Abstract
Sepsis is a life-threatening disease that results in excessive stimulation of the host’s immune cells. In the animal study, the purpose was to investigate the roles of fresh frozen plasma (FFP) transfusion in shaping the CD4+ T lymphocytes immune response through modulating the secreted exosome protein Galectin-9 in mice with severe sepsis. By using Western blot analysis, we first identified that the protein Galectin-9 is highly accumulated in the blood plasma of severe sepsis mice, and with transmission electron microscopy (TEM) and protein analysis, we found that Galectin-9 is a secreted exosome protein. Thereafter, we treated the severe sepsis mice with the antibiotic Cefuroxime Axetil; one group of mice received FFP transfusion and the other group of mice received normal saline. Surprisingly, the FFP transfusion reduced the secretion of exosome protein Galectin-9 and there was crosstalking between the exosome protein Galectin-9 and CD4+ T lymphocytes in mice with severe sepsis. Results showed that the proliferation of T helper (Th) cells (Th1 and Th17) was promoted, and regulatory T (Treg) cells’ maintenance was inhibited in the sepsis mice after receiving FFP transfusion. Correspondingly, this immune reprogrammed activity shaped the inflammatory cytokine secretion with an increase in the interleukin (IL)-1β, IL-6, and interferon-gamma levels, while it decreased IL-10 levels. Taken together, it was suggested that FFP transfusion promoted reprogramming of CD4+ T lymphocytes’ immune response through inhibiting the secretion of exosome protein Galectin-9 in mice with severe sepsis to relieve immunosuppression.
Keywords: fresh frozen plasma (FFP) transfusion, CD4+ T lymphocytes, exosome protein galectin 9, severe sepsis
Introduction
Sepsis is a life-threatening disease with an estimation of 27% morbidity and 26% mortality globally1. It is caused by a dysregulated host immune response to bacterial infection, which made the initial hyperinflammatory phase reverse to immunosuppression and immune paralysis status2. Studies had shown that the major histocompatibility complex II molecules, such as human leukocyte antigen-DR (HLA-DR) in antigen-presenting cells (APCs; dendritic cells [DCs], macrophage cells, etc.), played important roles in the innate immune response and activated adapted immune responses in sepsis3,4. While CD4+ T lymphocytes were the predominant effector cells in the adaptive immune response of sepsis5, in recent years, more and more researchers paid attention to the functions or roles of CD4+ T lymphocytes in sepsis6,7. The CD4+ T lymphocytes can differentiate into several subtypes, such as T helper (Th) cells (Th1, Th2, and Th17) and regulatory T (Treg) cells8. The Th1 cells are responsible for cell-mediated immunity and release of interleukin-2 (IL-2) and interferon-gamma (IFN-γ) to promote differentiation and enhance the endogenous phagocytosis or clearance of pathogens in monocytes and macrophages, while Th2 cells participate in humoral immune response and cleared the extracellular infections, such as parasite9. Moreover, the Th17 cells play important roles in the clearance of extracellular pathogens and recruiting and activating neutrophils by chemotaxis10. Besides, Treg cells could inhibit excessive inflammatory response by secreting IL-10 and tumor growth factor (TGF)-β, which also suppressed the activities of monocytes, DCs, and macrophages11. Studies show that the ratio of Th1/Th212 and Th17/Treg13 decreased in severe sepsis patients. Hence, maintaining the balance of Th cells and Treg cells is a way to alleviate immunosuppression in severe sepsis.
The protein Galectin-9 is an important immune modulator in both innate and adaptive immune responses14. It mediated the innate immune cells, such as macrophages and DCs via binding with the T cell immunoglobulin and mucin domain-containing molecule 3 (Tim-3)15 to initiate the inflammatory response. Conversely, it suppressed Th1 cell immune responses via binding with the Tim-3 receptor, which induced Th1 cell apoptosis and exhaustion16. Moreover, studies identified that protein Galectin-9 not only inhibited Th17 expansion but also promoted the proliferation of Treg cells17; it was found interacting with CD44 receptor to promote FOXP3 expression, which thus induced the differentiation and expansion of Treg (iTreg) cells18. Importantly, our preliminary experiment found the protein Galectin-9 existing in the blood samples of severe sepsis mice model, which participates in the immunosuppression activity in severe sepsis. Therefore, to our knowledge, we made further studies about it and revealed it to be an exosome protein in the blood samples of severe sepsis mice model.
Fresh-frozen plasma (FFP) transfusion and red blood cell transfusion are controversial in patients with septic shock19,20. Hence, in this study, we attempted to investigate the roles of FFP transfusion in severe sepsis mice model.
Materials and Methods
Ethics Statement
All animal experiments were performed according to the guidelines of the animal ethical organization and obtained the permission of Shanghai Gongli Hospital, the Second Military Medical University.
Materials and Reagents
Lipopolysaccharides from Escherichia coli 0111: B4 (catalog: L2630, Sigma, USA), antibiotic-Cefuroxime Axetil (Chengdu Beite Pharmaceutical Co. Ltd., China),
Total exosome isolation reagent (catalog: 4478359; Invitrogen, USA), ExoAb Antibody Kit (catalog: EXOAB-KIT-1; SBI, USA), antimouse Galectin 9 (catalog: ab69630; Abcam, Wuhan, China), transmission electron microscope (catalog: HT7700; HITACHI, Japan), mouse lymphocyte isolation medium (catalog: LTS1092; Shanghai Yanjin Bio. Co. Ltd., China), Roswell Park Memorial Institute (RPMI)-1640 medium (Thermo Fisher Scientific, USA), fetal bovine serum (FBS; Gibco, USA), fixation/permeabilization solution (catlog: 554722; Bioscience, BD, USA), wash buffer (catalog: 554723; Bioscience), flow cytometry staining buffer (catalog: 00-4222-57; eBioscience, USA), antimouse CD16/CD32 (catalog: MFCR00; Invitrogen, USA), antimouse CD4-FITC (catalog: 11-0040-85; eBioscience), antimouse IFN-γ-PE (catalog: 12-7319-42; eBioscience), antimouse IL-17-PE-Cy7 (catalog: 25-7042-82; eBioscience), antimouse CD25-APC (catalog: RM6005; Invitrogen), antimouse Foxp3-PE-Cy5.5 (catalog: 35-4776-41; eBioscience), mouse IL-1β ELISA kit (catlog: 70-EK212/3-96; MultiSciences, China), mouse IL-6 ELISA kit (catalog: 70-EK206/3-96; MultiSciences), mouse IL-10 ELISA kit (catalog: 70-EK210/3-96; MultiSciences), mouse IFN-γ high sensitivity ELISA kit (catalog: 70-EK280HS-96; MultiSciences)
Infectious Severe Sepsis Mice Model Establishment and FFP Transfusion
Forty C57/BL6 mice (aged 10–12 weeks) were brought from Shanghai Bangyao Biotechnology Co. Ltd. (No. A0001). They were maintained in specific pathogen-free conditions in the animal care facility. After 1 week of adapted cultivation, 10 mice were anesthetized with 2% isoflurane and sacrificed. The blood samples from the mice aorta abdominalis were collected immediately for FFP transfusion. The remaining 30 mice were administered with E. coli 0111: B4 (100 × 106 colony forming units) by intraperitoneal injection. The methods of bacteria cultivation are as follows21. The E. coli 0111: B4 were stored in 20% glycerol at −70°C. Then, some bacterial colonies were collected with inoculation loops and were cultured on LB agar (Sigma) for 12 h at 37°C. Afterward, the colonies were counted with the scratch inoculation method after overnight incubation. The breath, mobility, and food/water intake of the mice (to estimate endotoxemia) were recorded three times daily for up to 7 days; the mice were found to be in severe sepsis with the symptoms of trembles, high fever, and difficulty breathing. The infectious mice were gavaged with the antibiotic Cefuroxime Axetil (75 mg/day/mice) and were randomly divided into two groups: one group received FFP transfusion via the lateral tail vein while the other group received an equivalent saline injection. After FFP transfusion, the mice were recovered from endotoxemia, anesthetized with 2% isoflurane, sacrificed, and the blood was collected immediately.
Exosome Isolation and Identification
The collected blood samples of mice were centrifuged at 3000 × g/min to obtain the serum. The total exosome isolation reagent was added to the serum with reverse blending and placed for one night at 4°C. Afterward, the mixed medium was centrifuged three times at 3000 × g/min for 5 min. We extracted the proteins from the precipitates (exosome), transferred into three tubes, and analyzed for the marker proteins (CD63 and CD81) with the ExoAb antibody kit. The exosome protein, Galectin-9, was detected with Western blot analysis. The precipitates in other tubes were resuspended with PBS and visualized using a transmission electron microscope.
Flow Cytometry
The lymphocytes were isolated from the collected blood samples of mice with mouse lymphocyte isolation medium by centrifugation at 3000 × g/min. Then, the obtained lymphocytes were cultured in RPMI-1640 medium with FBS and counted. Later, 3–5 × 106 cells were put in each tube and permeabilized with a fixation/permeabilization solution for 30 min away from light. The antimouse CD16/CD32 (1 μg/106 cell) cells were added into each tube and kept for 30 min at 4°C away from light.
Afterward, the cells were washed with wash buffer. Double-staining antibodies dilution buffer was added equally into all except one tube: antimouse CD4-FITC mixed with antimouse IFN-γ-PE, antimouse CD4-FITC mixed with antimouse IL-17-PE-Cy7, antimouse CD25-APC mixed with antimouse Foxp3-PE-Cy5.5 for 30 min at 4°C incubation away from light, Thereafter, the cells were washed twice and resuspended in flow cytometry staining buffer for flow cytometry analysis using the Calibur FACS (Bioscience) instrument.
ELISA
The collected blood samples of mice were centrifuged three times at 3000 × g/min to obtain the plasma. The plasma was analyzed for the presence of cytokines IL-1β, IL-6, IL-10, and IFN-γ using ELISA. Finally, the microplate reader (Bio-Tek, Epoch) was used to measure the optical density at 450 nm. All the procedures were done according to the manufacturers’ instructions.
Statistical Analysis
The data were analyzed with a one-way analysis of variance. Statistical analyses were performed using SPSS19.0 software. The significance was defined as P < 0.05. All graphs were depicted with Graphpad prism 6.0 presenting data with mean ± standard error of the mean. Data are representative of at least two or three independent experiments.
Results
FFP Transfusion Inhibited the Secretion of Exosome Protein Galectin-9 in Infectious Severe Sepsis Mice Model
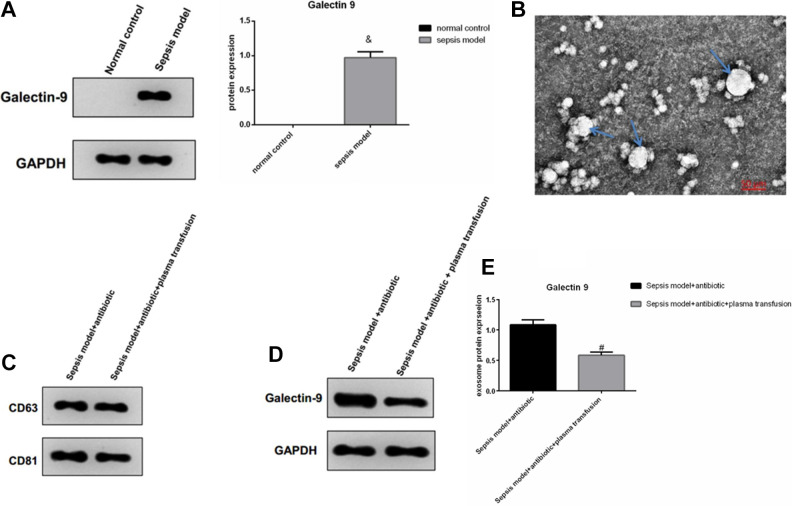
We found that the protein Galectin-9 was higher in the blood of the severe sepsis mice compared with the normal control mice (Fig. 1A). We also identified that the protein Galectin-9 was a secreted exosome protein (Fig. 1B, C). Also, the FFP transfusion into the sepsis mice model inhibited the secretion of exosome protein Galectin-9 compared with the sepsis mice without FFP transfusion (Fig. 1D, E).
Figure 1.
Western blot analyzed the protein Galectin-9 in sepsis mice blood samples. & P < 0.05 indicated significant difference versus the normal control group (A); transmission electron microscope detected protein Galectin-9 to be an exosome protein; the arrow points the exosomes (B) and the exosome surface protein CD63 and CD81 (C). Western blot analyzed exosome protein Galectin-9 in sepsis mice blood samples with or without plasma transfusion. # P < 0.05 indicated a significant difference versus sepsis model group (D, E).
FFP Transfusion Facilitated the Reprogramming of CD4+ T Cell Immune Response Through Regulating the Exosome Protein Galectin-9
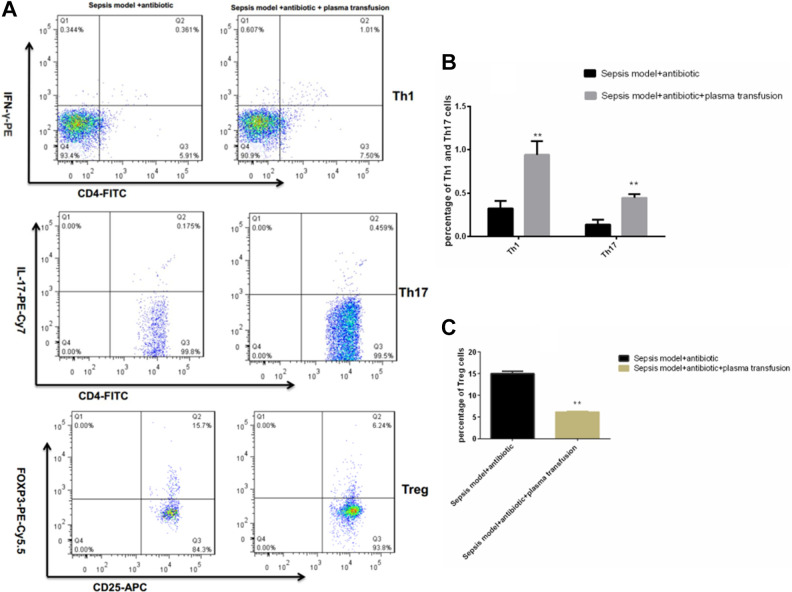
The protein Galectin-9 was a member of the β-galactoside binding lectin family located in the cell membrane, cytoplasm, and nucleus. Interestingly, in this study, the results showed that FFP transfusion promoted the proliferation of Th1 and Th17 cells (Fig. 2A, B) but inhibited the differentiation and expansion of Treg cells (Fig. 2A, C). Moreover, this immune reprogrammed activity mediated the secretion of inflammatory cytokines with an increase in the proinflammatory cytokine production of IL-1β, IL-6, and IFN-γ at different levels, while the anti-inflammatory cytokine IL-10 production was reduced (Fig. 3).
Figure 2.
The T cell subtypes of Th1, Th17, and Treg cells’ maintenance changed with or without plasma transfusion by flow cytometry analysis (A). **P < 0.05 of Th1, Th17, and Treg cells showed significant difference versus sepsis model group (B, C; data were presented as mean ± standard error of the mean. Th, T helper cells; Treg, regulatory T cells.
Figure 3.
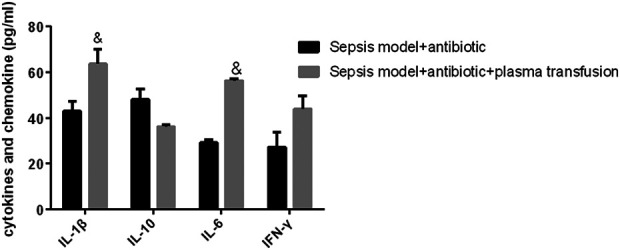
ELISA assay analyzed the secretion of inflammatory cytokines, the increase of IL-1β, IL-6, and IFN-γ, and the decrease of IL-10 in sepsis mice model with plasma transfusion versus sepsis mice model. & P < 0.05 indicated a significant difference; data were presented as mean ± standard error of the mean. IL, interleukin; IFN, interferon.
Discussion
In this study, we studied the sepsis disease in hypoinflammatory status. In the severe sepsis mice model, we identified the protein Galectin-9 to be an exosome protein, which was found exerting therapeutic effects on polymicrobial sepsis through expanding natural killer T (NKT) cells and pDC-like macrophages to modulate the inflammatory response22. The results showed that FFP transfusion facilitated the reprogramming of CD4+ T cell immune response through inhibiting the secretion of exosome protein Galectin-9. The differentiation and expansion of Th1 and Th17 cells were recovered after FFP transfusion, and this activity of Treg cells was evidently suppressed. This finding was consistent with the study of Wu HP23 and Li J24 that the proportion of Th1/Th2 was inversed and the counts of Th1, Th2, Th17, and Treg cells were all decreased in severe sepsis or septic shock. Furthermore, the inflammatory cytokines (IL-1β, IL-6, IFN-γ, and IL-10) were all modulated with FFP transfusion. The proinflammatory cytokines (IL-1β, IL-6, and IFN-γ) were reversed, while the production of IL-10 was reduced. This effect was possibly due to the activity of exosome protein Galectin-9 as it induced Th1 cell apoptosis and exhaustion and inhibited the Th17 cell expansions, and thus, IFN-γ mutually influenced Th1 cell differentiation and its secretion25, while IL-1β and IL-6 favored Th17 cells’ differentiation to suppress TGF-β-driven induction of Foxp3 T cells26,27. In addition, FFP transfusion reduced the secretion of exosome protein Galectin-9, which could indirectly reduce the differentiation and expansion of Treg (iTreg) cells18. Additionally, owing to the severe sepsis, all mice were treated with antibiotics, hence, it may also assist in balancing the destroyed immune system. While, during our study process, all the severe sepsis mice without antibiotic treatment died, and hence so we concluded that FFP transfusion facilitated the reprogramming of CD4+ T cell immune response through inhibiting the secretion of exosome protein Galectin-9. However, further studies are needed to reveal the individual function of antibiotic treatment and FFP transfusion in the reprogramming of CD4+ T cell immune response in sepsis.
Galectin-9 protein had been found highly expressed in eosinophils, DC, macrophages, T lymphocytes, endothelial cells, Kupffer cells, intestinal epithelial cells, and vascular endothelial cells28. Also, it is a highly modulatory molecule in immune function that interact with multiple receptors, such as, Tim-3, cell surface protein disulfide isomerase (PDI), IgE, 4-1BB, (CD137 and tumor necrosis factor receptor superfamily, member 9 (TNFRSF9)), and CD4416,28,29. Wang et al30 clearly proved Tim-3 to be a potential therapeutic target for the treatment of sepsis. Therefore, in our study, we should further investigate the interacted receptor of the Galectin-9 exosome in CD4+ T cells. In addition, the master transcription factor and secondary transcription factors8, such as Th1, T-bet/STAT4; Th2, GATA3/STAT5; Th17, RORγt/STAT3; and iTregs, Foxp3/ play important roles in the differentiation and maintenance of CD4+ T cells; hence, how the cytokines regulate the transcription factors in CD4+ T cells is yet to be found. On the other hand, in our study, FFP transfusion in sepsis mice did not induce unpredictable adverse effects, while FFP transfusion in the clinical study often shows adverse effects, such as acute lung injury31. This may be due to the species variation and the amount of FFP transfusion. Further study is necessary to identify whether the results are similar in clinical sepsis patients. Taken together, FFP transfusion promoted reprogramming of CD4+ T lymphocytes immune response through inhibiting the secretion of exosome protein Galectin-9 in mice with severe sepsis to relieve immunosuppression.
Consent to publish
All of the authors have consented to publish this research.
Authors’ Note: Lei Zhang, MD, Jian-Ping Zhang, MD, and Yang Liu, MD, Co-first authors.
Ethical Approval: All animal experiments were approved by the Animal Research Ethics Committee of The Shanghai Gongli Hospital, the Second Military Medical University.
Statement of Human and Animal Rights: All of the experimental procedures involving animals were conducted in accordance with the Institutional Animal Care guidelines of Shanghai Gongli Hospital, the Second Military Medical University, Shanghai, China, and approved by the Administration Committee of Experimental Animals, Shanghai, China.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by Key Disciplines Group Construction Project of Pudong Health Bureau of Shanghai (No. PWZxq 2017-10) and National Natural Science Foundation of China (No. 81870147).
ORCID iD: Jian-Rong Guo, MD, PhD  https://orcid.org/0000-0002-3995-2995
https://orcid.org/0000-0002-3995-2995
References
- 1. Thompson K, Venkatesh B, Finfer S. Sepsis and septic shock: current approaches to management. Intern Med J. 2019;49(2):160–170. [DOI] [PubMed] [Google Scholar]
- 2. Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13(3):260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cajander S, Bäckman A, Tina E, Strålin K, Söderquist B, Källman J. Preliminary results in quantitation of HLA-DRA by real-time PCR: a promising approach to identify immunosuppression in sepsis. Crit Care. 2013;17(5):R223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cajander S, Tina E, Bäckman A, Magnuson A, Strålin K, Söderquist B, Källman J. Quantitative real-time polymerase chain reaction measurement of HLA-DRA gene expression in whole blood is highly reproducible and shows changes that reflect dynamic shifts in monocyte surface HLA-DR expression during the course of sepsis. PLoS One. 2016;11(5):e0154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ammer-Herrmenau C, Kulkarni U, Andreas N, Ungelenk M, Ravens S, Hübner C, Kather A, Kurth I, Bauer M, Kamradt T. Sepsis induces long-lasting impairments in CD4+ T-cell responses despite rapid numerical recovery of T-lymphocyte populations. PLoS One. 2019;14(2):e0211716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramonell KM, Zhang W, Hadley A, Chen CW, Fay KT, Lyons JD, Klingensmith NJ, McConnell KW, Coopersmith CM, Ford ML. CXCR4 blockade decreases CD4+ T cell exhaustion and improves survival in a murine model of polymicrobial sepsis. PLoS One. 2017;12(12):e0188882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang P, Zhou Y, Liu Z, Zhang P. Interaction between ANXA1 and GATA-3 in Immunosuppression of CD4(+) T Cells. Mediators Inflamm. 2016;2016(10):1701059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 2010;28(3):445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10(4):225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Song X, Gao H, Qian Y. Th17 differentiation and their pro-inflammation function. Adv Exp Med Biol. 2014;841(9):99–151. [DOI] [PubMed] [Google Scholar]
- 11. Chen X, Oppenheim JJ. Th17 cells and Tregs: unlikely allies. J Leukoc Biol. 2014;95(5):723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferguson NR, Galley HF, Webster NR. T helper cell subset ratios in patients with severe sepsis. Intensive Care Med. 1999;25(1):106–109. [DOI] [PubMed] [Google Scholar]
- 13. Guo J, Tao W, Tang D, Zhang J. Th17/regulatory T cell imbalance in sepsis patients with multiple organ dysfunction syndrome: attenuated by high-volume hemofiltration. Int J Artif Organs. 2017;40(11):607–614. [DOI] [PubMed] [Google Scholar]
- 14. Li Y, Feng J, Geng S, Geng S, Wei H, Chen G, Li X, Wang L, Wang R, Peng H, Han G, et al. The N- and C-terminal carbohydrate recognition domains of galectin-9 contribute differently to its multiple functions in innate immunity and adaptive immunity. Mol Immunol. 2011;48(4):670–677. [DOI] [PubMed] [Google Scholar]
- 15. Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M, Bruce JN, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318(5853):1141–1143. [DOI] [PubMed] [Google Scholar]
- 16. Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245–1252. [DOI] [PubMed] [Google Scholar]
- 17. Seki M, Oomizu S, Sakata KM, Sakata A, Arikawa T, Watanabe K, Ito K, Takeshita K, Niki T, Saita N, Nishi N, et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008;127(1):78–88. [DOI] [PubMed] [Google Scholar]
- 18. Wu C, Thalhamer T, Franca RF, Xiao S, Wang C, Hotta C, Zhu C, Hirashima M, Anderson AC, Kuchroo VK. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity. 2014;41(2):270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reiter N, Wesche N, Perner A. The majority of patients in septic shock are transfused with fresh-frozen plasma. Dan Med J. 2013;60(4):A4606. [PubMed] [Google Scholar]
- 20. Holst LB. Benefits and harms of red blood cell transfusions in patients with septic shock in the intensive care unit. Dan Med J. 2016;63(2):B5209. [PubMed] [Google Scholar]
- 21. Okeke EB, Okwor I, Mou Z, Jia P, Uzonna JE. CD4+CD25+ regulatory T cells attenuate lipopolysaccharide-induced systemic inflammatory responses and promotes survival in murine Escherichia coli infection. Shock. 2013;40(1):65–73. [DOI] [PubMed] [Google Scholar]
- 22. Kadowaki T, Morishita A, Niki T, Hara J, Sato M, Tani J, Miyoshi H, Yoneyama H, Masaki T, Hattori T, Matsukawa A, et al. Galectin-9 prolongs the survival of septic mice by expanding Tim-3-expressing natural killer T cells and PDCA-1+ CD11c+ macrophages. Crit Care. 2013;17(6):R284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu HP, Chung K, Lin CY, Jiang BY, Chuang DY, Liu YC. Associations of T helper 1, 2, 17 and regulatory T lymphocytes with mortality in severe sepsis. Inflamm Res. 2013;62(8):751–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li J, Li M, Su L, Wang H, Xiao K, Deng J, Jia Y, Han G, Xie L. Alterations of T helper lymphocyte subpopulations in sepsis, severe sepsis, and septic shock: a prospective observational study. Inflammation. 2015;38(3):995–1002. [DOI] [PubMed] [Google Scholar]
- 25. Zheng J, Liu Y, Qin G, Lam KT, Guan J, Xiang Z, Lewis DB, Lau YL, Tu W. Generation of human Th1-like regulatory CD4+ T cells by an intrinsic IFN-γ- and T-bet-dependent pathway. Eur J Immunol. 2011;41(1):128–139. [DOI] [PubMed] [Google Scholar]
- 26. Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30(4):576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. [DOI] [PubMed] [Google Scholar]
- 28. Merani S, Chen W, Elahi S. The bitter side of sweet: the role of Galectin-9 in immunopathogenesis of viral infections. Rev Med Virol. 2015;25(3):175–186. [DOI] [PubMed] [Google Scholar]
- 29. Madireddi S, Eun SY, Lee SW, Nemčovičová I, Mehta AK, Zajonc DM, Nishi N, Niki T, Hirashima M, Croft M. Galectin-9 controls the therapeutic activity of 4-1BB-targeting antibodies. J Exp Med. 2014;211(7):1433–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang F, Hou H, Xu L, Jane M, Peng J, Lu Y, Zhu Y, Sun Z. Tim-3 signaling pathway as a novel negative mediator in lipopolysaccharide-induced endotoxic shock. Hum Immunol. 2014;75(5):470–478. [DOI] [PubMed] [Google Scholar]
- 31. Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327. [DOI] [PubMed] [Google Scholar]