Abstract
Previous studies in rodents have indicated that function and survival of transplanted islets can be substantially improved by mesenchymal stem cells (MSC). The few human islet studies to date have confirmed these findings but have not determined whether physical contact between MSC and islets is required or whether the benefit to islets results from MSC-secreted proteins. This study aimed to investigate the protective capacity of MSC-preconditioned media for human islets. MSC were cultured for 2 or 5 days in normoxia or hypoxia before harvesting the cell-depleted media for human islet culture in normoxia or hypoxia for 6–8 or 3–4 days, respectively. To characterize MSC-preconditioned media, proteomic secretome profiling was performed to identify angiogenesis- and inflammation-related proteins. A protective effect of MSC-preconditioned media on survival and in vitro function of hypoxic human islets was observed irrespective of the atmosphere used for MSC preconditioning. Islet morphology changed markedly when media from hypoxic MSC were used for culture. However, PDX-1 and insulin gene expression did not confirm a change in the genetic phenotype of these islets. Proteomic profiling of preconditioned media revealed the heterogenicity of the secretome comprising angiogenic and antiapoptotic as well as angiostatic or proinflammatory mediators released at an identical pattern regardless whether MSC had been cultured in normoxic or hypoxic atmosphere. These findings do not allow a clear discrimination between normoxia and hypoxia as stimulus for protective MSC capabilities but indicate an ambivalent character of the MSC angiogenesis- and inflammation-related secretome. Nevertheless, culture of human islets in acellular MSC-preconditioned media resulted in improved morphological and functional islet integrity suggesting a disbalance in favor of protective factors. Further approaches should aim to eliminate potentially detrimental factors to enable the production of advanced clinical grade islet culture media with higher protective qualities.
Keywords: cell culture, human islets, hypoxia, mesenchymal stem cells, preconditioning, secretome
Introduction
Human islet allotransplantation has been established as a successful and safe procedure for reversing life-threatening hypoglycemia unawareness and restoring normoglycemia in selected patients with type 1 diabetes mellitus1. Nevertheless, 72% of islet recipients still require two or more procedures to achieve these outcomes2. This low efficiency can be explained by a combination of multiple factors inducing a substantial loss of isolated islets during pretransplant culture and during engraftment, due to the lack of extracellular matrix3, hypoxic conditions4,5, and the shortage of essential nutrients6,7.
Mesenchymal stem cells (MSC) are undifferentiated multipotent stromal cells that can be found in any type of adult organs to maintain local tissue homeostasis and initiate tissue repair after damage8. Numerous studies have suggested that MSC have immunomodulatory9,10, regenerative11,12, anti-inflammatory13–15, antiapoptotic16–19, and angiogenic properties19,20 when cocultured or transplanted with rodent islets. These protective properties improve engraftment of nonhuman primate islets following transplantation into the liver21 and also increase graft survival when rodent islets are placed into the less well-vascularized subcutaneous site that is usually characterized by poor graft function22. Despite these promising findings in rodents and primates, only 2 out of 108 clinical trials involving islet transplantation as treatment for patients with type 1 diabetes are currently registered to use MSC as supportive co-grafts to enhance human islet graft function23. The cautious implementation of MSC in the clinical islet transplantation setting can be partially explained by concerns about the potential risk of chromosomal aberrations and spontaneous transformation in long-term cultured human MSC24,25. Nevertheless, as demonstrated by previous studies in mice, pretransplant coculture with MSC is an alternative way of improving islet graft function without having to simultaneously implant MSC into the recipient26. It is unclear, however, whether the protective effects of MSC result from their physical contact to the islets or whether secretory products from the MSC are mainly responsible. Previous comparative studies in rodents are inconclusive and do not clarify the most efficient mode for MSC-derived protection of islets13,17,18,27,28. Among the very few coculture studies that have used human islets, only one out of seven performed indirect culture in transwell plates29, while the other ones used direct coculture with physical contact to MSC30–35.
The anti-inflammatory, antiapoptotic, and regenerative properties of MSC may not only benefit transplanted islets but may also be protective for islets during pretransplant culture and during shipment from the islet processing facility to distant islet transplantation sites36. The aim of our study therefore was to test the hypothesis that the survival and function of cultured islets can be improved by cell-free MSC-preconditioned culture media. The study compared the efficiency of different protocols for preconditioning to protect the integrity of isolated human islets exposed to hypoxia.
Materials and Methods
MSC Isolation and Culture
Human MSC were isolated by the Celution system (Cytori Therapeutics, San Diego, CA, USA) from lipoaspirates of female donors after signing informed consent. Processing and use of the tissue was approved by the local ethical committee (2014/838).
Three batches of adipose tissue–derived MSC were used for this study. The cells were seeded at a density of 3,000 cells/cm2 and expanded using minimum essential medium-alpha (MEMα) with a CMRL 1066-identical glucose concentration of 5.5 mmol/l and supplemented with 2 mmol/l Glutamax, 10% bovine serum (Gibco, Thermo Fisher Scientific, Oslo, Norway), 20 mmol/l 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Gibco), and 50 µg/ml gentamycin (Braun, Frederiksberg, Denmark). Culture medium was changed every two to three days until 70%–80% of confluence was obtained.
After harvesting, MSC were stored in liquid nitrogen at −196°C until further use. Thawed MSC underwent one to two passages in normal atmosphere (21% O2, 5% CO2, 95% humidity) for cell attachment. Afterward, culture flasks were divided and incubated in normoxia for 2 or 5 days (21% O2–2d MEM, 21% O2–5d MEM) or in severe hypoxia (1% O2) for 2 days only (1% O2–2d MEM). During the 2- and 5-day culture periods for media preconditioning, medium change was not performed. After culture, media were collected, spun at 5,000 × g for 10 min, and the supernatant stored at −80°C until used for islet culture. Nonconditioned supplemented MEMα served as native control medium. In the first series of experiments (normoxic islet culture atmosphere), human islets were suspended in native MEM, 1% O2–2d MEM, and 21% O2–2d MEM. In addition, nonconditioned CMRL 1066, supplemented as described for MEM, was used as gold standard. In the second series (hypoxic islet culture atmosphere), human islets were incubated in native MEM, 1% O2–2d MEM, 21% O2–2d MEM, and 21% O2–5d MEM. The experimental design of the study is shown in Fig. 1.
Fig. 1.
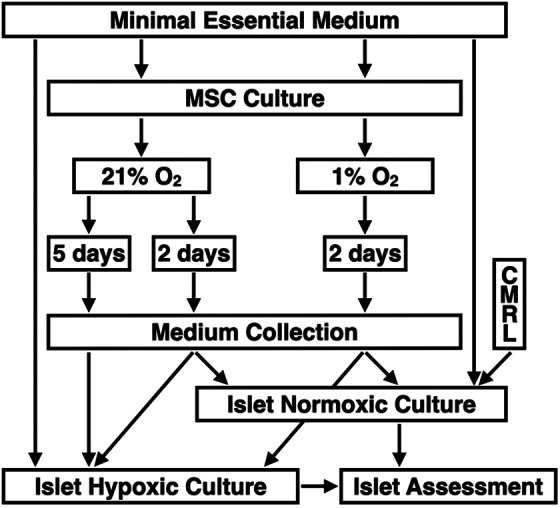
Experimental study design of human islet culture in MEMα preconditioned with MSC isolated from human adipose tissue. MSC were suspended in supplemented MEMα and cultured for 2 or 5 days in normoxia at 21% O2 or for 2 days in severe hypoxia at 1% O2 prior to medium collection. In the first and second trial, human islets were suspended in conditioned or nonconditioned MEMα and cultured for 6 to 8 days in normoxia (n = 6) or for 3 to 4 days in hypoxia at 2% O2 (n = 8), respectively, prior to islet assessment and characterization. In the normoxia trial, CMRL 1066 was additionally used as gold standard. MEMα: minimum essential medium-alpha; MSC: mesenchymal stem cells.
MSC Characterization
Characterization of MSC was performed before further experiments were initiated following the position statement of the International Society for Cellular Therapy37. Cell counting and viability assessment were performed in a hemocytometer by means of a dye exclusion test using 0.4% Trypan-blue solution (Gibco). Expression of key cell surface markers was analyzed using flow cytometry with a BD FACS Canto II (Becton Dickinson, San Diego, CA, USA) and BD Stemflow Human MSC Analysis Kit (BD Biosciences, San Jose, CA, USA) according to manufacturer’s instructions. Briefly, 5 × 105 cells/100 µl were incubated with the conjugated monoclonal or isotype matched IgG control antibodies, then analyzed by FACS to measure the levels of positive (CD105 PerCP-Cy™5.5, CD73 allophycocyanin, and CD90 fluorescein isothiocyanate) or negative (CD45, CD34, CD11b, CD19, and HLA-DR PE) markers of MSC.
Differentiation capacity was evaluated using StemPro chondrogenic, osteogenic, and adipogenic differentiation kits (Gibco) following the manufacturer’s instructions. Briefly, 1 × 104 cells/cm2 were seeded into 12-well tissue culture plates (Costar, Corning, NY, USA) and incubated for 14 days in tissue-specific medium changed every 3–4 days. While control cells were cultured for 14 days in tissue-specific basal media, cells treated for differentiation were cultured for the same period of time in tissue-specific media supplemented with corresponding stimulation factors. At the end of the differentiation period, all cultures were fixed with 4% formaldehyde (Chemi-teknik, Oslo, Norway) before differentiated cells were stained with Alcian Blue, Alizarin Red S, and Oil Red O (Sigma-Aldrich, St. Louis, MO, USA) to detect glucosaminoglycan deposition, punctate mineral deposition, and lipid droplet formation specific for chondrocytes, osteocytes, and adipocytes, respectively.
Islet Isolation and Culture
Nineteen human donor pancreases were retrieved with appropriate consent and ethical approval by the institutional review board. After a mean cold ischemia time of 6 h (range 4–8), islets were isolated and purified as previously described3. Aliquots of 300 islet equivalents (IEQ) were incubated in 24-well plates (Greiner Bio-One, Stonehouse, UK) and suspended in 300 µl of nonconditioned or preconditioned MEMα. In the first series of experiments (normoxic islet culture atmosphere, n = 6), human islets were suspended in native MEM, 1% O2–2d MEM, and 21% O2–2d MEM. Nonconditioned CMRL 1066, supplemented as described for MEM, was used as gold standard. In the second series (hypoxic islet culture atmosphere, n = 8), human islets were incubated in native MEM, 1% O2–2d MEM, 21% O2–2d MEM, and 21% O2–5d MEM. The experimental design of the study is shown in Fig. 1.
Islet Characterization
Before and after islet culture, islet number was quantified as islet particle number (IN) and IEQ as previously described in detail38. Islet yield (%) was calculated by normalizing IEQ, as counted post-culture, to pre-culture yield of IEQ. Islet morphological integrity was determined as fragmentation index by calculating the ratio of IN over IEQ. Islet viability was assessed utilizing 0.67 µmol/l fluorescein diacetate (Sigma-Aldrich, UK) and 4.0 µmol/l propidium iodide (Sigma-Aldrich) for staining of viable and dead cells, respectively39. Islet overall survival was utilized to consider the recovery of viable cells only. For this variable, normalized post-culture islet yield was multiplied by the proportion of viable cells. In vitro function of 20 hand-picked islets of similar size (150–200 µm) was assessed in duplicate during static glucose incubation. Islets were seeded on 0.8 µm pore size filter inserts, transferred into 24-well plates, and sequentially incubated for 45 min in 1 ml Krebs-Ringer buffer supplemented with 2.0 mmol/l glucose followed by 45 min at 20 mmol/l followed by a second period of 45 min at 2 mmol/l glucose. Afterward, islets were recovered and sonified in distilled water prior to insulin extraction in acid ethanol and for subsequent determination of DNA content. Intracellular and secreted insulin was determined utilizing an enzyme immunoassay for human insulin (Mercodia, Uppsala, Sweden) and normalized to islet DNA content measured by the Pico Green assay (Life Technologies, Paisley, UK). The glucose stimulation index was calculated by dividing the insulin release at 20 mmol/l glucose by the mean of the two basal periods.
Quantitative Real-time Polymerase Chain Reaction
Gene expression of cultured islets (n = 5) was measured using Taqman-based quantitative real-time polymerase chain reaction (qRT-PCR). Briefly, total RNA was extracted from 100 cultured handpicked islets of similar size (150–200 µm) using the RNeasy Micro kit (Qiagen, Hilden, Germany) before being run in triplicate for 35 cycles on a QuantStudio 7 (Applied Biosystems, Carlsbad, CA, USA) using the CellsDirect One-Step qRT-PCR kit (Invitrogen, Carlsbad, CA, USA). Duplex reactions were performed using TaqMan assays specific for the target genes BCL-2 associated X protein (BAX, Hs00180269_m1), B-cell lymphoma-2 (BCL-2, Hs00608023_m1), insulin (Hs00355773_m1), and pancreatic and duodenal homeobox-1 (PDX-1, Hs00236830_m1) normalized to 18 S ribosomal RNA (rRNA) (18 S rRNA, Hs99999901_s1). All primers were provided by Applied Biosystems (Warrington, UK). Quantitative values were obtained using the threshold cycle number and the x-fold change in expression using the ΔΔCT method40.
Proteomic Secretome Profiling
Three batches of freshly thawed MEM preconditioned at either 21% (21% O2–2d MEM) or 1% O2 (1% O2–2d MEM) were analyzed in duplicate utilizing a proteome profiler array kit for human angiogenesis (R&D Systems, Abingdon, UK) measuring the relative production of 55 human angiogenesis-related factors by chemiluminescence. The expression of each detectable protein was measured by densitometric analysis using ImageJ (National Institutes of Health, Bethesda, ML, USA) and normalized to its presence in native MEMα.
Statistical Analysis
Statistical analysis was performed utilizing Prism 7.0d for MacIntosh (GraphPad, San Diego, CA, USA). Data analysis was carried out by the nonparametric Friedman test followed by Dunn’s test for multiple comparisons or by the Wilcoxon test. Differences were considered significant at P less than 0.05. P-values more than 0.05 are termed nonsignificant. Results are expressed as mean ± standard error and are normalized to islet variables determined pre-culture if appropriate.
Results
MSC Characterization
The percentage of viable cells was >95% in all samples assessed. After culture, the surface marker expression of the cells fulfilled the criteria for MSC according to the guidelines of the International Society for Cellular Therapy37. As shown in Table 1, MSC-specific surface markers such as CD105, CD90, or CD73 were highly expressed while expression of negative markers such as CD45, CD34, CD19, CD11b, or HLA-DR was virtually absent.
Table 1.
Immunophenotyping of Human MSC After 2 Days of Culture (n = 3).
| CD105 (%) | CD90 (%) | CD73 (%) | CD45 (%) | CD34 (%) | CD19 (%) | CD11b (%) | HLA-DR |
|---|---|---|---|---|---|---|---|
| 94.8 ± 3.4 | 99.9 ± 0.04 | 99.9 ± 0.04 | 0.6 ± 0.13 | 2.9 ± 0.6 | 1.2 ± 0.6 | 2.3 ± 1.6 | 1.0 ± 0.4 |
MSC: mesenchymal stem cells.
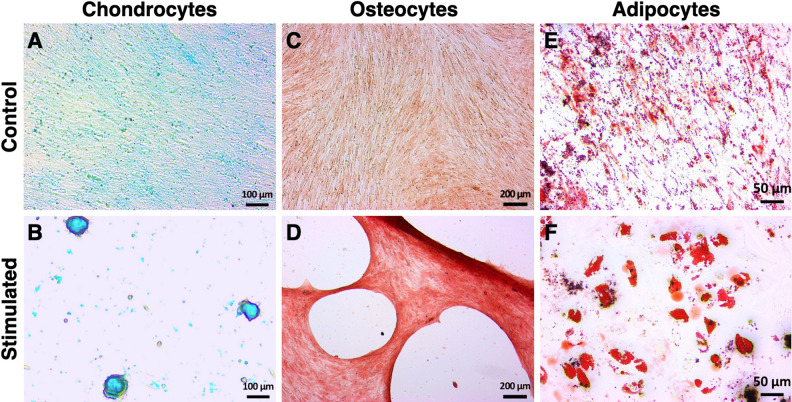
After 14 days of specific stimulation, MSC of all batches demonstrated the ability to differentiate into chondrocytes (Fig. 2A, B), osteocytes (Fig. 2C, D), and adipocytes (Fig. 2E, F).
Fig. 2.
Trilineage differentiation potential of human MSC isolated from human adipose tissue. MSC were stimulated over a culture period of 14 days using tissue-specific media supplemented with corresponding stimulation factors for chondrogenesis (B), osteogenesis (D), or adipogenesis (F). Control MSC were cultured in tissue-specific basal media (A, C, E). Differentiation of MSC was detected using staining with (A, B) Alcian Blue for glucosaminoglycan deposition, (C, D) Alizarin Red S for punctate mineral deposition, and (E, F) Oil Red O for lipid droplet formation specific for chondrocytes, osteocytes, and adipocytes, respectively. Actual size of the tissue is shown by scale bars. Pictures are representative for all batches assessed. MSC: mesenchymal stem cells.
Islet Characterization after Normoxic Islet Culture
The major characteristics of human islets cultured in native CMRL 1066, native MEM, or MEM preconditioned at either 21% (21% O2–2d MEM) or 1% O2 (1% O2–2d MEM) are shown in Table 2. Islet yield was not significantly different after 6–8 days of normoxic islet culture in native CMRL and native MEM or 21% O2–2d MEM. When islets were cultured in 1% O2–2d MEM, significantly improved recovery was obtained compared with native MEM. This corresponded with the lowest fragmentation index among all experimental groups. The improved preservation of islet integrity by means of preconditioned MEM is reflected by a significantly higher viability after culture in 1% O2–2d MEM and 21% O2–2d MEM. Calculation of overall survival demonstrated highest survival rates when islets were cultured in 1% O2–2d MEM.
Table 2.
Islet Culture Outcome After 6–8 Days of Islet Culture in Normoxic Atmosphere (n = 6).
| Media | IEQ yield (%) | Fragmentation (IN/IEQ) | Viability (%) | Overall survival (%) | Stimulation index | Insulin (µU/ng DNA) |
|---|---|---|---|---|---|---|
| Native CMRL | 39.5 ± 14.5 | 1.71 ± 0.26 | 76.9 ± 7.7 | 27.0 ± 6.4 | 1.27 ± 0.05 | 107.9 ± 23.4 |
| Native MEMα | 45.5 ± 12.1 | 1.65 ± 0.29 | 82.6 ± 7.2 | 35.4 ± 6.5 | 1.21 ± 0.03 | 140.7 ± 45.7 |
| 1% O2–2d MEM | 60.2 ± 16.6‡ | 1.35 ± 0.22‡ | 89.9 ± 7.7† | 50.2 ± 9.1† | 1.12 ± 0.02† | 201.8 ± 64.6‡ |
| 21% O2–2d MEM | 54.1 ± 17.3 | 1.54 ± 0.26 | 90.3 ± 6.7† | 45.9 ± 11.3 | 1.16 ± 0.02 | 172.6 ± 60.9 |
1% O2–2d MEM: medium preconditioned by hypoxic MSC culture for 2 days; 21% O2–2d MEM: medium preconditioned by normoxic MSC culture for 2 days; MSC: mesenchymal stem cells.
† P < 0.05, ‡ P < 0.01 versus native MEMα.
Overall survival inversely correlated with gene expression of proapoptotic BAX and antiapoptotic BCL-2 calculated as BAX-over-BCL-2 ratio (Fig. 3A). While no difference was found between CMRL and native MEM, a substantial reduction was observed in islets cultured in 1% O2–2d MEM (P < 0.01) or 21% O2–2d MEM (P = 0.086).
Fig. 3.
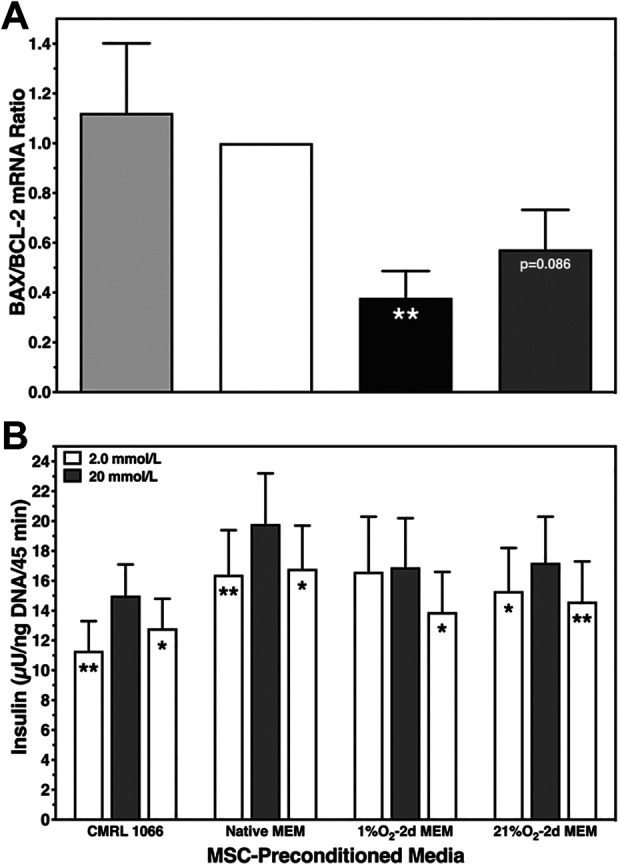
Human islet characterization after 6–8 days of normoxic islet culture. (A) Postculture BAX and BCL-2 mRNA expression calculated as BAX-over-BCL-2 ratio (n = 5) after culture in native CMRL 1066 (light grey bars), native MEMα (white), or MEMα preconditioned for 2 days at 1% O2 (black) or 21% O2 (dark grey); **P < 0.01 and P = 0.086 versus native MEMα. (B) Glucose-stimulated insulin release of 20 islets at 2 mmol/l (white bars), 20 mmol/l (grey), and again 2 mmol/l of glucose was normalized to islet DNA content (n = 6); *P < 0.05, **P < 0.01 for 2 versus 20 mmol/l of glucose. MEMα: minimum essential medium-alpha.
The in vitro function of islets varied only slightly between different media except in islets cultured in 1% O2–2d MEM, which demonstrated a marginal insulin response toward glucose (Fig. 3B). This treatment was also associated with a significantly reduced stimulation index corresponding to the significantly highest insulin content as shown in Table 2. Vice versa, islets cultured in CMRL were characterized by the highest stimulation index and the lowest insulin content.
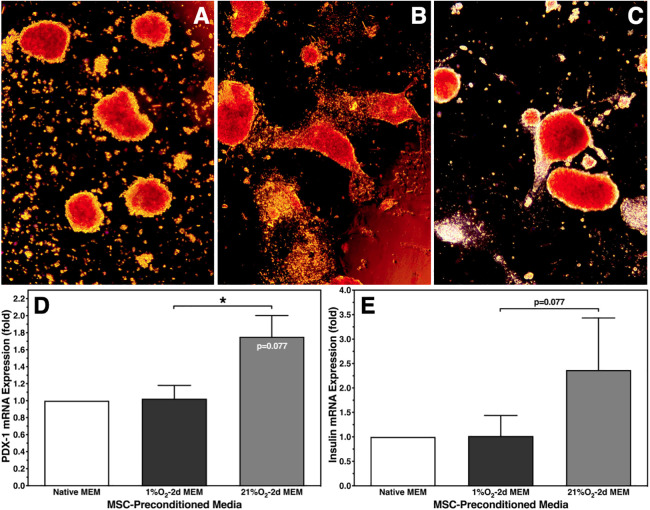
Despite these findings, microscopical assessment revealed a distinct change of islet morphology after normoxic islet culture in 1% O2–2d MEM. While control islets cultured for 6–8 days in native MEM had an ovoid shape (Fig. 4A), islets cultured for the same period of time in 1% O2–2d MEM transformed into a spindle-like form, which may indicate beginning of spreading (Fig. 4B). In contrast, islets cultured for 6–8 days in 21% O2–2d MEM showed only a marginal number of longitudinal flat extensions (Fig. 4C). To clarify whether change of islet morphology is associated with an alteration of the genetic phenotype, mRNA expression of PDX-1 and insulin was analyzed by qRT-PCR (n = 5). As demonstrated in Fig. 4D, PDX-1 gene expression of islets cultured in 1% O2–2d MEM was nearly identical with expression of control islets cultured in native MEM. Surprisingly, when islets were cultured in 21% O2–2d MEM, a higher PDX-1 mRNA expression was measured. A similar trend was observed for insulin gene expression without reaching statistical significance (Fig. 4E).
Fig. 4.
Morphology of dithizone-stained human islets and the corresponding genetic phenotype after 6–8 days of islet culture in normoxic atmosphere. Morphology was assessed after culture in (A) native MEMα or in MEMα preconditioned for 2 days at (B) 1% O2 or (C) 21% O2. Photographs at ×50 magnification are representative for all islet preparations assessed. mRNA expression (n = 5) of (D) PDX-1; *P < 0.05 as indicated, P = 0.077 versus native MEMα; and (E) insulin, P = 0.077 as indicated. MEMα: minimum essential medium-alpha.
Islet Characterization After Hypoxic Islet Culture
In the second trial (n = 8), islets were cultured for 3–4 days in hypoxia (2% O2) in order to estimate the protective potency of MSC-preconditioned MEM. For these experiments one additional aliquot of MSC was cultured for 5 days at 21% O2 (21% O2–5d MEM) to increase the release of potentially protective factors.
As shown in Table 3, islet yield after hypoxic islet culture was significantly improved in MEM preconditioned at 1% or 21% O2 compared with native MEM. A negative effect was observed when MEM preconditioning at 21% O2 had been extended to 5 days (21% O2–5d MEM). This is consistent with the increased fragmentation index of islets cultured in 21% O2–5d MEM, which was the highest among all experimental groups. The detrimental effect of extended preconditioning on islet morphology is also reflected by islet viability that was identical compared with native MEM. No effect on islet viability was noted comparing islets cultured in 1% O2–2d MEM or 21% O2–2d MEM. In contrast to the first series of experiments in normoxic islet culture atmosphere, alterations of islet morphology were not observed (data not shown).
Table 3.
Islet Culture Outcome After 3–4 Days of Islet Culture in Hypoxic Atmosphere (n = 8).
| Media | IEQ yield (%) | Fragmentation (IN/IEQ) | Viability (%) | Overall survival (%) | Stimulation index | Insulin (µU/ng DNA) |
|---|---|---|---|---|---|---|
| Native MEMα | 42.1 ± 2.4 | 1.55 ± 0.20 | 79.4 ± 3.8 | 33.5 ± 2.8 | 0.95 ± 0.08 | 40.4 ± 6.7 |
| 1% O2–2d MEM | 65.1 ± 6.2§ | 0.92 ± 0.05§ | 83.4 ± 4.4‡ | 55.0 ± 7.1§ | 1.52 ± 0.08§ | 59.1 ± 7.7¶ |
| 21% O2–2d MEM | 58.6 ± 5.5§ | 1.06 ± 0.09‡ | 83.9 ± 4.2‡ | 49.7 ± 6.1§ | 1.41 ± 0.08‡ | 72.4 ± 8.4§ |
| 21% O2–5d MEM | 53.1 ± 5.7¶ | 1.34 ± 0.14¶ | 79.5 ± 3.9# | 42.8 ± 5.9¶ | 1.34 ± 0.11† | 67.0 ± 9.9§ |
1% O2–2d MEM: medium preconditioned by hypoxic MSC culture for 2 days; 21% O2–2d MEM: medium preconditioned by normoxic MSC culture for 2 days; 21% O2–5d MEM: medium preconditioned by normoxic MSC culture for 5 days; MSC: mesenchymal stem cells.
† P < 0.05, ‡ P < 0.01, § P < 0.001 versus native MEMα; ¶ P < 0.05, # P < 0.01 versus 21%–2d MEM.
Calculation of overall survival significantly suggested best islet protection after hypoxic islet culture in 1% O2–2d MEM. A slightly lower grade of protection was reached using 21% O2–2d MEM. A protective effect of 21% O2–5d MEM was virtually not existing when compared with native MEMα (Table 3). In contrast, the BAX-over-BCL-2 mRNA ratio was substantially decreased in all treatment groups regardless whether hypoxic preconditioning or normoxic preconditioning for 2 or 5 days had been applied (Fig. 5A). No significant difference was found between 21% O2–2d MEM and 21% O2–5d MEM.
Fig. 5.
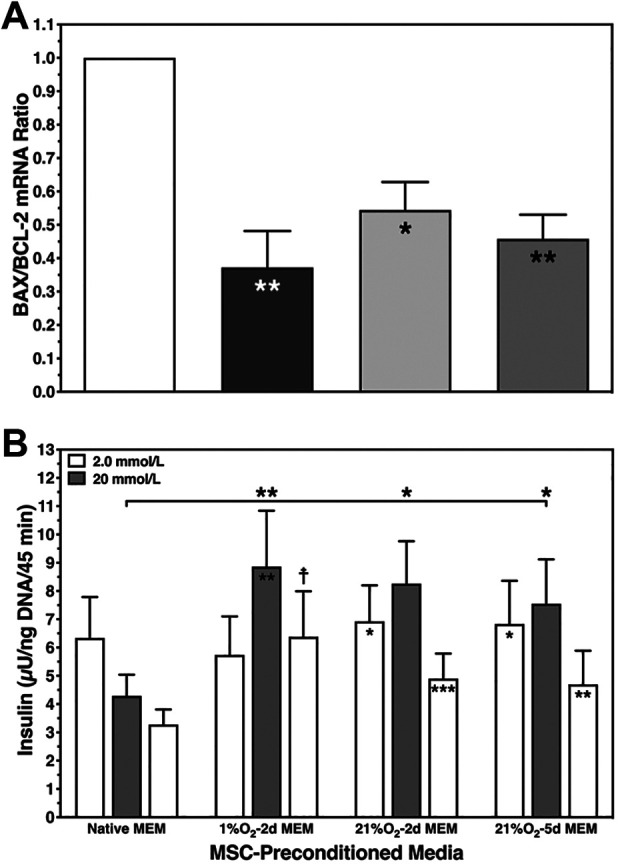
Human islet characterization after 3–4 days of hypoxic islet culture. (A) Postculture BAX and BCL-2 mRNA expression was calculated as BAX-over-BCL-2 ratio (n = 6) after culture in native MEMα (white bar) and in MEMα preconditioned at 1% O2 (black) or preconditioned at 21% O2 for 2 days (light grey) and 5 days (dark grey), *P < 0.05, **P < 0.01 versus native MEMα. (B) Glucose-stimulated insulin release of 20 islets at 2 mmol/l (white bars), 20 mmol/l (grey), and again 2 mmol/l of glucose was normalized to islet DNA content (n = 8); *P < 0.05, **P < 0.01, ***P < 0.001 for 2 versus 20 mmol/l of glucose; *P < 0.05, **P < 0.01 for 1%–2d medium, 21%–2d medium, and 21%–5d medium versus native MEMα as indicated. MEMα: minimum essential medium-alpha.
Islet responsiveness to glucose after hypoxic islet culture was significantly preserved by any mode of preconditioning applied. Stimulated insulin release was significantly increased incubating islets in 1% O2–2d MEM, 21% O2–2d MEM, or 21% O2–5d MEM compared with native MEMα (Fig. 5B). Hypoxic islets incubated in the latter medium did not show any stimulated insulin response, resulting in a stimulation index of <1, which was significantly reduced compared with 1% O2–2d MEM, 21% O2–2d MEM, or 21% O2–5d MEM (Table 3).
Proteomic Secretome Profiling
To clarify the effect of severe hypoxia on MSC secretion of angiogenesis- and inflammation-related proteins, a proteome profiler assay was applied on three lots of MEM preconditioned for 2 days in normoxia or hypoxia.
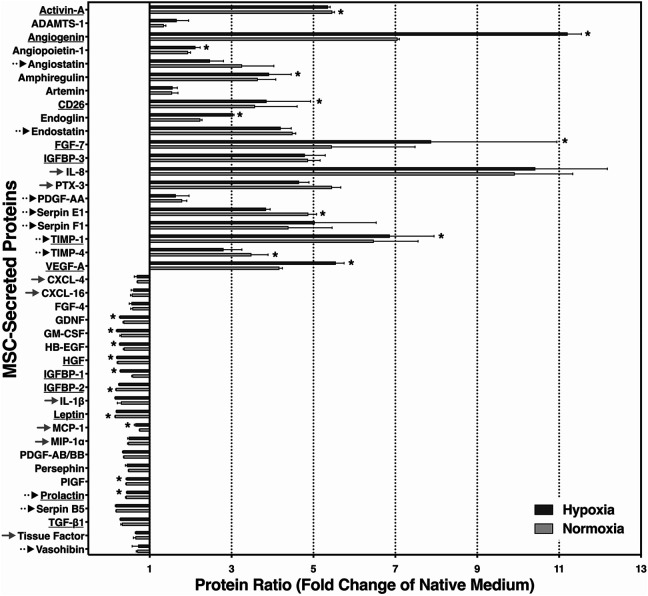
Twenty out of 41 soluble biomarkers that could be detected in measurable amounts were expressed on a higher level, while 21 factors were decreased compared with native MEMα (Fig. 6). Among those that were substantially increased by more than five-fold, we found the angiogenic or antiapoptotic factors Activin-A, angiogenin, fibroblast growth factor-7 (FGF-7), tissue inhibitor of metalloproteinases-1 (TIMP-1), and vascular endothelial growth factor-A (VEGF-A), but also proinflammatory, antiangiogenic, or antiproliferative mediators such as insulin-like growth factor binding protein-3 (IGFBP-3), interleukin-8 (IL-8), Pentraxin-3 (PTX-3), or Serpin F1. In contrast, the group of reduced factors included CXCL-4, CXCL-16, granulocyte macrophage colony-stimulating factor (GM-CSF), interleukin-1 beta (IL-1β), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), transforming growth factor-beta 1 (TGF-β1), and tissue factor as well as IGFBP-1 and IGFBP-2. However, protective, angiogenic, or proliferation-related factors such as FGF-4, heparin-binding epidermal growth factor (HB-EGF), hepatocyte growth factor (HGF), placental growth factor (PlGF), and prolactin were also reduced.
Fig. 6.
Proteomic secretome analysis of angiogenesis- and inflammation-related proteins in MSC-preconditioned media. MEMα was preconditioned by MSC for 2 days at 1% O2 (dark grey bars) or 21% O2 (light grey bars). Proteins related to islet protection and/or proliferation are underlined. Angiostatic and inflammation-related substances are indicated by dotted and grey arrows, respectively. Data are from six samples of preconditioned media and were normalized to native MEMα; *P < 0.05 for normoxic versus hypoxic preconditioning. MEMα: minimum essential medium-alpha; MSC: mesenchymal stem cells.
The amount of secreted proteins partially differed between 21% O2–2d MEM and 1% O2–2d MEM, particularly regarding angiogenin, FGF-7, and VEGF-A. Nevertheless, Fig. 6 demonstrates a similar pattern of MSC secretome release irrespective of whether normoxic or hypoxic atmosphere had been used for MSC culture.
Discussion
Our findings confirm previous observations that MSC-secreted proteins protect the integrity of isolated islets even when exposed to a hypoxic environment. However, the vast majority of these previous studies have been conducted with rodent islets41. The present study is one of the few to demonstrate this with human islets and is the first study to demonstrate the beneficial effect of media preconditioned by human adipose tissue-derived MSC on human islets exposed to hypoxia that is similar to the marginal oxygen supply encountered during pretransplant clinical islet culture, islet shipment, and after intraportal islet transplantation. The experimental design of this study purposely investigated various protocols for preconditioning to maximize reduction of hypoxia-induced damage as the key determinant for survival and integrity of cultured and/or transplanted islets. Importantly, a decreased expression of proapoptotic key markers, as well as increased islet survival and viability, were observed when human islets were suspended in MSC-preconditioned media and cultured in normoxic atmosphere. With regard to in vitro function, apoptosis, and viability of human islets, only minor differences were noted between media preconditioned during either normoxic or hypoxic MSC culture. The major difference between normoxic and hypoxic preconditioning protocols was an alteration of islet morphology into a spindle-like shape observed when culture in medium from hypoxic MSC was performed. The present secretome profiling was focused on angiogenesis- and inflammation-related proteins, thus analyzing a limited range of the entire MSC secretome. We therefore have to speculate whether a nonspecific growth factor, that is particularly produced and secreted by hypoxic MSC, may induce dedifferentiation of the islet phenotype as suggested by the nontypical shape of islets cultured in 1% O2–2d MEM.
Elements of the MSC secretome, that can theoretically be considered for islet dedifferentiation, are angiogenin, endoglin, and FGF-7, which are secreted in substantially higher quantities when MSC are exposed to hypoxia. Angiogenin plays a major role in endothelial cell proliferation and in the formation of tube-like structures42, but it is unknown whether it has an impact on islets apart from its angiogenic properties. The proliferative and insulinotropic effect of FGF-7 is documented only in human beta cells from fetal origin43,44. Therefore, endoglin might be one of the most likely candidates to be involved in downregulation of insulin gene expression. Serving as receptor for TGF-β45, endoglin has the potential to promote the inhibitory effect of TGF-β on insulin gene transcription46. On the other hand, TGF-β belongs to the downregulated secretome group.
Despite the change in morphology, islets still positively stained with dithizone. In addition, PDX-1 and insulin mRNA expression could not confirm a change of the β-cell phenotype when compared with islets cultured in native MEMα. Interestingly, the gene expression of PDX-1 and insulin was significantly higher after culture in 21% O2–2d MEM when compared with 1% O2–2d MEM suggesting a specific stimulatory effect on beta cells rather than dedifferentiation of islets. In this context, we have to underline the disadvantage of the present approach to focus solely on the gene expression of PDX-1 and insulin. PDX-1 is a major regulator of numerous genes expressed in β-cells47. Its tight control of insulin transcription and translation means that PDX-1 has been used as a specific marker of human mature β-cells48,49 in the majority of β-cell profiling studies performed so far50. From this perspective, an altered expression of PDX-1 and insulin could reflect an adaptation to metabolic demands, rather than a change in islet cell phenotype. Because PDX-1 is a significant but not an exclusive regulator gene that controls viable β-cell functions in collaboration with other transcription factors51, a simultaneous up- or downregulation of a broad range of genes can be observed52,53. To address this dilemma in the future, subsequent studies should include the assessment of multiple β-cell-related genes54.
The morphological alterations we exclusively observed in islets cultured in normoxic atmosphere may also reflect the major physiological stresses that islets experience in hypoxic atmosphere. The lack of oxygen prevents efficient mitochondrial ATP generation and results in a rapid depletion of resources that would be essentially needed for remodeling of islet morphology55.
As noted with normoxic human islet culture, only marginal differences were observed after islet culture in hypoxia comparing the protective effect of MSC media preconditioned at normoxia or hypoxia. The attempt to increase the protective capability of preconditioned medium by prolonged MSC culture did not result in any improvement of islet survival after hypoxia. Moreover, a significant decrease of all quality parameters assessed was observed, when islets were cultured in 21% O2–5d MEM. So far, we can only speculate whether a less frequent medium change during MSC expansion most likely results in an accumulation of metabolic waste products that affect islet integrity or whether the amount of proinflammatory mediators reaches a toxic level as discussed below.
The proteomic secretome profiling revealed that the large majority of the proteins was secreted at a very similar pattern irrespective of the atmosphere used for MSC culture. Increased secretion, which was observed in approximately half of all factors assessed, was significantly higher in 8 out of 20 (40%) cell products when MSC were stimulated by hypoxia, while 3 out of 20 (15%) factors were significantly enhanced after normoxic MSC culture. One increased member of the latter group is Activin A. In contrast to the other two compounds Serpin-E1 and TIMP-4, characterized by angiostatic properties56,57, Activin A has been shown to have a stimulatory effect on insulin secretion of adult human islets58.
As expected, VEGF was detected in significantly higher levels among the hypoxia-stimulated proteins (1% O2–2d MEM) in comparison with normoxia-preconditioned medium (21% O2–2d MEM). Together with angiogenin, it is a decisive factor for revascularization of islets42,59,60. In this context it is important to consider recent findings, which demonstrate that newly formed vessels in transplanted rat islets occupy approximately 20% of the intraislet volume61. This may explain why the MSC-induced neo-formation of vessels in human islets results in increased islet size, therefore contributing to increased yield of IEQ32. Apart from its role as proangiogenic factor, VEGF seems to have an islet-protective effect that is independent of revascularization and may contribute to human islet survival during hypoxia62. The same applies to Angiopoietin-1 that mediates protective effects on cytokine-induced apoptosis in isolated islets63.
Approximately half of the angiogenesis- and inflammation-related secretome factors were decreased in preconditioned media compared with the native medium. Among these are biomarkers with proven growth or angiogenic benefits for human adult or fetal islet tissue. These include FGF-4, HB-EGF, HGF, GM-CSF, glial-derived neurotrophic factor (GDNF), PDGF-AB/BB, or prolactin64–70. Nevertheless, several proinflammatory mediators such as CXCL-4, CXCL-16, MCP-1, MIP-1α, tissue factor, and IL-1β71–73 but also potent angiostatic substances like angiostatin, endostatin, PDGF-AA, or Serpin-E1 and Serpin-F156,74 decreased as well during MSC culture. It is quite likely that the reduction of proinflammatory or angiostatic proteins may have positive implications for early survival of intraportally transplanted islets. The reasons for the reduction of certain proteins are unknown and may be attributed to degradation or to metabolization through expanding MSC.
The decline of proinflammatory mediators in preconditioned media could not be confirmed for all proteins of this category. Besides PTX-3, involved in tissue repair but also identified as activator and regulator of the complement system75, IL-8 is another chemokine that was massively increased in preconditioned media. Together with angiogenin and FGF-7, IL-8 is among the top three of secreted MSC proteins. Although IL-8 belongs to the group of proangiogenic chemokines that can stimulate neovascularization in isolated islets76, it is involved, together with MCP-1, in proinflammatory pathways in transplanted islets contributing to early loss of islet viability and post-transplant function77. The ambivalence of IL-8 is shared by tissue inhibitor of metalloproteinases-1 (TIMP-1) and TIMP-4, proteins with antiangiogenic attributes57 that were secreted in relatively large amounts by hypoxic as well as normoxic MSC. Apart from its angiostatic properties, TIMP-1 has been characterized as an antiapoptotic compound protecting rat islets from cytokine-induced dysfunction and cell death78. The properties of IL-8, TIMP-1, and TIMP-4 seem to be representative for the ambivalent character of MSC-preconditioned media as reflected by the secretome profile. The potential problems related to this ambiguity do not only apply to acellular preconditioned media but also to the approach to perform MSC-islet cotransplantation in patients with type 1 diabetes.
In conclusion, the findings of this study suggest that media preconditioned by human adipose tissue-derived MSC exert a beneficial effect on survival and function of hypoxic human islets irrespective of the atmosphere used for MSC preconditioning. Proteomic profiling of preconditioned media revealed the heterogenicity of the secretome comprising angiogenic and antiapoptotic as well as angiostatic or proinflammatory mediators. The ambivalence of MSC-secreted proteins is a substantial obstacle that has to be overcome before the application of MCS-derived products or MSC-islet cotransplantation can be translated into clinical practice. Further approaches should aim to identify and to eliminate potentially detrimental factors to enable the production of advanced clinical grade islet culture media with higher protective qualities as an initial step for future clinical applications.
Acknowledgments
The authors would like to thank all members of the Oxford Human Islet Isolation Team for isolating and providing human islets for this study.
Footnotes
Ethical Approval: Processing and use of lipoaspirates for MSC isolation has been approved by the local ethical committee of the Oslo University Hospital (2014/838). Human islet isolation and utilization of isolated islets for research purposes has been approved by the local institutional review board of the Nuffield Department of Surgical Sciences at the Oxford University (09/H0605/2).
Statement of Human and Animal Rights: This article does not contain any studies with human subjects or animals.
Statement of Informed Consent: Lipoaspirates were collected from female donors after donors signed informed consent. Pancreases from human multiorgan donors were retrieved and processed after informed consent was given by the relatives of the donor.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the European Union’s Cooperation Programme FP7 (HEALTH-F2-2012-305746). Isolation of human islets for research was supported by the Oxford NIHR Biomedical Research Centre and a Juvenile Diabetes Research Foundation (JDRF) award (31-2008-617). Members of the Oxford islet isolation team are funded by the Diabetes Research and Wellness Foundation (DRWF).
ORCID iD: Daniel Brandhorst  https://orcid.org/0000-0002-2932-6595
https://orcid.org/0000-0002-2932-6595
References
- 1. Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, Kaufman DB. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2016;39(7):1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. CITR. Ninth annual report of the Collaborative Islet Transplant Registry (CITR). 2016:1–204. [Google Scholar]
- 3. Cross SE, Vaughan RH, Willcox AJ, McBride AJ, Abraham AA, Han B, Johnson JD, Maillard E, Bateman PA, Ramracheya RD, Rorsman P. Key matrix proteins within the pancreatic islet basement membrane are differentially digested during human islet isolation. Am J Transplant. 2017;17(2):451–461. [DOI] [PubMed] [Google Scholar]
- 4. Giuliani M, Moritz W, Bodmer E, Dindo D, Kugelmeier P, Lehmann R, Gassmann M, Groscurth P, Weber M. Central necrosis in isolated hypoxic human pancreatic islets: evidence for postisolation ischemia. Cell Transplant. 2005;14(1):67–76. [DOI] [PubMed] [Google Scholar]
- 5. Avgoustiniatos ES, Hering BJ, Rozak PR, Wilson JR, Tempelman LA, Balamurugan AN, Welch DP, Weegman BP, Suszynski TM, Papas KK. Commercially available gas-permeable cell culture bags may not prevent anoxia in cultured or shipped islets. Transplant Proc. 2008;40(2):395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Avgoustiniatos ES, Scott WE, Suszynski TM, Schuurman HJ, Nelson RA, Rozak PR, Mueller KR, Balamurugan AN, Ansite JD, Fraga DW, Friberg AS. Supplements in human islet culture: human serum albumin is inferior to fetal bovine serum. Cell Transplant. 2012;21(12):2805–2814. [DOI] [PubMed] [Google Scholar]
- 7. Kerr-Conte J, Vandewalle B, Moerman E, Lukowiak B, Gmyr V, Arnalsteen L, Caiazzo R, Sterkers A, Hubert T, Vantyghem MC, Pattou F. Upgrading pretransplant human islet culture technology requires human serum combined with media renewal. Transplantation 2010;89(9):1154–1160. [DOI] [PubMed] [Google Scholar]
- 8. Heldring N, Mager I, Wood MJA, Le Blanc K, Andaloussi SEL. Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Human Gene Therapy. 2015;26(8):506–517. [DOI] [PubMed] [Google Scholar]
- 9. Boumaza I, Srinivasan S, Witt WT, Feghali-Bostwick C, Dai Y, Garcia-Ocana A, Feili-Hariri M. Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. J Autoimmun. 2009;32(1):33–42. [DOI] [PubMed] [Google Scholar]
- 10. Ben Nasr M, Vergani A, Avruch J, Liu L, Kefaloyianni E, D’Addio F, Tezza S, Corradi D, Bassi R, Valderrama-Vasquez A, Usuelli V. Co-transplantation of autologous MSCs delays islet allograft rejection and generates a local immunoprivileged site. Acta Diabetol. 2015;52(5):917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li L, Li F, Gao F, Yang Y, Liu Y, Guo P, Li Y. Transplantation of mesenchymal stem cells improves type 1 diabetes mellitus. Cell Tissue Res. 2016;364(2):345–355. [DOI] [PubMed] [Google Scholar]
- 12. Xin Y, Jiang X, Wang Y, Su X, Sun M, Zhang L, Tan Y, Wintergerst KA, Li Y, Li Y. Insulin-producing cells differentiated from human bone marrow mesenchymal stem cells in vitro ameliorate streptozotocin-induced diabetic hyperglycemia. PLoS One. 2016;11(1):e0145838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jung EJ, Kim SC, Wee YM, Kim YH, Choi MY, Jeong SH, Lee J, Lim DG, Han DJ. Bone marrow-derived mesenchymal stromal cells support rat pancreatic islet survival and insulin secretory function in vitro. Cytotherapy. 2011;13(1):19–29. [DOI] [PubMed] [Google Scholar]
- 14. Rahavi H, Hashemi SM, Soleimani M, Mohammadi J, Tajik N. Adipose tissue-derived mesenchymal stem cells exert in vitro immunomodulatory and beta cell protective functions in streptozotocin-induced diabetic mice model. J Diabetes Res. 2015;16:878535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoshimatsu G, Sakata N, Tsuchiya H, Minowa T, Takemura T, Morita H, Hata T, Fukase M, Aoki T, Ishida M, Motoi F. The co-transplantation of bone marrow derived mesenchymal stem cells reduced inflammation in intramuscular islet transplantation. PLoS One. 2015;10(2):e0117561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu Y, Jin X, Chen Y, Li S, Yuan Y, Mai G, Tian B, Long D, Zhang J, Zeng L, Li Y. Mesenchymal stem cells protect islets from hypoxia/reoxygenation-induced injury. Cell Biochem Funct. 2010;28(8):637–643. [DOI] [PubMed] [Google Scholar]
- 17. Park KS, Kim YS, Kim JH, Choi B, Kim SH, Tan AH, Lee MS, Lee MK, Kwon CH, Joh JW, Kim SJ. Trophic molecules derived from human mesenchymal stem cells enhance survival, function, and angiogenesis of isolated islets after transplantation. Transplantation. 2010;89(5):509–517. [DOI] [PubMed] [Google Scholar]
- 18. Shin JY, Jeong JH, Han J, Bhang SH, Jeong GJ, Haque MR, Al-Hilal TA, Noh M, Byun Y, Kim BS. Transplantation of heterospheroids of islet cells and mesenchymal stem cells for effective angiogenesis and antiapoptosis. Tissue Eng Part A. 2015;21(5–6):1024–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cao XK, Li R, Sun W, Ge Y, Liu BL. Co-combination of islets with bone marrow mesenchymal stem cells promotes angiogenesis. Biomed Pharmacother. 2016;78:156–164. [DOI] [PubMed] [Google Scholar]
- 20. Fumimoto Y, Matsuyama A, Komoda H, Okura H, Lee CM, Nagao A, Nishida T, Ito T, Sawa Y. Creation of a rich subcutaneous vascular network with implanted adipose tissue-derived stromal cells and adipose tissue enhances subcutaneous grafting of islets in diabetic mice. Tissue Eng Part C. 2009;15(3):437–444. [DOI] [PubMed] [Google Scholar]
- 21. Berman DM, Willman MA, Han D, Kleiner G, Kenyon NM, Cabrera O, Karl JA, Wiseman RW, O’Connor DH, Bartholomew AM, Kenyon NS. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes. 2010;59(10):2558–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hirabaru M, Kuroki T, Adachi T, Kitasato A, Ono S, Tanaka T, Matsushima H, Sakai Y, Soyama A, Hidaka M, Yamanouchi K. A method for performing islet transplantation using tissue-engineered sheets of islets and mesenchymal stem cells. Tissue Eng Part C. 2015;21(12):1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. NIH. Clinical trials with mesenchymal stem cells: an update. 2019. https://clinicaltrials.gov [DOI] [PubMed]
- 24. Ben-David U, Mayshar Y, Benvenisty N. Large-scale analysis reveals acquisition of lineage-specific chromosomal aberrations in human adult stem cells. Cell Stem Cell. 2011;9(2):97–102. [DOI] [PubMed] [Google Scholar]
- 25. Pan Q, Fouraschen SM, de Ruiter PE, Dinjens WN, Kwekkeboom J, Tilanus HW, van der Laan LJ. Detection of spontaneous tumorigenic transformation during culture expansion of human mesenchymal stromal cells. Exp Biol Med. 2014;239(1):105–115. [DOI] [PubMed] [Google Scholar]
- 26. Rackham CL, Dhadda PK, Le Lay AM, King AJ, Jones PM. Preculturing islets with adipose-derived mesenchymal stromal cells is an effective strategy for improving transplantation efficiency at the clinically preferred intraportal site. Cell Med. 2014;7(1):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rackham CL, Dhadda PK, Chagastelles PC, Simpson SJ, Dattani AA, Bowe JE, Jones PM, King AJ. Pre-culturing islets with mesenchymal stromal cells using a direct contact configuration is beneficial for transplantation outcome in diabetic mice. Cytotherapy. 2013;15(4):449–459. [DOI] [PubMed] [Google Scholar]
- 28. Yamada S, Shimada M, Utsunomiya T, Ikemoto T, Saito Y, Morine Y, Imura S, Mori H, Arakawa Y, Kanamoto M, Iwahashi S. Trophic effect of adipose tissue-derived stem cells on porcine islet cells. J Surg Res. 2014;187(2):667–672. [DOI] [PubMed] [Google Scholar]
- 29. Park KS, Kim YS, Kim JH, Choi BK, Kim SH, Oh SH, Ahn YR, Lee MS, Lee MK, Park JB, Kwon CH. Influence of human allogenic bone marrow and cord blood-derived mesenchymal stem cell secreting trophic factors on ATP (adenosine-5’-triphosphate)/ADP (adenosine-5’-diphosphate) ratio and insulin secretory function of isolated human islets from cadaveric donor. Transplant Proc. 2009;41(9):3813–3818. [DOI] [PubMed] [Google Scholar]
- 30. Luo L, Badiavas E, Luo JZ, Maizel A. Allogeneic bone marrow supports human islet beta cell survival and function over six months. Biochem Biophys Res Commun. 2007;361(4):859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johansson U, Rasmusson I, Niclou SP, Forslund N, Gustavsson L, Nilsson B, Korsgren O, Magnusson PU. Formation of composite endothelial cell-mesenchymal stem cell islets: a novel approach to promote islet revascularization. Diabetes. 2008;57(9):2393–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luo JZ, Xiong F, Al-Homsi AS, Roy T, Luo LG. Human BM stem cells initiate angiogenesis in human islets in vitro. Bone Marrow Transplant. 2011;46(8):1128–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luo JZ, Xiong F, Al-Homsi AS, Ricordi C, Luo L. Allogeneic bone marrow cocultured with human islets significantly improves islet survival and function in vivo. Transplantation. 2013;95(6):801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buitinga M, Janeczek Portalska K, Cornelissen DJ, Plass J, Hanegraaf M, Carlotti F, de Koning E, Engelse M, van Blitterswijk C, Karperien M, Van Apeldoorn A. Coculturing human islets with proangiogenic support cells to improve islet revascularization at the subcutaneous transplantation site. Tissue Eng Part A. 2016;22(3–4):375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arzouni AA, Vargas-Seymour A, Rackham CL, Dhadda P, Huang GC, Choudhary P, Nardi N, King AJF, Jones PM. Mesenchymal stromal cells improve human islet function through released products and extracellular matrix. Clin Sci (Lond). 2017;131(23):2835–2845. [DOI] [PubMed] [Google Scholar]
- 36. Ichii H, Sakuma Y, Pileggi A, Fraker C, Alvarez A, Montelongo J, Szust J, Khan A, Inverardi L, Naziruddin B, Levy MF. Shipment of human islets for transplantation. Am J Transplant. 2007;7(4):1010–1020. [DOI] [PubMed] [Google Scholar]
- 37. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 38. Ricordi C, Gray DW, Hering BJ, Kaufman DB, Warnock GL, Kneteman NM, Lake SP, London NJ, Socci C, Alejandro R, Zeng Y. Islet isolation assessment in man and large animals. Acta Diabetol Lat. 1990;27(3):185–195. [DOI] [PubMed] [Google Scholar]
- 39. London NJ, Contractor H, Lake SP, Aucott GC, Bell PR, James RF. A fluorometric viability assay for single human and rat islets. Horm Metab Res Suppl. 1990;25:82–87. [PubMed] [Google Scholar]
- 40. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 41. Dietrich I, Crescenzi A, Chaib E, D’Albuquerque LA. Trophic effects of adipose derived stem cells on Langerhans islets viability--review. Transplant Rev (Orlando). 2015;29(3):121–126. [DOI] [PubMed] [Google Scholar]
- 42. Shestenko OP, Nikonov SD, Mertvetsov NP. Angiogenin and its functions in angiogenesis [in Russian]. Mol Biol (Mosk). 2001;35(3):294–314. [PubMed] [Google Scholar]
- 43. Otonkoski T, Beattie GM, Rubin JS, Lopez AD, Baird A, Hayek A. Hepatocyte growth factor/scatter factor has insulinotropic activity in human fetal pancreatic cells. Diabetes. 1994;43(7):947–953. [DOI] [PubMed] [Google Scholar]
- 44. Movassat J, Beattie GM, Lopez AD, Portha B, Hayek A. Keratinocyte growth factor and beta-cell differentiation in human fetal pancreatic endocrine precursor cells. Diabetologia. 2003;46(6):822–829. [DOI] [PubMed] [Google Scholar]
- 45. Ollauri-Ibanez C, Lopez-Novoa JM, Pericacho M. Endoglin-based biological therapy in the treatment of angiogenesis-dependent pathologies. Expert Opin Biol Ther. 2017;17(9):1053–1063. [DOI] [PubMed] [Google Scholar]
- 46. Lin HM, Lee JH, Yadav H, Kamaraju AK, Liu E, Zhigang D, Vieira A, Kim SJ, Collins H, Matschinsky F, Harlan DM. Transforming growth factor-beta/Smad3 signaling regulates insulin gene transcription and pancreatic islet beta-cell function. J Biol Chem. 2009;284(18):12246–12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Keller DM, McWeeney S, Arsenlis A, Drouin J, Wright CV, Wang H, Wollheim CB, White P, Kaestner KH, Goodman RH. Characterization of pancreatic transcription factor Pdx-1 binding sites using promoter microarray and serial analysis of chromatin occupancy. J Biol Chem. 2007;282(44):32084–32092. [DOI] [PubMed] [Google Scholar]
- 48. Benner C, van der Meulen T, Caceres E, Tigyi K, Donaldson CJ, Huising MO. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics. 2014;15(1):620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu JS, Hebrok M. All mixed up: defining roles for beta-cell subtypes in mature islets. Genes Dev. 2017;31(3):228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vieira A, Druelle N, Avolio F, Napolitano T, Navarro-Sanz S, Silvano S, Collombat P. Beta-cell replacement strategies: the increasing need for a “beta-Cell Dogma”. Front Genet. 2017;8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Aramata S, Han SI, Yasuda K, Kataoka K. Synergistic activation of the insulin gene promoter by the beta-cell enriched transcription factors MafA, Beta2, and Pdx1. Biochim Biophys Acta. 2005;1730(1):41–46. [DOI] [PubMed] [Google Scholar]
- 52. Gao T, McKenna B, Li C, Reichert M, Nguyen J, Singh T, Yang C, Pannikar A, Doliba N, Zhang T, Stoffers DA. Pdx1 maintains beta cell identity and function by repressing an alpha cell program. Cell Metab. 2014;19(2):259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gutierrez GD, Bender AS, Cirulli V, Mastracci TL, Kelly SM, Tsirigos A, Kaestner KH, Sussel L. Pancreatic beta cell identity requires continual repression of non-beta cell programs. J Clin Invest. 2017;127(1):244–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Negi S, Jetha A, Aikin R, Hasilo C, Sladek R, Paraskevas S. Analysis of beta-cell gene expression reveals inflammatory signaling and evidence of dedifferentiation following human islet isolation and culture. PLoS One. 2012;7(1):e30415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hellman B, Idahl LA, Danielsson A. Adenosine triphosphate levels of mammalian pancreatic B cells after stimulation with glucose and hypoglycemic sulfonylureas. Diabetes. 1969;18(8):509–516. [DOI] [PubMed] [Google Scholar]
- 56. Tillmar L, Welsh N. In vitro cultured rat islets express genes that both prevent and promote angiogenesis. JOP. 2004;5(2):81–91. [PubMed] [Google Scholar]
- 57. Mannello F, Gazzanelli G. Tissue inhibitors of metalloproteinases and programmed cell death: conundrums, controversies and potential implications. Apoptosis. 2001;6(6):479–482. [DOI] [PubMed] [Google Scholar]
- 58. Florio P, Luisi S, Marchetti P, Lupi R, Cobellis L, Falaschi C, Sugino H, Navalesi R, Genazzani AR, Petraglia F. Activin A stimulates insulin secretion in cultured human pancreatic islets. J Endocrinol Invest. 2000;23(4):231–234. [DOI] [PubMed] [Google Scholar]
- 59. Lai Y, Schneider D, Kidszun A, Hauck-Schmalenberger I, Breier G, Brandhorst D, Brandhorst H, Iken M, Brendel MD, Bretzel RG, Linn T. Vascular endothelial growth factor increases functional beta-cell mass by improvement of angiogenesis of isolated human and murine pancreatic islets. Transplantation. 2005;79(11):1530–1536. [DOI] [PubMed] [Google Scholar]
- 60. Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J, Baldwin HS. Pancreatic islet production of vascular endothelial growth factor--a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55(11):2974–2985. [DOI] [PubMed] [Google Scholar]
- 61. Vlahos AE, Cober N, Sefton MV. Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets. Proc Natl Acad Sci USA. 2017;114(35):9337–9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cross SE, Richards SK, Clark A, Benest AV, Bates DO, Mathieson PW, Johnson PR, Harper SJ, Smith RM. Vascular endothelial growth factor as a survival factor for human islets: effect of immunosuppressive drugs. Diabetologia. 2007;50(7):1423–1432. [DOI] [PubMed] [Google Scholar]
- 63. Su D, Zhang N, He J, Qu S, Slusher S, Bottino R, Bertera S, Bromberg J, Dong HH. Angiopoietin-1 production in islets improves islet engraftment and protects islets from cytokine-induced apoptosis. Diabetes. 2007;56(9):2274–2283. [DOI] [PubMed] [Google Scholar]
- 64. Arany E, Hill DJ. Ontogeny of fibroblast growth factors in the early development of the rat endocrine pancreas. Pediatr Res. 2000;48(3):389–403. [DOI] [PubMed] [Google Scholar]
- 65. Beattie GM, Montgomery AM, Lopez AD, Hao E, Perez B, Just ML, Lakey JR, Hart ME, Hayek A. A novel approach to increase human islet cell mass while preserving beta-cell function. Diabetes. 2002;51(12):3435–3439. [DOI] [PubMed] [Google Scholar]
- 66. Zarrouki B, Benterki I, Fontes G, Peyot ML, Seda O, Prentki M, Poitout V. Epidermal growth factor receptor signaling promotes pancreatic beta-cell proliferation in response to nutrient excess in rats through mTOR and FOXM1. Diabetes. 2014;63(3):982–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Contreras JL, Smyth CA, Eckstein C, Bilbao G, Thompson JA, Young CJ, Eckhoff DE. Peripheral mobilization of recipient bone marrow-derived endothelial progenitor cells enhances pancreatic islet revascularization and engraftment after intraportal transplantation. Surgery. 2003;134(2):390–398. [DOI] [PubMed] [Google Scholar]
- 68. Abadpour S, Gopel SO, Schive SW, Korsgren O, Foss A, Scholz H. Glial cell-line derived neurotrophic factor protects human islets from nutrient deprivation and endoplasmic reticulum stress induced apoptosis. Sci Rep. 2017;7(1):1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen H, Gu X, Liu Y, Wang J, Wirt SE, Bottino R, Schorle H, Sage J, Kim SK. PDGF signalling controls age-dependent proliferation in pancreatic beta-cells. Nature. 2011;478(7369):349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Terra LF, Garay-Malpartida MH, Wailemann RA, Sogayar MC, Labriola L. Recombinant human prolactin promotes human beta cell survival via inhibition of extrinsic and intrinsic apoptosis pathways. Diabetologia. 2011;54(6):1388–1397. [DOI] [PubMed] [Google Scholar]
- 71. Piemonti L, Leone BE, Nano R, Saccani A, Monti P, Maffi P, Bianchi G, Sica A, Peri G, Melzi R, Aldrighetti L. Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes. 2002;51(1):55–65. [DOI] [PubMed] [Google Scholar]
- 72. Johansson H, Lukinius A, Moberg L, Lundgren T, Berne C, Foss A, Felldin M, Kallen R, Salmela K, Tibell A, Tufveson G. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54(6):1755–1762. [DOI] [PubMed] [Google Scholar]
- 73. Loweth AC, Williams GT, James RF, Scarpello JH, Morgan NG. Human islets of Langerhans express Fas ligand and undergo apoptosis in response to interleukin-1beta and Fas ligation. Diabetes. 1998;47(5):727–732. [DOI] [PubMed] [Google Scholar]
- 74. Johansson A, Olerud J, Johansson M, Carlsson PO. Angiostatic factors normally restrict islet endothelial cell proliferation and migration: implications for islet transplantation. Transpl Int. 2009;22(12):1182–1188. [DOI] [PubMed] [Google Scholar]
- 75. Doni A, Garlanda C, Mantovani A. Innate immunity, hemostasis and matrix remodeling: PTX3 as a link. Semin Immunol. 2016;28(6):570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Movahedi B, Gysemans C, Jacobs-Tulleneers-Thevissen D, Mathieu C, Pipeleers D. Pancreatic duct cells in human islet cell preparations are a source of angiogenic cytokines interleukin-8 and vascular endothelial growth factor. Diabetes. 2008;57(8):2128–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Melzi R, Mercalli A, Sordi V, Cantarelli E, Nano R, Maffi P, Sitia G, Guidotti LG, Secchi A, Bonifacio E, Piemonti L. Role of CCL2/MCP-1 in islet transplantation. Cell Transplant. 2010;19(8):1031–1046. [DOI] [PubMed] [Google Scholar]
- 78. Han X, Sun Y, Scott S, Bleich D. Tissue inhibitor of metalloproteinase-1 prevents cytokine-mediated dysfunction and cytotoxicity in pancreatic islets and beta-cells. Diabetes. 2001;50(5):1047–1055. [DOI] [PubMed] [Google Scholar]