form; (2) no changes in the antidiabetic, antithrombotic, antihypertensive medication, or a therapeutic agent for dyslipidemia management for at least 12 weeks before signing the consent form; (3) age 30 to 74 years at the time of giving consent; and (4) able to provide informed consent. Furthermore, the following exclusion criteria were applied: (1) type 1 or secondary diabetes; (2) in the perioperative period or with a serious infection or injury; (3) a history of myocardial infarction, angina, stroke, or cerebral infarction; (4) severe renal dysfunction (estimated glomerular filtration rate [eGFR] of < 30 mL/min/1.73 m2); (5) serious liver functional impairment; 6) moderate to severe heart failure (class 3 or worse based on the New York Heart Association Functional Classification); (7) urinary tract or genital infection; (8) pregnant, possibly pregnant, nursing, or planning to conceive a child; (9) history of hypersensitivity to the study drug; (10) present or past history of a malignant tumor (exceptions: patients not on medication for malignant tumors and no recurrence of the disease so far without recurrence risks during this study were allowed to participate); (11) prohibited to use tofogliflozin; and (12) other ineligibility determined by an investigator.
The patients were screened consecutively at 24 institutions in Japan (Additional file 1). Patients who met the above eligibility criteria were asked to participate, and those who agreed were included in the study. Measurements of baPWV were performed at 10 out of 24 institutions, and the individuals who completed baPWV measurements were subjected to this analysis.
The protocol was approved by the Osaka University Clinical Research Review Committee and the institutional review board of each participating institution in compliance with the Declaration of Helsinki and current legal regulations in Japan. Written informed consent was obtained from all patients after a complete explanation of the study.
Randomization and study intervention
As previously reported, patient registration was performed at the administration office of the UTOPIA trial using the Internet. Once enrolled, the patients were randomly and equally assigned to either tofogliflozin treatment or conventional treatment group using drugs other than SGLT2 inhibitors [22, 23].
Treatment was continued to achieve the target value specified in the Japanese treatment guide for diabetes [24] in both groups. In the conventional treatment group, either the current therapy dosage was increased or a concomitant oral glucose-lowering drug (excluding any other SGLT2 inhibitor) was added 12 weeks after randomization. In the tofogliflozin group, 20 mg of tofogliflozin once daily was started in addition to the ongoing therapy. However, the addition of an alternative antidiabetic agent (excluding another SGLT2 inhibitor) was permitted 12 weeks after randomization. In the case of hypoglycemia, the dosage of the concomitant oral glucose-lowering drug was titrated. The use of antihyperlipidemic and antihypertensive drugs was allowed during the study.
Measurement of baPWV
The baPWV was measured using the same volume plethysmography apparatus as for ABI (BP-203RPE II form PWV/ABI, Omron Healthcare Co., Ltd., Kyoto, Japan), with patients in the supine position after at least 5 min of rest, as previously reported [25–29]. Specifically, four oscillometric cuffs, each connected to a plethysmographic sensor that determined the volume pulse from and to an oscillometric pressure sensor that measured BP, were wrapped on both the brachia and ankle; two electrocardiogram electrodes were placed on each wrist. The cuffs were simultaneously pressurized to the approximate value of the patient’s diastolic pressure such that the pulse volume waveforms could be recorded using semiconductor pressure sensors. The distance between the sampling points of baPWV was calculated automatically according to the height of the subject. The path length from the suprasternal notch to the ankle (La) was calculated as follows: La = 0.8129 × height (in cm) + 12.328. The path length from the suprasternal notch to the brachium (Lb) was calculated as follows: Lb = 0.2195 × height − 2.0734. The baPWV was calculated according to the following formula: baPWV = (La – Lb)/Tba, where Tba is the time interval between the wavefront of the brachial waveform and that of the ankle waveform [25]. Two simultaneous measurements of baPWV were recorded on the right and left sides. Investigations were conducted at the beginning of the study and 52 and 104 weeks.
Biochemical tests
Blood samples were collected after overnight fasting. HbA1c, glucose, insulin, serum lipids, and creatinine were measured using standard techniques. Urinary albumin excretion was measured by the improved bromocresol purple method using a spot urine sample. The eGFR was calculated using the following formula: eGFR (mL/min per 1.73 m2) = 194 × age − 0.287 × serum creatinine − 0.1094 (× 0.739 for females) [30].
Statistical analysis
All enrolled patients, except those without baseline baPWV measurements, were analyzed. As for baseline and follow-up variables, group comparisons were performed using Student’s t-test or the Wilcoxon rank-sum test for continuous variables and Fisher’s exact test or the chi-square test for categorical variables. Changes from the baseline to treatment visits were assessed with a one-sample t-test and Wilcoxon signed-rank test within the group.
To confirm the effect of tofogliflozin on changes in baPWV, post-hoc analyses were performed using the mixed-effects model for repeated measures, with the treatment group, time (week), interactions between treatment group and time (week), age, sex, use of insulin at baseline, and baseline baPWV as fixed effects. An unstructured covariate was used to model the covariance of within-subject variability. Sensitivity analysis assessed differences in the delta change in baPWV from the baseline between the two groups using analysis of covariance (ANCOVA) models that included the treatment group, age, sex, baseline baPWV, systolic blood pressure (SBP), and administration of antihypertensive agents.
All statistical tests were two-sided with a 5% significance level. All analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
Role of the funding source
The sponsor had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had complete access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Study population
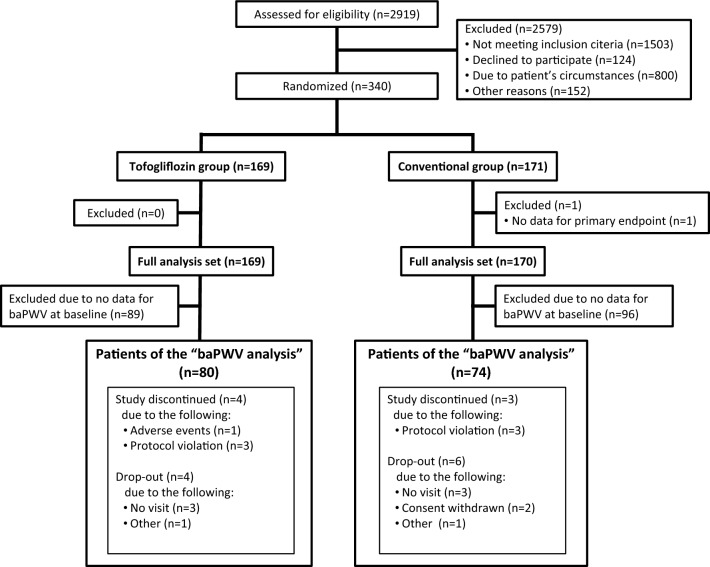
Initially, 340 patients were enrolled and randomly allocated into either the tofogliflozin treatment group (n = 169) or the conventional treatment group (n = 171). Among the study patients in the original UTOPIA trial, 154 individuals (80 belonging to the tofogliflozin group and 74 belonging to the conventional treatment group) completed baPWV measurement at baseline (Fig. 1). There were significant differences in certain variables, such as body mass index (BMI), waist circumference, SBP, total cholesterol, use of glucose-lowering agents, and use of antithrombotic agents, between those who underwent baPWV measurements and those who did not (Additional file 2).
Fig. 1.
Study design flowchart
Baseline clinical characteristics, such as sex, age, smoking habit, BMI, waist circumference, duration of diabetes, glycated hemoglobin (HbA1c), fasting blood glucose, BP, the prevalence of dyslipidemia, serum total cholesterol levels, low-density lipoprotein (LDL) cholesterol levels, high-density lipoprotein (HDL) cholesterol levels, the prevalence of diabetic retinopathy, prevalence of diabetic nephropathy, and use of glucose-lowering agents, lipid-lowering agents, and antithrombotic agents, were comparable between the tofogliflozin and conventional treatment groups, whereas serum triglyceride levels, the prevalence of hypertension, and use of antihypertensive drugs were significantly lower in the tofogliflozin treatment group than in the conventional treatment group (Table 1).
Table 1.
Baseline clinical characteristics of patients in both treatment groups
| Parameters | Tofogliflozin group (n = 80) | Conventional group (n = 74) | p value |
|---|---|---|---|
| Sex (males) (%) | 48 (60.0) | 48 (64.9) | 0.53 |
| Age (years) | 61.5 ± 9.2 | 62.5 ± 9.3 | 0.50 |
| Current smoking | 16 (20.0) | 14 (18.9) | 0.87 |
| Body mass index (kg/m2) | 26.4 ± 5.4 | 26.2 ± 4.2 | 0.83 |
| Waist circumference (cm) | 91.2 ± 12.3 (n = 75) | 91.7 ± 10.7 | 0.79 |
| Duration of diabetes (years) | 11.9 ± 8.2 | 14.1 ± 8.7 | 0.11 |
| HbA1c (%) | 7.5 ± 0.7 | 7.4 ± 0.8 | 0.32 |
| Fasting blood glucose (mmol/L) | 8.0 ± 1.8 | 7.9 ± 1.8 (n = 73) | 0.65 |
| C-peptide (ng/mL) | 1.91 ± 1.11 (n = 79) | 1.92 ± 0.89 (n = 73) | 0.96 |
| Hypertension | 38 (47.5) | 49 (66.2) | 0.019 |
| Systolic blood pressure (mmHg) | 130.9 ± 14.4 | 132.4 ± 18.8 | 0.58 |
| Diastolic blood pressure (mmHg) | 79.0 ± 10.0 | 79.6 ± 12.0 | 0.70 |
| Dyslipidemia | 48 (60.0) | 51 (68.9) | 0.31 |
| Total cholesterol (mmol/L) | 5.01 ± 0.77 (n = 79) | 5.07 ± 0.79 (n = 73) | 0.63 |
| LDL cholesterol (mmol/L) | 2.88 ± 0.69 | 3.01 ± 0.66 | 0.25 |
| HDL cholesterol (mmol/L) | 1.47 ± 0.39 | 1.37 ± 0.27 | 0.09 |
| Triglyceride (mmol/L) | 1.07 [0.84, 1.61] | 1.46 (1.02, 1.90) | 0.011 |
| Diabetic retinopathy | 14 (17.9) | 17 (23.3) | 0.43 |
| eGFR (mL/min/1.73 m2) | 79.1 ± 19.9 | 82.0 ± 26.6 | 0.45 |
| Urinary albumin excretion (mg/g/cre) | 9.6 (5.7, 36.8) (n = 75) | 18.7 (5.3, 65.9) (n = 69) | 0.38 |
| Diabetic nephropathy | 24 (30.0) | 27 (36.5) | 0.49 |
| Use of glucose-lowering agents | 69 (86.3) | 61 (82.4) | 0.66 |
| Metformin | 36 (45.0) | 38 (51.4) | 0.52 |
| Sulfonylurea | 16 (20.0) | 14 (18.9) | 1.00 |
| Glinides | 4 (5.0) | 4 (5.4) | 1.00 |
| Thiazolidinediones | 8 (10.0) | 10 (13.5) | 0.62 |
| α-Glucosidase inhibitor | 14 (17.5) | 15 (20.3) | 0.68 |
| DPP-4 inhibitors | 36 (45.0) | 34 (45.9) | 1.00 |
| GLP-1 R agonists | 9 (11.3) | 4 (5.4) | 0.25 |
| Insulin | 12 (15.0) | 16 (21.6) | 0.30 |
| Use of antihypertensive drugs | 36 (45.0) | 46 (62.2) | 0.037 |
| Angiotensin-converting enzyme inhibitors | 1 (1.3) | 4 (5.4) | 0.20 |
| Angiotensin II receptor blockers | 25 (31.3) | 41 (55.4) | 0.003 |
| Direct renin inhibitor | 1 (1.3) | 0 (0.0) | 1.00 |
| Calcium channel blocker | 24 (30.0) | 24 (32.4) | 0.86 |
| Diuretic drugs | 4 (5.0) | 6 (8.1) | 0.52 |
| α-Adrenergic receptor antagonist | 1 (1.3) | 0 (0.0) | 1.00 |
| β-Adrenergic receptor antagonist | 3 (3.8) | 2 (2.7) | 1.00 |
| Others | 0 (0.0) | 0 (0.0) | – |
| Use of lipid-lowering agents | 39 (48.8) | 39 (52.7) | 0.63 |
| Statins | 33 (41.3) | 32 (42.3) | 0.87 |
| Ezetimibe | 4 (5.0) | 5 (6.8) | 0.74 |
| Resins | 0 (0.0) | 0 (0.0) | – |
| Fibrates | 4 (5.0) | 2 (2.7) | 0.68 |
| Use of antithrombotic agents | 11 (13.8) | 11 (14.9) | 1.00 |
| Antiplatelet agents | 10 (12.5) | 9 (12.2) | 1.00 |
| Anticoagulants | 1 (1.3) | 2 (2.7) | 0.61 |
| Others | 0 (0.0) | 0 (0.0) | – |
| Right baPWV (cm/s) | 1690.1 ± 399.2 | 1680.4 ± 323.6 | 0.87 |
| Left baPWV (cm/s) | 1696.2 ± 432.7 | 1672.8 ± 338.0 | 0.71 |
| Mean baPWV (cm/s) | 1693.2 ± 410.3 | 1676.6 ± 324.6 | 0.78 |
Data are presented as the number (%) of patients, mean ± standard deviation (SD) values, or median (the 25th and 75th percentiles) values
HbA1C glycated hemoglobin, SD standard deviation, LDL low-density lipoprotein, HDL high-density lipoprotein, DPP-4 dipeptidyl peptidase, GLP glucagon-like peptide-1, baPWV brachial-ankle pulse wave velocity
Post-hoc between-group comparison of changes in clinical parameters during the treatment period was performed in 154 individuals who completed baPWV measurement at baseline. Within 104 weeks, the reductions (value at study end-value at the baseline) in HbA1c (– 0.37 ± 0.73% vs. 0.00 ± 0.68%, p = 0.002), fasting blood glucose (− 0.8 ± 2.0 mmol/L vs. 0.1 ± 2.1 mmol/L, p = 0.011), BMI (– 1.1 ± 1.1 kg/m2 vs. − 0.1 ± 0.8 kg/m2, p < 0.001), and waist circumference (− 0.9 ± 6.7 cm vs. 1.5 ± 3.9 cm, p = 0.015) were significantly higher in the tofogliflozin group than in the conventional group. There was no significant difference between the groups regarding changes in other clinical parameters including lipid profile and BP (Additional file 3). Over the course of the study, the concomitantly used antidiabetic agents were balanced between the conventional and tofogliflozin groups (Additional file 4). However, antihypertensive drugs, especially angiotensin II receptor blockers, were significantly more frequently used in the conventional group than in the tofogliflozin group during the study (Additional file 5).
Brachial-ankle pulse wave velocity
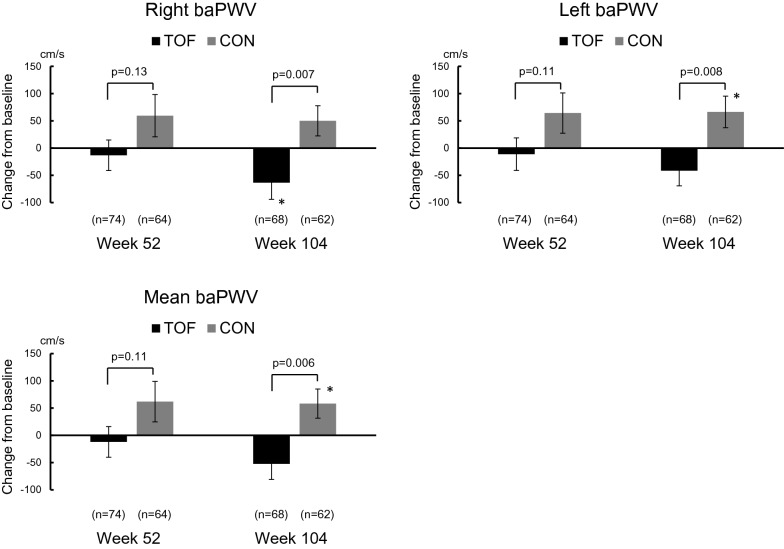
Figure 2 shows the change in baPWV from the baseline to weeks 52 and 104 in the tofogliflozin and conventional treatment groups. There were significant differences in delta change in the right, left, and mean baPWV from the baseline to week 104 between the treatment groups (p = 0.007, 0.008, 0.006, respectively). The progression of baPWV values was significantly attenuated in the tofogliflozin treatment group compared to the conventional treatment group.
Fig. 2.
Delta change in baPWV from the baseline to weeks 52 and 104. Data are presented as the mean ± SE. Comparisons of baPWV values during treatment with those at baseline were performed using a one-sample t-test. *p < 0.05, #p < 0.01, §p < 0.001. Differences in delta change in baPWV from baseline to weeks 52 and 104 between the groups at each point (treatment effect) were analyzed using Student’s t-test. baPWV, brachial-ankle pulse wave velocity; TOF, tofogliflozin treatment group; CON, conventional treatment group
In a mixed-effects model for repeated measures, the progression in the right, left, and mean baPWV during week 104 was significantly attenuated in the tofogliflozin group compared to that in the conventional treatment group (− 109.3 [− 184.3, − 34.3], mean change [95% CI], p = 0.005; –98.3 [− 172.6, –24.1], p = 0.010; − 104.7 [− 177.0, − 32.4], p = 0.005, respectively) (Table 2). An additional analysis, where individuals with all three measurements (baseline, week 52, and week 104) alone were included, showed similar results (Additional file 6).
Table 2.
Effects of tofogliflozin on brachial-ankle pulse wave velocity
| Tofogliflozin group (n = 74) | Conventional group (n = 66) | Treatment effect (tofogliflozin–conventional treatment) (mean change [95% CI]) | p value between groups | |
|---|---|---|---|---|
| Right baPWV | ||||
| Change from baseline at week 52 (cm/s) | − 15.4 (38.1) | 50.0 (38.3) | − 65.4 (–163.1, 32.2) | 0.19 |
| Change from baseline at week 104 (cm/s) | − 70.6 (31.3) * | 38.7 (30.2) | − 109.3 (–184.3, –34.3) | 0.005 |
| Left baPWV | ||||
| Change from baseline at week 52 (cm/s) | 1.5 (36.9) | 63.7 (37.3) | − 62.2 (–157.1, 32.7) | 0.20 |
| Change from baseline at week 104 (cm/s) | − 32.6 (30.7) | 65.8 (29.8)* | − 98.3 (–172.6, –24.1) | 0.010 |
| Mean baPWV | ||||
| Change from baseline at week 52 (cm/s) | − 7.7 (36.7) | 56.7 (37.0) | − 64.4 (–158.8, 29.9) | 0.18 |
| Change from baseline at week 104 (cm/s) | − 52.5 (30.1) | 52.2 (29.1) | − 104.7 (–177.0, –32.4) | 0.005 |
Data are presented as mean (SE) unless stated otherwise. Comparisons of baPWV values during treatment with those at baseline were performed using a one-sample t-test based on the mixed-effects model for repeated measures. *p < 0.05, #p < 0.01, §p < 0.001. Differences in delta change in baPWV from baseline to weeks 52 and 104 between groups at each point (treatment effect) were analyzed with the mixed-effects model for repeated measures. Treatment group, week, interactions between treatment group and week, age, sex, use of insulin at baseline, and baseline baPWV were included as fixed effects
baPWV brachial-ankle pulse wave velocity
Similar findings were obtained even after adjusting the mixed-effects models for traditional cardiovascular risk factors, including BMI, HbA1c, total cholesterol, HDL cholesterol, triglycerides, SBP, presence of hypertension, and smoking, and/or the administration of drugs, including hypoglycemic agents, antihypertensive agents, statins, and anti-platelets at baseline (Table 3). Moreover, the findings produced by ANCOVA models, as a sensitivity analysis, resembled those generated by the mixed-effects models (Table 4).
Table 3.
Effects of tofogliflozin on brachial-ankle pulse wave velocity after adjusting for traditional CV risk factors and/or administration of drugs
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Right baPWV | ||||
| Week 52 | − 78.3 (− 173.1, 16.6) | − 87.1 (− 186.0, 11.9) | − 90.9 (− 189.9, 8.1) | − 84.6 (− 180.6, 11.4) |
| Week 104 | − 126.5 (− 211.0, − 42.0)# | − 136.9 (− 226.5, − 47.2)# | − 140.5 (− 230.2, − 50.7)# | − 134.4 (− 221.3, − 47.4)# |
| Left baPWV | ||||
| Week 52 | − 77.2 (− 171.0, 16.7) | − 92.2 (− 190.0, 5.6) | − 94.6 (− 192.7, 3.4) | − 82.2 (− 178.3, 14.0) |
| Week 104 | − 115.6 (− 196.3, − 34.9)# | − 132.8 (− 219.3, –46.3)# | − 134.6 (− 221.4, –47.8)# | − 121.4 (− 205.7, − 37.2)# |
| Mean baPWV | ||||
| Week 52 | − 77.9 (− 170.1, 14.2) | − 89.7 (− 185.8, 6.4) | − 93.0 (− 189.2, 3.3) | − 83.6 (− 177.6, 10.3) |
| Week 104 | − 121.1 (− 200.5, − 41.6)# | − 134.7 (− 219.3, –50.0)# | − 137.6 (− 222.5, − 52.7)# | − 128.0 (− 210.3, − 45.6)# |
Treatment effect (tofogliflozin–conventional treatment) is expressed as mean change (95% CI). Differences in delta change in baPWV from the baseline at weeks 52 and 104 between the groups at each point (treatment effect) were analyzed with a mixed-effects model for repeated measures. *p < 0.05, #p < 0.01, §p < 0.001. Model 1: treatment group, week, interactions between treatment groups and week, body mass index, HbA1c, total cholesterol, high-density lipoprotein cholesterol, triglyceride, and systolic blood pressure were included as fixed effects. Model 2: Model 1 plus smoking, DPP-4 inhibitors, pioglitazone, angiotensin-converting enzyme/angiotensin II receptor blocker, statins, and anti-platelets were included as fixed effects. Model 3: Model 1 plus smoking, hypoglycemic agents, angiotensin-converting enzyme/angiotensin II receptor blocker, statins, and anti-platelets were included as fixed effects. Model 4: Model 1 plus hypertension, smoking, hypoglycemic agents, antihypertensive agents, statins, and anti-platelets were included as fixed effects
Table 4.
Effects of tofogliflozin on brachial-ankle pulse wave velocity analyzed using covariance models
| Tofogliflozin treatment group | Conventional treatment group | Treatment effect (tofogliflozin–conventional treatment) (mean change [95% CI]) | |
|---|---|---|---|
| Right baPWV (cm/s) (mean change; SE) | |||
| Week 52 (n = 138) | 1.3 (39.9) | 77.0 (38.8) | − 75.5 (− 171.1, 19.7), p = 0.12 |
| Week 104 (n = 130) | − 67.9 (32.5) | 34.7 (31.5) | − 102.7 (− 182.1, − 23.2), p = 0.012 |
| Left baPWV (cm/s) (mean change; SE) | |||
| Week 52 (n = 138) | − 7.1 (40.1) | 76.2 (39.1) | − 83.3 (− 179.3, 12.8), p = 0.09 |
| Week 104 (n = 130) | − 33.7 (32.2) | 67.6 (31.4) | − 101.3 (− 180.3, − 22.3), p = 0.012 |
| Mean baPWV (cm/s) (mean change; SE) | |||
| Week 52 (n = 138) | − 4.2 (39.2) | 76.9 (38.1) | − 81.2 (− 174.9, 12.6), p = 0.09 |
| Week 104 (n = 130) | − 51.9 (31.4) | 51.2 (30.5) | − 103.1 (− 180.1, − 26.2), p = 0.009 |
Differences in delta change in baPWV from the baseline between the two groups were analyzed using analysis of covariance models that included treatment group, age, sex, use of insulin, baseline mean baPWV, systolic blood pressure, presence of hypertension, and antihypertensive drug administration
Subgroup analysis was performed according to whether the reductions in SBP (value at week 104-value at the baseline < 0 mmHg) was observed or did not show similar results. In the subgroup without a reduction in SBP during the observation period, tofogliflozin (n = 39) significantly attenuated the progression of right, left, and mean baPWV compared to those in the conventional treatment (n = 39) (− 101.4 [− 173.0, –29.8], mean change [95%CI], p = 0.006; − 103.8 [− 182.2, − 25.5], p = 0.010; − 102.6 [− 173.6, − 31.7], p = 0.005, respectively). In the subgroup with the reduction in SBP during the observation period (tofogliflozin; n = 37, conventional treatment [n = 29], similar results were observed; however, they did not reach statistical significance (− 108.9 [− 260.2, 42.3], p = 0.15; − 99.1 [− 243.6, 45.4], p = 0.18; − 104.0 [− 246.9, 38.9], p = 0.15, respectively). The results of other subgroup analyses resembled those produced by whole-group analyses (Additional file 7).
Discussion
Arterial stiffness is one of the earliest detectable signs of functional and structural changes in the vascular wall. baPWV, an established quantitative indicator of arterial stiffness, is linked to CV risk factors [31, 32] and is a promising predictor of future CV events independent of conventional CV risk factors [29, 33–39]. However, the effect of SGLT2 inhibitors on the progression of baPWV has not yet been completely elucidated.
To the best of our knowledge, this is the first study to demonstrate the suppression of baPWV increase by SGLT2 inhibitors in patients with T2DM without a history of CVD. Interestingly, our findings were consistent with those of several previous studies indicating the beneficial effect of SGLT2 inhibitors on the carotid-femoral PWV (cfPWV), which is currently the gold standard for measuring aortic stiffness and has been shown to predict outcomes. A single-arm, prospective clinical trial in 42 patients with T1DM revealed that 8-week treatment with empagliflozin was associated with a decline in arterial stiffness as assessed by cfPWV and carotid-radial PWV [8]. Similarly, another pilot study including 16 patients with T2DM revealed that 2-day treatment with dapagliflozin significantly improved cfPWV, systemic endothelial function, and renal resistive index [9, 10]. Furthermore, a recent, small randomized clinical trial demonstrated that canagliflozin improved cfPWV in 30 patients with T2DM and hypertension, independent of the BP effect [11]. Although the cfPWV primarily reflects aortic stiffness, baPWV is an index of arterial stiffness based on the measurements performed at peripheral sites, reflecting the wall properties not only of the aorta but also of muscle arteries. The baPWV is thus influenced by several factors modulating small- and medium-sized arteries, such as central sympathetic drive, smooth muscle tone, and spontaneous vasomotion, other than those known to influence large arteries and cfPWV. The clinical significance could be different between baPWV and cfPWV, and thus, our study adds novel findings regarding the effect of SGLT2 inhibitors on arterial stiffness.
The beneficial effects of SGLT2 inhibitors, such as tofogliflozin [12], empagliflozin [13–15], and canagliflozin [16], on arterial stiffness have been revealed in clinical studies using other indices of arterial stiffness, such as ambulatory arterial stiffness index [13], pulse pressure, mean arterial pressure, double product [16], and cardio-ankle vascular index (CAVI) [12]. However, several studies did not find beneficial effects of this class of antidiabetics on arterial stiffness [18]. Patoulias et al. recently reviewed and summarized clinical studies on this topic and concluded that evidence on the effect of SGLT2 inhibitors on arterial stiffness remains limited and controversial [19].
In addition, these clinical findings were consistent with those of an experimental study on animal models of arteriosclerosis that showed that an SGLT2 inhibitor suppressed arteriosclerosis [40–43]. Furthermore, our findings were consistent with the results of several previous studies that indicated the suppression of CV events by SGLT2 treatment in patients with high-risk T2DM [3, 4].
Interestingly, as we recently reported in a previous study, there was no significant difference in carotid IMT between the tofogliflozin and conventional treatment groups in the UTOPIA trial [23]. Several possible reasons explain the different outcomes between the observed changes in the carotid IMT (the primary outcome) and that of baPWV (a secondary outcome). One possible explanation is that the UTOPIA trial was not powered to detect significant differences between the treatment groups when evaluating IMT. Furthermore, it could be possible that the inhibition of IMT progression following tofogliflozin treatment might have been masked by the analogous effects of other drugs such as metformin, dipeptidyl peptidase (DPP)-4 inhibitors, antihypertensive drugs, and lipid-lowering agents [23]. Another possible explanation is that tofogliflozin could have different effects on IMT and baPWV. Both IMT and baPWV are indicators of atherosclerosis; however, it is believed that they reflect different aspects of atherosclerosis. Generally, atherosclerotic changes occur in the large arteries (including the carotid arteries), whereas CIMT primarily reflects the degree of atherosclerosis, particularly structural changes, developed in the large arteries [44]. In contrast, baPWV reflects a decrease in the elasticity of the arterial wall due to functional as well as structural arteriosclerotic changes that occur in the area extending from the aorta to the arteries of the extremities [20, 21]. Such a decrease in the elasticity of the arterial wall is not solely associated with atherosclerotic changes. Loss of elasticity of the arterial wall increases the vascular wall stress, resulting in atherosclerosis, an increase in the left ventricular afterload, left ventricular diastolic dysfunction, and coronary perfusion disorder. In asymptomatic patients with CV risk factors, deterioration in arterial stiffness was associated with hospitalization for new–onset heart failure [7]. Based on this, baPWV may reflect not only atherosclerotic changes but also risks of an extensive range of cardiovascular disorders including cardiac dysfunction. Therefore, changes in the baPWV, rather than in the carotid IMT, might be a better indicator of the whole CV system.
Although the beneficial effect of tofogliflozin on baPWV progression was independent of the conventional risk factors assessed at baseline, improvements in multiple CV risk factors by tofogliflozin could have inhibited the progression of arterial stiffness. Arterial stiffness reportedly improved after an intervention of classic CV risk factors (diabetes, obesity, hypertension, and hyperlipidemia). SGLT2 inhibitors have a favorable effect on these risk factors, such as lowering blood glucose levels and blood pressure as well as improving obesity and lipid profile [1, 2]. Moreover, the original UTOPIA trial showed a significant decrease in HbA1c, blood glucose levels, BMI, abdominal circumference, and SBP. Furthermore, high-density lipoprotein (HDL) cholesterol levels significantly increased [23]. Similar results were observed in this study, where patients with baPWV data at baseline were subjected (Additional file 3). Thus, improvements in the classic risk factors via an intervention could inhibit the progression of arterial stiffness.
It is believed that BP is a strong contributing factor to PWV [45]. To assess the effect of BP, we assigned patients to two groups based on their SBP during the observation period (i.e., decreased BP and unchanged/increased BP groups) for subgroup analysis. Interestingly, a positive effect of tofogliflozin on baPWV was observed in the group where patients’ SBP remained unchanged or increased during the observation period. In other words, such a favorable effect cannot be explained by the decrease in BP alone.
The major pathophysiologic mechanisms underlying increased arterial stiffness in DM include hyperglycemia, insulin resistance, the formation of advanced glycation end products (AGEs), enhanced oxidative stress, chronic inflammation, and increased activity of the renin–angiotensin–aldosterone system [43]. In addition, our previous study indicated that elevated levels of plasma indoxyl sulfate, a uremic toxin, was related to increased baPWV [46]. Although it has been demonstrated that SGLT2 inhibition ameliorates insulin resistance, decreases the expression of AGEs, and suppresses oxidative stress and inflammatory response [19], these aspects were not elucidated in the present study.
Limitations
This study had several limitations. First, the number of study subjects was small and the change in baPWV over time was a secondary outcome in the UTOPIA trial. In addition, not all patients who were enrolled in the UTOPIA trial underwent baPWV evaluation. There were significant differences in certain variables at baseline between those who underwent baPWV measurements and those who did not (Additional file 2), which was not irrelevant to bias. Therefore, results should be interpreted with caution and further investigation in a large-scale study that uses changes in baPWV over time as the primary outcome is required.
Second, rates of complication with hypertension and the use of antihypertensive drugs were lower in the tofogliflozin than in the normal treatment group at baseline (Additional file 5). Such a tendency was observed throughout the study period and may have affected the study results. However, multivariate analysis after adjusting for concomitant drugs and several CV risk factors, including BP, confirmed a significant association between tofogliflozin treatment and the inhibition of baPWV progression (Tables 3 and 4).
Third, several studies have demonstrated baPWV as an independent risk factor for CVDs. In addition, various studies have been conducted for antihypertensive [47, 48] and antidiabetic agents [49, 50] using changes in baPWV over time as an outcome. However, there is insufficient evidence about whether inhibiting the increase in baPWV reflects a lowered risk of the onset of CVDs. Hence, it would be too early to conclude that tofogliflozin reduces the risk of onset of CVDs in patients with T2DM without a history of CVD.
Finally, the patients in this study were Japanese with T2DM, a cohort with relatively low CV risks. Therefore, it would be premature to generalize our findings to other racial or ethnic groups.
Conclusion
This study showed that tofogliflozin significantly inhibited the increase in baPWV in patients with T2DM without a history of CVD, and suggested that tofogliflozin suppressed the progression of arterial stiffness.
Supplementary Information
Additional file 1: UTOPIA trial site investigators.
Additional file 2: Table S1. Clinical characteristics of patients with and without brachial-ankle pulse wave velocity data.
Additional file 3: Table S2. Between-group comparison of changes in clinical parameters during the treatment period.
Additional file 4: Table S3. Changes in concomitantly used anti-diabetic agents.
Additional file 5: Table S4. Changes in concomitantly used cardiovascular medications.
Additional file 6: Table S5. Effects of tofogliflozin on brachial-ankle pulse wave velocity in individuals with all three measurements (baseline, week 52, and week 104).
Additional file 7: Table S6. Effects of tofogliflozin on brachial-ankle pulse wave velocity change in subgroups.
Acknowledgments
The authors thank all the staff and patients who participated in this study. The authors gratefully acknowledge the assistance of D. Takayama and H. Yamada (Soiken Holdings Inc., Tokyo, Japan) and Editage (https://www.editage.com) for English language editing.
Abbreviations
- ANCOVA
Analysis of covariance
- BMI
Body mass index
- eGFR
Estimated glomerular filtration rate
- IMT
Intima–media thickness
- SGLT2
Sodium-glucose cotransporter 2
- UTOPIA
Using Tofogliflozin for possible better intervention against atherosclerosis for type 2 diabetes patients
Authors’ contributions
The authors meet the criteria for authorship recommended by the International Committee of Medical Journal Editors and take complete responsibility for all contents of the manuscript and editorial decisions. All authors contributed to the study design and were involved at all stages of manuscript development. NK drafted the manuscript. YS contributed to the analysis of research data. All authors were involved in the analysis and interpretation of data, reviewed and edited the manuscript, and approved the final manuscript. IS and HW are the principal guarantors of this work and have complete access to all the data and take responsibility for the integrity of the data and accuracy of data analysis. All authors read and approved the final manuscript.
Funding
Financial support for this study, including the fees for assistance and processing of the article by the journal, was provided by Kowa Co., Ltd., Tokyo, Japan.
Availability of data and materials
The datasets generated and/or analyzed during our study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and as per the Helsinki Declaration of 1964 (revised in 2013). Informed consent was obtained from all patients for inclusion in the study.
Consent for publication
Not applicable.
Competing interests
Naoto Katakami is a staff member of the endowed chair established by funds from Kowa Co., Ltd., and has received research funds from MSD and lecture fees from Astellas Pharma Inc., AstraZeneca K.K., Boehringer Ingelheim, Daiichi Sankyo Inc., Eli Lilly, Kowa Pharmaceutical Co., Kyowa Hakko Kirin Co. Ltd., Mitsubishi Tanabe Pharma Co., Novartis Pharmaceuticals, Novo Nordisk Pharma, Ono Pharmaceutical Co., Taisho Toyama Pharmaceutical Co., Takeda Pharmaceutical Co., Sanofi-Aventis, and Shionogi & Co.
Tomoya Mita has received lecture fees from Astellas Pharma Inc., Daiichi Sankyo Inc., Eli Lilly, Kowa Pharmaceutical Co., Kyowa Hakko Kirin Co. Ltd., Mitsubishi Tanabe Pharma Co., Novo Nordisk Pharma, Ono Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Company Ltd. and Sanofi-Aventis; scholarship donations from MSD K.K., Astellas Pharma Inc., AstraZeneca K.K., Ono Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co. Ltd., Sanofi-Aventis K.K., Daiichi Sankyo Company, Limited, Sumitomo Dainippon Pharma Co., Ltd., Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, Terumo Corporation, Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Pfizer Japan Inc., Benefit One Health Care Inc., Mochida Pharmaceutical Co., Ltd., and Nitto Boseki Co., Ltd.; and endowed chair funding from MSD K.K., Takeda Pharmaceutical Company Limited.
Toshihiko Shiraiwa has received lecture fees from Sanofi-Aventis K.K. and Takeda Pharmaceutical Company Limited, and research funding from Novo Nordisk Pharma Ltd., Sanofi-Aventis K.K., Takeda Pharmaceutical Company Limited, AstraZeneca K.K., Nippon Boehringer Ingelheim Co., Ltd., and Mitsubishi Tanabe Pharma Corporation.
Tetsuyuki Yasuda received lecture fees from Nippon Boehringer Ingelheim Co., Ltd. and Sanofi-Aventis K.K.
Yosuke Okada has received lecture fees from Astellas Pharma Inc., MSD K.K., Ono Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Bayer Holding Ltd., Novartis Pharmaceuticals Corp., Novo Nordisk Pharma Ltd., Eli Lilly Japan K.K., and Kissei Pharmaceutical Co., Ltd.; and research funding from Kowa Pharmaceutical Co. Ltd. and Mitsubishi Tanabe Pharma Corporation.
Hideaki Kaneto has received lecture fees from Nippon Boehringer Ingelheim Co., Ltd., Sanofi-Aventis K.K., Novo Nordisk Pharma Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited, Daiichi Sankyo Company, Limited, Mitsubishi Tanabe Pharma Corporation, AstraZeneca K.K., Astellas Pharma Inc., Novartis Pharmaceuticals Corp., and Sumitomo Dainippon Pharma Co.; scholarship donations from Novo Nordisk Pharma Ltd., Nippon Boehringer Ingelheim Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd., Sanofi-Aventis K.K., Eli Lilly Japan K.K., Astellas Pharma Inc., Daiichi Sankyo Company, Limited, Mitsubishi Tanabe Pharma Corporation, MSD K.K., Takeda Pharmaceutical Company Limited, AstraZeneca K.K., Mochida Pharmaceutical Co., Ltd., Taisho Toyama Pharmaceutical Co., Kissei Pharmaceutical Co., Ltd., and Kyowa Hakko Kirin Co. Ltd; and research funding from Taisho Pharmaceutical Co., Sumitomo Dainippon Pharma Co. Nippon Boehringer Ingelheim Co.
Takeshi Osonoi has received lecture fees from Takeda Pharmaceutical Company Limited, Astellas Pharma Inc., Novo Nordisk Pharma Ltd., Sanwa Kagaku Kenkyusho Co., Ltd.; manuscript fees from Sanwa Kagaku Kenkyusho Co., Ltd.; and research funding from Takeda Pharmaceutical Company Limited, Novo Nordisk Pharma Ltd., Astellas Pharma Inc., Sanwa Kagaku Kenkyusho Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Taisho Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Daiichi Sankyo Company, Limited, Bayer Holding Ltd., Kowa Pharmaceutical Co. Ltd., and AbbVie Inc.
Nobuichi Kuribayashi has received lecture fees from Takeda Pharmaceutical Company Limited, Sanofi-Aventis K.K., Novo Nordisk Pharma Ltd., MSD K.K., and Mitsubishi Tanabe Pharma Corporation.
Satoru Sumitani has received lecture fees from Sumitomo Dainippon Pharma Co., Ltd.
Yasunori Sato has received lecture fees from Mochida Pharmaceutical Co., Ltd.
Hirotaka Watada has received lecture fees from Sumitomo Dainippon Pharma Co., Ltd., Bayer Yakuhin, Ltd. Sanofi-Aventis K.K., MSD K.K., Astellas Pharma Inc., Takeda Pharmaceutical Company Ltd., Mitsubishi Tanabe Pharma Co., AstraZeneca K.K., Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Kowa Co., Ltd., Novartis Pharmaceuticals Corp., Daiichi Sankyo Company, Ltd, Kyowa Hakko Kirin Co. Ltd., Ono Pharmaceutical Co., Ltd., and Kissei Pharmaceutical Co., Ltd.; and research support from Novartis Pharmaceuticals Corp., Otsuka Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., MSD K.K., Astellas Pharma Inc., Bayer Yakuhin, Ltd. Teijin Pharma Ltd., Ono Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co. Ltd., Kowa Pharmaceutical Co. Ltd., Sanofi-Aventis K.K., Sanwa Kagaku Kenkyusho Co., Ltd., Daiichi Sankyo Company, Ltd., Sumitomo Dainippon Pharma Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Pfizer Japan Inc., Shionogi & Co., Ltd. Yakult, and Kissei Pharmaceutical Co., Ltd.
Iichiro Shimomura has received lecture fees from Astellas Pharma Inc., AstraZeneca K.K., MSD K.K., Ono Pharmaceutical Co., Kyowa Kirin Co., Ltd., Kowa Company, Ltd., Sanofi K.K., Sanwa Kagaku Kenkyusho Co., Daiichi Sankyo Co., Takeda Pharma K.K., Mitsubishi Tanabe Pharma Co., Teijin Pharma, Eli Lilly Japan K.K., Nippon Boehringer Ingelheim Co., Novartis Pharma K.K., Novo Nordisk Pharma, Mochida Pharmaceutical Co., Taisho Pharmaceutical Co. Ltd., Nippon Chemiphar Co., Ltd., Covidien Japan Inc., Amgen Astellas Biopharma K.K., KOBAYASHI Pharmaceutical Co., Ltd., Dainippon Sumitomo Pharma Co., Rohto Pharmaceutical Co., Ltd.; research funds from Astellas Pharma Inc., MSD K.K, Ono Pharmaceutical Co., Kaken Pharmaceutical Co., Kyowa Kirin Co., Ltd., Sanofi K.K., Shionogi & Co., Daiichi Sankyo Co., Dainippon Sumitomo Pharma Co., Takeda Pharma K.K., Mitsubishi Tanabe Pharma Co., Teijin Pharma, Novartis Pharma K.K., Novo Nordisk Pharma, Eli Lilly Japan K. K, Kowa Company, Ltd.; and consulting fees from AstraZeneca K.K., MSD K.K., Taisho Pharmaceutical Co. Ltd., Novo Nordisk Pharma, Lotte Co., Ltd.
Hidenori Yoshii, Yutaka Umayahara, Tsunehiko Yamamoto, Kazuhisa Maeda, Hiroki Yokoyama, Keisuke Kosugi, Kentaro Ohtoshi, Isao Hayashi, Mamiko Tsugawa, Kayoko Ryomoto, Hideki Taki, Tadashi Nakamura, and Satoshi Kawashima declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-020-01206-1.
References
- 1.Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, et al. Sodium glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262–274. doi: 10.7326/0003-4819-159-4-201308200-00007. [DOI] [PubMed] [Google Scholar]
- 2.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 3.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:211721–211728. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 4.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, CANVAS Program Collaborative Group et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 5.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, DECLARE–TIMI 58 Investigators et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 6.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 7.Aisu H, Saito M, Inaba S, Morofuji T, Takahashi K, Sumimoto T, et al. Association of worsening arterial stiffness with incident heart failure in asymptomatic patients with cardiovascular risk factors. Hypertens Res. 2017;40:173–180. doi: 10.1038/hr.2016.116. [DOI] [PubMed] [Google Scholar]
- 8.Cherney DZ, Perkins BA, Soleymanlou N, Har R, Fagan N, Johansen OE, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28. doi: 10.1186/1475-2840-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solini A, Giannini L, Seghieri M, Vitolo E, Taddei S, Ghiadoni L, et al. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16:138. doi: 10.1186/s12933-017-0621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batzias K, Antonopoulos AS, Oikonomou E, Siasos G, Bletsa E, Stampouloglou PK, et al. Effects of newer antidiabetic drugs on endothelial function and arterial stiffness: a systematic review and meta-analysis. J Diabetes Res. 2018;2018:1232583. doi: 10.1155/2018/1232583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramirez AJ, Sancheza MJ, Sanchez RA. Diabetic patients with essential hypertension treated with amlodipine: blood pressure and arterial stiffness effects of canagliflozin or perindopril. J Hypertens. 2019;37:636–642. doi: 10.1097/HJH.0000000000001907. [DOI] [PubMed] [Google Scholar]
- 12.Bekki M, Tahara M, Tahara A, Igata S, Honda A, Sugiyama Y, et al. Switching dipeptidyl peptidase-4 inhibitors to tofogliflozin, a selective inhibitor of sodium-glucose cotransporter 2 improve arterial stiffness evaluated by cardio-ankle vascular index in patients with type 2 diabetes: a pilot study. Curr Vasc Pharmacol. 2019;17:411–420. doi: 10.2174/1570161116666180515154555. [DOI] [PubMed] [Google Scholar]
- 13.Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17:1180–1193. doi: 10.1111/dom.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Striepe K, Jumar A, Ott C, Karg MV, Schneider MP, Kannenkeril D, et al. Effects of the selective sodium-glucose cotransporter 2 inhibitor empagliflozin on vascular function and central hemodynamics in patients with type 2 diabetes mellitus. Circulation. 2017;136:1167–1169. doi: 10.1161/CIRCULATIONAHA.117.029529. [DOI] [PubMed] [Google Scholar]
- 15.Bosch A, Ott C, Jung S, Striepe K, Karg MV, Kannenkeril D, et al. How does empagliflozin improve arterial stiffness in patients with type 2 diabetes mellitus? Sub analysis of a clinical trial. Cardiovasc Diabetol. 2019;18:44. doi: 10.1186/s12933-019-0839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfeifer M, Townsend RR, Davies MJ, Vijapurkar U, Ren J. Effects of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on blood pressure and markers of arterial stiffness in patients with type 2 diabetes mellitus: a post hoc analysis. Cardiovasc Diabetol. 2017;16:29. doi: 10.1186/s12933-017-0511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ott C, Jumar A, Striepe K, Friedrich S, Karg MV, Bramlage P, et al. A randomised study of the impact of the SGLT2 inhibitor dapagliflozin on microvascular and macrovascular circulation. Cardiovasc Diabetol. 2017;16:26. doi: 10.1186/s12933-017-0510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kario K, Okada K, Murata M, Suzuki D, Yamagiwa K, Abe Y, et al. Effects of luseogliflozin on arterial properties in patients with type 2 diabetes mellitus: The multicenter, exploratory LUSCAR study. Clin Hypertens. 2020;22:1585–1593. doi: 10.1111/jch.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patoulias D, Papadopoulos C, Stavropoulos K, Zografou I, Doumas M, Karagiannis A. Prognostic value of arterial stiffness measurements in cardiovascular disease, diabetes, and its complications: The potential role of sodium-glucose co-transporter-2 inhibitors. J Clin Hypertens. 2020;22:562–571. doi: 10.1111/jch.13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005;18:3S–10S. doi: 10.1016/j.amjhyper.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 22.Katakami N, Mita T, Yoshii H, Shiraiwa T, Yasuda T, Okada Y, UTOPIA Study Investigators et al. Rationale, design, and baseline characteristics of the UTOPIA trial for preventing diabetic atherosclerosis using an SGLT2 inhibitor: a prospective, randomized, open-label, parallel-group comparative study. Diabetes Ther. 2017;8:999–1013. doi: 10.1007/s13300-017-0292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katakami N, Mita T, Yoshii H, Shiraiwa T, Yasuda T, Okada Y, on behalf of the UTOPIA study investigators et al. Tofogliflozin does not delay progression of carotid atherosclerosis in patients with type 2 diabetes: a prospective, randomized, open-label, parallel-group comparative study. Cardiovasc Diabetol. 2020;19:110. doi: 10.1186/s12933-020-01079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Japan Diabetes Society . Treatment guide for diabetes. 2014–2015. Tokyo: Bunkodo Co., Ltd.; 2014. [Google Scholar]
- 25.Suzuki E, Kashiwagi A, Nishio Y, Egawa K, Shimizu S, Maegawa H, et al. Increased arterial wall stiffness limits flow volume in the lower extremities in type 2 diabetic patients. Diabetes Care. 2001;24:2107–2114. doi: 10.2337/diacare.24.12.2107. [DOI] [PubMed] [Google Scholar]
- 26.Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359–364. doi: 10.1291/hypres.25.359. [DOI] [PubMed] [Google Scholar]
- 27.Turin TC, Kita Y, Rumana N, Takashima N, Kadota A, Matsui K, et al. Brachial-ankle pulse wave velocity predicts all-cause mortality in the general population: findings from the Takashima Study. Japan Hypertens Res. 2010;33:922–925. doi: 10.1038/hr.2010.103. [DOI] [PubMed] [Google Scholar]
- 28.Miyano I, Nishinaga M, Takata J, Shimizu Y, Okumiya K, Matsubayashi K, et al. Association between brachial-ankle pulse wave velocity and 3-year mortality in community-dwelling older adults. Hypertens Res. 2010;33:678–682. doi: 10.1038/hr.2010.56. [DOI] [PubMed] [Google Scholar]
- 29.Katakami N, Osonoi T, Takahara M, Saitou M, Matsuoka TA, Yamasaki Y, et al. Clinical utility of brachial-ankle pulse wave velocity in the prediction of cardiovascular events in diabetic patients. Cardiovasc Diabetol. 2014;13:128. doi: 10.1186/s12933-014-0128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 31.Won KB, Chang HJ, Kim HC, Jeon K, Lee H, Shin S, et al. Differential impact of metabolic syndrome on subclinical atherosclerosis according to the presence of diabetes. Cardiovasc Diabetol. 2013;12:41. doi: 10.1186/1475-2840-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohnishi H, Saitoh S, Takagi S, Ohata J, Isobe T, Kikuchi Y, et al. Pulse wave velocity as an indicator of atherosclerosis in impaired fasting glucose: the Tanno and Sobetsu study. Diabetes Care. 2003;26:437–440. doi: 10.2337/diacare.26.2.437. [DOI] [PubMed] [Google Scholar]
- 33.Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, Ioakeimidis N, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: a systematic review and meta-analysis. Hypertension. 2012;60:556–562. doi: 10.1161/HYPERTENSIONAHA.112.194779. [DOI] [PubMed] [Google Scholar]
- 34.Han JY, Choi DH, Choi SW, Kim BB, Ki YJ, Chung JW, et al. Predictive value of brachial-ankle pulse wave velocity for cardiovascular events. Am J Med Sci. 2013;346:92–97. doi: 10.1097/MAJ.0b013e318268c05a. [DOI] [PubMed] [Google Scholar]
- 35.Nagai K, Shibata S, Akishita M, Sudoh N, Obara T, Toba K, et al. Efficacy of combined use of three non-invasive atherosclerosis tests to predict vascular events in the elderly; carotid intima-media thickness, flow-mediated dilation of brachial artery and pulse wave velocity. Atherosclerosis. 2013;231:365–370. doi: 10.1016/j.atherosclerosis.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 36.Takashima N, Turin TC, Matsui K, Rumana N, Nakamura Y, Kadota A, et al. The relationship of brachial-ankle pulse wave velocity to future cardiovascular disease events in the general Japanese population: the Takashima Study. J Hum Hypertens. 2014;28:323–327. doi: 10.1038/jhh.2013.103. [DOI] [PubMed] [Google Scholar]
- 37.Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, Collaborative Group for J-BAVEL (Japan Brachial-Ankle Pulse Wave Velocity Individual Participant Data Meta-Analysis of Prospective Studies) et al. Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta-analysis. Hypertension. 2017;69:1045–52. doi: 10.1161/HYPERTENSIONAHA.117.09097. [DOI] [PubMed] [Google Scholar]
- 38.Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, et al. Endothelial dysfunction, increased arterial stiffness, and cardiovascular risk prediction in patients with coronary artery disease: FMD-J (Flow-Mediated Dilation Japan) study A. J Am Heart Assoc. 2018;7:e008588. doi: 10.1161/JAHA.118.008588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JM, Kim SS, Kim IJ, Kim JH, Kim BH, Kim MK, Lee SH, Lee CW, Kim MC, Ahn JH, Kim J, for the Relationship between Cardiovascular disease and Brachial-ankle Pulse Wave Velocity (baPWV) in Patients with Type 2 Diabetes (REBOUND) Study Group Arterial stiffness is an independent predictor for risk of mortality in patients with type 2 diabetes mellitus: the REBOUND study. Cariovasc Diabetol. 2020;19:143. doi: 10.1186/s12933-020-01120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin B, Koibuchi N, Hasegawa Y, Sueta D, Toyama K, Uekawa K, et al. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol. 2014;13:148. doi: 10.1186/s12933-014-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han JH, Oh TJ, Lee G, Maeng HJ, Lee DH, Kim KM, et al. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE−/− mice fed a western diet. Diabetologia. 2017;60:364–376. doi: 10.1007/s00125-016-4158-2. [DOI] [PubMed] [Google Scholar]
- 42.Nasiri-Ansari Ν, Dimitriadis GK, Agrogiannis G, Perrea D, Kostakis ID, Kaltsas G, et al. Canagliflozin attenuates the progression of atherosclerosis and inflammation process in APOE knockout mice. Cardiovasc Diabetol. 2018;17:106. doi: 10.1186/s12933-018-0749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aroor AR, Das NA, Carpenter AJ, Habibi J, Jia G, Ramirez-Perez FI, et al. Glycemic control by the SGLT2 inhibitor empagliflozin decreases aortic stiffness, renal resistivity index and kidney injury. Cardiovasc Diabetol. 2018;17:108. doi: 10.1186/s12933-018-0750-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, American College of Cardiology Foundation. American Stroke Association. American Association of Neurological Surgeons. American College of Radiology; American Society of Neuroradiology. Congress of Neurological Surgeons. Society of Atherosclerosis Imaging and Prevention. Society for Cardiovascular Angiography and Interventions. Society of Interventional Radiology. Society of NeuroInterventional Surgery; Society for Vascular Medicine. Society for Vascular Surgery et al. ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation. 2011;124:489–32. doi: 10.1161/CIR.0b013e31820d8d78. [DOI] [PubMed] [Google Scholar]
- 45.Baier D, Teren A, Wirkner K, Loeffler M, Scholz M. Parameters of pulse wave velocity: determinants and reference values assessed in the population-based study LIFE-Adult. Clin Res Cardiol. 2018;107:1050–1061. doi: 10.1007/s00392-018-1278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katakami N, Omori K, Taya N, Arakawa S, Takahara M, Matsuoka TA, et al. Plasma metabolites associated with arterial stiffness in patients with type 2 diabetes. Cardiovasc Diabetol. 2020;19:75. doi: 10.1186/s12933-020-01057-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahimastos AA, Aggarwal A, D’Orsa KM. Effect of perindopril on large artery stiffness and aortic root diameter in patients with Marfan syndrome. JAMA. 2007;298:1539–1547. doi: 10.1001/jama.298.13.1539. [DOI] [PubMed] [Google Scholar]
- 48.Redon J, Pichler G. Comparative study of the efficacy of olmesartan/amlodipine vs. Perindopril/amlodipine in peripheral and central blood pressure parameters after missed dose in type 2 diabetes. Am J Hypertens. 2016;29:1055–62. doi: 10.1093/ajh/hpw033. [DOI] [PubMed] [Google Scholar]
- 49.de Boer SA, Heerspink HJL, Juárez Orozco LE, van Roon AM, Kamphuisen PW, Smit AJ, et al. Effect of linagliptin on pulse wave velocity in early type 2 diabetes: a randomized, double-blind, controlled 26-week trial (RELEASE) Diabetes Obes Metab. 2017;19:1147–1154. doi: 10.1111/dom.12925. [DOI] [PubMed] [Google Scholar]
- 50.Kishimoto S, Kinoshita Y, Matsumoto T, Maruhashi T, Kajikawa M, Matsui S, et al. Effects of the dipeptidyl peptidase 4 inhibitor alogliptin on blood pressure in hypertensive patients with type 2 diabetes mellitus. Am J Hypertens. 2019;32:695–702. doi: 10.1093/ajh/hpz065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: UTOPIA trial site investigators.
Additional file 2: Table S1. Clinical characteristics of patients with and without brachial-ankle pulse wave velocity data.
Additional file 3: Table S2. Between-group comparison of changes in clinical parameters during the treatment period.
Additional file 4: Table S3. Changes in concomitantly used anti-diabetic agents.
Additional file 5: Table S4. Changes in concomitantly used cardiovascular medications.
Additional file 6: Table S5. Effects of tofogliflozin on brachial-ankle pulse wave velocity in individuals with all three measurements (baseline, week 52, and week 104).
Additional file 7: Table S6. Effects of tofogliflozin on brachial-ankle pulse wave velocity change in subgroups.
Data Availability Statement
The datasets generated and/or analyzed during our study are available from the corresponding author upon reasonable request.