Abstract
Background
Tocilizumab has been proposed as a treatment for the new disease COVID-19, however, there is not enough scientific evidence to support this treatment. The objective of this study is to analyze whether the use of tocilizumab is associated with respiratory improvement and a shorter time to discharge in patients with COVID-19 and lung involvement.
Methods
Observational study on a cohort of 418 patients, admitted to three county hospitals in Catalonia (Spain). Patients admitted consecutively were included and followed until discharge or up to 30 days of admission. A sub-cohort of patients treated with tocilizumab and a sub-cohort of control patients were identified, matched by a large number of risk factors and clinical variables. Sub-cohorts were also matched by the number of other treatments for COVID-19 that patients received. Increment in SAFI (inspired oxygen fraction / saturation) 48 h after the start of treatment, and time to discharge, were the primary outcomes. Mortality, which was a secondary outcome, was analyzed in the total cohort, by using logistic regression models, adjusted by confounders.
Results
There were 96 patients treated with tocilizumab. Of them, 22 patients could be matched with an equivalent number of control patients. The increment in SAFI from baseline to 48 h of treatment, was not significantly different between groups (tocilizumab: −0.04; control: 0.09; p = 0.636). Also, no difference in time to discharge was found between the two sub-cohorts (logrank test: p = 0.472). The logistic regression models, did not show an effect of tocilizumab on mortality (OR 0.99; p = 0.990).
Conclusions
We did not find a clinical benefit associated with the use tocilizumab, in terms of respiratory function at 48 h of treatment, or time to discharge.
Keywords: Tocilizumab, Efficacy, Coronavirus, Sars-cov-2, Observational study, Cohort
1. Introduction
A novel coronavirus (severe acute respiratory syndrome coronavirus 2, SARS-CoV-2) causing an epidemic outbreak was reported in Wuhan (China) in December 2019 [1], being declared the outbreak as a pandemic on March 12, 2020 [2]. Although in most cases the symptomatology is mild (approx. 80%) [3], the extent and total number of people affected have resulted in a considerable number of fatalities [4].
A significant proportion of mortality in these patients is linked to severe respiratory deterioration (acute respiratory distress syndrome) caused by an excessive immune system activation, similar to the cytokine storm syndrome [5]. Interkeukin-6, which production may be increased by SARS-CoV-2, plays a potential role in this syndrome. As a result, it has been hypothesized that tocilizumab, a recombinant humanized anti-interleukin-6 receptor monoclonal antibody, may have a beneficial effect on COVID-16 lung disease, especially in the most severe cases [5,6].
So far, the results of a single clinical trial investigating the efficacy of Tocilizumab in COVID-19 have been published [7]. In this trial, no benefit was observed in time to intubation or death, although the authors acknowledge that the confidence intervals of the results are too wide to rule out some benefit or harm associated with the use of Tocilizumab in this disease.
Regarding observational studies, only a few have considered a robust adjustment method for confounders [[8], [9], [10], [11], [12], [13]], being the methods based on the Propensity Score, the most robust [14,15]. These methods, although more effective than multivariate analysis in reducing the probability of biases, decrease their matching performance when confounders change over time (time-dependent) [15], such as oxygen saturation or temperature, which are relevant in the case of the evaluation of patients affected by Covid- 19.
In this observational study on the efficacy of tocilizumab on COVID-19 hospitalized patients, we measured the improvement in lung function and time to discharge, as primary outcomes. We have used brute force algorithms to determine the moment of maximum similarity between groups, with respect to variables that change over time, which has allowed us to maximize the quality of matching between comparison groups.
2. Design and methods
2.1. Study design and settings
Patients analyzed in this study are part of the COVID-19 cohort of the Consorci Sanitari de l'Alt Penedès i Garraf (CSAPG). CSAPG is a consortium which brings together three regional hospitals in Catalonia, Spain, with a total reference population of 247,357 inhabitants from the regions of Alt Penedès and Garraf. The COVID-19 cohort of the CSAPG is made up of 418 consecutive patients admitted from March 12, 2020, to May 2, 2020. In this cohort, patients without respiratory symptoms and patients with negative nucleic acid test for SARS-CoV-2 in a sample obtained by nasal smear, were excluded.
The investigators of the COVID-19 research group of CSAPG collected all data from the electronic medical records. The investigators collected sociodemographic data, comorbidities, previous chronic treatments and symptoms of presentation of COVID-19 disease. Moreover, every day since admission, the researchers recorded the vital signs of the patients, the inspired fraction of oxygen (FiO2), the peripheral pulse-oximetry values and all treatments given during the hospital stay. FiO2 values were recorded from the clinical history; FiO2 was always considered to be 28% for low flow nasal cannula and 100% for non-rebreathing masks. All blood tests and chest radiographs performed were also included in the database. Data were collected with the aid of a structured form created in the OpenClinica, version 3.1. (Copyright © OpenClinica LLC and collaborators, Waltham, MA, USA), following a common procedure on which all participants were previously trained. During the data collection process, quality controls were established on the data collected, correcting errors and retraining researchers, when necessary.
2.2. Exposure and outcome variables
In the study hospitals, Tocilizumab was used both as a single dose of 600 mg intravenously, as well as in multidose regimens: initial dose of 600 mg, second dose of 400–600 mg at 12 h, and a third optional dose of 400 mg (depending on the evolution of the patient). For the purposes of this study, all patients who received at least one dose were considered exposed to tocilizumab.
The primary outcomes were time to discharge and SAFI at 48 h after start of treatment. SAFI is a parameter related with respiratory function, which was calculated using the following formula: saturation (%) / FiO2(%). As secondary outcomes we studied the SAFI in the first 96 h after treatment, C-reactive protein (CRP) in the first 96 h after the start of treatment, and mortality.
2.3. Matched features
In the statistical analysis, a subcohort of patients treated with tocilizumab was formed, and a control subcohort was matched with it (1:1 match ratio). The patients were matched by the following basal characteristics, which were identified as prognostic markers in previous bivariate analyses and multivariate models: sex, age, obesity, heart failure, chronic renal failure, and sleep apnea–hypopnea syndrome (SAHS). In addition the patients were matched by SAFI, vital signs (blood pressure, heart rate, and temperature), radiological involvement, CRP, and other COVID-19 treatments at the time of comparison. Vital signs were recorded by hospital nurses, as part of their routine daily practice, using the standard validated instruments of the hospital.
2.4. Matching time frame
Follow-up of each patient started the day the patient took the first dose of a study drug. Follow-up of each control started the day after admission on which SAFI, vital signs (blood pressure, heart rate, and temperature), radiological involvement, and CRP were similar to those of the patient with whom they were matched. For this purpose, the CRP on day 1 of follow-up of the patient or, failing that, the day before the start of treatment, was taken as reference. Likewise, the radiological involvement on the treatment started, or any previous time up to a maximum of two days before the start of treatment, was considered. Missing data on radiological involvement were imputed in the following way: It was assumed that the radiological involvement on the days between two equal radiographs was the same as on the days of said radiographs (e.g., if a patient had an X-ray with three affected quadrants on day 1 and another with three affected quadrants on day 6, it was assumed that on all intervening days they had three affected quadrants). This interpolation was allowed up to a maximum interval of 6 days between radiographs. No missing data were imputed for other variables. Patients who received the study treatment and their controls were matched only if they had received the same other treatments for COVID-19, including hydroxychloroquine, lopinavir/ritonavir or azithromycin. A margin of 3 days of lag at the start of the other treatments was tolerated between the patients under study and the matched controls. In the study hospitals, corticosteroids were also used as disease's treatment (mainly high doses of metilpredinosolone); in previous analysis we found no relationship between the use of these drugs and the study outcomes [16], thus we allowed the subcohorts to not be paired by corticosteroids (which facilitated greater availability of controls who were more similar other characteristics of interest).
2.5. Matching technic
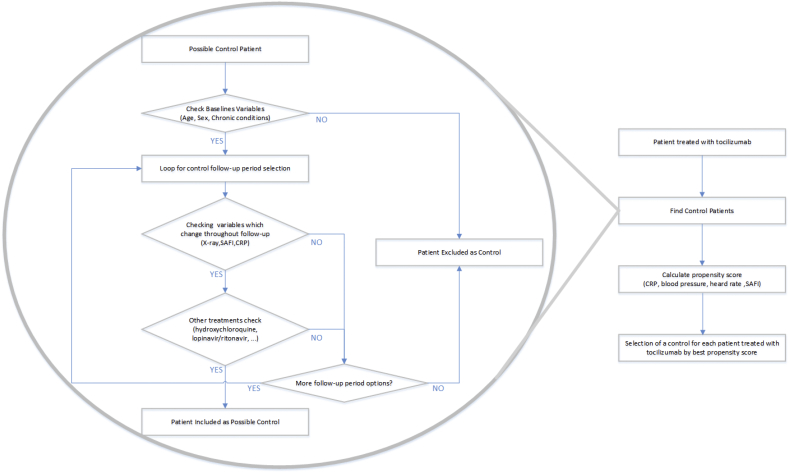
For pairing, a first step was performed using brute-force computing algorithms, which identified all possible controls in the database for each of the patients who received the study treatment. In this first step, controls were chosen who had the same sex and state of obesity (“yes” vs “no”, according to the clinical history), the same radiological involvement (number of affected quadrants on anteroposterior radiography: 0–4) and an age difference not exceeding 15 ears. The control was allowed to have a SAFI from 1.1 points lower to 2 points higher than the treated patient and a CRP from 6 mg/dL lower to 4 mg/dL higher than the treated patient. The matching was then refined, choosing from among the previously identified potential controls the most similar in terms of SAFI, blood pressure, heart rate, and CRP by the propensity score. The same matching methodology has been used for the analysis of efficacy of other COVID-19 drugs in the CSAPG cohort [[16], [17]]. The complete process of selecting patient pairs, including the procedures performed by the brute force algorithms, are summarized in Fig. 1.
Fig. 1.
Selection process for patient-matched controls.
The success of the matching was verified by comparing means or percentages between groups. Although the cohorts were paired by all relevant variables the first day of comparison, we wanted to rule out a different trend in the evolution of patients such day (improvement in one group and worsening in another). To do so, we verified that in both groups, the difference in the SAFI was similar between day 1 of analysis and the day before entering the analysis.
2.6. Statistical analysis
In the matched subcohorts, the SAFI was studied at 48, 72, and 96 h using Student's t-test for independent samples and the time to discharge was analyzed using the log-rank test. In the SAFI analyses, patients with palliative sedation were excluded because in these patients SAFI is not related to the severity of the disease. In the analysis of time to discharge, deceased patients were excluded.
Due to the small sample size of the matched cohorts, association between tocilizumab use and mortality (proportion of events) was studied in the complete cohort, fitting logistic regression models, adjusted for other COVID medications (corticosteroids, hidroxicloroquin, azithromycin, lopinavir/ritonavir, interferon) and the following confounders: sex, age, obesity, heart failure, chronic renal failure, SAHS, baseline saturation in the emergency room, CRP in the emergency room, and quadrants affected in the emergency radiography.
For the statistical analysis, R software version 3.6.1 (R Project for Statistical Computing) and IBM SPSS statistics version 26 were used.
2.7. Ethics
Authors confirm that all methods were carried out in accordance with relevant guidelines and regulations, including the Declaration of Helsinki in its latest version and Regulation (EU) 2016/679 of the European Parliament and of the Council of April 27, 2016 on Data Protection (RGPD) and other concordant rules. The research ethics committee of the Hospital de Bellvitge (CSAPG's tertiary referral hospital) reviewed the study and accepted the waiver of the patient's informed consent, as it was an observational and ambispective review of clinical data, and the patient's personal data were anonymized for its publication (Ethics committee reference number: PR252/20).
3. Results
Of the 418 patients included in the COVID-19 cohort of the CSAPG, a total of 96 (23%) patients were treated with tocilizumab. Of them, 22 patients could be paired with matched controls. The characteristics of the matched subcohorts are shown in Table 1. The characteristics of the matched subcohorts, after excluding deceased patients, and their controls, are shown in Table 2.
Table 1.
Baseline characteristics of patients treated with tocilizumab and the matched controls.
| Tocilizumab (n22) | Control (n22) | p | |
|---|---|---|---|
| Age (years) | 68.4 | 66.1 | 0.572 |
| Men (n) | 18 | 18 | 1.000 |
| Obesity (n) | 3 | 3 | 1.000 |
| CHF (n) | 0 | 0 | – |
| CRF (n) | 3 | 1 | 0.604 |
| SAHS (n) | 1 | 1 | 1.000 |
| Saturation (%) | 95.0 | 95.1 | 0.533 |
| Systolic BP (mmHg) | 125.9 | 131.7 | 0.337 |
| Diastolic BP (mmHg) | 72.1 | 74.3 | 0.483 |
| HR (bpm) | 72.5 | 75.3 | 0.314 |
| Temperature (°C) | 36.4 | 36.7 | 0.191 |
| SAFIa | 2.8 | 3.1 | 0.249 |
| SAFI trendb | −0.2 | 0.0 | 0.120 |
| Intubated patients | 0 | 0 | – |
| Radiographic involvementc | 2.3 | 2.3 | 1.000 |
| CRP (mg/dL) | 10.8 | 12.7 | 0.345 |
| Urea (mg/dL) | 35.4 (n12) | 32.7 (n11) | 0.600 |
| Hydroxychloroquine (n) | 21 | 21 | 1.000 |
| Lop/Rit (n) | 21 | 21 | 1.000 |
| Interferon (n) | 2 | 0 | 0.488 |
| Tocilizumab (n) | – | – | – |
| Methylprednisolone (n) | 10 | 7 | 0.537 |
| Dexamethasone (n) | 6 | 1 | 0.095 |
| Azithromycin (n) | 16 | 18 | 0.721 |
CHF: congestive heart failure. CRF: chronic renal failure. SAHS: sleep apnea–hypopnea syndrome. BP: blood pressure. HR: heart rate. SAFI: saturation (%)/fraction of inspired O2 (%). CRP: C-reactive protein.
Maximum value 4.76, corresponding to 100% saturation with FiO2 of 21%.
Change in SAFI with respect to the day before the start of the follow-up period.
Number of affected quadrants in an anteroposterior chest radiograph. Range: 0–4 (0: no involvement; 4: involvement of the upper and lower lobes of both lungs).
Table 2.
Baseline characteristics of patients treated with tocilizumab and the matched controls, after exclusion of deceased patients.
| Tocilizumab (n19) | Control (n19) | p | |
|---|---|---|---|
| Age (years) | 64.8 | 66.1 | 0.766 |
| Men (n) | 15 | 15 | 1.000 |
| Obesity (n) | 3 | 3 | 1.000 |
| CHF (n) | 0 | 1 | 0.312 |
| CRF (n) | 3 | 4 | 0.680 |
| SAHS (n) | 1 | 2 | 0.550 |
| Saturation (%) | 95.2 | 95.6 | 0.533 |
| Systolic BP (mmHg) | 127.5 | 130.5 | 0.568 |
| Diastolic BP (mmHg) | 71.8 | 72.9 | 0.702 |
| HR (bpm) | 72.9 | 76.7 | 0.198 |
| Temperature (°C) | 36.4 | 36.6 | 0.300 |
| SAFIa | 2.8 | 3.0 | 0.429 |
| SAFI trendb | −0.2 | 0.0 | 0.092 |
| Intubated patients (n) | 0 | 0 | – |
| Radiographic involvementc | 2.4 | 2.4 | 0.504 |
| CRP (mg/dL) | 11.5 | 13.3 | 0.512 |
| Urea (mg/dL) | 37.6 (n14) | 38.6 (n13) | 0.900 |
| Hydroxychloroquine (n) | 21 | 21 | 1.000 |
| Lop/Rit (n) | 21 | 21 | 1.000 |
| Interferon (n) | 2 | 0 | 0.488 |
| Tocilizumab (n) | – | – | – |
| Methylprednisolone (n) | 10 | 7 | 0.537 |
| Dexamethasone (n) | 6 | 1 | 0.095 |
| Azithromycin (n) | 16 | 18 | 0.721 |
CHF: congestive heart failure. CRF: chronic renal failure. SAHS: sleep apnea–hypopnea syndrome. BP: blood pressure. HR: heart rate. SAFI: saturation (%)/fraction of inspired O2 (%). CRP: C-reactive protein.
Maximum value 4.76, corresponding to 100% saturation with FiO2 of 21%.
Change in SAFI with respect to the day before the start of the follow-up period.
Number of affected quadrants in an anteroposterior chest radiograph. Range: 0–4 (0: no involvement; 4: involvement of the upper and lower lobes of both lungs).
Compared with the rest of the patients who received tocilizumab, the patients selected for the matched cohort (matched control available), were older and had less severe disease (Table 3).
Table 3.
Baseline characteristics of patients treated with tocilizumab, included in the matched cohort, compared with those treated with tocilizumab, for whom no matched control was found (excluded from the matched cohort).
| Unmatched patients (n74) | Matched patients (n22) | p | |
|---|---|---|---|
| Age (years) | 58.4 | 66.1 | 0.020 |
| Men (%) | 74.3 | 81.8 | 0.470 |
| Obesity (%) | 31.1 | 13,6 | 0.106 |
| CHF (%) | 1.4 | 0 | 1.000 |
| CRF (%) | 9.5 | 13.6 | 0.691 |
| SAHS (%) | 8.1 | 4.5 | 1.000 |
| Saturation (%) | 94.3 | 96.0a | 0.023 |
| Systolic BP (mmHg) | 124.8 | 124.6a | 0.951 |
| Diastolic BP (mmHg) | 71.3 | 70.0a | 0.628 |
| HR (bpm) | 82.5 | 79.1a | 0.262 |
| Temperature (°C) | 37.0 | 37.1a | 0.898 |
| SAFIb | 2.8 | 3.1a | 0.249 |
| Intubated patients (%) | 18.9 | 0 | 0.035 |
| Radiographic involvementc | 2.6 | 1.7a | 0.001 |
| CRP (mg/dL) | 17.6 | 8.9a | 0.001 |
| Urea (mg/dL) | 37.6 | 33.4a | 0.347 |
CHF: congestive heart failure. CRF: chronic renal failure. SAHS: sleep apnea–hypopnea syndrome. BP: blood pressure. HR: heart rate. SAFI: saturation (%)/fraction of inspired O2 (%). CRP: C-reactive protein.
Bold indicates statistically significant results.
First day of admission values (may not coincide with those of the first day entering the matched cohort, sown in Table 1).
Maximum value 4.76, corresponding to 100% saturation with FiO2 of 21%.
Number of affected quadrants in an anteroposterior chest radiograph. Range: 0–4 (0: no involvement; 4: involvement of the upper and lower lobes of both lungs).
Mean changes in saturation, FiO2, and SAFI, in the first 48, 72 and 96 h after the start of the treatment, both in treated and untreated subcohorts, are shown in Table 4.
Table 4.
Increase in respiratory function parameters with respect to the first day of follow-up in patients treated with azithromycin, corticosteroids, and tocilizumab.
| Tocilizumab | Control | p | |
|---|---|---|---|
| Saturation increase | |||
| 48 h | 0.67 (n22) | −0.63 (n22) | 0.104 |
| 72 h | 0.44 (n20) | −0.64 (n21) | 0.205 |
| 96 h | 0.53 (n18) | −1.54 (n19) | 0.124 |
| FiO2 increase | |||
| 48 h | 7.64 (n22) | 1.71 (n22) | 0.428 |
| 72 h | 0.81(n21) | −2.30 (n21) | 0.652 |
| 96 h | −1.85 (n20) | −2.35 (n19) | 0.954 |
| SAFI increase | |||
| 48 h | −0.04 (n22) | 0.09 (n22) | 0.636 |
| 72 h | 0.37 (n20) | 0.31 (n20) | 0.824 |
| 96 h | 0.39 (n19) | 0.26 (n19) | 0.699 |
FiO2: fraction of inspired oxygen.
Statistical power was estimated to be 79.7% for detecting an improvement of 3% of saturation in Tocilizumab group; 62.3% for detecting a 15% FiO2 reduction in Tocilizumab group; and 81.4% for detecting an increase of 0.7 points in SAFI.
Comparing with control group, patients treated with tocilizumab presented a sustained CRP decrease after treatment. The absolute difference in CRP levels between groups were as follow: 199 mg/L at 48 h (p < 0.032), 118 mg/L at 72 h (p < 0.010) and 45.7 mg/L at 96 h (p < 0.006).
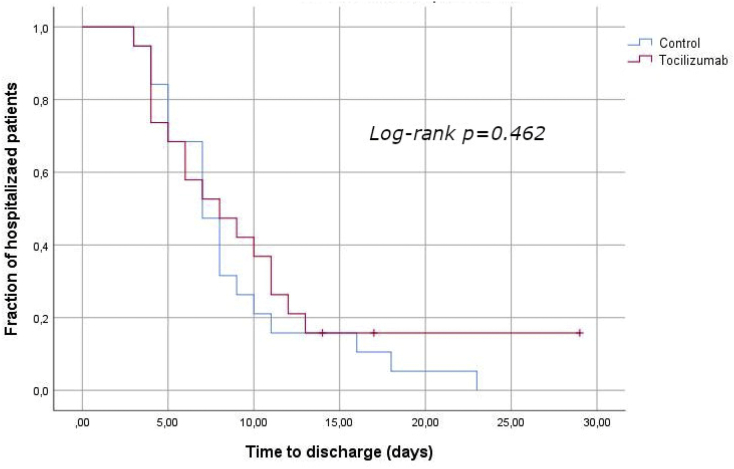
The mean of follow up of the total patients included in the analysis was 8 days (interquartile range: 5 days). In average, patients in tocilizumab group were discharge in 9.3 days, this time was of 8.7 days for patients in control group. Time to discharge was not significantly different between treated and untreated patients (logrank: p = 0.462). Kaplan-Meier curves for the time to discharge in the treated and control subchorts, are shown in Fig. 2. Statistical power was calculated to be 46.4% for a reduction of 3 days in hospital stay in tocilizumab group.
Fig. 2.
Time to discharge in tocilizumab treated patients and paired controls. Kaplan-Meier comparison curves and log-rank test.
Of the total cohort (N418), 79 patients died in the first 30 days after admission (18.9%). Adjusted logistic regression models, did not show an association between the use of tocilizumab and mortality: adjusted OR: 0.99 (IC95: 0.30–3.27); p = 0.990.
4. Discussion
We were unable to find a benefit associated to the use of tocilizumab in terms of respiratory function (SAFI) or time to discharge. However, we observed a significant decrease in CRP in patients treated with tocilizumab, which did not correlate with an improvement in the studied clinical parameters. This observation may indicate that the increased synthesis of CRP, which Tocilizumab reduces through IL-6 blocking, represents more of a host reaction against infection, rather than components of a self-amplifying inflammatory loop that would benefit from CRP suppression [7].
None of the patients included in our paired cohort were intubated, so our results cannot be compared with those obtained in critically ill patients [9,10,12,13]. We found two observational studies, conducted on less severe patients, which used correct matching techniques to avoid biases [8,11]. None of them reported results on improvement in lung function, or time to discharge, therefore we cannot compare our primary endpoints with them. These studies found benefit in mortality / need for intubation with the use of tocilizumab in their respective cohorts of 778 and 196 hospitalized patients, with clinical criteria of relevant systemic inflammation; their results do not coincide with the mortality results obtained by us, by using Cox regression analysis. Nor do the results of these observational studies coincide with the data released from the COVACTA randomized clinical trial, whose results has not been published, but informally disseminated in ta press release [18]; the COVACTA trial found no benefit in terms of mortality, in patients hospitalized for coronavirus pneumonia. This may be due to the fact that these observational studies, unlike ours, and unlike the COVACTA trial, used a specific inflammatory markers level as inclusion criteria, therefore, both our patients and those in the COVACTA trial may have a less significant inflammatory state.
Our results support those of the only clinical trial published to date, which found no benefit in time to intubation (or time to death, if it happened before intubation), in patients hospitalized for COVID-19. In line with our results, the authors also found that CRP decreased at a faster rate in patients in the tocilizumab group compared to the control group.
Given the observational nature of our study, the existence of residual confounders cannot be ruled out, as there is a greater probability that patients treated with tocilizumab, would have a higher-risk factors or disease severity. However, the exhaustive matching method used for studying the primary outcomes, and the verification of the comparability of the groups lead us to assume that this confounding effect was unlikely and, if there, was small. The fact that we included the patients in the matched cohort on the first day of treatment, avoids immortality time bias. Furthermore, the verification of the trend in SAFI, with respect to the previous day, and the vital signs matching, reasonably rules out biases related to disease's course time.
The use of secondary data (obtained from the clinical history) might have led to information biases, as clinical data were not originally recorded for the purpose of this research. Nevertheless, given that the main variables were quantitative parameters, which were little influenced by the observers or their expertise in measurement, and given that these parameters are routinely and properly collected in clinical practice, we consider unlikely the existence of a relevant bias of this type.
Due to exhaustive matching, the final sample size of our comparison cohorts is small. This can lead to problems of generability, since our patients may not represent well the common of patients hospitalized for COVID-19. Specifically, our results cannot be applied to the most severe cases, which were not represented in our matched cohort. In addition, the small sample size leads to lack of statistical power, especially with regard to time to discharge calculations, which may prevent appreciating existing differences between groups. The lack of statistical power also affected the study of mortality in the entire cohort, as can be seen in the width of the confidence intervals. However, given the similarity of the results means (whose differences are not clinically significant, and tend to favor control group), the authors believe that, if tocilizumab has a beneficial effect on SAFI in the first 96 h, on the time until discharge, or in mortality, this effect is not prominent (and, in any case, this effect should be less than its biological effect on PCR, which we have been able to detect in our sample). Thus, the sample size and observational nature of our study warrant waiting for the results of randomized clinical trials to confirm ours.
In conclusion, in this observational study, we did not find evidence of a clinical benefit on post-treatment lung function (SAFI in the first 96 h) or on length of the hospital stay, associated to the use of tocilizumab in patients hospitalized with COVID-19.
Author contributions
ARM designed the study, contributed to data analysis and interpretation, drafted the manuscript and approved the final version.
CP designed algorithms for patient matching and aproved the final versión of the manuscirpt.
CG contributed to the study design, data interpretation and drafting the manuscript.
AM did the statistical analysis, reviewed and approved the final version of the manuscript.
OM, GFL, MTR, MDD, SM, ER, IC and the COVID-19 research group of CSAPG, collected the data and reviewed and aproved the final versión of the manuscirpt.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgment
This work was supported by the Consorci Sanitari de l'Alt Penedès i Garraf.
We would like to thank Gloria Moes for her invaluable help in coordinating the fieldwork. Gloria Alba, Nuria Pola and Anna María Soler, for their initial help in collecting drug data, and to Montserrat Pérez and Rosa Guilera, for their help with the electronic medical record. David Blancas and Lourdes Gabarró for their work in the hospital protocols for COVID-19, and their initial supply of bibliography. We also should thank the CSAPG informatics team, for their support during the study. We want to thank the hospital manager, José Luis Ibáñez, and the management team, for making this study possible.
Contributor Information
Alejandro Rodríguez-Molinero, Email: arodriguez@csapg.cat.
COVID-19 research group of CSAPG:
Anna Alberti Casas, Jose L. Avalos Garcia, Manel Borrego Ruiz, Gemma Añaños Carrasco, Pedro L. Campo Pisa, Ana M. Capielo Fornerino, Antonio Chamero Pastilla, Andreu Fenollosa Artés, Clara Gris Ambros, Lourdes Hernandez Martinez, Antonio Hidalgo García, Mireia Martín Puig, Núria Milà Ràfols, José C. Molina Hinojosa, Ernesto E. Monaco, Laura Peramiquel Fonollosa, Italo G. Pisani Zambrano, Juan P. Rives, Enric Sabria Bach, Yris M. Sanchez Rodriguez, Maria del Mar Segura Martin, Gemma Tremosa Llurba, Ester Ventosa Gili, Florencia I. Venturini Cabanellas, and Natàlia Vidal Meler
References
- 1.Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020 Mar;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Director-General'’s opening remarks at the media briefing on COVID-19 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [Internet]. [cited 2020 May 20]. Available from.
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 CASES FROM THE Chinese center for disease control and prevention. JAMA. 2020 07;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.WHO Coronavirus Disease (COVID-19) Situation Reports. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports [Internet]. [cited 2020 Oct 5]. Available from.
- 5.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020 May 1;55(5) doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J. Transl. Med. 2020 14;18(1):164. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S. Tocilizumab trial investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. N. Engl. J. Med. 2020 Dec 10;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikulska M., Nicolini L.A., Signori A., Di Biagio A., Sepulcri C., Russo C. Tocilizumab and steroid treatment in patients with COVID-19 pneumonia. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Somers E.C., Eschenauer G.A., Troost J.P., Golob J.L., Gandhi T.N., Wang L. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin. Infect. Dis. 2020 Jul 11 doi: 10.1093/cid/ciaa954. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ip A., Berry D.A., Hansen E., Goy A.H., Pecora A.L., Sinclaire B.A. Hydroxychloroquine and tocilizumab therapy in COVID-19 patients-an observational study. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez-Baño J., Pachón J., Carratalà J., Ryan P., Jarrín I., Yllescas M. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: a multicentre cohort study (SAM-COVID-19) Clin. Microbiol. Infect. 2020 Aug 27 doi: 10.1016/j.cmi.2020.08.010. Epud ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colaneri M., Bogliolo L., Valsecchi P., Sacchi P., Zuccaro V., Brandolino F. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE) Microorganisms. 2020 May 9;8(5) doi: 10.3390/microorganisms8050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biran N., Ip A., Ahn J., Go R.C., Wang S., Mathura S. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020 Oct;2(10):e603–e612. doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenbaum P.R., Rubin D.B. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 15.Coscia Requena C., Muriel A., Peñuelas O. Analysis of causality from observational studies and its application in clinical research in intensive care medicine. Med. Int. 2018 Jul;42(5):292–300. doi: 10.1016/j.medin.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez-Molinero A., Pérez-López C., Gálvez-Barrón C., Miñarro A., Rodríguez Gullello E.A., Collado Pérez I., Milà Ràfols N., Mónaco E.E., Hidalgo García A., Añaños Carrasco G., Chamero Pastilla A., en representación del grupo de investigadores para la COVID-19 del Consorci Sanitari de l'Alt Penedès i Garraf (CSAPG) Association between high-dose steroid therapy, respiratory function, and time to discharge in patients with COVID-19: Cohort study. Med Clin (Engl Ed). 2020 Nov:27. doi: 10.1016/j.medcle.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez-Molinero A., Pérez-López C., Gálvez-Barrón C., Miñarro A., Macho O., López G.F., Robles M.T., Dapena M.D., Martínez S., Rodríguez E., Collado I. COVID-19 research group of CSAPG. Observational study of azithromycin in hospitalized patients with COVID-19. PLoS One. 2020 Sep 3;15(9) doi: 10.1371/journal.pone.0238681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anonymous Roche Provides an Update on the Phase III COVACTA Trial of Actemra/RoActemra in Hospitalized Patients with Severe COVID-19 Associated Pneumonia. 2020. https://www.roche.com/investors/updates/inv-update-2020-07-29.htm [Internet]. [cited 2020 Oct 5]. Available from: