Presentation
A 32-year-old female intravenous drug abuser with hepatitis C infection presented to our institution complaining of 1 day of worsening shortness of breath. Coagulation panel was within normal limits and troponin-I < 0.01 ng/mL. Chest x-ray showed bilateral infiltrates. Baseline 12-lead electrocardiogram showed sinus tachycardia with no acute ischemic changes (Figure 1 ). Transthoracic echocardiogram demonstrated normal biventricular function. Ultimately, SARS-CoV-2 RT-PCR was detected in a nasal specimen. She rapidly developed acute hypoxemic respiratory failure due to COVID-19 requiring intubation. Basic metabolic panel at the time demonstrated hypokalemia to 3.5 mEq/L and hypernatremia to 149 mEq/L. She was started on heparin 5000 units 3 times daily for thromboembolism prophylaxis and transferred to the intensive care unit. On day 6 of admission, she self-extubated and developed pulseless electrical activity with return of spontaneous circulation within 10 minutes of cardiopulmonary resuscitation. She was reintubated and started on vasopressors with targeted temperature management.
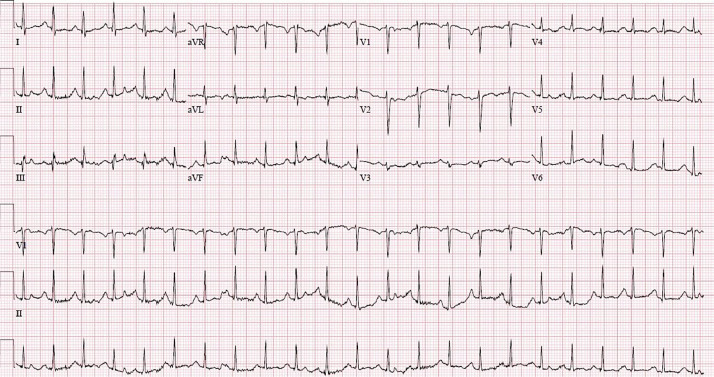
Figure 1.
Baseline electrocardiogram upon admission showing sinus tachycardia.
Assessment
Immediate resuscitation panel showed respiratory acidosis with metabolic alkalosis, hypokalemia to 2.7 mEq/L, and hypernatremia to 164 mEq/L. Troponin-I was elevated to 1.32 ng/mL. After normalization of electrolyte and pH derangements, a post-cardiac arrest electrocardiogram was performed (Figure 2 ), which showed a triangular QRS-ST-T waveform with an amplitude > 1mV in leads I, II, and III, and aVL, also known as a “shark fin-like” pattern. Repeat echocardiogram now showed a moderately depressed left ventricular ejection fraction of 35%-40% with severe hypokinesis of only the mid-ventricular walls.
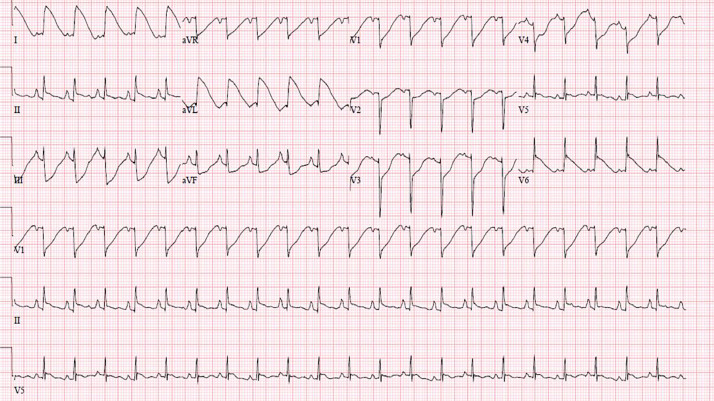
Figure 2.
Post-cardiac arrest electrocardiogram showing a “shark fin-like” pattern in leads I, II, III, and aVL.
Diagnosis
The differential diagnosis included post-cardiac arrest ischemia with myopathy, mid-ventricular variant Takotsubo cardiomyopathy, acute coronary thrombosis, and extensive myocarditis. The “shark fin-like” pattern has traditionally been associated with acute coronary syndromes caused by a complete occlusion of a major epicardial coronary artery.
Management
Interventional cardiology evaluation recommended against emergent coronary angiography given her critically ill clinical picture. She was started on aspirin and a bolus of unfractionated heparin 60 units/kg followed by 12 units/kg/hour infusion with appropriate partial thromboplastin time (PTT) response. Troponins subsequently trended downward at 24 hours post-arrest and a repeat electrocardiogram demonstrated improvement of previous abnormal findings (Figure 3 ). Four days later, a repeat transthoracic echocardiogram showed resolution of left ventricular dysfunction with normalization of wall motion and left ventricular ejection fraction to 55%-60%. Unfortunately, new onset right ventricular dilation and dysfunction was instead noted, worrisome for right ventricular strain.
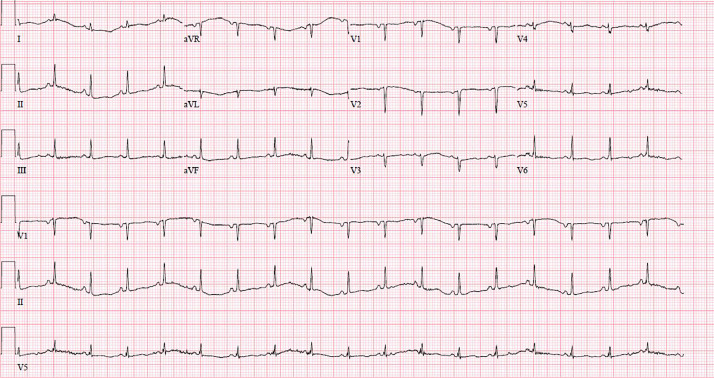
Figure 3.
Electrocardiogram performed 24-hours post-cardiac arrest with triangular pattern resolution after antiplatelet and anticoagulation initiation.
Subsequent computed tomography angiography of the chest showed segmental and sub-segmental pulmonary emboli despite systemic anticoagulation and antiplatelet therapy, consistent with an underlying severe hypercoagulable state. Despite all interventions, her clinical condition deteriorated, resulting in transition to palliative care management strategy. She ultimately succumbed to her disease.
The “shark fin-like” pattern is an uncommon electrocardiographic finding that typically results from the fusion of the QRS complex, ST segment, and T wave. Similar patterns have been reproduced in experimental animal models after ligation of a large coronary artery.1 Furthermore, mounting evidence has associated its presence with increased risk of ventricular fibrillation and cardiogenic shock.2 Although traditionally associated with ST-segment elevation in the setting of acute coronary ischemia, this unusual tracing has been found in a small percentage of Takotsubo cardiomyopathy patients, with a higher incidence of hemodynamic instability and shock.3 A proposed mechanism of ST-segment elevation in Takotsubo cardiomyopathy is an endo-epicardial wall tension mismatch with activation of the stretch activated channels eventually leading to repolarization transmural gradient, and thence to ST-segment elevation.3
Takotsubo cardiomyopathy is a syndrome of transient and acute regional wall motion abnormality with non-obstructive coronary arteries in the absence of pheochromocytoma or myocarditis. Although the pathophysiology of Takotsubo cardiomyopathy is not yet fully understood, it is believed to be related to a stress-induced catecholamine surge several times higher than those in patients with ST-segment elevation acute coronary syndrome.4 The most common presentation is that of apical ballooning of the left ventricle during systole; however, there are additional morphological variants including the basal, focal, mid-ventricular, biventricular (apical and right ventricle), and isolated right ventricular focal wall motion abnormalities.5
Although there are reports of confirmed Takotsubo cardiomyopathy in patients with COVID-19,6 absence of coronary angiography makes definitive diagnosis challenging. The above-mentioned dynamic ST-segment changes in the setting of new and dynamic mid-wall hypokinesis may suggest a mid-ventricular variant Takotsubo cardiomyopathy. Although ischemia from acute coronary thrombosis cannot be excluded without a coronary angiogram, the pattern of hypokinesis on echocardiogram was not in keeping with a coronary vascular territory (or territories if more than one artery were involved). Severe acute respiratory syndrome coronavirus 2 infection has been associated with increased risk for arterial and venous thromboembolic complications. Prompt resolution of regional wall motion abnormalities after unfractionated heparin and newly developed pulmonary embolism do not allow us to exclude the possibility of coronary thrombosis from an underlying hypercoagulable state due to COVID-19. Although diffuse demand ischemia and myopathy from prolonged resuscitation for cardiac arrest remains in the differential, we may have expected a more global pattern of hypokinesis instead of the focal mid-wall pattern seen in this patient.
Mathew D. Hutchinson, MD, Section Editor
Footnotes
Funding: None.
Conflict of Interest: None.
Authorship: All authors had access to the data and a role in writing this manuscript.
References
- 1.Ekmekci A, Toyoshima H, Kwoczynski JK, Nagaya T, Prinzmetal M. Angina pectoris: V. Giant R and receding S wave in myocardial ischemia and certain nonischemic conditions. Am J Cardiol. 1961;7(4):521–532. doi: 10.1016/0002-9149(61)90510-0. [DOI] [PubMed] [Google Scholar]
- 2.Cipriani A, D'Amico G, Brunello G, Perazzolo Marra M, Migliore F, Cacciavillani L, et al. The electrocardiographic “triangular QRS-ST-T waveform” pattern in patients with ST-segment elevation myocardial infarction: incidence, pathophysiology and clinical implications. J Electrocardiol. 2018;51(1):8–14. doi: 10.1016/j.jelectrocard.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Tarantino N, Santoro F, Brunetti ND. Triangular “shark fin-like” ST modification in takotsubo syndrome: challenging the concept of ST-elevation patterns without coronary occlusion? J Electrocardiol. 2018;51(6):1157–1158. doi: 10.1016/j.jelectrocard.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Lima G, Trejo-Paredes MC, Cardoso E. A mid-ventricular variant of Takotsubo cardiomyopathy: a case study and review of literature. Cureus. 2020;12(7):e9403. doi: 10.7759/cureus.9403. Accessed October 7, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hafeez Y, Gala K. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL: 2020 Jan. Mid-ventricular Takotsubo cardiomyopathy.https://www.ncbi.nlm.nih.gov/books/NBK557506/ [Updated August 10, 2020] Accessed October 7, 2020. [PubMed] [Google Scholar]
- 6.Giustino G, Croft LB, Oates CP, et al. Takotsubo cardiomyopathy in COVID-19. J Am Coll Cardiol. 2020;76(5):628–629. doi: 10.1016/j.jacc.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]