Abstract
Transplantation of pancreatic islets within a biomaterial device is currently under investigation in clinical trials for the treatment of patients with type 1 diabetes (T1D). Patients’ preferences on such implants could guide the designs of next-generation implantable devices; however, such information is not currently available. We surveyed the preferences of 482 patients with T1D on the size, shape, visibility, and transplantation site of islet containing implants. More than 83% of participants were willing to receive autologous stem cells, and there was no significant association between implant fabricated by one’s own stem cell with gender (χ 2 (1, n = 468) = 0.28; P = 0.6) or with age (χ 2 (4, n = 468) = 2.92; P = 0.6). Preferred location for islet transplantation within devices was under the skin (52.7%). 48.3% preferred microscopic disks, and 32.3% preferred a thin device (like a credit card). Moreover, 58.4% preferred the implant to be as small as possible, 25.4% did not care about visibility, and 16.2% preferred their implants not to be visible. Among female participants, 81% cared about the implant visibility, whereas this number was 64% for male respondents (χ 2 test (1, n = 468) = 16.34; P < 0.0001). 22% of those younger than 50 years of age and 30% of those older than 50 did not care about the visibility of implant (χ 2 test (4, n = 468) = 23.69; P < 0.0001). These results suggest that subcutaneous sites and micron-sized devices are preferred choices among patients with T1D who participated in our survey.
Keywords: implantable devices, biomaterials, islet transplantation, type 1 diabetes, patients’ preferences
Introduction
With the advancement of diabetes medical technology, the use of medical devices has quickly reached the forefront of how care is provided to patients with type 1 diabetes (T1D). Thus far, Food and Drug Administration-approved devices have included glucometers, continuous subcutaneous insulin infusions, and continuous glucose monitors (CGMs) leading to reductions in hypoglycemia, complications, and improvements in overall glycemic control 1 . Additionally, upon introduction 2 and a multicenter trial 3 of clinical islet transplantation, many efforts have been devoted to further optimize transplantation of islets into humans with T1D as a long-term treatment. Critical barriers have impeded long-term efficacy of islet transplantation. For instance, islets are prone to Anoikis (detachment of islets from extracellular matrix after transplantation) 4 as well as instant blood-mediated inflammatory reaction 5 , which eventually eliminates the transplanted islets from a recipients’ body. To counteract these effects, immunosuppressive drugs have been employed in islet allotransplantation and xenotransplantation; however, the amount of immunosuppressive regimen required after islet transplantation may make patients more prone to infection, mouth ulcers, diarrhea, and acne 6 . To partly address these challenges, the use of a protective shield around the islets 7 has helped the functional longevity of the transplant through the protection of islets from the host’s immune response, while still providing the diffusion of insulin and glucose across the membrane 8,9 .
At least three factors are known to play vital roles in the success of islet transplantation within an immune-isolating device 10 : islet survival and function after transplantation, lack of immune response against the implant, and functional integration of the transplant with the host. While extensive research has been devoted to modulating the physical, chemical, immunological, and mechanical properties of implantable devices, there is a lack of understanding regarding patients’ preferences on devices. Such information is critical because it could guide the designs of next-generation implantable devices, as T1D patients are the ultimate users of these products. These devices could generally be classified into two categories, that is, macro-devices 11 –20 and microdevices 9,21 –27 . In most cases, macro-devices are few centimeter-sized made of biomaterials with channels or chambers that could hold islets within. The fundamental aspect of these devices is the protection of islets from immune attack while allowing the diffusion of nutrients, glucose, cellular waste, and insulin. Microdevice, also known as encapsulation device, refers to encapsulating islets within a semipermeable biomaterial, mostly with a spherical shape and micron-scale diameters. Similarly, microencapsulation has also been developed to block the immune attack against islets 28 , while allowing the diffusion of nutrients, glucose, cellular waste, and insulin. These implants are now under preclinical and clinical investigations with the goal of providing long-term glycemic control for patients with T1D 29,30 .
While these efforts and discoveries are setting the stage for successful clinical outcomes, patient’s preferences on the devices have not been explored to the best of our knowledge. We therefore aimed to reach out to patients with T1D to gain a better understanding of their perspectives regarding implants through an online survey. Such information may influence the design of future implantable devices to better suit the needs of patients with T1D. In a broader context, this study aids the cell transplantation and implantable biomedical devices fields to consider and implement the preferences of their endpoint users.
Materials and Methods
Respondent Recruitment and Inclusion Criteria
The inclusion criteria for this survey required respondents to be any individual diagnosed with T1D residing in the United States. Respondents were recruited from the Juvenile Diabetes Research Foundation (JDRF) Orange County chapter, Savvy Diabetic, and Close Concern Communities panel of patients with T1D, who are engaged in a web-based community. Our study was conducted in accordance with the relevant guidelines stated by the Declaration of Helsinki. Based on the nature of this survey that includes the use of anonymous responses, there was no requirement for the involvement of an ethics board and informed consent from participants, and we received an IRB Exemption Approval from the University of California Irvine, Irvine, CA, USA (Exemption Category 2b Approval: 12-04-2018) for the same.
Data Handling
We set the best scientific practice by defining a minimum requirement for the validity of the responses. We eliminated responses where respondents did not complete the survey or completed the survey in an unpractically short time (defined as less than 1.5 min based on the minimum amount of time required to read and answer all questions). These results were removed to produce a final validated dataset (overall, 13 participants [∼3%] of surveys were excluded from subsequent analysis). No specific product or brand names were mentioned in the survey. Data were categorized based on age and gender, and χ 2 analysis was performed to identify statistical significance using SAS 9.4 software.
Results were reported as χ 2 (degree of freedom, sample size) = test statistic value; P value.
Results
Cohort Characteristics
We first classified patients and identified our participants based on age, sex, duration of T1D, and current treatment they use (Table 1). The cohort that participated in our survey was of different ages. One hundred and sixty-three people (34.7%) were aged between 31 and 50, 115 people (24.5%) between 51 and 64, 76 people (16.2%) were 65+, 59 people (12.6%) were between 18 and 30, and 56 people (11.9%) were 16–18 years of age. Among the participants, 297 were female (63.3%), and more than 86.5% of the cohort were diagnosed with T1D for more than 5 years. Moreover, 34 (7.3%) and 29 (6.2%) patients were diagnosed with T1D between 2 and 5 and less than 2 years, respectively. More than 82.3% of patients are using both CGMs and insulin pump for managing their blood glucose levels. In addition, 77 participants are using multiple daily injections (MDI); 12 of whom are using CGM simultaneously. One of the patients was transplanted with the pancreas, and three others used MDI and flash glucose monitors.
Table1.
Participants in the Survey.
| Choices | Number | |
|---|---|---|
| Age | 16–18 | 56 |
| 18–30 | 59 | |
| 31–50 | 163 | |
| 51–64 | 115 | |
| 65+ | 76 | |
| Sex | Female | 297 |
| Male | 172 | |
| T1D duration | <2 years | 29 |
| 2–5 years | 34 | |
| >5 years | 405 | |
| Current treatment | MDI | 65 |
| Insulin pump + CGM | 386 | |
| Others | 18 |
CGM, continuous glucose monitoring; MDI, multiple daily injections; T1D, type 1 diabetes.
Responses to Survey
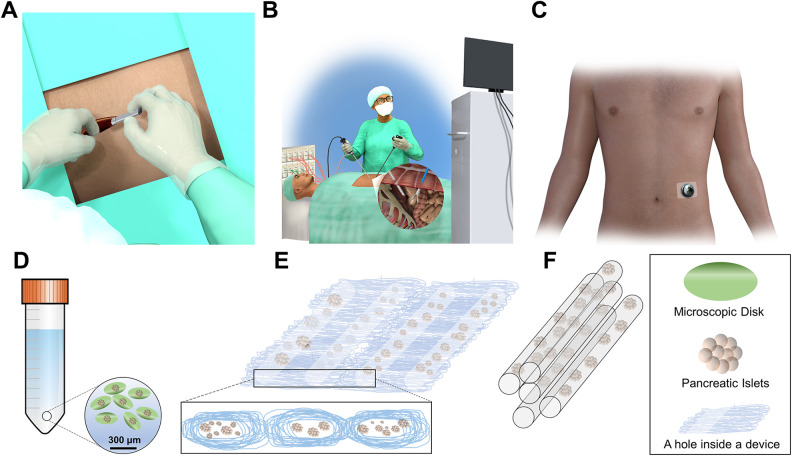
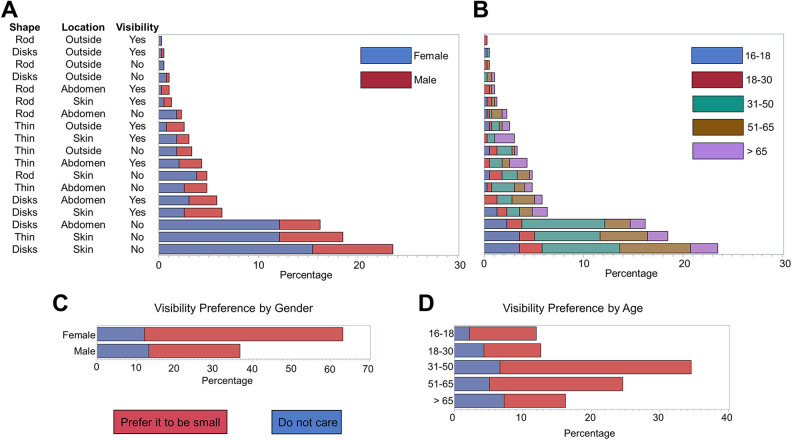
We first sought to investigate patients’ preference for the physical characteristics of the implants (Table 2). Figure 1 depicts the schematic representation of the implants and their transplantation location asked in the survey. We chose these shapes and locations because they resemble devices currently under investigation in clinical trials and research laboratories 30 . These preferences were defined as implant location (under the skin, in the abdomen, and outside of the body, schematically presented in Figs. 1A, B, and C, respectively), shape (multiple microscopic small disks deposited in a fluid, a thin device which is soft and the size of a credit card, and rod shaped of the size of a 3″ pencil, schematically presented in Figs. 1D, E, and F, respectively), and visibility (small or invisible, do not care about visibility). To evaluate these data and find associations between implants’ physical characteristics with age and gender, we examined the responses by gender (Fig. 2A) and age (Fig. 2B). We asked patients “If you receive an implant containing insulin-secreting cells, where do you prefer it to be? (Assume that size and shape are comfortable in each case)”. Two hundred and forty-seven (52.7%) preferred the device to be transplanted under their skin. The next most popular location was intra-abdominal with 141 patients (30.1%) choosing this location. Interestingly, only 34 (7.2%) participants responded, “outside my body.” Moreover, 47 patients (10.0%) commented on the question and chose the “Others” option. Some comments pointed out that depending on the longevity of the device’s activity, their preferences could vary, where longer-acting devices would be preferred to be transplanted into the abdomen. Interestingly, 16 patients noted that as long as the device functions, they are open to any location. Considering age and gender of participants, we found no significant association between implant location and gender (χ 2 (3, n = 468) = 2.08; P = 0.6), and age group (χ 2 (12, n = 468) = 13.74; P = 0.3).
Table 2.
Preferences of Individuals with Type 1 Diabetes Regarding Implants Characteristics.
| Question | Choices | Percentage |
|---|---|---|
| Implantation site | Under the skin | 52.7 |
| Inside abdomen | 30.1 | |
| Outside of body | 7.2 | |
| Size and shape of implants | Multiple microscopic disks deposited in a fluid | 48.3 |
| A thin device (soft and size of the credit card) | 32.3 | |
| Rod-shaped device (size of a 3″ pencil) | 9.8 | |
| Visibility importance | Not at all | 25.4 |
| Prefer it to be as small as possible | 58.4 | |
| Not visible at all | 16.2 | |
| Immunoregulatory implants | Implants that only regulate blood glucose | 30.6 |
| Implants that regulate blood glucose and immune system | 69.4 | |
| Implants with stem cell | Prefer an implant fabricated with my own stem cells | 83.7 |
| DO NOT prefer an implant fabricated with my own stem cells | 16.3 |
Figure 1.
Schematic representations of the implants and possible transplantation sites. Implants could be transplanted (A) under the skin (subcutaneously), (B) into the peritoneal cavity through laparoscopic surgery, or (C) outside of the body. Implant shapes could be (D) multiple microscopic small disks deposited in a fluid, (E) a thin device which is soft and the size of a credit card, and (F) rod shaped of the size of a 3″ pencil.
Figure 2.
Dependence of (A) gender and (B) age on respondents’ preference with respect to implant location (under the skin, in the abdomen, and outside of the body), shape (multiple microscopic small disks deposited in a fluid, a thin device which is soft and the size of a credit card, and rod shaped of the size of a 3″ pencil), and visibility (very small or invisible, do not care about visibility). Note that for the visibility, “Yes” means the respondent is fine with the implant to be visible, and “No” means otherwise. There is a significant association between age and implants’ shape (χ 2 (12, n = 468) = 25.59; P = 0.01). However, no significant association was found between age and implant location preference (χ 2 (12, n = 468) = 13.74; P = 0.3). Gender had no significant association with shape preference (χ 2 (3, n = 468) = 2.74; P = 0.4) and implant location (χ 2 (3, n = 468) = 2.08; P = 0.6). There was also an association of implant’s visibility with (C) gender and (D) age. Male participants were more indifferent regarding implant visibility (χ 2 (1, n = 468) = 16.34; P < 0.0001). Among male participants, 36% (62 out of 172) were indifferent about implant’s visibility, while this ratio was 19% (57 out of 296) for female participants. The age also was a determinant factor in the implant’s visibility preference among respondents. Around 22% of those younger than 50 (61 out of 277) do not care about the implant visibility compared with 30% (58 out of 191) of those older than 50 (χ 2 (4, n = 468) = 23.69; P < 0.0001).
We then asked our participants their preferences on the device’s shape. The top choice was “multiple microscopic disks deposited in a fluid” (n = 226, i.e., 48.3% of participants). A thin device (soft and size of the credit card) was the second top choice with 151 (32.3%) votes. A rod shape device (size of a 3″ pencil) was the least popular choice (n = 46, i.e., 9.8%). Nearly 10% (n = 45) had comments on the question and chose the “Others” option, 14 of which again noted that if functional, the shape is irrelevant. The majority of the other 31 patients pointed out the visibility in their comments. In the next question, we asked them “how much do you care about the visibility of the implant on your body”? More than 58.4% (n = 274) replied that they prefer the device to be as small as possible; 25.4% (n = 119) did not care about the visibility, while 16.2% (n = 76) preferred the device to be not visible at all. We found no significant association between shape preference and gender (χ 2 (3, n = 468) = 2.74; P = 0.4), but the association between shape preference and age group was significant (χ 2 (12, n = 468) = 25.59; P = 0.01). Among the four options (multiple microscopic small disks deposited in a fluid, thin device which is the size of a credit card under the skin, rod-shaped device of the size of 3″ pencil, and other), multiple microscopic small disks deposited in a fluid is the most preferred shape among all age group (46%–52%) except for the those 65 or older (38%) where they preferred thin device which is the size of a credit card under the skin (43%).
We also sought to further understand the possible role of gender and age on the implants’ visibility (Figs. 2C, D). Evaluating these results, we found a significant association (χ 2 (1, n = 468) = 16.34; P < 0.0001) between gender and participant’s preference on implant visibility. Among female participants, 81% (239 out of 296) cared about the implant visibility, whereas this number was 64% (110 out of 172) for male respondents. Around 22% of those younger than 50 did not care about the implant visibility (61 out of 277), as compared with 30% (58 out of 191) for our respondents older than 50. There was a significant association between age and the participants’ preference on implant visibility (χ 2 (4, n = 468) = 23.69; P < 0.0001).
In addition to generic details about implants, we further attempted to understand participants’ opinion on emerging features of implants. Among recent developments, immune-regulatory devices are showing promise in long-term glycemic control in rodents and nonhuman primates 21,24 . Future developments may also consider other immune-regulatory devices, where an implant could reduce and/or regulate the immune insults against β-cells, providing β-cells regeneration capability. We followed the survey question by gaining knowledge about patients’ willingness to receive immunoregulatory devices after islet transplantation. We asked “if you have type 1 diabetes, your immune system is dysregulated. Which option would you prefer?” There were two choices “1. An implant that only regulates my blood glucose” and “2. An implant that not only controls my glucose but also capable of regulating my immune system to a healthy state.” Among responders, 143 (30.6%) chose the former, and 325 (69.4%) preferred the latter. There was no significant association between the preference on immune-regulatory implants with gender (χ 2 (1, n = 468) = 0.98; P = 0.3) or with age (χ 2 (4, n = 468) = 7.6; P = 0.14).
Lack of allogeneic cell donors is one of the main impediments for pancreatic islet transplantation. Much effort has been devoted to develop islets from xenosources 31 . In addition, reprogramming stem cells into insulin-producing cells has been under investigation 32,33 . To understand our participants’ perspective on such developments, we asked them “do you prefer to receive an implant fabricated with stem cells derived from your own body?”. More than 83.6% (390 people) answered yes, while 16.3% (n = 76) responded no, implying the acceptability of stem cell research and development among our cohort. We found no significant association between implant fabricated by one’s own stem cell with gender (χ 2 (1, n = 468) = 0.28; P = 0.6) or with age (χ 2 (4, n = 468) = 2.92; P = 0.6).
Discussion
Islet transplantation has been the focus of 50 years of research to treat T1D 28,29 . The ultimate goal of islet transplantation is to routinely restore insulin independence with no (or minimal) immunosuppression in T1D patients. However, two main barriers are yet to be overcome for the successful clinical islet transplantation. The first challenge is the scarcity of the allogeneic islet source, which heavily relies on the deceased donors. The second issue is the death of transplanted islets shortly after transplantation due to immune reactions and ischemia, which limits the intended long-term benefits of islet transplantation 10 . These two challenges have been under extensive investigations, where xenotransplantation and stem cell research are mainly under development for the former barrier. For the latter, biomedical devices with a variety of sizes, shapes, and materials have been under development. While recent advances in stem cell engineering and biomaterials science have paved the way to breakdown these barriers, patients’ preferences on the outcome products are largely unknown. Such information bridges the gap between science-oriented thinking paradigms in designing the biomedical devices to patient-oriented or at least patient-considered designs. This consideration could guide the designs of next-generation implantable devices, as T1D patients are the ultimate users of these products. We therefore aimed to target a small population of the T1D community and get an understanding of their preferences for such devices and stem cells. We designed a short online survey and distributed among patients with T1D engaged in a web-based community.
In the first section of the survey, we attempted to get information on the characteristic of our participant cohort. Our participants’ age followed a normal distribution curve, and their duration of diabetes was weighted toward >5 years (∼86%). The most widely used current treatment among our cohort was CGM and insulin pump (82.3%), implying their familiarity with external devices. Interestingly, only about 7% preferred an implant outside their bodies, which may imply the patients’ discomfort. The subcutaneous (under the skin) spot was the top choice for our respondents. Ease of implantation and explantation, along with less surgical complexity make subcutaneous transplantations a good choice for implantable devices. In the case of islet transplantation, however, subcutaneous implantation is challenging due to poor vascularity, which causes islets to suffer from hypoxic conditions 34 . Therefore, strategies have been developed to vascularize and prevascularize the subcutaneous space for islet transplantations.
The size and shape of the implants were another aspect about which we sought to understand patients’ perspectives. Classically, islet transplantation devices have been classified into microdevices and macro-devices. Multiple microscopic disks were preferred by ∼48% of patients. This option was phrased to suggest the microencapsulation technologies to the participants. Fortunately, some recent microencapsulation technologies have shown promise for islet transplantation in rodents and nonhuman primates 21,24 . Participants’ preference for receiving microencapsulated islets matches their other preference regarding the implant’s visibility, where 58.4% of patients prefer the implant to be as small as possible. The second choice of our participants was a “thin soft device in the size of a credit card,” which fits the specifications most of the current macro-devices under development.
The use of an immunoregulatory implant is an emerging concept, where the implant “engineers” the immune system of the host. A few examples are antigen-releasing scaffolds that enhance vascularization 35 , biological scaffolds that inhibit tumor growth through alterations in immunocyte recruitments 36,37 , and bioresponsive biomaterials for immune checkpoint blockade 38 . Immunoregulatory implants could be envisioned from two perspectives in the treatment of T1D. First, any implant including microcapsules or macro-devices need not elicit an immune response, that is, no immunocyte activation due to the implant itself. Second, T1D is inherently a disease of immune system dysregulation. Therefore, it is expected that immunoregulatory implants will get more attention in T1D research. Accordingly, ∼70% of participants preferred implants that would address this issue.
The paucity of cell donors remains one of the main impediments for the islet transplantation into patients. Much effort has been devoted to xenotransplantation 31,39 and stem cell research 33 . We asked participants their willingness to receive autologous stem cells (i.e., generated from their own body). More than 83% of participants preferred to receive devices with stem cells derived from their own body, implying that autologous stem cell transplantations may be an acceptable approach among patients with T1D.
This study represents the first attempt in reflecting the perspectives of a small cohort of patients with T1D on their preferences to receive an implantable islet containing device. This work aids bridging the gap between fully science-oriented designs to patient-considered implantable devices. This study gives not only insight to the future developers of biomedical devices for islet transplantation but also inspires the general biomedical devices field to seek and implement their users’ perspectives.
Supplemental Material
Supplementary_Info for Preferences of Type 1 Diabetic Patients on Devices for Islet Transplantation by M. Rezaa Mohammadi, Farideh Dehkordi-Vakil, Joni Ricks-Oddie, Robert Mansfield, Himala Kashmiri, Mark Daniels, Weian Zhao and Jonathan RT Lakey in Cell Transplantation
Acknowledgments
The authors greatly acknowledge Joanne Milo (Savvy Diabetic) and Kelly Close (Close Concerns) for their support on the online survey distribution.
Footnotes
Author Contributions: MRM designed and led the study. JL guided the direction and facilitated the study. FDL and JRO conducted the statistical analyses. RM, MD, and WZ contributed scientifically to the concept and critical aspects of the manuscript. HK contributed to the manuscript editing.
Ethical Approval: This study received an IRB Exemption Approval from the University of California Irvine, Irvine, CA, USA (Exemption Category 2b Approval: 12-04-2018).
Statement of Human Rights: Our study was conducted in accordance with the relevant guidelines stated by the Declaration of Helsinki.
Statement of Human Rights: Based on the nature of this survey that includes the use of anonymous responses, there was no requirement for the involvement of an ethics board and informed consent from participants.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been supported by the Newkirk Foundation at the University of California Irvine. We also acknowledge the supports from the Department of Surgery and Padre foundation.
ORCID iDs: M. Rezaa Mohammadi  https://orcid.org/0000-0002-9370-3228
https://orcid.org/0000-0002-9370-3228
Farideh Dehkordi-Vakil  https://orcid.org/0000-0002-0990-2436
https://orcid.org/0000-0002-0990-2436
Jonathan RT Lakey  https://orcid.org/0000-0001-8553-4287
https://orcid.org/0000-0001-8553-4287
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Kathleen Dungan NV. Monitoring technologies – continuous glucose monitoring, mobile technology, biomarkers of glycemic control. In: Feingold KR E-i-c, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, et al. editors. Endotext. MDText.com, Inc; 2000. –2018. [PubMed] [Google Scholar]
- 2. Shapiro AMJ, Lakey JRT, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. [DOI] [PubMed] [Google Scholar]
- 3. Shapiro AMJ, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, et al. International trial of the edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–1330. [DOI] [PubMed] [Google Scholar]
- 4. Thomas FT, Contreras JL, Bilbao G, Ricordi C, Curiel D, Thomas JM. Anoikis, extracellular matrix, and apoptosis factors in isolated cell transplantation. Surgery. 1999;126(2):299–304. [PubMed] [Google Scholar]
- 5. Bennet W, Groth C-G, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci. 2000;105(2):125–133. [DOI] [PubMed] [Google Scholar]
- 6. Ryan EA, Paty BW, Senior PA, Shapiro AMJ. Risks and side effects of islet transplantation. Curr Diab Rep. 2004;4(4):304–309. [DOI] [PubMed] [Google Scholar]
- 7. Rezaa Mohammadi M, Rodrigez S, Cao R, Alexander M, Lakey JRT. Immune response to subcutaneous implants of alginate microcapsules. Mater Today: Proceedings. 2018;5(7):15580–15585. [Google Scholar]
- 8. Bochenek MA, Veiseh O, Vegas AJ, McGarrigle JJ, Qi M, Marchese E, Omami M, Doloff JC, Mendoza-Elias J, Nourmohammadzadeh M, Khan A, et al. Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques. Nat Biomed Eng. 2018;2(11):810–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, Li J, Bader AR, Langan E, Olejnik K, Fenton P, et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat Biotechnol. 2016;34(3):345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kopan C, Tucker T, Alexander M, Mohammadi MR, Pone EJ, Lakey JRT. Approaches in immunotherapy, regenerative medicine, and bioengineering for type 1 diabetes. Front Immunol. 2018;9:1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. So V, Martinson L, Green C, Scott M. 3-dimensional large capacity cell encapsulation device assembly. US Patent No 9,526,880. 2013.
- 12. Carlsson PO, Espes D, Sedigh A, Rotem A, Zimerman B, Grinberg H, Goldman T, Barkai U, Avni Y, Westermark GT, Carlbom L, et al. Transplantation of macroencapsulated human islets within the bioartificial pancreas βAir to patients with type 1 diabetes mellitus. Am J Transplant. 2018;18(7):1735–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boettler T, Schneider D, Cheng Y, Kadoya K, Brandon EP, Martinson L, Von Herrath M. Pancreatic tissue transplanted in theracyte™ encapsulation devices is protected and prevents hyperglycemia in a mouse model of immune-mediated diabetes. Cell Transplant. 2016;25(3):609–614. [DOI] [PubMed] [Google Scholar]
- 14. Pepper AR, Pawlick R, Gala-Lopez B, MacGillivary A, Mazzuca DM, White DJG, Toleikis PM, Shapiro AMJ. Diabetes is reversed in a murine model by marginal mass syngeneic islet transplantation using a subcutaneous cell pouch device. Transplantation. 2015;99(11):2294–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smink AM, Li S, Hertsig DT, de Haan BJ, Schwab L, van Apeldoorn AA, de Koning E, Faas MM, Lakey JRT, de Vos P. The efficacy of a prevascularized, retrievable poly(D, L,-lactide-co-∊-caprolactone) subcutaneous scaffold as transplantation site for pancreatic islets. Transplantation. 2017;101(4):e112–e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Graham JG, Zhang X, Goodman A, Pothoven K, Houlihan J, Wang S, Gower RM, Luo X, Shea LD. PLG scaffold delivered antigen-specific regulatory T cells induce systemic tolerance in autoimmune diabetes. Tissue Eng Part A. 2013;19(11–12):1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berman DM, O’Neil JJ, Coffey LCK, Chaffanjon PCJ, Kenyon NM, Ruiz P, Jr, Pileggi A, Ricordi C, Kenyon NS. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant. 2009;9(1):91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Najjar M, Manzoli V, Abreu M, Villa C, Martino MM, Molano RD, Torrente Y, Pileggi A, Inverardi L, Ricordi C, Hubbell JA, et al. Fibrin gels engineered with pro-angiogenic growth factors promote engraftment of pancreatic islets in extrahepatic sites in mice. Biotechnol Bioeng. 2015;112(9):1916–1926. [DOI] [PubMed] [Google Scholar]
- 19. Kette F, Rojas-Canales D, Drogemuller C, McInnes S, Toby Coates P. Modification of polyurethane scaffolds for localised immunosuppression of subcutaneous islet transplantation. Transplantation. 2018;102:S77. [Google Scholar]
- 20. An D, Chiu A, Flanders JA, Song W, Shou D, Lu Y-C, Grunnet LG, Winkel L, Ingvorsen C, Christophersen NS, Fels JJ, et al. Designing a retrievable and scalable cell encapsulation device for potential treatment of type 1 diabetes. Proc Natl Acad Sci USA. 2018;115(2):E263–E272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farah S, Doloff JC, Müller P, Sadraei A, Han HJ, Olafson K, Vyas K, Tam HH, Hollister-Lock J, Kowalski PS, Griffin M, et al. Long-term implant fibrosis prevention in rodents and non-human primates using crystallized drug formulations. Nat Mater. 2019;18(8):892–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manzoli V, Villa C, Bayer AL, Morales LC, Molano RD, Torrente Y, Ricordi C, Hubbell JA, Tomei AA. Immunoisolation of murine islet allografts in vascularized sites through conformal coating with polyethylene glycol. Am J Transplant. 2018;18(3):590–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weaver JD, Headen DM, Coronel MM, Hunckler MD, Shirwan H, García AJ. Synthetic poly(ethylene glycol)-based microfluidic islet encapsulation reduces graft volume for delivery to highly vascularized and retrievable transplant site. Am J Transplant. 2019;19(5):1315–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sremac M, Lei J, Penson MFE, Schuetz C, Lakey JRT, Papas KK, Varde PS, de Vos P, Brauns T, Markmann J, Poznansky MC, et al. Preliminary studies of the impact of CXCL12 on the foreign body reaction to pancreatic islets microencapsulated in alginate in nonhuman primates. Transplant Direct. 2019;5(5):e447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hwa AJ, Weir GC. Transplantation of macroencapsulated insulin-producing cells. Curr Diab Rep. 2018;18(8):50. [DOI] [PubMed] [Google Scholar]
- 26. Kozlovskaya V, Zavgorodnya O, Chen Y, Ellis K, Tse HM, Cui W, Thompson JA, Kharlampieva E. Ultrathin polymeric coatings based on hydrogen-bonded polyphenol for protection of pancreatic islet cells. Adv Funct Mater. 2012;22(16):3389–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc Natl Acad Sci USA. 2012;109(11):4245–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210(4472):908–910. [DOI] [PubMed] [Google Scholar]
- 29. Desai T, Shea LD. Advances in islet encapsulation technologies. Nat Rev Drug Discov. 2016;16(5):338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Orive G, Emerich D, Khademhosseini A, Matsumoto S, Hernandez RM, Pedraz JL, Desai T, Calafiore R, de Vos P. Engineering a clinically translatable bioartificial pancreas to treat type I diabetes. Trends Biotechnol. 2018;36(4):445–456. [DOI] [PubMed] [Google Scholar]
- 31. Park C-G, Bottino R, Hawthorne WJ. Current status of islet xenotransplantation. Int J Surg. 2015;23(Pt B):261–266. [DOI] [PubMed] [Google Scholar]
- 32. Sneddon JB, Tang Q, Stock P, Bluestone JA, Roy S, Desai T, Hebrok M. Stem cell therapies for treating diabetes: progress and remaining challenges. Cell Stem Cell. 2018;22(6):810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pagliuca Felicia W, Millman Jeffrey R, Gürtler M, Segel M, Van Dervort A, Ryu Jennifer H, Peterson Quinn P, Greiner D, Melton Douglas A. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159(2):428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vlahos AE, Cober N, Sefton MV. Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets. Proc Natl Acad Sci USA. 2017;114(35):9337–9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kwee BJ, Seo BR, Najibi AJ, Li AW, Shih TY, White D, Mooney DJ. Treating ischemia via recruitment of antigen-specific T cells. Sci Adv. 2019;5(7):eaav6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wolf MT, Ganguly S, Wang TL, Anderson CW, Sadtler K, Narain R, Cherry C, Parrillo AJ, Park BV, Wang G, Pan F, et al. A biologic scaffold–associated type 2 immune microenvironment inhibits tumor formation and synergizes with checkpoint immunotherapy. Sci Transl Med. 2019;11(477):eaat7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuai R, Sun X, Yuan W, Xu Y, Schwendeman A, Moon JJ. Subcutaneous nanodisc vaccination with neoantigens for combination cancer immunotherapy. Bioconjug Chem. 2018;29(3):771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu S, Wang C, Yu J, Wang J, Lu Y, Zhang Y, Zhang X, Hu Q, Sun W, He C, Chen X, et al. Injectable bioresponsive gel depot for enhanced immune checkpoint blockade. Adv Mater. 2018;30(28):e1801527. [DOI] [PubMed] [Google Scholar]
- 39. Lau H, Corrales N, Alexander M, Mohammadi MR, Li S, Smink AM, Vos Pd, Lakey JRT. Necrostatin-1 supplementation enhances young porcine islet maturation and in vitro function. Xenotransplantation. 2020;27(1):e12555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Info for Preferences of Type 1 Diabetic Patients on Devices for Islet Transplantation by M. Rezaa Mohammadi, Farideh Dehkordi-Vakil, Joni Ricks-Oddie, Robert Mansfield, Himala Kashmiri, Mark Daniels, Weian Zhao and Jonathan RT Lakey in Cell Transplantation