Graphical abstract
Keywords: Protoporphyrin IX (PPIX), Lipid biolayer-targeting, Broad-spectrum antivirals, Enveloped virus
Highlights
-
•
PPIX possesses broad antiviral activities in vitro against a panel of enveloped viruses.
-
•
PPIX interacts with the lipids of enveloped virions, thereby inhibiting the entry of enveloped viruses into host cells.
-
•
PPIX shows the antiviral effect in vivo by testing mice infected with the influenza A/Puerto Rico/8/34 (H1N1) virus.
Abstract
Severe emerging and re-emerging viral infections such as Lassa fever, Avian influenza (AI), and COVID-19 caused by SARS-CoV-2 urgently call for new strategies for the development of broad-spectrum antivirals targeting conserved components in the virus life cycle. Viral lipids are essential components, and viral-cell membrane fusion is the required entry step for most unrelated enveloped viruses. In this paper, we identified a porphyrin derivative of protoporphyrin IX (PPIX) that showed broad antiviral activities in vitro against a panel of enveloped pathogenic viruses including Lassa virus (LASV), Machupo virus (MACV), and SARS-CoV-2 as well as various subtypes of influenza A viral strains with IC50 values ranging from 0.91 ± 0.25 μM to 1.88 ± 0.34 μM. A mechanistic study using influenza A/Puerto Rico/8/34 (H1N1) as a testing strain showed that PPIX inhibits the infection in the early stage of virus entry through biophysically interacting with the hydrophobic lipids of enveloped virions, thereby inhibiting the entry of enveloped viruses into host cells. In addition, the preliminary antiviral activities of PPIX were further assessed by testing mice infected with the influenza A/Puerto Rico/8/34 (H1N1) virus. The results showed that compared with the control group without drug treatment, the survival rate and mean survival time of the mice treated with PPIX were apparently prolonged. These data encourage us to conduct further investigations using PPIX as a lead compound for the rational design of lipid-targeting antivirals for the treatment of infection with enveloped viruses.
1. Introduction
Outbreaks of severe pathogenic viral infections such as Avian influenza (AI), severe acute respiratory syndrome-coronavirus, Middle East Respiratory Syndrome- coronavirus, and Ebola virus have posed a significant threat to public health. Among them, the outbreaks this year of Lassa fever in Nigeria caused by the deadly Lassa virus has infected 472 people and led to >70 deaths; influenza induced by influenza virus in the United States has infected >25 million people and caused 14,000 deaths. Pneumonia due to infection with the novel coronavirus SARS-CoV-2 (also named COVID-19), which is currently circulating globally, has caused >576,000 deaths. These outbreaks highlight the urgent need for new strategies and approaches to developing efficient antiviral drugs with broad-spectrum activities for prophylactic and therapeutic usage. Notably, many of these viruses are enveloped RNA viruses, and a third of the currently emerging and re-emerging infections are caused by this type of pathogen [1]. As such, we speculated that it might be possible to develop an arsenal of broad-spectrum antivirals by targeting conserved components involved in the life cycle of these viruses.
Structurally, an enveloped virus particle comprises a genome and a protein coat or capsid that are surrounded by a lipid bilayer envelope, where the genome encoding viral elements is enclosed inside the capsid compartment [2], [3]. In the virus life cycle, the entry process is the first step, which involves multiple scenarios, including attachment to the surfaces of host cells, internalization into host cell compartments by exploitation of cellular uptake machineries [4], transport to the lumen of endosomes or the endoplasmic reticulum (ER) [5], and fusion of viral and host cell membranes [6]. Due to their crucial roles in mediating the entry of a virus, each of these steps is a promising target for antivirals, which can intervene by blocking the infection in the early stage. Among these steps, the fusion of the virus envelope with the cell membrane is a known essential step for all enveloped viruses [7] and some molecules targeting this step have shown broad antiviral activity [8], [9], [10].
Porphyrins and their derivatives are a group of conjugated planar molecules that possess broad applications in the field of biomedical science, materials chemistry, and electrochemistry due to their unique spectroscopic properties and electrochemical performance [11], [12], [13], [14]. Of particular interest for biomedical science is their significant role in therapies that are associated with microbial infection and tumor therapies [15], [16]. In our previous work, through a bioassay-guided approach, we identified a porphyrin derivative of pyropheophorbide a (PPa) from the marine mussel Musculus senhousei. The biological activity test showed that PPa exhibited good antiviral activity against a panel of influenza A viruses. The mechanism of action study indicated that PPa exerted its inhibitory effect in the early stage of virus infection by interacting with the lipid bilayer function of the virion, blocking the entry of enveloped viruses into host cells [17].
Considering the proposed drug target of PPa, we decided to conduct extensive research by synthesizing a number of derivatives possessing the same skeleton of porphyrin as that of PPa to study its broad antiviral activities in addition to those exhibited against influenza A viruses. Hence, an easily accessed material, protoporphyrin IX (PPIX) (Fig. 1 A), was adopted as a starting material, and derivatives were designed by increasing the hydrophobicity of PPIX with the expectation of increasing the interactions between PPIX and the lipid of the virus, although these variations might structurally decrease the amphipathicity of PPIX, which as a result, decreases the antiviral activity based on the proposed modes of action in our previous work [17]. These molecules were then tested for antiviral activities against a broad panel of enveloped viruses including the highly pathogenic Lassa virus (LASV) and Machupo virus (MACV) as well as SARS-CoV-2 which causes COVID-19 in China currently. In addition, various subtypes of influenza A viruses (IAV) were also included. Herein, we report the antiviral activities as well as the possible modes of action of these molecules.
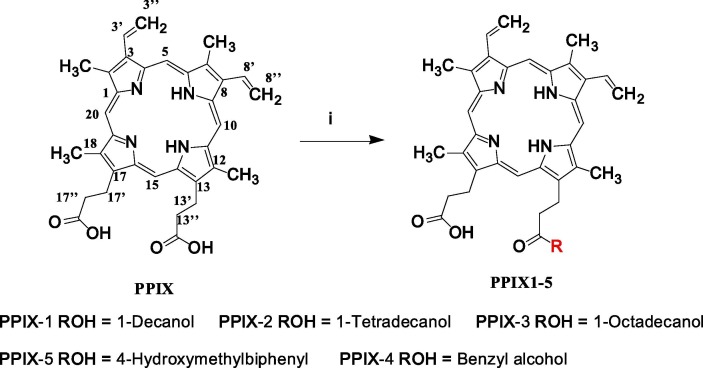
Fig. 1.
The structure of PPIX and its derivatives. Reagents and conditions: (i) DMF, HBTU, DMAP, DCC, DIEA, 0℃ for the first 30 min, then turned to room temperature.
2. Materials and methods
2.1. Anti-influenza A virus assay
The protocol was adopted as previously reported [18]. MDCK cells were seeded in 96-well plates of 100 μL/well (2 × 104 cells) and cultured in a 5% CO2 incubator at 37℃ for 24 h. Influenza A/Puerto Rico/8/34 (H1N1), A/FM/1/47 (H1N1), A/Puerto Rico/8/34 (H1N1) with the NA-H274Y mutation, and A/Aichi/2/68 (H3N2) viruses at 100 TCID50 were each mixed with twofold-diluted PPIX solutions and incubated at 37 °C for 30 min. After washing twice with PBS (Sigma, USA), the virus-compound mixtures were added to these cells and incubated for another 1 h. After that, DMEM (Sigma, USA) supplemented with 1 μg/mL of TPCK-trypsin (Sigma, USA) was added to the MDCK cells. At 48 h postinfection, cell viability was measured by the MTT method. S-KKWK was reported as an IAV virus entry inhibitor by interaction with HA2 subunit to inhibit the fusion between virus envelop and host cells’ membrane under acidic condition [18], and the experiment was repeated at least three times independently.
2.2. Anti- Lassa or Machupo pseudotyped viruses assay
Vero-E6 cells were seeded in 96-well plates with 1.5 × 104 cells/well overnight. Lassa or Machupo pseudotyped viruses were incubated with gradient-diluted compounds at 37℃ for 30 min, then the mixture was added to cells and incubated at 37℃ for 1 h. After that, the supernatants were completely removed, cells were washed with PBS and cultured for 24 h before being subjected to luciferase activity detection [19]. The experiment was repeated at least three times independently.
2.3. Anti- SARS-CoV-2 assay
Vero-E6 cells were seeded in 48-well plates with 5 × 104 cells/well overnight. For ‘pretreatment of virus’ mode, SARS-CoV-2 were processed as the same way as Lassa or Machupo pseudotyped viruses. For ‘full-time treatment’ mode, gradient-diluted compounds were firstly, incubated with cells for 1 h, then SARS-CoV-2 were added and incubated with cells for another 1 h. Then the supernatants were completely removed, cells were washed with PBS and supplemented with medium containing gradiently-diluted compounds. After 24 h, cell supernatants were collected and subjected to viral RNA isolation, then viral genome copies were detected by qRT-PCR with primers targeting S gene. At the same time, cells were fixed with 4% formaldehyde and viruses were detected by indirect immunofluorescence with polyantibodies against NP [20]. The experiment was repeated at least three times with a similar result each time.
2.4. Polykaryon formation inhibition assay
Following a procedure previously reported by Lai et al. [21], Briefly, MDCK cells (2 × 105 cells per well; 12-well plate) incubated for 16 h. According to the manufacturer’s instructions transfected with plasmid-encoded HA (from influenza A/Thai/Kan353/ 2004 virus) (2 μg DNA/well) using PEI. After 8–10 h of culture, fresh DMEM containing 10% fetal bovine serum was used instead of transfection medium. At 48 h after transfection, the cells were washed twice with PBS and treated with TPCK-trypsin (5 μg/mL) for 15 min at 37℃. Then, the cells were rinsed with PBS solution twice and pretreated with 0.5 mL of PPIX or 1% methanol for 15 min at 37℃ followed by an incubation with 0.5 mL of pH 5.0 PBS or 1% methanol at 37℃ for another 15 min. After the reaction, the cells were washed twice with PBS, added 1 mL of DMEM containing 10% FBS incubated at 37℃ to form polykaryon. 3 h later, the cells were fixed with methanol and incubated with Giemsa stain (Sigma, USA), and visualized under a microscope. Syncytium formation was quantified by counting the number of polynuclei (containing 5 or more nuclei) under a microscope, and the percentage of syncytium formation was determined relative to methanol. S-KKWK was used as a positive control, and the experiment was repeated at least three times independently.
2.5. Anti-influenza virus test in vivo
2.5.1. Animals
Four-week-old male KM mice without specific pathogen (SPF), with an average weight of 22 g, were purchased from the Experimental Animal Center of Southern Medical University (permission number: SYXK(粤)2016-0167, Guangzhou, China). In animal experiments, all mice were fed a standard laboratory diet and provided with water. The animal experiments are conformed to the standard operating procedures of the facility and the Animal Welfare Act.
The mice were randomly divided into five groups, including the uninfected and water-treated group (blank), the infected and water-treated group (viruses), the group that was infected and treated with 10 mg/mL oseltamivir phosphate , and the groups that were infected and treated with PPIX at concentrations of 10 mg/kg (PPIX-H) and 2.5 mg/kg (PPIX-L), of which, the blank group was 5 mice and the other groups were 10 mice. Oseltamivir phosphate was dissolved in water, whereas PPIX was initially dissolved in polyethylene glycol 400 and then diluted fourfold with water. Infected mice were anesthetized with ethyl ether and then intranasally challenged twice with influenza A/PR/8/34 (H1N1) virus at the titer of 2 × 105 TCID50 in a volume of 30 μL. The mice then took a different drug orally once a day for five days. The mice were given food and water ad libitum on the sixth day. Body weight, mortality, and the general behaviors of the mice were recorded for fifteen consecutive days.
2.5.2. Lung index
Using the same protocol as the in vivo anti-influenza virus test described above, on the fourth day after infection drug administration to the stomach was stopped in each group of three mice, and the animals were sacrificed. Their lungs were harvested, washed with normal saline, dried with gauze, and weighed. Meanwhile, the lung images were obtained (Fig. 6 D). The lung index was calculated using the following equation:
| Lung index = lung weight/body weight × 100% |
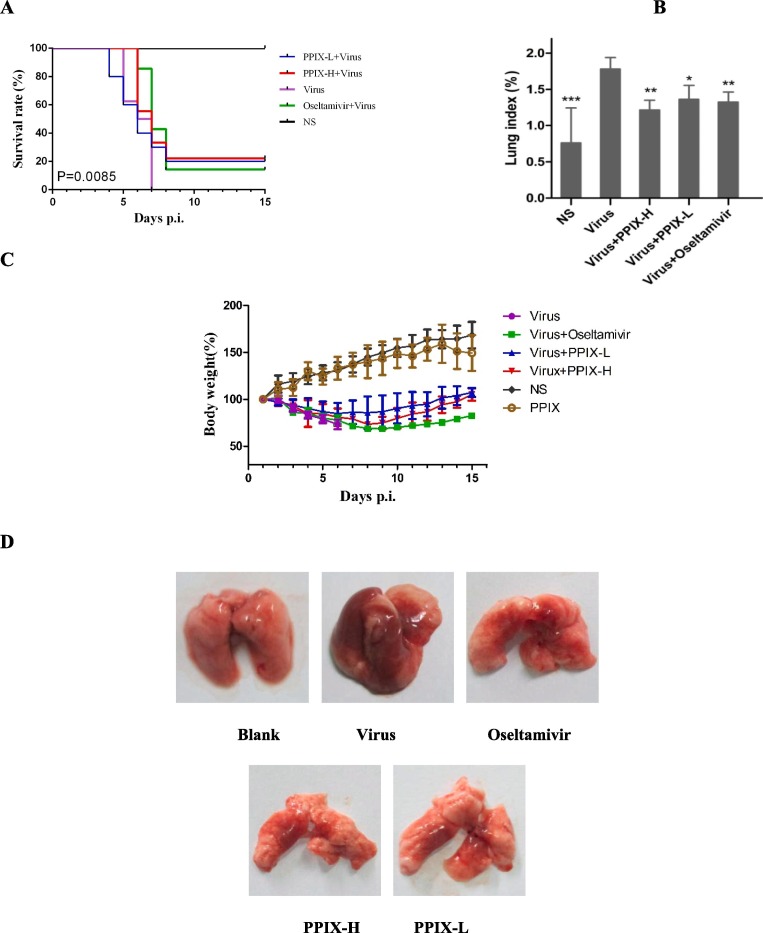
Fig. 6.
The anti-IAV activity of PPIX in mice. 6A The survival rate of mice after infection with IAV and treatment with PPIX or oseltamivir compared with the infected mice without drug treatment. 2 × 105 TCID50 of influenza virus A/PR/8/34 (H1N1) in a volume of 30 µL of normal saline (NS) was used to infect the mice. The mice were intranasally challenged with virus twice and subsequently orally administered PPIX (2.5 and 10 mg/kg) or oseltamivir phosphate (10 mg/kg). The drugs were administered once a day until the fifth day. The mortality rates were analyzed with GraphPad Prism 6 software by a log-rank (Mantel-Cox) test (P < 0.01). 6B Lung index of infected mice treated with PPIX or oseltamivir. After the viral challenging and PPIX or oseltamivir treatment for 3 days, the lung index of the mice was measured. Data were analyzed with one-way ANOVA, *p < 0.05, **p < 0.01, and ***p < 0.001. 6C The body weight of the infected mice was recorded. 6D Lung images of the influenza virus-infected mice in each group after treated with different agents for three days.
2.5.3. Statistical analysis
The half-cytotoxic concentration (CC50) and half-inhibitory concentration (IC50) values of the PPIX were calculated with GraphPad Prism 5 software (San Diego, CA). Each data point, expressed as the means ± standard deviation (SD), was repeated at least three to five times. Fluorescent images were greyscale quantized using ImageJ. The mortality rates were analyzed with GraphPad Prism 6 software by a log-rank (Mantel-Cox) test. Lung index were determined by one-way ANOVA using SPSS 22.0 software. Statistical significance was defined as *p < 0.05, ** p < 0.01, *** p < 0.001.
3. Results
3.1. Protoporphyrin IX derivatives were synthesized by reacting with various alcohols
Protoporphyrin IX (PPIX) is a heterocyclic molecule consisting of four pyrrole rings and two symmetric carboxyl groups (Fig. 1), which were then used as a functional group to conjugate with other alcohols. Considering the possible target of PPa as the lipid bilayer of the virion, alcohols with various lengths of aliphatic chains or aromatic rings were used to react with PPIX to enhance the hydrophobicity and steric effects, thereby studying the antiviral activities of these compounds.
As a result, two types of PPIX derivatives (1–5) including three aliphatic and two aromatic alcohols were synthesized, as indicated in Fig. 1. The obtained derivatives were purified, their structures were confirmed by NMR and MS spectroscopic data, and they were used for the following antiviral activity evaluation.
3.2. PPIX showed broad-spectrum antiviral activities against a panel of unrelated enveloped viruses
Compounds 1–5 together with PPIX were tested for their antiviral activity against a number of enveloped viruses including pseudotyped Old World and New World human arenaviruses Lassa virus (LASV) and Machupo virus (MACV), respectively, and various subtypes of influenza A viruses (IAV), such as A/Puerto Rico/8/34 (H1N1), A/FM/1/47 (H1N1) mouse-adapted viral strain, A/Puerto Rico/8/34 (H1N1) with the NA-H274Y mutation, and A/Aichi/2/68 (H3N2) viral strains, as indicated in Table 1 . By using a ‘pretreatment of virus’ approach [22], the antiviral efficacies were assessed by measuring the survival of the host cells using MTT assay in an anti-IAV activity test and measuring the luciferase activity toward the pseudotyped LASV and MACV, as described before [19].
Table 1.
Inhibitory activity of PPIX and its derivatives against various viruses and cellular toxicities.
| Name | IC50 ± SD (μM)a |
CC50 ± SD (μM)b |
MWh | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PR8c | FM-1d | 274e | H3N2f | LASVg | MACVg | MDCK | Vero-E6 | ||
| PPIXi | 1.88 ± 0.85 | 1.88 ± 0.34 | 1.24 ± 0.53 | 1.49 ± 0.66 | 0.91 ± 0.25 | 1.07 ± 0.27 | 229.59 ± 4.53 | 120.80 ± 0.95 | 562.66 |
| PPIX-1 | 7.2 ± 3.23 | 12.12 ± 2.68 | 13 ± 1.36 | 8.95 ± 1.68 | 4.63 ± 2.16 | 17.34 ± 3.88 | 121.23 ± 2.18 | 76.39 ± 2.29 | 720.94 |
| PPIX-2 | 21.27 ± 3.5 | >25.74 | 21.12 ± 2.16 | 13.63 ± 2.01 | >25.74 | >25.74 | 128.32 ± 0.54 | 111.90 ± 3.58 | 777.05 |
| PPIX-3 | >21.93 | >21.93 | >21.93 | >21.93 | >21.93 | >21.93 | 139.56 ± 1.58 | 127.970 ± 1.46 | 912.16 |
| PPIX-4 | >29.82 | >29.82 | >29.82 | >29.82 | >29.82 | >29.82 | 198.57 ± 1.62 | 137.70 ± 2.89 | 670.79 |
| PPIX-5 | >26.78 | >26.78 | >26.78 | >26.78 | >26.78 | >26.78 | 237.49 ± 2.72 | 215.93 ± 2.18 | 746.89 |
| PPa | 0.32 ± 0.28 | 1.65 ± 0.56 | 1.05 ± 0.99 | 1.23 ± 1.10 | NTi | NT | 139.98 ± 3.61 | NT | 534.66 |
| S-KKWKj | 1.85 ± 0.92 | 1.96 ± 0.23 | NT | NT | NT | NT | NT | NT | 1014.84 |
The anti-influenza A virus (IAV) activity was determined after the virus was pretreated with PPIX or its derivatives.
The toxicities of MDCK or Vero-E6 cells were assessed using MTT assay.
A/Puerto Rico/8/34,
A/FM/1/47 mouse-adapted viral strain,
A/Puerto Rico/8/34 with the NA-H274Y mutation,
A/Aichi/2/68.
The activity was assessed by measuring the luciferase activity toward the pseudotyped LASV and MACV.
MW: molecular weight, INT: not tested.
The IC90 values of PPIX for each viral strain were calculated as: 9.10 ± 1.81 (PR8), 5.01 ± 0.75 (FM-1), 3.52 ± 0.59 (2 7 4), 5.31 ± 1.80 (H3N2) µM; while the cytotoxicity of CC90 was 316.40 ± 2.75 (MDCK) µM.
S-KKWK an IAV entry inhibitor was used as a positive control, as reported previously [18].
The data in Table 1 showed that the prototype of PPIX was the most active one among all tested porphyrins, displaying broad activity against all viral strains with IC50 values ranging from 0.91 to 1.88 μM. Significantly, PPIX was also active toward the oseltamivir-resistant virus of influenza A/Puerto Rico/8/34 (H1N1) with the NA-H274Y mutation.
To confirm the antiviral effect of PPIX, we focused on influenza A virus and employed ‘pretreatment of the virus’ and ‘treatment during infection’ methods to test the inhibitory activity of PPIX using indirect immunofluorescence staining, qRT-PCR and plaque reduction assays. As shown in Fig. 2 A, the data from the indirect immunofluorescence staining on the expression of the viral nucleoprotein (NP) in MDCK cells showed that the green fluorescence associated with NP expression from the A/Puerto Rico/8/34 (H1N1) influenza virus was apparently decreased after treatment with PPIX. Moreover, the ‘Pretreatment’ group showed more potent than the ‘During infection’ group, which was further qualified as indicated in Fig. 2 B using ImageJ software [23].
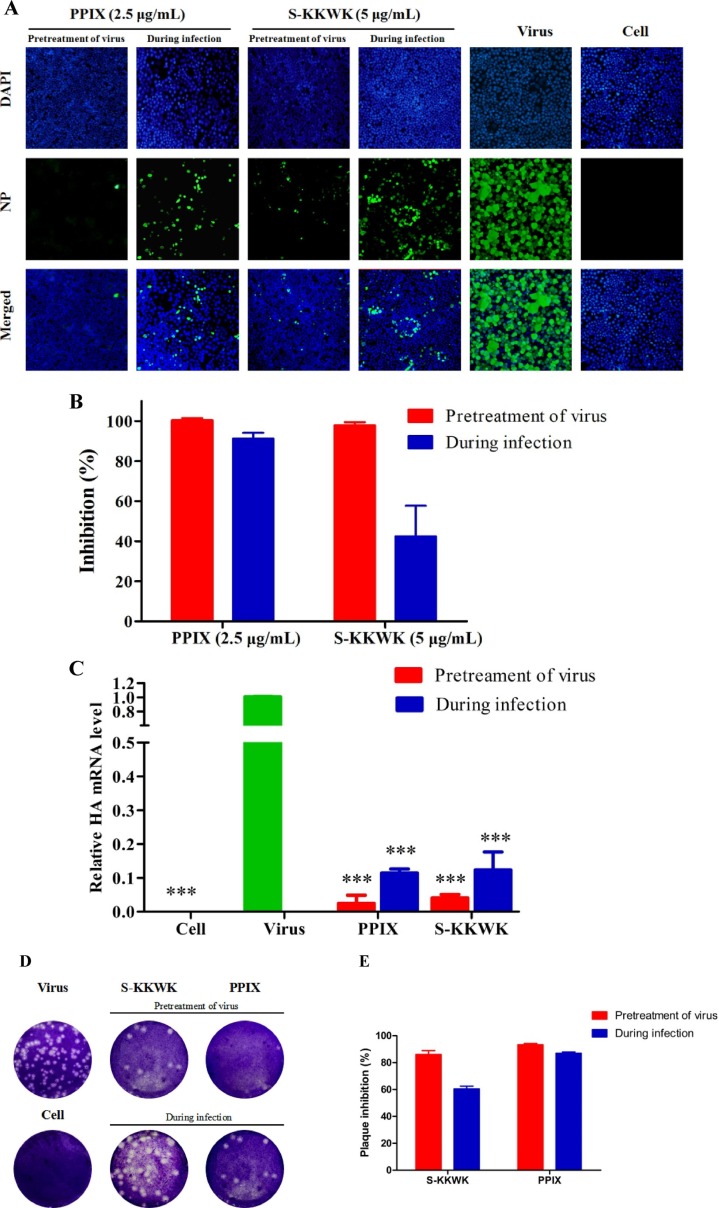
Fig. 2.
A The inhibitory effect of PPIX on the expression of NP in MDCK cells using indirect immunofluorescence assay. In this assay. 100 TCID50 of influenza A/PR/8/34(H1N1) virus and 2.5 μg/mL PPIX were used to treat MDCK cells by ‘pretreatment of virus’ and ‘during infection’ approaches, respectively. At 24 h p.i, the expression of NP (green fluorescence) in the cytoplasm of MDCK cells was immunostained and observed under a fluorescence microscope. S-KKWK (5 μg/mL) was used as a positive control, as reported previously [18]. DAPI staining (blue fluorescence) was employed to indicate the position of the nucleus. 2B The NP expression (green fluorescence) in each group was quantified using ImageJ software, and the inhibitory effect of PPIX on NP expression was thus calculated. 2C The mRNA levels of the HA gene after treatment with PPIX using the ‘pretreatment of the virus’ or ‘during infection’ drug administration approaches were used to evaluate the antiviral effects of PPIX on influenza A/PR/8/34 (H1N1). The significance of the differences in the data between the virus-infected groups was determined using one-way ANOVA: * p < 0.05, ** p < 0.01, and *** p < 0.001. 2D Plaque reduction assay to test the inhibitory effects of PPIX (5 μg/mL) to reduce the number of plaques of the influenza A/Puerto Rico/8/34 (H1N1) virus using the ‘pretreatment of virus’ and ‘during infection’ approaches. In the first approach, PPIX was preincubated with 100 TCID50 of the virus for 30 min at 37℃ before being added to the cells, whereas in the latter approach virus mixed with PPIX was added to the cells simultaneously. After 48 h p.i, the cells were fixed and stained. 2E The number of plaques in each group were counted, from which the plaque inhibition rate of PPIX against the influenza A/Puerto Rico/8/34 (H1N1) virus was obtained. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Moreover, both measurements of the mRNA level of the HA gene from influenza A/PR/8/34 (H1N1) after treatment with PPIX (qRT-PCR) (Fig. 2 C) and the plaque reduction assay (Fig. 2 D and 2E) showed similar results as the indirect immunofluorescence staining (Fig. 2 A), which again confirmed the antiviral effects of PPIX.
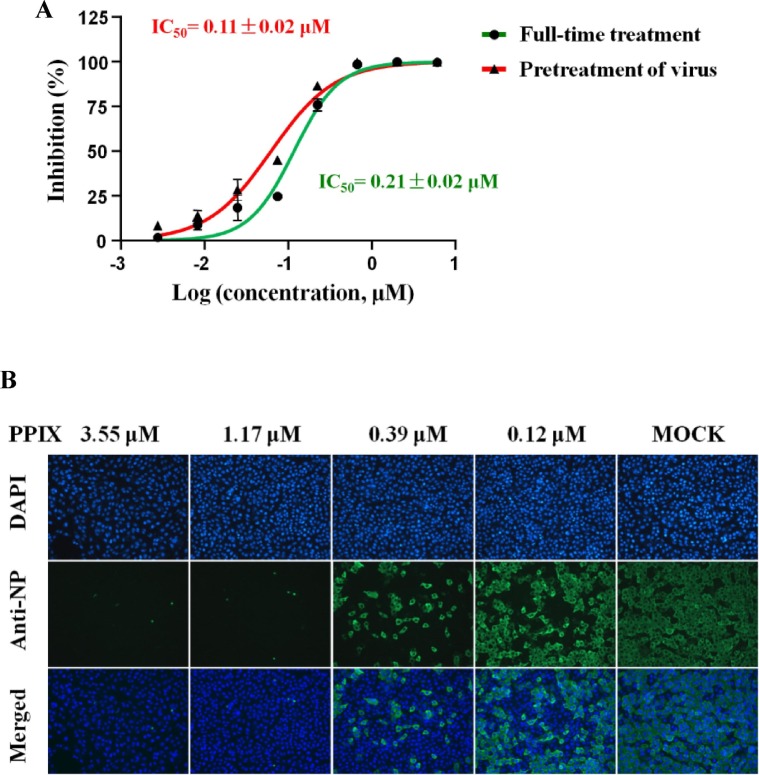
Furthermore, to confirm the broad antiviral activity of PPIX, we tested its activity toward the recently emerged deadly virus Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Two drug administration approaches including ‘Full-time treatment’ and ‘Pretreatment of virus’ were employed to test the inhibitory effect of PPIX toward this virus. As shown in Fig. 3 A, after postinfection for 24 h, the obtained IC50 values were 0.11 ± 0.02 μM and 0.21 ± 0.02 μM, respectively, compared with 3.7 μM for Remdesivir and 10 μM for chloroquine under the same ‘Full-time treatment’ testing condition after incubation for 48 h [20]. Moreover, the in vitro anti-SARS-CoV-2 activity was again assessed by immunofluorescence microscopy toward this virus using the ‘Full-time treatment’ condition and detected at 48 h p.i (Fig. 3 B).
Fig. 3.
The inhibitory effect of PPIX against SARS-CoV-2. 3A Two drug administration approaches including ‘Full-time treatment’ and ‘Pretreatment of virus’ were used in the experiment. In the first method, 5 × 104 overnight cultured Vero E6 cells/well were pretreated with the serially diluted PPIX for 1 h, followed by the addition of virus (MOI of 0.05) and incubation for 2 h. After removal of the virus-PPIX mixture, the cells were cultured with fresh PPIX-containing medium for 24 h postinfection. In the ‘Pretreatment of virus’ approach, the serially diluted PPIX was preincubated with virus (MOI of 0.05) for 1 h at 37 °C prior to being added into the cells. After infection for 1 h, the virus-PPIX mixture was removed and fresh medium was added to the cells, which were allowed to incubate at 37℃ in a 5% CO2 atmosphere for 24 h. In both approaches, at 24 h p.i the cell supernatant was collected and the S gene of the virus was quantified with qPCR [20]; then, the inhibitory efficacies represented as IC50 values were calculated using MOCK as a blank control. 3B The anti-SARS-CoV-2 activity on the expression of NP in Vero E6 cells using indirect immunofluorescence assay. Following the same full-time treatment protocol and after incubation of cells for 24 h of postinfection, the cells were fixed and stained. The expression of NP (as green fluorescence) in the Vero E6 cells was detected under a fluorescence microscope. MOCK (2% DMSO) was used as a blank control, and the position of the nucleus was indicated using DAPI staining (blue fluorescence). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. PPIX displayed its antiviral effect in the early stage of infection of influenza a virus
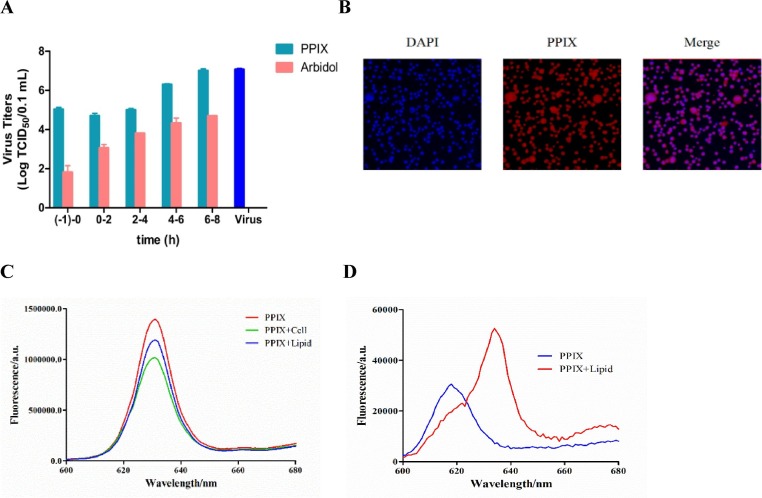
A time-of-addition experiment was then performed to study the detailed antiviral effect of PPIX on the influenza virus life cycle. In the experiment, PPIX was added to MDCK cells that were infected with the influenza A/PR/8/34 (H1N1) virus (100 TCID50) at the indicated time intervals, and the antiviral effects were then evaluated by measuring viral titers in the supernatant at 48 h postinfection. As a result, a better inhibitory effect was observed when PPIX was added at the early stage of infection, e.g., at the interval of (-1)-0h and 0–2 h of treatment with the virus (Fig. 4 A).
Fig. 4.
A Time-of-addition assay. First, 5 μg/mL PPIX was added to MDCK cells infected with A/PR/8/34 (H1N1) (100 TCID50) at the indicated time intervals. After treatment, the cells were incubated for 48 h postinfection, and the TCID50 values of the virus titer in the supernatant were then measured. Arbidol was used as a positive control as indicated in our previous work [24]. The significance of the differences in the data was determined using one-way ANOVA: *p < 0.05, **p < 0.01, and ***p < 0.001. 4B Fluorescence microscopic images of PPIX in MDCK cells. The MDCK cells in PBS were incubated with PPIX at a final concentration of 5 μg/mL at 37℃ for 45 min, and then unbound PPIX was washed off twice with PBS. DAPI was used to indicate the position of the nucleus. Cells were observed under the fluorescence microscope. 4C PPIX at the final concentration of 5 μg/mL was added to the lipid suspension in PBS and incubated at 37℃ for 45 min; then, the unbound PPIX was removed and the lipid was washed with PBS. The PPIX bound to the lipid was then extracted with 1 mL of methanol for 1 h, followed by the measurements of the fluorescence intensity at the excitation wavelength of 365 nm. 4D Lipids were suspended in PBS at a concentration of 1 mg/mL; then, PPIX was added to a final concentration of 1 μg/mL. After incubation for 2 h, the fluorescence intensity in either the presence or absence of lipids was measured at the excitation wavelength of 365 nm.
This observation prompted us to further investigate the inhibitory effect of PPIX using four different drug administration approaches, including pretreatment of the cells, pretreatment of virus, during infection, and after infection, as reported previously [18]. After drug treatment, the cells were incubated for 48 h postinfection, and then the antiviral effect was evaluated by measuring the cell survival with an MTT assay. Again, the data in Table 2 showed that the IC50 value of the ‘pretreatment of the virus’ approach was the most active drug administration with the IC50 value of 1.88 ± 0.85 µM compared with the other approaches.
Table 2.
The anti-Influenza virus activity of PPIX using four different drug treatment approachesa.
| Name | IC50 ± SD (μg/mL) |
|||
|---|---|---|---|---|
| Pretreatment of cell | Pretreatment of virus | During infection | After infection | |
| S-KKWK | >9.85 | 1.85 ± 0.92 | 5.75 ± 1.86 | >9.85 |
| PPIX | >17.77 | 1.88 ± 0.85 | 8.87 ± 3.07 | >17.77 |
The virus used in this experiment was influenza virus A/PR/8/34 (H1N1)
3.4. The possible mechanism of PPIX was interaction with the lipid bilayer of enveloped virus
It is known that hemagglutinin (HA) plays a critical role in the early stage of infection by mediating the entry of IAV into host cells [25]. We therefore employed two assays including the hemagglutinin inhibition assay and the hemolysis inhibition assay to study the possible interactions between HA and PPIX to determine whether PPIX can inhibit viral adsorption into target cells or inhibit the hemolytic effect on chicken erythrocytes [22]. Both experiments showed negative results in the test range, indicating that hemagglutinin (HA) may not be the primary target of PPIX (Fig. S1 A and S1B).
Due to the significant role of the viral lipid bilayer in the entry process of viruses [26], we next examined whether PPIX binds to the surface of the lipid bilayer. Because the lipids of the enveloped virus are derived from the host cell membrane [27], we then extracted lipids directly from MDCK cells and used them for the following study. PPIX at a final concentration of 5 μg/mL was added to the suspended lipid or MDCK cells in PBS and incubated for 45 min. After removal of the unbound PPIX, the red fluorescence of PPIX was detected under the fluorescence microscope (Fig. 4 B), and its intensity was quantified using a fluorescence spectrophotometer at an excitation wavelength of 365 nm and a scanning range of 600 to 680 nm (Fig. 4 C). PPIX showed apparent binding toward the lipid bilayer, as indicated in Fig. 4 B and 4C.
We further investigated the interactions between PPIX and lipids using fluorescence spectroscopy. The experiment was conducted by measuring the emission fluorescence from 600 to 680 nm when excited at 365 nm of 1 µg/mL of PPIX either in PBS or mixed with 1 mg/mL of lipids. As shown in Fig. 4 D, a significant redshift from 618 to 634 nm in the maximum emission wavelength was detected when measurements were conducted in the presence of lipids.
Next, proton nuclear magnetic resonance (1H NMR) spectroscopic technology was used to study the interactions between PPIX and lipids. As indicated in Table S1, in the presence of lipids, the chemical shifts of a number of protons of PPIX moved downfield to a different extent with or without the presence of water. Moreover, the C-5, C-10, C-15 and C-20 positions of porphyrin showed more chemical shifts than the other positions (Fig. 1).
To quantitatively measure the lipid-PPIX interactions, isothermal titration calorimetry (ITC) technology was employed to measure the thermodynamic curves resulting from the binding of PPIX to lipids [28]. First, 50 μg/mL PPIX was added to lipids at a concentration of 1 mg/mL, and the thermal changes were then measured. The thermogram in Fig. S1 C shows that the interactions between PPIX and lipids were exothermic due to the negative ITC peaks, and the binding affinity Ka (association constant) resulting from the binding of PPIX to lipids was 0.01 µM−1 as calculated from the measured ΔH and TΔS values (Table S2).
3.5. The major antiviral effect of PPIX was not resulted from the activation of PPIX irritated by the red-light
PPIX possesses a porphyrin skeleton which is well known for its role as a photosensitizer in the photodynamic therapy (PDT) against cancers and microbial infections including bacterial, fungal and viral infections [29]. When exposure at a specific wavelength, PPIX may be excited from the ground state to the excited state, which as a result, generates active radicals or oxygen molecules in the excited single state [30]. These active particles will instantaneously react with biological components such as plasma membrane, proteins, and nucleic acids [31], leading to the death of cancer cells or microbial invaders.
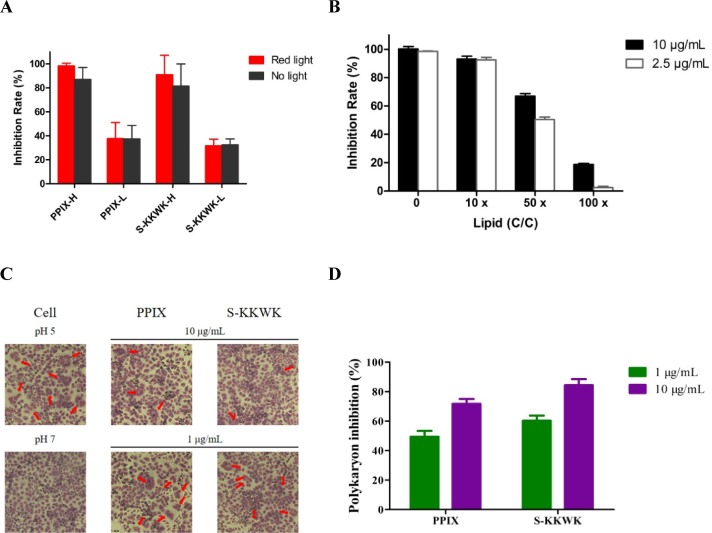
To test whether the antiviral activity of PPIX was due to the irritation effect by visible light, we performed a comparison experiment by respectively testing its antiviral activity with and without the irritation of red light. The concentrations of PPIX were 5 and 1 µg/mL respectively, and the experiment was performed by pretreatment of influenza A/Puerto Rico/8/34 (H1N1) at 100 TCID50 mixed with PPIX for 30 min under the irritation of red light or wrapped with an aluminum (no light), prior to transferring to the MDCK cells for another 1 h, which was also wrapped with the aluminum in the whole process. As shown in Fig. 5 A, no significant difference in their antiviral activity was observed, indicating the major antiviral activity of PPIX was not resulted from the active radicals or oxygen molecules.
Fig. 5.
A The antiviral activity of PPIX under the controlled light condition. The red bar indicates that the mixture of virus and compounds was irritated with red light for 30 min prior to adding to the MDCK cells, while the black bar indicates the mixture of virus and compounds were wrapped with aluminum for 30 min before transferring into MDCK cells. The MDCK cells were then wrapped with aluminum and infected with the mixture of virus and compounds for another 1 h before post-infection at 37 ℃ for 48 h and measuring the antiviral effect as indicated in the experimental section. The influenza A/Puerto Rico/8/34 (H1N1) virus at 100 TCID50 and concentrations of 5 and 1 µg/mL of compounds were respectively used in this experiment, while virus entry inhibitor S-KKWK was used as a control [18]. 5B The inhibition rate of PPIX against influenza A virus in the presence of lipids. PPIX was used at 10 and 2.5 µg/mL while the lipid concentration was 10×, 50×, and 100 × that of PPIX. The antiviral activity was assessed using the ‘pretreatment of the virus’ approach. 5C Polykaryon formation inhibition assay. PPIX or a positive control of S-KKWK [18] was added to the MDCK cells expressing HA from influenza A/Thailand/Kan353/2004, of which the positive control was set to be the same as that reported in our previous work [17]; subsequently, the culture medium was acidified to a pH of 5.0 and allowed to incubate for 15 min at 37℃. After neutralization, the cells were incubated for another 3 h. Then, the cells were fixed and stained with Giemsa solution. Syncytium formation was visualized, and the number of syncytia was indicated by red arrowheads under a microscope. 5D The number of syncytia was counted and quantified in whole fields of the plate. Each polykaryon containing at less three nuclei was counted. The syncytium formation inhibition rate was determined by comparing with the pH 5.0 control. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.6. The addition of lipids reduces the anti-IAV activity of PPIX
The virus lipid bilayer as the possible target of PPIX prompted us to speculate whether the addition of lipids to PPIX would affect the anti-IAV activity of PPIX. The results as displayed in Fig. 5 A show that the antiviral activity of PPIX was decreased along with the increase of lipids, further confirming the interactions between lipids and PPIX.
Due to the early inhibitory effect of PPIX toward the infection of influenza A virus and because the experimental data showed that PPIX may interact with the viral lipid bilayer to interfere with the entry of virus into host cells, we then employed a polykaryon formation inhibition assay to study the effects of PPIX on the formation of syncytium mediated by HA under acidic conditions [21]. As shown in Fig. 5 B and 5C, the number of syncytia was significantly decreased when tested in the presence of PPIX, especially at a higher concentration of 10 µg/mL, where the number of syncytia was reduced by up to 80% compared with the control without drug treatment, again confirming the possible interactions between PPIX/HA or PPIX/cell membranes.
In addition, considering that these molecules are working through interactions with membrane bilayer thereby blocking the fusion of viral-host cells’ membrane, it is critical to understand the resistance profile of these compounds. To this end, the influenza A/PR/8/34 (H1N1) virus (100 TCID50) was then passed through ten generations in the presence of sublethal concentration of PPIX (1 µg/mL) with each generation for three days. As a result, the IC50 value was 2.18 ± 0.71 µg/mL (Fig. S1 D), indicating that no significant reduction toward this viral strain was observed after passage of ten generations.
3.7. PPIX displays antiviral activity in vivo
To explore the preliminary antiviral effect of PPIX in vivo, we next tested the anti-IAV activity of PPIX using mice infected with comparatively high titers of IAV. In this experiment, 4-week-old (ca. 19–22 g) male Kunming mice were divided into five groups with 10 mice each and intranasally challenged twice each with 2 × 105 TCID50 of influenza A/PR/8/34 (H1N1) virus in a volume of 30 µL of normal saline (NS), as indicated in Fig. 6 A. The drugs, either a high (10 mg/kg) or low dose (2.5 mg/kg) of PPIX or oseltamivir phosphate (10 mg/kg), were orally administered once a day starting from the first day after viral challenging until the fifth day. The in vivo antiviral effect of PPIX was then determined by measuring the mean number of survival days, and the survival rate was measured up to 15 days.
The data in Fig. 6 A show that the survival rate and mean survival time of the infected mice treated with high and low dosages of PPIX were apparently increased compared with the virus control group without drug treatment (p < 0.01), in which all mice were dead within seven days. In addition, the lung indices measurements showed a significant increase for all virus-challenged mice compared with the control group without virus challenging (p < 0.001). Moreover, the average lung indices for compound-treated groups were lower than for the virus control group (p < 0.01) (Fig. 6 B), which was further supported by the lung images where a dramatic difference in their lung colors was observed between compound-treated groups and virus control group (Fig. 6 D).
In contrast, with the slow increase for the blank control group and PPIX-treatment group without virus challenging, the body weight of all virus-infected mice decreased until the eighth day, where the body weights of both the PPIX- and oseltamivir phosphate-treated groups slowly increased. Meanwhile, all mice in the virus control group died within seven days (Fig. 6 C).
Furthermore, to evaluate the antiviral effect of PPIX in virus infected mice, we measured the concentrations of PPIX in plasma of mice within 24 h. As shown in Fig. S2 and Table S4, the data showed that the maximum peak of PPIX in plasma was about 2 h after orally administered 0.2 mL of drug at 10 mg/kg, and the concentration was ca. 0.0517 ± 0.030 µg/mL. After that, the levels of PPIX were gradually decreased to 0.0179 ± 0.010 µg/mL in between 4 and 8 h, and was reduced to almost undetectable level of 0.0057 ± 0.002 µg/mL at 18 h (Fig. S2 and Table S4).
4. Discussion
Among a number of emerging and re-emerging contagious viruses, enveloped RNA viruses account for many outbreaks [1], [32], including SARS in 2003, the H1N1 epidemics in 2009, and the current COVID-19 outbreak. In addition, many viruses, such as the Lassa and Ebola viruses, that lead to severe hemorrhagic fever are highly pathogenic; thus far, there are no FDA-approved drugs available for treating these diseases [19]. Therefore, the purpose of this study is to develop broad antivirals by targeting the essential components in the virus life cycle for prophylaxis and treatment of these diseases [33].
Herein, we report a porphyrin derivative of PPIX that showed broad anti-IAV activity against several unrelated RNA viruses, including the highly pathogenic Lassa virus (LASV) and Machupo virus (MACV) as well as SARS-CoV-2, which is responsible for the current worldwide outbreak of COVID-19. In addition, PPIX is also active against various subtypes of influenza A viruses (IAV), such as A/Puerto Rico/8/34 (H1N1), the A/FM/1/47 (H1N1) mouse-adapted viral strain, A/Puerto Rico/8/34 (H1N1) with the NA-H274Y mutation, and the A/Aichi/2/68 (H3N2) viral strain, with IC50 values ranging from 0.91 ± 0.25 μM to 1.88 ± 0.34 μM. These results indicate that PPIX may interact with a key component involved in the life cycle of these viruses.
To investigate the possible modes of action of PPIX, by using influenza A/Puerto Rico/8/34 (H1N1) virus as a testing strain, we employed a set of experiments involving different drug administration approaches, indirect immunofluorescence staining, qRT-PCR and plaque reduction assays (Fig. 2). The results show that the antiviral effect of PPIX was mainly observable in the early stage of infection. Based on these results, we further identified that the activity may have resulted from the interactions of PPIX with the lipid bilayer of the virus envelope, thereby inhibiting the entry of virus; this was deduced from fluorescence spectroscopy (Fig. 3 B, Fig. 4 A and Fig. S1 A), NMR experiments (Table S1), and semiquantitative ITC analyses (Table S2 and Fig. S1 C). This conclusion is consistent with the results of our previous study, showing that the antiviral activity of Pyropheophorbide a (PPa) was due to the interaction with the viral membrane or biophysical interference with the virus-cell membrane fusion process, which resulted in blocking the entry of an enveloped virus into cells [17].
Nevertheless, considering the pivotal role of hemagglutinin (HA) of influenza A virus in mediating the entry of the virus, the results of the fusion inhibition assay (Fig. 4 C and 4D) cannot exclude the possibility that the antiviral activity may have resulted from the interaction of PPIX with the surface glycoprotein HA, blocking the entry of the virus.
However, (1) both the negative results of the hemagglutinin inhibition assay and hemolysis inhibition assay (Fig. 2 D and 2E) showed that PPIX was unable to either block the absorption of virus onto host cells or inhibit the fusion of virus with the host cell membrane [20], [22]; (2) PPIX showed broad antiviral activities against a number of unrelated enveloped viruses belonging to different families; and (3) PPIX showed interactions with the lipid bilayer. Therefore, we reasonably deduced that the major antiviral properties were not mediated by a specific receptor of the viral particles. Instead, the observed inhibitory effect in the fusion inhibition assay (Fig. 4 C) may be attributed to the interaction of PPIX with the lipid bilayer.
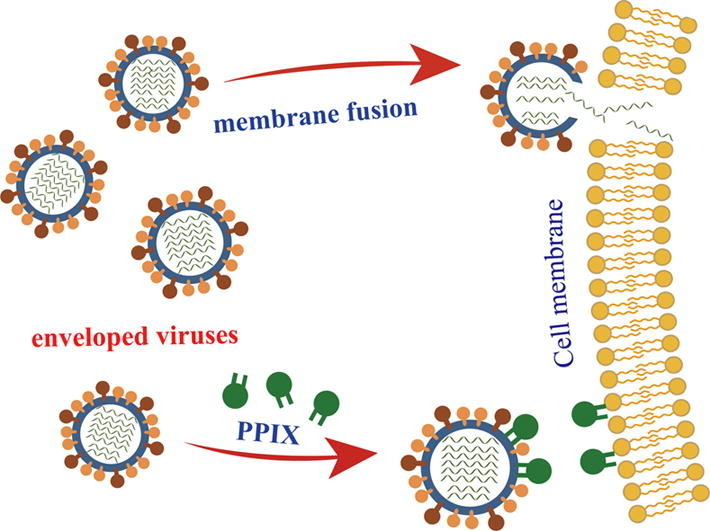
From the structural point of view (Fig. 1), PPIX possesses a rigid amphipathic skeleton with two polar carboxy groups and a nonpolar moiety of aromatic rings. These features enable PPIX to intercalate with hydrophobic lipids of enveloped virions or cell membrane [34], [35], thereby inhibiting the formation of the negative curvature required for fusion [36] or inhibiting virion-cell lipid mixing [34]; as a result, these features inhibit infectivity of a broad variety of unrelated viruses [36]. When PPIX was modified with a large nonpolar group, as indicated in Fig. 1, the structural integrity as well as the amphipathicity of PPIX was compromised, correspondingly diminishing the antiviral effectivity.
With respect to the selective virion-to-cell activity of PPIX over that of cellular fusion, based on the model proposed by Colpitts et al, it could be deduced that PPIX functions by increasing the hemifusion stalk energy barrier when intercalated in lipids [34], [35]. Compared with virions that are metabolically inert, cells are able to use metabolic energy to reshape lipids and bring membranes together. These different properties between virions and cells may lead to the observed selectivity of PPIX when interacting with virions and host cells [34], [36], [37], [38]. Therefore, it can be deduced that the fusion inhibitory activity of PPIX resulted from the biophysical interactions rather than the chemical interactions with lipids [36]. However, the proposed mechanism remains to be investigated.
To assess the antiviral effect of PPIX in vivo, we respectively compared the survival rate, lung indices, and body weights of the drug-treated group with those of no drug-treated group using PR8 virus infected mice. The data indicated that PPIX showed the antiviral effect to some extent, however, were not so dramatically different from those of control group. In this regard, we measured the levels of PPIX in plasma of mice, which showed that the maximum amount of ca. 0.0517 ± 0.030 µg/mL of PPIX was appeared after orally administered drug for 2 h. Based on these preliminary data and considering the high virus titer [2 × 105 TCID50 of influenza A/PR/8/34 (H1N1) virus] that was used to infect mice, we deduce that this might be the reason for the animal experiment results were not so impressing, although the antiviral effect of PPIX was observable to some extent. Therefore, to accurately exhibit the antiviral effect of drugs, the mouse model in this experiment should be further improved.
In this study, we report the antiviral properties of PPIX. Structurally, it possesses a porphyrin core with two aliphatic carboxy groups; therefore, PPIX is a new rigid amphipathic fusion inhibitor (RAFIs) [37]. It showed broad-spectrum antiviral activities toward a panel of unrelated enveloped viruses, including highly pathogenic Lassa virus (LASV) and Machupo virus (MACV) as well as SARS-CoV-2. The mechanism study indicated that PPIX biophysically interacted with the lipid bilayer of the enveloped virus, inhibiting the membrane-associated functions required for virion-to-cell fusion. Because membrane fusion is an essential step for most enveloped viruses, PPIX showed a broad spectrum of antiviral activity toward many unrelated enveloped viruses, Nevertheless, detailed information on how the interaction with the lipid bilayer inhibits the fusion process is still unknown. Even so, PPIX exerts broad antiviral activity and can still be used as a lead compound for further rational modification to obtain more potent candidates for the treatment of infections with enveloped viruses.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors wish to thank Dr. Guang Yang for his measurements of ITC data. This work was funded by the National Natural Science Foundation of China (81773556), China, Science and Technology Department of Guangdong Province of China (2015A020211010), and Southern Medical University (B1040903).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioorg.2020.104619.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Howard C.R., Fletcher N.F. Emerging virus diseases: can we ever expect the unexpected? Emerg. Microbes. Infect. 2012;1 doi: 10.1038/emi.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith A.E., Helenius A. How viruses enter animal cells. Science. 2004;304:237–242. doi: 10.1126/science.1094823. [DOI] [PubMed] [Google Scholar]
- 3.Marsh M., Helenius A. Virus entry: Open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamauchi Y., Helenius A. Virus entry at a glance. J. Cell. Sci. 2013;126:1289–1295. doi: 10.1242/jcs.119685. [DOI] [PubMed] [Google Scholar]
- 5.Mercer J., Schelhaas M., Helenius A. Virus Entry by Endocytosis. Annu. Rev. Biochem. 2010;79:803–833. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- 6.Edinger T.O., Pohl M.O., Stertz S. Entry of influenza A virus: host factors and antiviral targets. J. Gen. Virol. 2014;95:263–277. doi: 10.1099/vir.0.059477-0. [DOI] [PubMed] [Google Scholar]
- 7.Martens S., Mcmahon H.T. Mechanisms of membrane fusion: disparate players and common principles. Nat. Rev. Mol. Cell. Biol. 2008;9:543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- 8.Badani H., Garry R.F., Wimley W.C. Peptide entry inhibitors of enveloped viruses: the importance of interfacial hydrophobicity. Biochim. Biophys. Acta. 1838;2014:2180–2197. doi: 10.1016/j.bbamem.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bobardt M.D., Cheng G., de Witte L., Selvarajah S., Chatterji U., Sanders-Beer B.E., Geijtenbeek T.B., Chisari F.V., Gallay P.A. Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus. Proc. Natl. Acad. Sci. U S A. 2008;105:5525–5530. doi: 10.1073/pnas.0801388105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng G., Montero A., Gastaminza P., Whitten-Bauer C., Wieland S.F., Isogawa M., Fredericksen B., Selvarajah S., Gallay P.A., Ghadiri M.R., Chisari F.V. A virocidal amphipathic α-helical peptide that inhibits hepatitis C virus infection in vitro. Natl. Acad. Sci. U S A. 2008;105:3088–3093. doi: 10.1073/pnas.0712380105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mody T.D. Pharmaceutical development and medical applications of porphyrin-type macrocycles. J. Porphyr. Phthalocya. 2000;4:362–367. [Google Scholar]
- 12.Ballut S., Naud-Martin D., Loock B., Maillard P. A Strategy for the targeting of photosensitizers. Synthesis, characterization, and photobiological property of porphyrins bearing glycodendrimeric moieties. J. Org. Chem. 2011;76:2010–2028. doi: 10.1021/jo102185d. [DOI] [PubMed] [Google Scholar]
- 13.Chen S., Fetzer J.C., Meyerhoff M.E. Pharmaceutical development and medical applications of porphyrin-type macrocycles. Fresenius. J. Anal. Chem. 2001;369:385–392. doi: 10.1007/s002160000580. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong N.R. Phthalocyanines and porphyrins as materials. J. Porphyr. Phthalocya. 2000;4:414–417. [Google Scholar]
- 15.Choi S.R., Britigan B.E., Narayanasamy P. Dual inhibition of Klebsiella pneumonia and Pseudomonas aeruginosa iron metabolism using gallium porphyrin and gallium nitrate. ACS. Infect. Dis. 2019;5:1559–1569. doi: 10.1021/acsinfecdis.9b00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X.B., Wang Y., Wang P., Cheng X.X., Liu Q.H. Sonodynamically induced anti-tumor effect with protoporphyrin IX on hepatoma-22 solid tumor. Ultrasonics. 2010;51:539–546. doi: 10.1016/j.ultras.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Chen D., Lu S., Yang G., Pan X., Fan S., Xie X., Chen Q., Li F., Li Z., Wu S., He J. The seafood Musculus senhousei shows anti-influenza A virus activity by targeting virion envelope lipids. Biochem Pharmacol. 2020;16 doi: 10.1016/j.bcp.2020.113982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin D.G., Luo Y.Z., Yang G., Li F.F., Xie X.K., Chen D.W., He L.F., Wang J.Y., Ye C.F., Lu S.S., Lv L., Liu S.W., He J. Potent influenza A virus entry inhibitors targeting a conserved region of hemagglutinin. Biochem. Pharmacol. 2017;144:35–51. doi: 10.1016/j.bcp.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Wang P.L., Liu Y., Zhang G.S., Wang S.B., Guo J., Cao J.Y., Jia X.Y., Zhang L.K., Xiao G.F., Wang W. Screening and identification of Lassa virus entry inhibitors from an FDA-approved drug library. J. Virol. 2018;92:e00954–e1018. doi: 10.1128/JVI.00954-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M.L., Cao R.Y., Zhang L.K., Yang X.L., Liu J., Xu M.Y., Shi Z.L., Hu Z.H., Zhong W., Xiao G.F. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell. Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai K.K., Cheung N.N., Yang F., Dai J., Liu L., Chen Z.W., Sze K.H., Chen H.L., Yuen K.Y., Kao R.Y.T. Identification of novel fusion inhibitors of influenza A virus by chemical genetics. J. Virol. 2015;90:2690–2701. doi: 10.1128/JVI.02326-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin D.G., Li F.F., Wu Q.Y., Xie X.K., Wu W.J., Wu J., Chen Q., Liu S.W., He J. A ‘building block’ approach to the new influenza A virus entry inhibitors with reduced cellular toxicities. Sci. Rep. 2016;6:22790. doi: 10.1038/srep22790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zu M., Yang F., Zhou W.L., Liu A.L., Du G.H., Zheng L.S. In vitro anti-influenza virus and anti-inflammatory activities of theaflavin derivatives. Antiviral. Res. 2012;94:217–224. doi: 10.1016/j.antiviral.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Fangfang Li, Shengsheng Lu, Xi Xie, Sheng Fan, Daiwei Chen, Shaohua Wu, Jian He, Antiviral properties of extracts of Streptomyces sp. SMU 03 isolated from the feces of Elephas maximus. Fitoterapia. 143 (2020) 104600. [DOI] [PubMed]
- 25.Gamblin SJ, Haire LF, Russell RJ, Stevens DJ, Xiao B, Ha Y, Vasisht N, Steinhauer DA, Daniels RS, Elliot A, Wiley DC, Skehel JJ. The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science. 303 (2004) 1838–1842. [DOI] [PubMed]
- 26.Wu W.J., Lin D.G., Shen X.T., Li F.F., Fang Y.X., Li K.Q., Xun T.R., Yang G., Yang J., Liu S.W., He J. New influenza A virus entry inhibitors derived from the viral fusion peptides. Plos. One. 2015;10 doi: 10.1371/journal.pone.0138426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhn R.J., Strauss J.H. Enveloped viruses. Adv. Protein. Chem. 2003;64:363–377. doi: 10.1016/s0065-3233(03)01010-6. [DOI] [PubMed] [Google Scholar]
- 28.Myszka D.G. Kinetic, equilibrium, and thermodynamic analysis of macromolecular interactions with BIACORE. Method. Enzymol. 2000;323:325–340. doi: 10.1016/s0076-6879(00)23372-7. [DOI] [PubMed] [Google Scholar]
- 29.Rajesh S., Koshi E., Philip K., Mohan A. Antimicrobial photodynamic therapy: An overview. J. Indian Soc. Periodontol. 2011;15:323–327. doi: 10.4103/0972-124X.92563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castano A.P., Demidova T.N., Hamblin M.R. Mechanisms in photodynamic therapy: Part one-Photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004;1:279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma J., Jiang L. Photogeneration of singlet oxygen (1O2) and free radicals (Sen·−, O·−2) by tetra-brominated hypocrellin B derivative. Free Radic. Res. 2001;35:767–777. doi: 10.1080/10715760100301271. [DOI] [PubMed] [Google Scholar]
- 32.Yee H.Y., Luo D.H. RIG-I-Like receptors as novel targets for pan-antivirals and vaccine adjuvants against emerging and re-emerging viral infections. Front. Immunol. 2018;9:1379. doi: 10.3389/fimmu.2018.01379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halfon P., Sarrazin C. Future treatment of chronic hepatitis C with direct acting antivirals: is resistance important? Liver. Int. 2012;1:79–87. doi: 10.1111/j.1478-3231.2011.02716.x. [DOI] [PubMed] [Google Scholar]
- 34.Colpitts C.C., Ustinov A.V., Epand R.F., Epand R.M., Korshun V.A., Schang L.M. 5-(Perylen-3-yl) ethynyl-arabino-uridine (aUY11), an arabino-based rigid amphipathic fusion inhibitor, targets virion envelope lipids to inhibit fusion of influenza virus, hepatitis C virus, and other enveloped viruses. J. Virol. 2013;87:3640–3654. doi: 10.1128/JVI.02882-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.St Vincent M.R., Colpitts C.C., Ustinov A.V., Muqadas M., Joyce M.A., Barsby N.L., Epand R.F., Epand R.M., Khramyshev S.A., Valueva O.A., Korshun V.A., Tyrrell D.L., Schang L.M. Rigid amphipathic fusion inhibitors, small molecule antiviral compounds against enveloped viruses. Proc. Natl. Acad. Sci. U S A. 2010;107:17339–17344. doi: 10.1073/pnas.1010026107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Speerstra S., Chistov A.A., Proskurin G.V., Aralov A.V., Ulashchik E.A., Streshnev P.P., Shmanai V.V., Korshun V.A., Schang L.M. Antivirals acting on viral envelopes via biophysical mechanisms of action. Antiviral. Res. 2018;149:164–173. doi: 10.1016/j.antiviral.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 37.Vigant F., Hollmann A., Lee J., Santos N.C., Jung M.E., Lee B. The rigid amphipathic fusion inhibitor dUY11 acts through photosensitization of viruses. J. Virol. 2014;88:1849–1853. doi: 10.1128/JVI.02907-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vigant F., Santos N.C., Lee B. Broad-spectrum antivirals against viral fusion. Nat. Rev. Microbiol. 2015;13:426–437. doi: 10.1038/nrmicro3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.