Abstract
Spinal cord injury (SCI) remains among the most challenging pathologies worldwide and has limited therapeutic possibilities and a very bleak prognosis. Biomaterials and stem cell transplantation are promising treatments for functional recovery in SCI. Seven patients with acute complete SCI diagnosed by a combination of methods were included in the study, and different lengths (2.0–6.0 cm) of necrotic spinal cord tissue were surgically cleaned under intraoperative neurophysiological monitoring. Subsequently, NeuroRegen scaffolds loaded with autologous bone marrow mononuclear cells (BMMCs) were implanted into the cleaned site. All patients participated in 6 months of rehabilitation and at least 3 years of clinical follow-up. No adverse symptoms associated with stem cell or functional scaffold implantation were observed during the 3-year follow-up period. Additionally, partial shallow sensory and autonomic nervous functional improvements were observed in some patients, but no motor function recovery was observed. Magnetic resonance imaging suggested that NeuroRegen scaffold implantation supported injured spinal cord continuity after treatment. These findings indicate that implantation of NeuroRegen scaffolds combined with stem cells may serve as a safe and promising clinical treatment for patients with acute complete SCI. However, determining the therapeutic effects and exact application methods still requires further study.
Keywords: acute complete spinal cord injury, NeuroRegen scaffold, autologous bone marrow mononuclear cells, regeneration
Introduction
Spinal cord injury (SCI) is a severe traumatic injury characterized by the loss of sensory and motor function below the level of injury. The incidence of SCI is approximately 40 per million worldwide1. Complete SCI is the most serious type of SCI and leads to various physical and psychological problems; additionally, neurological function is almost impossible to recover; complete SCI accounts for 9.4%–56.7% of all SCI cases2–4. Nevertheless, traditional treatment strategies, including surgical intervention and symptomatic therapy followed by rehabilitation, are currently used to relieve secondary damage and improve patients’ independent living ability5,6. However, existing treatment options cannot effectively improve neurological recovery in SCI patients, especially in patients with complete SCI. Fortunately, various promising neuroregenerative strategies, including the application of stem cells and biomaterials, have been widely developed and have achieved some progress.
Stem cells, including induced embryonic stem cells, bone marrow mononuclear cells (BMMCs), cord blood stem cells, and mesenchymal stem cells (MSCs), have been widely tested in animal models of SCI7–11. Limitations based on ethics, safety, and source materials mean that only a few stem cell types, including allogeneic primary neural stem cells, BMMCs, adipose stem cells, and umbilical cord MSCs, are approved for the clinical treatment of SCI. Among them, BMMCs have been demonstrated to effectively regulate the spinal microenvironment and have the potential to differentiate into neural cells, as well as the advantages of easy access, easy in vitro expansion, low immunogenicity, and no ethical controversy. The efficacy and safety of BMMC implantation for the treatment of patients with acute or chronic SCI have been fully evaluated8,9,12–15, although the individual treatment effects are still limited.
Biological scaffolds consist of natural polymers composed of repeating units that can promote axonal growth and bridge the site of SCI by providing a platform for stem cells and functional biomolecules and reconstructing the microenvironment16–18. Biological scaffolds of different materials with different spatial structures, biodegradability, and mechanical strength have been developed and demonstrated to have therapeutic effects on SCI animal models16,19. However, only a few biomaterials have been approved for clinical trials. NeuroRegen is a linearly ordered collagen scaffold functionalized with multiple functional nerve regeneration molecules. In previous experiments, the implantation of NeuroRegen scaffolds with MSCs significantly inhibited scar formation and promoted axonal growth and motor function recovery in animal models of complete SCI17,20–22. Based on preclinical studies, we conducted two clinical studies with implantation of NeuroRegen scaffolds loaded with BMMCs or human umbilical cord MSCs for the treatment of 13 patients with chronic complete SCI. During the 1-year follow-up period, no adverse reactions associated with the NeuroRegen scaffold grafts or scar resection were observed23,24.
These studies provide initial evidence that NeuroRegen scaffolds loaded with stem cells can be applied as a safe alternative clinical treatment for chronic SCI23,24. However, the actual therapeutic effect in chronic SCI is still limited, perhaps because of neuromuscular atrophy in the lower limbs over long periods after SCI25,26. In this study, we explored the application of NeuroRegen scaffolds combined with BMMCs via implantation in acute complete SCI patients in terms of both efficacy and safety.
Materials and Methods
Patient Selection
The criteria for inclusion, exclusion, and withdrawal were as follows:
- Inclusion criteria
- The patient was male or female and 18–65 years old.
- The patient had a severely compressed and injured spinal cord at the thoracic level (T1–T12) accompanied by loss of physiological function confirmed by neuroelectrophysiology, magnetic resonance imaging (MRI), and neurological examination below the injured segment; the American Spinal Injury Association (ASIA) grade was A and the spinal cord shock period had passed (one of the following conditions occurred: the foot and plantar stimulus induced a retraction action, the bulbar body reflex, or the testicular reflex, or the anal contraction reflex recovered, but the motor and sensory functions were still completely lost under the injury level); and the period from injury to surgery was less than 4 weeks.
- The patient was in a generally good condition without serious complications affecting other systems and could tolerate surgery; the peripheral muscles and nerve function below the level of damage were normal.
- The patient and the patient’s family showed good compliance, were willing to accept the risks involved, and provided written informed consent for BMMC acquisition and implantation, clinical validation, or surgery. The patient was also willing and able to go to the hospital for follow-up examinations on a regular basis.
- Exclusion criteria
- The patient had incomplete SCI, the location and size of SCI sites were difficult to identify, or whether spinal shock had passed could not be determined.
- The patient had a serious complication that prevented the toleration of surgery or affected SCI recovery and rehabilitation (such as multiple organ failure, severe bleeding tendency or coagulopathy, brain injury, cerebral hemorrhage, cognitive impairment, or other central nervous system–related complications).
- The patient had a serious underlying disease, such as diabetes, an autoimmune disease, a tumor, or severe hypertension.
- The patient had a history of a life-threatening immune-mediated reaction or allergy.
- Clinically obvious abnormalities in routine laboratory examinations were found, such as positivity for HIV antibody, HBsAg, HTLV-1 antibody, or HCV antibody.
- The patient participated in another clinical trial within 3 months before enrollment.
- The patient was lactating or pregnant.
- The patient was in a poor general condition or a condition that made it difficult to participate in the study.
Preparation of NeuroRegen Scaffolds and BMMCs
NeuroRegen scaffolds were prepared from bovine aponeurosis and assessed for biosafety in accordance with the previously described protocol23,24,27. BMMCs were obtained from patients 2 h before surgery, prepared as previously described23, and applied during surgery. Briefly, bone marrow blood (200 ml) was harvested from the posterior iliac crest and immediately heparinized. Mononuclear cells were extracted from bone marrow blood using a nucleated cell processing kit (AVIC Biology, Ningxia, China) according to the instructions. Briefly, blood was centrifuged under sterile conditions at 2,000 r/min for 10 min, and then the supernatant plasma was removed. An equal volume of normal saline was added and thoroughly mixed with the cells, 6.0% hydroxyethyl starch was mixed in a ratio of 1:4, and then the mixture was allowed to stand at room temperature for 40 min. The supernatant was collected and centrifuged at 2,000 r/min for 5 min to obtain a cell pellet, which was washed twice with physiological saline. After the cell suspension was separated and purified, it was diluted to a final volume of 0.5–1.5 ml (≥1 × 109 cells). The BMMCs were placed at 4°C for short-term storage and prepared for implantation with NeuroRegen scaffolds. The BMMCs were identified as previously suggested23, and CD34+ monocytes accounted for more than 1.0% of the cells.
Surgical Procedures
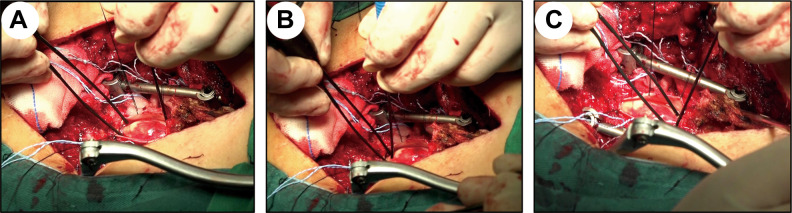
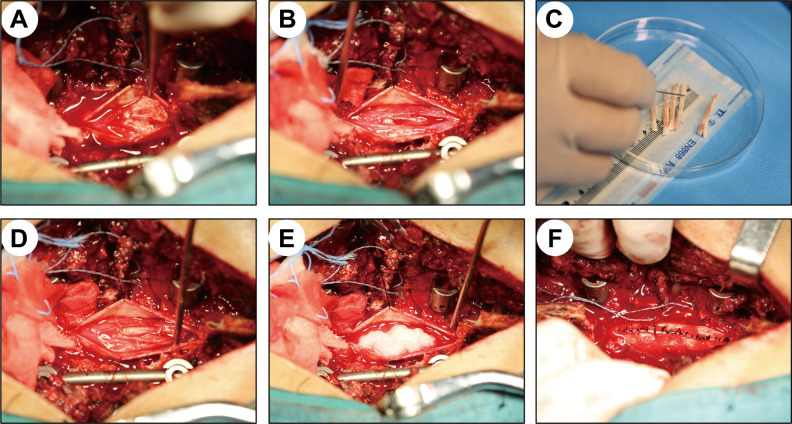
After anesthesia, the patient was placed in the prone position and sterilized via a routine protocol. The longitudinal surgical incision was made with the surgical segment as the center. After the routine induction of anesthesia, spinal fractures were routinely treated by reduction and internal fixation, and the spinal canal was opened through the interlaminar approach. Then, the lamina and dura mater were opened to expose the injured spinal cord. The boundaries of the necrotic spinal cord tissue were distinguished by visual observation and intraoperative neuroelectrophysiological monitoring (16-channel neurophysiological monitor, Nicolet, Madison, Wisconsin, USA) (Fig. 1) as previously described23,24. Briefly, neuroelectrophysiology was used to detect the somatosensory-evoked potential (SSEP) and motor-evoked potential (MEP) signals of the injured site and the cephalad and caudal segments of the spinal cord to determine the boundary between the normal and injured spinal cord (Fig. 1). The signal-receiving electrode was placed on the normal spinal cord, and the stimulation electrode was moved slowly from the injured side to the normal side until the normal spinal cord range was determined (Fig. 1). Then, the injured and necrotic spinal cord tissue was carefully cleaned (Fig. 2A, B). Subsequently, NeuroRegen scaffolds loaded with BMMCs (Fig. 2C) and biogel were implanted into the cleaned area of injured spinal cord tissue (Fig. 2D, E) and the dura mater was tightly sutured (Fig. 2F). During surgery, the mean arterial pressure was maintained at greater than 85 mmHg to maintain effective perfusion of the spinal cord.
Fig. 1.
Intraoperative neuroelectrophysiological monitoring was used to determine the extent of spinal cord injury (the left side shows the caudal segments, and the right side shows cephalad segments). (A, B) The signal-receiving electrode was placed on the normal spinal cord, and the stimulation electrode was moved slowly from the injured side to the normal side until the extent of the normal spinal cord in the caudal segments was determined. (C) The same method was used to determine the extent of spinal cord injury in the cephalad segments.
Fig. 2.
Injured spinal cords were cleaned, followed by functional NeuroRegen scaffold implantation. (A) Diffuse hyperemia, edema, and tissue necrosis were observed at the site of spinal cord injury after the dura mater was opened. (B) After determining the boundaries of the injured spinal cord by neuroelectrophysiological monitoring, the necrotic spinal cord tissue was cleaned. (C, D) Extracted autologous bone marrow mononuclear cells were evenly spread over the surface of the NeuroRegen scaffolds and grafted into the cleaned spinal cord gap. (E) Biogel was evenly injected onto the scaffolds. (F) The dura mater was tightly sutured.
Postoperative Rehabilitation and Follow-up Program
Patients participated in 6 months of standardized rehabilitation after the surgery to promote exercise and sensory function recovery and improve the patients’ ability for self-care24. The main rehabilitation program includes routine care, hyperbaric oxygen therapy, neurotrophic therapy, acupuncture, neuromuscular electrical stimulation therapy, upper limb muscle strength training, self-care training, etc. The patients underwent complete clinical and neurological assessments before the surgery and at each follow-up time point (1, 3, 6, 12, 18, 24, 30, and 36 months) after the surgery, including a neurological examination and assessments by radiography and the functional independence measure (FIM), activities of daily living (ADL) score, modified Ashworth spasticity scale (MAS) score, visual analogue scale (VAS) score, ASIA Impairment grade, and ASIA sensory and motor index score. MEP and SSEP tests were used to evaluate neurological recovery. Computed tomography (CT) was used to judge compression of the spinal cord by spinal fractures, and MRI was used to estimate the injury size and the continuity of the injured spinal cord.
Ethical Approval
This clinical study was registered in the National Institutes of Health database (ClinicalTrials.gov: NCT02510365) and approved by the ethics committee of Xinqiao Hospital affiliated with the Army Medical University. All steps were performed in strict accordance with the ethical standards of human clinical research. All patients and their families are fully aware of the possible risks and benefits of the treatment process. Informed consent was obtained for patient participation and publication of the results.
Statistical Analysis
Statistical analyses were performed using SPSS 20.0 software. The data are presented as the means ± standard errors. Quantitative variables were analyzed using one-way analysis of variance. A value of P <0.05 was considered significant.
Results
Patient Information
Seven male patients with acute complete SCI who met the inclusion criteria were included in the study. As shown in Table 1, all patients were classified as ASIA grade A and were primarily injured at the thoracic level (T5–T12). The main cause of SCI injury was falling. The average duration from injury to surgery was approximately 8.86 days (3–27 days). The average age of the patients was 46.57 years (29–61 years). Other trauma-related complications in the acute complete SCI patients included pulmonary contusion (five cases), pleural effusion (five cases), and rib fracture (three cases).
Table 1.
Patient Demographic and Clinical Features.
| Patient | Sex | Age (years) | Surgery time after SCI (days) | SCI levels | Cause of damage | ASIA grade |
|---|---|---|---|---|---|---|
| 1 | Male | 42 | 4 | T11–12 | Heavy bruise | A |
| 2 | Male | 53 | 5 | T11 | Falling | A |
| 3 | Male | 40 | 7 | T11 | Falling | A |
| 4 | Male | 52 | 6 | T11 | Falling | A |
| 5 | Male | 49 | 27 | T5 | Falling | A |
| 6 | Male | 29 | 9 | T12 | Falling | A |
| 7 | Male | 61 | 3 | T6–7 | Falling | A |
ASIA: American Spinal Injury Association; SCI: spinal cord injury.
Surgical Process
After opening the spinal canal, the dura mater was found to be intact in five patients, and the dura mater was partially torn in two patients. After the dura mater was opened and the spinal cord was exposed, the spinal cord was observed to be severely compressed and contused, and injured spinal cord tissue exhibited diffuse hyperemia, edema, and necrosis (Fig. 2A). However, the physical continuity of the spinal cord of all patients remained basically intact. The boundaries of the SCI were determined using neuroelectrophysiological monitoring combined with MRI and intraoperative observation. Necrohemorrhagic spinal cord tissue was carefully cleaned (Fig. 2B), and the length of the defect was measured. Then, NeuroRegen scaffolds were loaded with BMMCs (Fig. 2C) and implanted into the cleaned site of the injured spinal cord (Fig. 2D). The biogel was evenly injected onto the scaffolds (Fig. 2E); then, the dura mater was tightly sutured (Fig. 2F). The length of spinal cord tissue cleaning ranged from 2.0 to 6.0 cm, the length of NeuroRegen scaffold implantation ranged from 1.0 to 6.0 cm, and the quantity of implanted NeuroRegen scaffolds ranged from 3 to 6 bundles. Three of the seven patients had shorter lengths of implanted scaffolds (1.0 cm) than the length of injured spinal cord cleaning, which was mainly achieved by shortening internal fixation. The average operative time was 295.71 min (240–355 min), and the average bleeding volume was 1357.14 ml (400–3,800 ml) (Table 2). All patients successfully completed the operation.
Table 2.
Surgery-related Information.
| Patient | Length of spinal cord cleaning (cm) | Implant length (cm)/quantity | Time (min) | Bleeding (ml) |
|---|---|---|---|---|
| 1 | 6.0 | 6.0 (3) | 320 | 600 |
| 2 | 5.0 | 5.0 (5) | 285 | 1,500 |
| 3 | 2.0 | 2.0 (4) | 300 | 700 |
| 4 | 4.0 | 3.0 (5) | 240 | 700 |
| 5 | 5.0 | 4.0 (5) | 240 | 1,800 |
| 6 | 4.0 | 4.0 (6) | 355 | 400 |
| 7 | 2.0 | 1.0 (3) | 330 | 3,800 |
Functional Recovery of Acute Complete SCI Patients after Surgery
Among all the patients who underwent surgery, only one patient requested to withdraw from the study. This patient (No. 7) developed a stress ulcer and lung infection 1 week after the surgery and withdrew from the study at the request of the patient’s family. The remaining patients participated in 6 months of standardized rehabilitation and at least 3 years of follow-up (36–40 months). During the 3 years of neurological function observation after the surgery, different degrees of improvement were observed in terms of the sensory level (two cases), defecation sensation (four cases), physiological erection (five cases), enhanced sweating (three cases), superficial sensation recovery (one case), and deep sensation recovery (one case) (Table 3). In particular, one patient exhibited obvious recovery of deep sensory localization and feelings of numbness to pain with hot water stimulation but no clear localization. Additionally, compared with preoperative scores, the FIM score and ADL score of the patient at 6 months and 36 months after surgery were significantly increased (all P < 0.05), while the VAS score was significantly reduced (P < 0.05), indicating that the independent living ability and pain of the patient were significantly improved after surgery (Table 4). Five of the six patients had a slight improvement in the ASIA sensory score, while one patient had a decreased score (Table 4). According to the MAS scoring scale, four of six patients had varying degrees of muscle spasm during the follow-up (Table 4). Unfortunately, no obvious improvement in the ASIA grade, ASIA motor score, motor function, SSEPs, or MEPs was observed.
Table 3.
Summary of Neural Function Changes in the Patients.
| Patient | Sensation level | Defecation sensation | Physiological erection | Enhanced sweating | Superficial sensation recovery | Deep sensation recovery | Motor function recovery | |
|---|---|---|---|---|---|---|---|---|
| Before | After | |||||||
| 1 | T11 | T11 | Yes | Yes | No | Yes | Yes | No |
| 2 | T11 | T11 | Yes | Yes | No | No | No | No |
| 3 | T10 | T11 | Yes | Yes | Yes | No | No | No |
| 4 | T11 | T11 | Yes | Yes | Yes | No | No | No |
| 5 | T4 | T5 | No | Yes | No | No | No | No |
| 6 | T10 | T8 | No | No | Yes | No | No | No |
Table 4.
Scale Evaluation Results in SCI Patients after Surgery.
| Patient | Functional independence score | Activities of daily living | Visual pain score | Modified Ashworth spasticity scale score | ASIA sensory index score (right/left) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | 6 months | 36 months | Before | 6 months | 36 months | Before | 6 months | 36 months | Before | 6 months | 36 months | Before | 6 months | 36 months | |
| 1 | 48 | 75 | 82 | 0 | 25 | 45 | 80 | 10 | 10 | 0 | 0 | 0 | 68/68 | 66/62 | 70/70 |
| 2 | 48 | 74 | 94 | 0 | 30 | 40 | 80 | 30 | 10 | 0 | 12 | 12 | 66/66 | 70/70 | 70/70 |
| 3 | 50 | 89 | 82 | 0 | 30 | 35 | 80 | 30 | 10 | 0 | 24 | 18 | 66/66 | 70/70 | 72/72 |
| 4 | 48 | 72 | 88 | 0 | 35 | 35 | 80 | 20 | 10 | 0 | 0 | 6 | 68/68 | 69/69 | 70/66 |
| 5 | 50 | 66 | 71 | 0 | 30 | 35 | 70 | 20 | 30 | 0 | 0 | 6 | 40/40 | 43/42 | 42/42 |
| 6 | 49 | 92 | 91 | 0 | 40 | 45 | 80 | 0 | 20 | 0 | 0 | 0 | 62/62 | 54/56 | 54/56 |
| Mean | 48.83 ± 0.98 | 78.00 ± 10.22 | 84.6 ± 8.24 | 0.00 ± 0.00 | 31.67 ± 2.11 | 39.17 ± 2.01 | 78.30 ± 4.08 | 18.33 ± 11.69 | 15.00 ± 8.37 | ||||||
| P | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||||||
P: 6 months, 36 months versus before surgery. SCI: spinal cord injury.
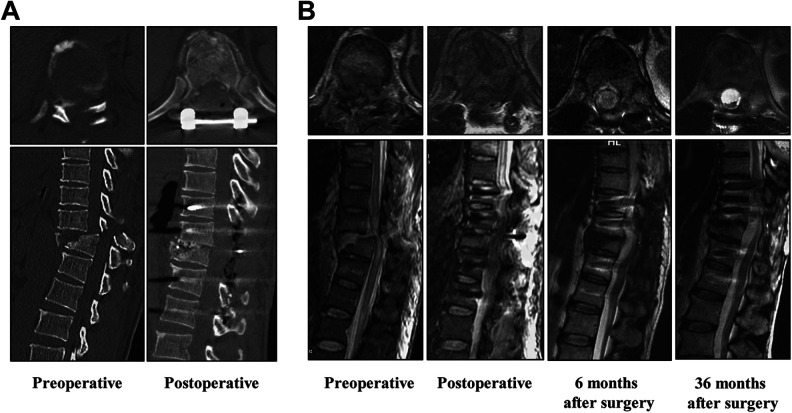
CT and MRI examinations were used to estimate the area and continuity of the injured spinal cord. Before surgery, the spinal cord medullary cavity showed an obviously narrowed cross-section, and spinal cord tissue was severely compressed by spinal fractures (Fig. 3A, B). However, the medullary cavity and continuity of the spinal cord returned to a coherent state after spinal reduction and internal fixation and the implantation of NeuroRegen scaffolds loaded with stem cells, as observed at 1 week, 6 months, and 36 months after the surgery (Fig. 3B). Additionally, no obvious abnormalities, such as proliferation or cavitation formation, were observed in the implantation area (Fig. 3B).
Fig. 3.
Preoperative and postoperative CT and MRI images of patients with acute complete spinal cord injury who underwent functional NeuroRegen scaffold implantation. (A) Preoperative and postoperative CT images. (B) MRI images of preoperatively, postoperatively, and 6 and 36 months after surgery. CT: computed tomography; MRI: magnetic resonance imaging.
Postoperative Complications in Patients with SCI
To date, all patients are alive and healthy. During the 3-year follow-up period, some postoperative complications related to SCI were observed. As shown in Table 5, one patient (No. 7) developed a stress ulcer and lung infection at 1 week after the surgery and withdrew from the study at the request of the patient’s family. One patient (No. 6) developed transient hyperthermia (38.5°C) that did not recur after antipyretic treatment. One patient developed a shallow wound that did not heal after discharge but healed successfully after local debridement. Other related complications included spasm (four cases), paraplegic neuralgia (three cases), pressure ulcers (one case), and lower limb amyotrophy (one case).
Table 5.
Postoperative Complications in SCI Patients.
| Patient | Stress ulcer | Pneumonia | Fever | Spasm | Paraplegic neuralgia | Pressure ulcers | Impaired wound healing | Myatrophy |
|---|---|---|---|---|---|---|---|---|
| 1 | No | No | No | No | Yes | No | No | No |
| 2 | No | No | No | Yes | Yes | No | No | No |
| 3 | No | No | No | Yes | Yes | No | No | No |
| 4 | No | No | No | Yes | No | Yes | No | No |
| 5 | No | No | No | Yes | No | No | Yes | No |
| 6 | No | No | Yes | No | No | No | No | Yes |
| 7 | Yes | Yes | No | No | No | No | No | No |
SCI: spinal cord injury.
However, no obvious early or late adverse events related to NeuroRegen scaffold or BMMC implantation, such as infection, uncontrollable high fever, allergic reaction, aggravation of neurological status, and cancer, were observed immediately after the surgery or during the 36 months of follow-up.
Discussion
The mechanisms of SCI have been extensively studied in past decades6,28–30. First, the initial physical trauma causes direct damage to blood vessels and cell membranes at the site of SCI. Subsequently, cascades of secondary damage occur, including excitotoxicity, vascular dysfunction, edema, ischemia, and free radical production, resulting in the necrosis and apoptosis of adjacent nerve cells and eventually leading to glial scarring and cystic cavity formation31,32. Scarring and cystic cavitation formation are major obstacles to nerve regeneration in patients with advanced SCI, resulting in permanent loss of sensory and motor function below the level of the SCI. Thus, intervention during the acute phase may provide maximum prevention of secondary damage and a chance to improve the functional recovery of SCI patients. Surgical cleaning of damaged spinal cord tissue may be the most direct method for eliminating the inhibitory effects of scar tissue, but reconstructing the structure and function of the spinal cord remains an insurmountable obstacle; one solution might be to implant stem cells in combination with biomaterials8,9,16,17,19,30.
The implantation of NeuroRegen scaffolds loaded with stem cells or neurotrophic factors has been shown to effectively rebuild the SCI microenvironment and promote nerve regeneration in animal models of complete SCI20,21,33. Then, the safety and feasibility of the implantation of NeuroRegen scaffolds loaded with autologous BMMCs or human umbilical cord mesenchymal stem cell (hUC-MSC) was demonstrated in patients with chronic complete SCI23,24. In some patients with acute complete SCI, partial cleaning of the injured spinal cord followed by scaffold implantation effectively improves sensory and motor function34,35. However, the number of reported cases and the follow-up periods are limited, and determination of the efficacy and safety requires more samples and longer observation periods. In this study, necrotic spinal cord tissue was cleaned in seven patients with acute complete SCI, and then NeuroRegen scaffolds combined with biogel and BMMCs were implanted into the cleaned sites. Except for some common complications of SCI, no obvious adverse reactions related to scaffold or stem cell implantation were observed. Additionally, sensation level expansion and autonomic nerve function recovery (defecation sensation, physiological erection, enhanced sweating) were observed in some patients, and one patient even exhibited erratic recovery of superficial sensation and deep sensation (No. 1). All patients showed significant improvements in the FIM, ADL score, and VAS score at 6 and 36 months after the surgery. MRI during the postoperative follow-up also showed that NeuroRegen scaffold implantation improved the continuity of the injured spinal cord. Cystic cavitation formation is an important factor hindering spinal cord regeneration. During the 3-year follow-up period, no obvious cystic cavitation formation or malignant proliferation was observed. Due to a lack of reliable controls, the specific clinical significance still requires further confirmation. Moreover, in addition to some conventional complications, no obvious early or late adverse events related to NeuroRegen scaffold or BMMCs implantation were observed. These findings indicate that implantation of NeuroRegen scaffolds combined with stem cells may serve as a safe clinical treatment for patients with acute complete SCI. Unfortunately, no significant improvement in the ASIA grade, ASIA motor score, or motor function was achieved.
Although this study failed to achieve the expected surgical results, several points are worthy of further discussion, and we hope to provide a reference and insight for similar research in the future. First, there is a lack of fully accurate standard clinical procedures for accurately diagnosing patients with acute complete SCI. Large studies have cited that 10%–20% of patients diagnosed with complete SCI are converted to a diagnosis of incomplete motor injury within 12 months36–38. One explanation for this phenomenon may be the misdiagnosis of incomplete SCI as complete SCI due to spinal shock. Spinal shock refers to the phenomenon when the spinal cord temporarily loses its ability to reflex and enters an unresponsive state, which often starts within a few minutes after a severe SCI and recovers after several days to several weeks. Early in spinal shock, although the nervous system is unable to effectively transmit neuronal signals, anatomical neural connections may still exist. Therefore, more stringent criteria should be used to identify patients with acute complete SCI before surgically cleaning injured spinal cord tissue. First, the patient must be confirmed to have passed the spinal cord shock stage without recovery of sensory and motor function below the injury level. Additionally, preoperative examination and intraoperative observation should suggest that the spinal cord is severely compressed and injured. Finally, preoperative and intraoperative neuroelectrophysiological monitoring should indicate no signal transmission in the injured segment, with further confirmation of complete SCI. In this study, seven patients were comprehensively confirmed to have acute complete SCI by a combination of methods, but the potential risks cannot be completely ignored. Perhaps patients with a completely severed spinal cord may be more clinically meaningful for this study. Furthermore, a well-designed controlled study may be more convincing, and more efficient diagnostic techniques and more rigorous diagnostic criteria may be required39.
The second issue is determining the length and extent of inactivated spinal cord tissue. Due to secondary injury after SCI, the area of spinal cord inactivation may be further enlarged. A variety of techniques, such as physical examination, MRI, and neuroelectrophysiology, have been used to determine the extent and segments of SCI39. In this study, based on preoperative imaging examinations, intraoperative neuroelectrophysiology monitoring was used to identify the rostral and caudal edges of the injured spinal cord23,35. In this study, the length of the injured spinal cord requiring cleaning ranged from 2.0 to 6.0 cm, and the length of NeuroRegen scaffold implantation ranged from 1.0 to 6.0 cm. Due to difficulties in nerve regeneration, the length of a spinal cord defect may also affect nerve recovery. Therefore, in three patients, we attempted to shorten the length (1.0 cm) of spinal cord cleaning via internal fixation and compression (Table 2). However, since no obvious motor or sensory recovery was observed in any patients, the significance of this approach requires further research.
In addition, under the premise of avoiding further damage to the spinal cord, to eliminate the inhibition of nerve repair by necrotic spinal cord tissue, the injured spinal cord tissue is fully cleaned under intraoperative neuroelectrophysiological monitoring. However, determining the extent of injured spinal cord cleaning still involves many technical difficulties. Some case reports have suggested that in patients with acute complete SCI, partial cleaning of the injured spinal cord followed by functional scaffold implantation may improve a patient’s sensory and motor function34,35, but these studies did not explain how to define the scope of cleaning. Our recommendation is to clean necrotic spinal cord tissue as precisely as possible based on the degree of SCI using intraoperative neuroelectrophysiology monitoring. However, determining the specific cleaning range of injured spinal cord tissue still needs further research. Additionally, due to the dynamic changes observed in the pathophysiology of SCI, the specific timing of surgical intervention is also worth exploring19,40.
Third, the therapeutic effect of scaffolds combined with stem cells in SCI is still limited. It has been widely confirmed that collagen scaffolds can induce stem cell differentiation, form a neural bridge across spinal cord defects, and reconnect the spinal cord in animal models16. The safety and availability of the scaffolds used in this study have also been confirmed in a variety of SCI animal models in past decades17,20–22. However, even with sufficient nerve regeneration and axonal myelination to form a neural bridge across the defect, motor function sometimes does not recover as expected or even becomes worse41. One possible explanation for the contradiction between the formation of a neural bridge and the deterioration of motor function may be the failure or insufficiency of regenerated axons to form correct synaptic connections with downstream neurons19. Therefore, promoting the formation of correct and effective connections between new axons and downstream neurons during the creation of a neural bridge is an important issue that must be considered in the future.
Finally, SCI causes substantial trauma physiologically, as well as in terms of psychological and social behavior. Most SCI patients develop pain syndrome, depression, and related mood disorders in the long term 42–44. Therefore, whether an SCI patient regains their confidence and actively cooperates with treatment and rehabilitation may have an enormous impact on their prognosis. All patients in this study participated in 6 months of standard rehabilitation, and their self-care ability was significantly improved. However, less attention was paid to the psychological state of the patients, but its importance cannot be ignored.
Conclusion
In conclusion, the use of NeuroRegen scaffolds combined with stem cells has broad prospects in the treatment of acute complete SCI. Since this study belongs to a non-controlled phase 1 clinical study, the effectiveness of recovery outcomes is still limited. We hope to provide more ideas and inspiration for similar research in the future by sharing the results and our experience in this study openly and transparently.
Acknowledgments
The NeuroRegen scaffold was developed and provided by the team of professor Jianwu Dai at the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences. The authors thank the Department of Blood Transfusion, Xinqiao Hospital of the Army Medical University, and the Department of Rehabilitation, Southwest Hospital of the Army Medical University, for their support and help in this study.
Footnotes
Ethical Approval: This clinical study has been registered in the National Institutes of Health database (ClinicalTrials.gov: NCT02510365) and approved by the ethics committee of Xinqiao Hospital affiliated with the Army Medical University.
Statement of Human and Animal Rights: Guideline provisions from the Helsinki Declaration were followed. The patients were informed of their right to withdraw consent at any time without reprisal.
Statement of Informed Consent: Written informed consent to participate in the study and publish the results was provided. The patients were fully aware of the treatment process and possible adverse outcomes.
Declaration of Conflicting Interests: The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Key Research and Development Plan Project (2016YFC1101504), the Special Project of Strategic Priority Research Program of the Chinese Academy of Sciences (XDA01030501), and the Clinical Research Project of the Second Affiliated Hospital of the Army Medical University (2015YLC03).
ORCID iD: Tongwei Chu  https://orcid.org/0000-0003-0309-7082
https://orcid.org/0000-0003-0309-7082
References
- 1. Kumar R, Lim J, Mekary RA, Rattani A, Dewan MC, Sharif SY, Osorio-Fonseca E, Park KB. Traumatic spinal injury: global epidemiology and worldwide volume. World Neurosurg. 2018;113:e345–e363. [DOI] [PubMed] [Google Scholar]
- 2. Ning GZ, Mu ZP, Shangguan L, Tang Y, Li CQ, Zhang ZF, Zhou Y. Epidemiological features of traumatic spinal cord injury in Chongqing, China. J Spinal Cord Med. 2016;39(4):455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. J Neurotrauma. 2004;21(10):1355–1370. [DOI] [PubMed] [Google Scholar]
- 4. Aito S, Tucci L, Zidarich V, Werhagen L. Traumatic spinal cord injuries: evidence from 30 years in a single centre. Spinal Cord. 2014;52(4):268–271. [DOI] [PubMed] [Google Scholar]
- 5. Hachem LD, Ahuja CS, Fehlings MG. Assessment and management of acute spinal cord injury: from point of injury to rehabilitation. J Spinal Cord Med. 2017;40(6):665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, Choi D, Fehlings MG. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017;80(3S):S9–S22. [DOI] [PubMed] [Google Scholar]
- 7. Forostyak S, Jendelova P, Sykova E. The role of mesenchymal stromal cells in spinal cord injury, regenerative medicine and possible clinical applications. Biochimie. 2013;95(12):2257–2270. [DOI] [PubMed] [Google Scholar]
- 8. Donnelly EM, Lamanna J, Boulis NM. Stem cell therapy for the spinal cord. Stem Cell Res Ther. 2012;3(4):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gazdic M, Volarevic V, Harrell CR, Fellabaum C. Stem Cells therapy for spinal cord injury. Int J Mol Sci. 2018;19(4):1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DeBrot A, Yao L. The combination of induced pluripotent stem cells and bioscaffolds holds promise for spinal cord regeneration. Neural Regen Res. 2018;13(10):1677–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kanekiyo K, Nakano N, Homma T, Yamada Y, Tamachi M, Suzuki Y, Fukushima M, Saito F, Ide C. Effects of multiple injection of bone marrow mononuclear cells on spinal cord injury of rats. J Neurotrauma. 2017;34(21):3003–3011. [DOI] [PubMed] [Google Scholar]
- 12. Sykova E, Homola A, Mazanec R, Lachmann H, Konradova SL, Kobylka P, Padr R, Neuwirth J, Komrska V, Vavra V, Stulík J, et al. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 2006;15(8-9):675–687. [DOI] [PubMed] [Google Scholar]
- 13. Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7(10):e47559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Satti HS, Waheed A, Ahmed P, Ahmed K, Akram Z, Aziz T, Satti TM, Shahbaz N, Khan MA, Malik SA. Autologous mesenchymal stromal cell transplantation for spinal cord injury: a phase i pilot study. Cytotherapy. 2016;18(4):518–522. [DOI] [PubMed] [Google Scholar]
- 15. Saini R. 181 therapeutic application of autologous bone marrow mononuclear stem cell in complete spinal cord injury in human. Neurosurgery. 2018;65(CN_suppl_1):109–109. [Google Scholar]
- 16. Haggerty AE, Oudega M. Biomaterials for spinal cord repair. Neurosci Bull. 2013;29(4):445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan C, Li X, Xiao Z, Zhao Y, Liang H, Wang B, Han S, Li X, Xu B, Wang N, Liu S, et al. A modified collagen scaffold facilitates endogenous neurogenesis for acute spinal cord injury repair. Acta Biomater. 2017;51:304–316. [DOI] [PubMed] [Google Scholar]
- 18. Wang M, Zhai P, Chen X, Schreyer DJ, Sun X, Cui F. Bioengineered scaffolds for spinal cord repair. Tissue Engineering Part B Reviews. 2011;17(3):177–194. [DOI] [PubMed] [Google Scholar]
- 19. Shrestha B, Coykendall K, Li Y, Moon A, Priyadarshani P, Yao L. Repair of injured spinal cord using biomaterial scaffolds and stem cells. Stem Cell Res Ther. 2014;5(4):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han S, Wang B, Jin W, Xiao Z, Li X, Ding W, Kapur M, Chen B, Yuan B, Zhu T, Wang H, et al. The linear-ordered collagen scaffold-BDNF complex significantly promotes functional recovery after completely transected spinal cord injury in canine. Biomaterials. 2015;41:89–96. [DOI] [PubMed] [Google Scholar]
- 21. Li X, Han J, Zhao Y, Ding W, Wei J, Han S, Shang X, Wang B, Chen B, Xiao Z, Dai J. Functionalized collagen scaffold neutralizing the myelin-inhibitory molecules promoted neurites outgrowth in vitro and facilitated spinal cord regeneration in vivo . ACS Appl Mater Interfaces. 2015;7(25):13960–13971. [DOI] [PubMed] [Google Scholar]
- 22. Li X, Tan J, Xiao Z, Zhao Y, Han S, Liu D, Yin W, Li J, Li J, Wanggou S, Chen B, et al. Transplantation of hUC-MSCs seeded collagen scaffolds reduces scar formation and promotes functional recovery in canines with chronic spinal cord injury. Sci Rep 2017;7:43559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xiao Z, Tang F, Tang J, Yang H, Zhao Y, Chen B, Han S, Wang N, Li X, Cheng S, Han G, et al. One-year clinical study of neuroregen scaffold implantation following scar resection in complete chronic spinal cord injury patients. Sci China Life Sci. 2016;59(7):647–655. [DOI] [PubMed] [Google Scholar]
- 24. Zhao Y, Tang F, Xiao Z, Han G, Wang N, Yin N, Chen B, Jiang X, Yun C, Han W, Zhao C, et al. Clinical study of neuroregen scaffold combined with human mesenchymal stem cells for the repair of chronic complete spinal cord injury. Cell Transplant. 2017;26(5):891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dietz V. Behavior of spinal neurons deprived of supraspinal input. Nat Rev Neurol. 2010;6(3):167–714. [DOI] [PubMed] [Google Scholar]
- 26. Dietz V. Neuronal plasticity after a human spinal cord injury: positive and negative effects. Exp Neurol. 2012;235(1):110–115. [DOI] [PubMed] [Google Scholar]
- 27. Lin H, Chen B, Wang B, Zhao Y, Sun W, Dai J. Novel nerve guidance material prepared from bovine aponeurosis. J Biomed Mater Res. 2006;79(3):591–598. [DOI] [PubMed] [Google Scholar]
- 28. Tator CH. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995;5(4):407–413. [DOI] [PubMed] [Google Scholar]
- 29. Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4(4):451–464. [DOI] [PubMed] [Google Scholar]
- 30. Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25(5):E2. [DOI] [PubMed] [Google Scholar]
- 31. Kong X, Gao J. Macrophage polarization: a key event in the secondary phase of acute spinal cord injury. J Cell Mol Med. 2017;21(5):941–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lukovic D, Stojkovic M, Moreno-Manzano V, Jendelova P, Sykova E, Bhattacharya SS, Erceg S. Concise review: reactive astrocytes and stem cells in spinal cord injury: good guys or bad guys?. Stem Cells. 2015;33(4):1036–1041. [DOI] [PubMed] [Google Scholar]
- 33. Han Q, Jin W, Xiao Z, Ni H, Wang J, Kong J, Wu J, Liang W, Chen L, Zhao Y, Chen B, et al. The promotion of neural regeneration in an extreme rat spinal cord injury model using a collagen scaffold containing a collagen binding neuroprotective protein and an EGFR neutralizing antibody. Biomaterials. 2010;31(35):9212–9220. [DOI] [PubMed] [Google Scholar]
- 34. Theodore N, Hlubek R, Danielson J, Neff K, Vaickus L, Ulich TR, Ropper AE. First human implantation of a bioresorbable polymer scaffold for acute traumatic spinal cord injury: a clinical pilot study for safety and feasibility. Neurosurgery. 2016;79(2):E305–E312. [DOI] [PubMed] [Google Scholar]
- 35. Xiao Z, Tang F, Zhao Y, Han G, Yin N, Li X, Chen B, Han S, Jiang X, Yun C, Zhao C, et al. Significant improvement of acute complete spinal cord injury patients diagnosed by a combined criteria implanted with neuroregen scaffolds and mesenchymal stem cells. Cell Transplant 2018;27(6):907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coleman WP, Geisler FH. Injury severity as primary predictor of outcome in acute spinal cord injury: retrospective results from a large multicenter clinical trial. Spine J. 2004;4(4):373–378. [DOI] [PubMed] [Google Scholar]
- 37. Lee BA, Leiby BE, Marino RJ. Neurological and functional recovery after thoracic spinal cord injury. J Spinal Cord Med. 2016;39(1):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burns AS, Lee BS, Ditunno JF, Tessler A. Patient selection for clinical trials: the reliability of the early spinal cord injury examination. J Neurotrauma. 2003;20(5):477–482. [DOI] [PubMed] [Google Scholar]
- 39. Armstrong AJ, Clark JM, Ho DT, Payne CJ, Nolan S, Goodes LM, Harvey LA, Marshall R, Galea MP, Dunlop SA. Achieving assessor accuracy on the international standards for neurological classification of spinal cord injury. Spinal Cord. 2017;55(11):994–1001. [DOI] [PubMed] [Google Scholar]
- 40. Saghazadeh A, Rezaei N. The role of timing in the treatment of spinal cord injury. Biomed Pharmacother. 2017;92:128–139. [DOI] [PubMed] [Google Scholar]
- 41. Lu P, Jones LL, Tuszynski MH. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp Neurol. 2005;191(2):344–360. [DOI] [PubMed] [Google Scholar]
- 42. Krause JS, Kemp B, Coker J. Depression after spinal cord injury: relation to gender, ethnicity, aging, and socioeconomic indicators. Arch Phys Med Rehabil. 2000;81(8):1099–1109. [DOI] [PubMed] [Google Scholar]
- 43. Al-Owesie RM, Moussa NM, Robert AA. Anxiety and depression among traumatic spinal cord injured patients. Neurosciences (Riyadh). 2012;17(2):145–150. [PubMed] [Google Scholar]
- 44. Craig A, Tran Y, Guest R, Gopinath B, Jagnoor J, Bryant RA, Collie A, Tate R, Kenardy J, Middleton JW, Cameron I. Psychological impact of injuries sustained in motor vehicle crashes: systematic review and meta-analysis. BMJ Open. 2016;6(9):e011993. [DOI] [PMC free article] [PubMed] [Google Scholar]