Abstract
Myocardial infarction (MI) is one of the most serious cardiovascular diseases associated with myocardial ischemia/reperfusion (I/R) injury. Glaucocalyxin A (GLA) is a biologically active ent-kauranoid diterpenoid that has been found to ameliorate myocardial I/R injury in mice. However, the mechanism has not been fully investigated. In the present study, we aimed to investigate the effect of GLA on rat cardiomyocytes H9c2 cells exposed to hypoxia/reoxygenation (H/R). The results showed that GLA treatment improved cell viability of H/R-stimulated H9c2 cells. Administration with GLA suppressed the H/R-stimulated reactive oxygen species (ROS) production in H9c2 cells. GLA also elevated the activities of antioxidant enzymes, including superoxide dismutase and glutathione peroxidase in H/R-stimulated H9c2 cells. Moreover, GLA prevented H/R-stimulated cell apoptosis in H9c2 cells, as evidenced by increased bcl-2 expression, decreased bax expression, as well as reduced caspase-3 activity. Furthermore, GLA enhanced the activation of protein kinase B (Akt)/nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling pathway in H9c2 cells exposed to H/R. Additionally, treatment with LY294002 reserved the protective effects of GLA on H/R-stimulated oxidative injury in H9c2 cells. In conclusion, these findings suggested that GLA protected H9c2 cells from H/R-stimulated oxidative damage, which was mediated by the Akt/Nrf2/HO-1 signaling pathway. Thus, GLA might be a promising therapeutic agent for the prevention and treatment of myocardial I/R.
Keywords: myocardial infarction (MI), glaucocalyxin A (GLA), myocardial ischemia/reperfusion (I/R) injury, oxidative stress, Akt/Nrf2/HO-1 signaling pathway
Introduction
Myocardial infarction (MI) is one of the most serious cardiovascular diseases which is resulted from the formation of plaques in the interior walls of arteries1,2. During the MI process, reduced blood flow to the heart causes a lack of oxygen supply and heart attack. MI is a leading cause of death among all cardiovascular diseases3,4. A comprehensive understanding of the molecular mechanisms may be helpful for the development of an effective intervention strategy for MI.
Acute myocardial ischemia/reperfusion (I/R) injury is a potent event in the development of MI5. A variety of pathological processes and mediators are involved in the I/R-related cell injury, including the generation of reactive oxygen species (ROS), changes in the pH, acute inflammatory response, and intracellular calcium overload6. Among these, overproduction of ROS is proposed to be the major intracellular event that occurred during I/R injury7. ROS are the most likely contributors to reperfusion-induced oxidative stress, which may contribute to myocardial injury and cardiomyocyte death through a variety of mechanisms, such as inducing the activation of proapoptotic pathways8,9. Therefore, attenuating I/R-mediated oxidative stress and apoptosis may be beneficial for preventing MI.
Glaucocalyxin A (GLA) is a biologically active ent-kauranoid diterpenoid that has been widely studied for some important biological activities, including antitumor, antibacterial, antioxidative, anti-inflammatory, anticoagulative, antithrombotic, and immune regulatory activities10–13. In addition, GLA might be an attractive candidate for improving the prognosis of acute MI by controlling cardiac fibrosis14. Additionally, GLA was reported to ameliorate myocardial I/R injury in mice through the attenuation of microvascular thrombosis15. However, the effects of GLA on I/R-mediated oxidative stress and apoptosis in cardiomyocytes remain unclear. The aim of this study was to investigate the role of GLA in H9c2 cells exposed to hypoxia/reoxygenation (H/R) and explore the underlying mechanism.
Materials and Methods
Cell Culture
The rat cardiomyocyte-derived H9c2 cell line (Cell Bank of the Chinese Academy of Sciences, Shanghai, China) were cultivated in Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco) and 1% (v/v) penicillin/streptomycin (Gibco) at 37 °C in a 5% carbon dioxide (CO2) atmosphere.
Establishment of H/R Model
To simulate myocardial I/R injury in vitro, H9c2 cells were cultured in a hypoxic environment with 1% oxygen (O2), 94% nitrogen (N2), and 5% CO2 in modular gas chambers for 24 h, followed by reoxygenation for 2 h in a 21% O2, 5% CO2, and 74% N2 incubator at 37 °C. Cells were pretreated with or without GLA for 2 h before H/R stimulation.
Cell Cytotoxicity Assay
After incubation with a series concentration of GLA (0, 5, 10, 20, 40 μM) for 24 h, the culture supernatants were collected for the determination of lactate dehydrogenase (LDH) leakage using a commercial LDH detection kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s directions.
Cell Viability Assay
Cell viability was evaluated using the cell counting kit-8 (CCK-8) solution assay (Dojindo, Kumamoto, Japan). Briefly, H9c2 cells were seeded at a density of 1 × 104 cells per well in 96-well plates. After various treatments, 10 µl of CCK-8 solution was added to the cells and incubated for 2 h following the manufacturer’s specifications. The cell viability was assessed by measuring the optical density at 450 nm using a microplate reader (BioTek, Winooski, VT, USA).
Measurement of Cellular ROS Production
The production of intracellular ROS was tested by flow cytometry using 2′-7′dichlorofluorescin diacetate (DCFH-DA) as the fluorescence probe. In short, H9c2 cells with different treatments were washed with phosphate-buffered saline and then incubated with 10 µM DCFH-DA (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C for 30 min in the dark. H9c2 cells were then analyzed by the flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) using a 488 nm excitation filter and a 525 nm emission filter.
ELISA
H9c2 cells were lysed in lysis buffer (Invitrogen, Carlsbad, CA, USA) for 30 min at 4 °C, and then the cell lysates were collected for the determination of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities with relevant detection kits (Nanjing Jiancheng Bioengineering Institute) per the manufacturer’s instructions.
Western Blot Analysis
Total and nuclear proteins of H9c2 cells were, respectively, extracted by protein extraction kits (Beyotime Institute of Biotechnology, Shanghai, China) following the manufacturer’s protocol. After the determination of the protein concentration using a bicinchoninic acid kit (Beyotime), protein fractions were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then blotted onto polyvinylidene difluoride membranes (pore size: 0.45 µm; Millipore Billerica, MA, USA). The membranes were blocked with 5% (w/v) skim milk dissolved in Tris-buffered saline with tween 20 (TBST) buffer for 2 h at room temperature. Subsequently, membranes were incubated overnight at 4 °C with primary antibodies against nuclear factor erythroid 2-related factor 2 (Nrf2), lamin B1 (Abcam, Cambridge, MA, USA); β-actin, heme oxygenase 1 (HO-1), bax, bcl-2 (Invitrogen), protein kinase B (Akt) or p-Akt (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:1000 dilution in TBST. Then, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz) for 1 h at room temperature. The signals were visualized by Image Lab software (Bio-Rad Laboratories, Hercules, CA, USA) after exposing the membranes to enhanced chemiluminescence solution (Millipore).
Caspase-3 Activity
The caspase-3 activity in H9c2 cells was analyzed using a caspase-3 activity assay kit (Beyotime Biotechnology, China) based on the catalytic activity of caspase-3 on Ac-DEVD-pNA. The formation of pNA was detected using a microplate reader (BioTek) at 405 nm to reflect the caspase-3 activity.
Statistical Analysis
Results from three independent experiments were analyzed by SPSS software 19.0 (SPSS Inc, Chicago, IL, USA) and presented as means ± SD. Comparisons among groups were determined by one-way analysis of variance followed by the least significant difference test. Differences were proposed to be statistically significant when P < 0.05.
Results
GLA Improves the Cell Viability in H/R-Stimulated H9c2 Cells
To assess the cytotoxic effect of GLA on H9c2 cells, the cells were incubated with a series concentration of GLA (0, 5, 10, 20, 40 μM) for 24 h. LDH leakage assay showed that GLA at the concentration of 40 μM caused a significant increase in LDH leakage (Fig. 1A). Therefore, the concentrations of 5, 10, and 20 μM were selected in the following experiments. Subsequently, we examined the effect of GLA on cell viability of H/R-stimulated H9c2 cells. Results from 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay demonstrated that preincubation with GLA (5, 10, and 20 μM) attenuated H/R-caused decreased cell viability of H9c2 cells (Fig. 1B).
Fig. 1.
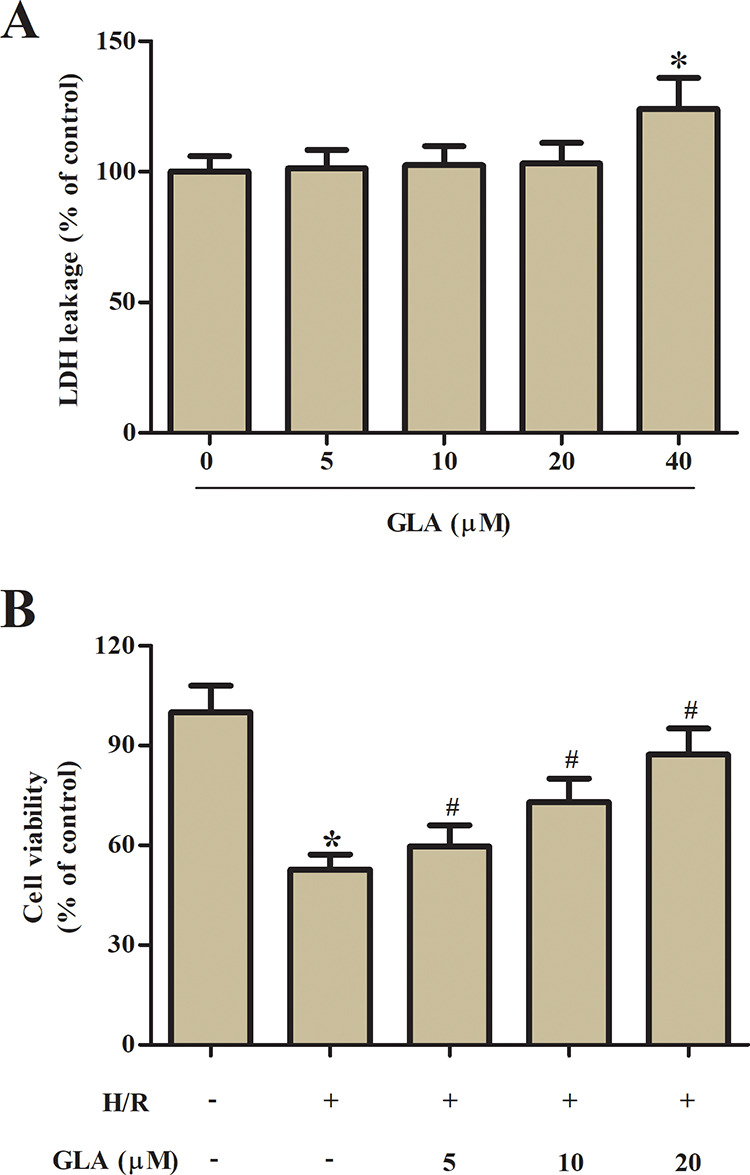
Effect of GLA on cell viability of H9c2 cells. (A) LDH leakage assay was performed to assess the cytotoxic effect of GLA on H9c2 cells after incubation with a series concentration of GLA (0, 5, 10, 20, and 40 μM) for 24 h. (B) MTT assay was performed to assess the protective effect of GLA on H/R-stimulated H9c2 cells. H9c2 cells were preincubated with GLA (5, 10, and 20 μM) for 2 h, and then subjected to H/R stimulation. *P < 0.05 indicates the significant difference compared with control H9c2 cells. # P < 0.05 indicates the significant difference compared with H/R-stimulated H9c2 cells. GLA: glaucocalyxin A; H/R: hypoxia/reoxygenation; LDH: lactate dehydrogenase; MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
GLA Represses Oxidative Stress in H9c2 Cells Exposed to H/R Treatment
As shown in Fig. 2A, the ROS level in H9c2 cells exposed to H/R treatment was markedly increased as compared with control H9c2 cells, while the induction of ROS production was mitigated by GLA. Moreover, H/R-caused decrease in SOD and GSH-Px activities, which were blocked by pretreatment with GLA (Fig. 2B, C).
Fig. 2.
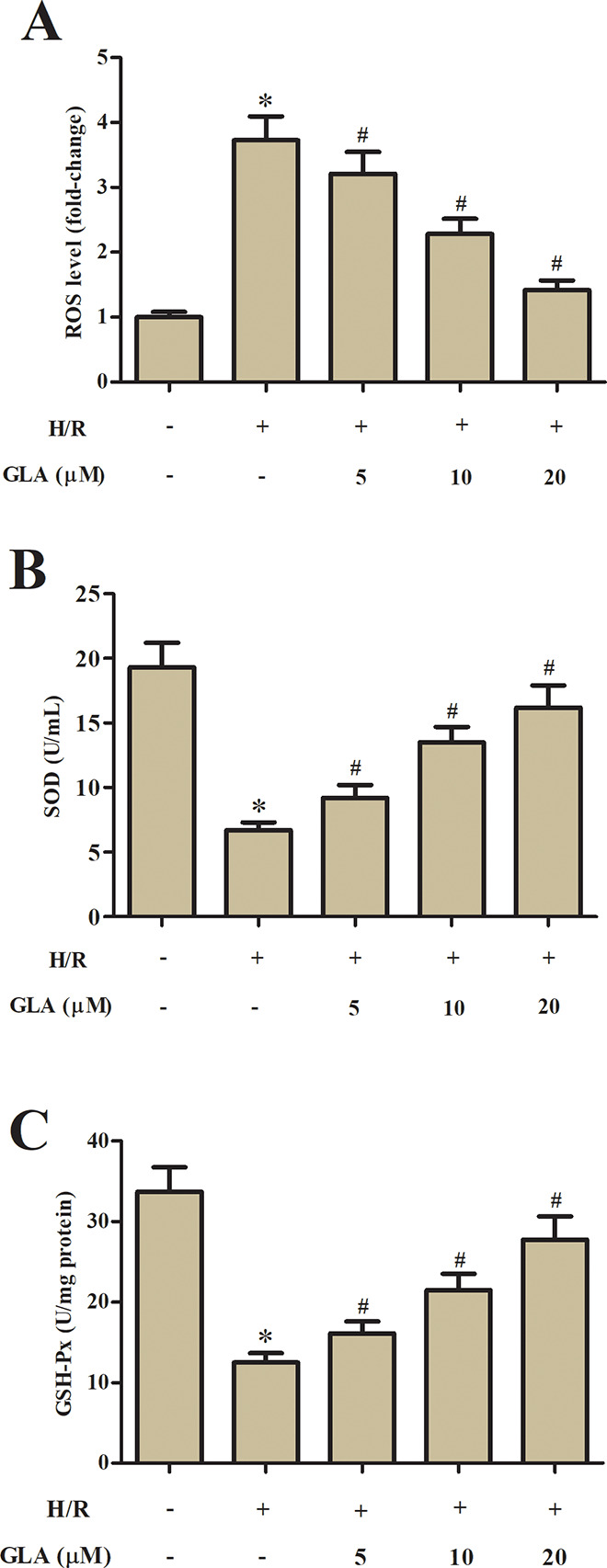
Effect of GLA on oxidative stress in H9c2 cells. H9c2 cells were preincubated with GLA (5, 10, and 20 μM) for 2 h, and then subjected to H/R stimulation. (A) Flow cytometry was performed to evaluate the ROS production in H9c2 cells using the fluorescence probe DCFH-DA. (B, C) ELISA was carried out to assess the SOD and GSH-Px activities in H9c2 cells. *P < 0.05 indicates the significant difference compared with control H9c2 cells. # P < 0.05 indicates the significant difference compared with H/R-stimulated H9c2 cells. DCFH-DA: 2′-7′dichlorofluorescin diacetate; GLA: glaucocalyxin A; GSH-Px: glutathione peroxidase; H/R: hypoxia/reoxygenation; ROS: reactive oxygen species; SOD: superoxide dismutase.
GLA Suppresses Apoptosis in H9c2 Cells Exposed to H/R Treatment
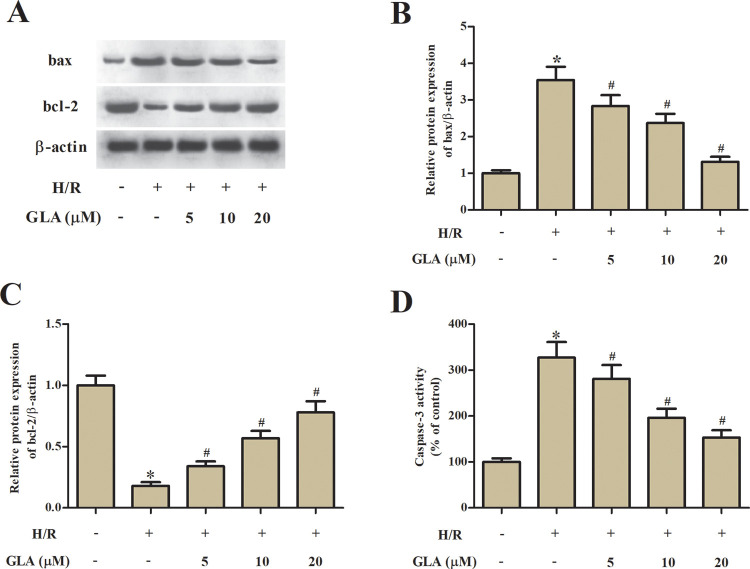
Subsequently, cell apoptosis was assessed by detecting the expression levels of bax and bcl-2. H/R treatment caused a significant increase in bax expression and decrease in bcl-2 expression in H9c2 cells. The H/R-caused changes in the expression levels of bax and bcl-2 were prevented by GLA (Fig. 3A–C). In addition, the caspase-3 activity was significantly increased in H/R-stimulated H9c2 cells. However, the increased caspase-3 activity was decreased in H9c2 cells pretreated with GLA (Fig. 3D).
Fig. 3.
Effect of GLA on apoptosis in H9c2 cells. H9c2 cells were preincubated with GLA (5, 10, and 20 μM) for 2 h, and then subjected to H/R stimulation. (A) Western blot analysis was performed to detect the expression levels of apoptosis-related proteins, including bax and bcl-2. (B, C) Quantification analysis of bax and bcl-2. (D) Colorimetric method was used to determine the caspase-3 activity with the substrate peptide Ac-DEVD-pNA. *P < 0.05 indicates the significant difference compared with control H9c2 cells. # P < 0.05 indicates the significant difference compared with H/R-stimulated H9c2 cells. GLA: glaucocalyxin A; H/R: hypoxia/reoxygenation.
GLA Induces the Activation of Akt/Nrf2/HO-1 Signaling Pathway in H/R-Stimulated H9c2 Cells
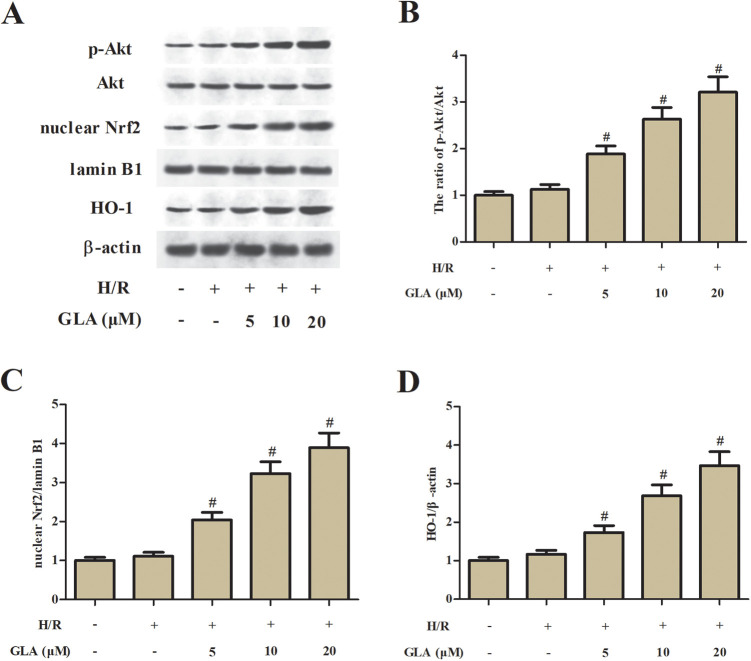
It has been proven that the Akt/Nrf2/HO-1 signaling pathway is associated with the I/R-induced oxidative injury. To uncover the mechanism underlying the protective effect of GLA, the expression levels of nuclear Nrf2, HO-1, Akt, and p-Akt were detected using Western blot. As shown in Fig. 4, GLA treatment significantly induced the expression levels of p-Akt, nuclear Nrf2, and HO-1 in H/R-stimulated H9c2 cells.
Fig. 4.
Effect of GLA on the Akt/Nrf2/HO-1 signaling pathway in H9c2 cells. H9c2 cells were preincubated with GLA (5, 10, and 20 μM) for 2 h, and then subjected to H/R stimulation. (A) Western blot analysis was performed to detect the expression levels of p-Akt, Akt, nuclear Nrf2, and HO-1. (B) The ratio of p-Akt/Akt. (C) The ratio of nuclear Nrf2/lamin B1. (D) The ratio of HO-1/β-actin. # P < 0.05 indicates the significant difference compared with H/R-stimulated H9c2 cells. Akt: protein kinase B; GLA: glaucocalyxin A; HO-1: heme oxygenase-1; Nrf2: nuclear factor erythroid 2-related factor 2.
Treatment with LY294002 Reserved the Effects of GLA on Cell Viability, Oxidative Stress, and Apoptosis in H/R-Stimulated H9c2 Cells
Then, the H9c2 cells were treated with LY294002 to block the activation of the Akt/Nrf2/HO-1 signaling pathway. Treatment with LY294002 caused a reduction in cell viability of H9c2 cells, as compared with the H9c2 cells pretreated with 20 μM GLA (Fig. 5A). The decreased ROS production caused by GLA was prevented by LY294002 (Fig. 5B). In addition, the GLA-caused decrease in caspase-3 activity was elevated after treatment with LY294002 (Fig. 5C).
Fig. 5.
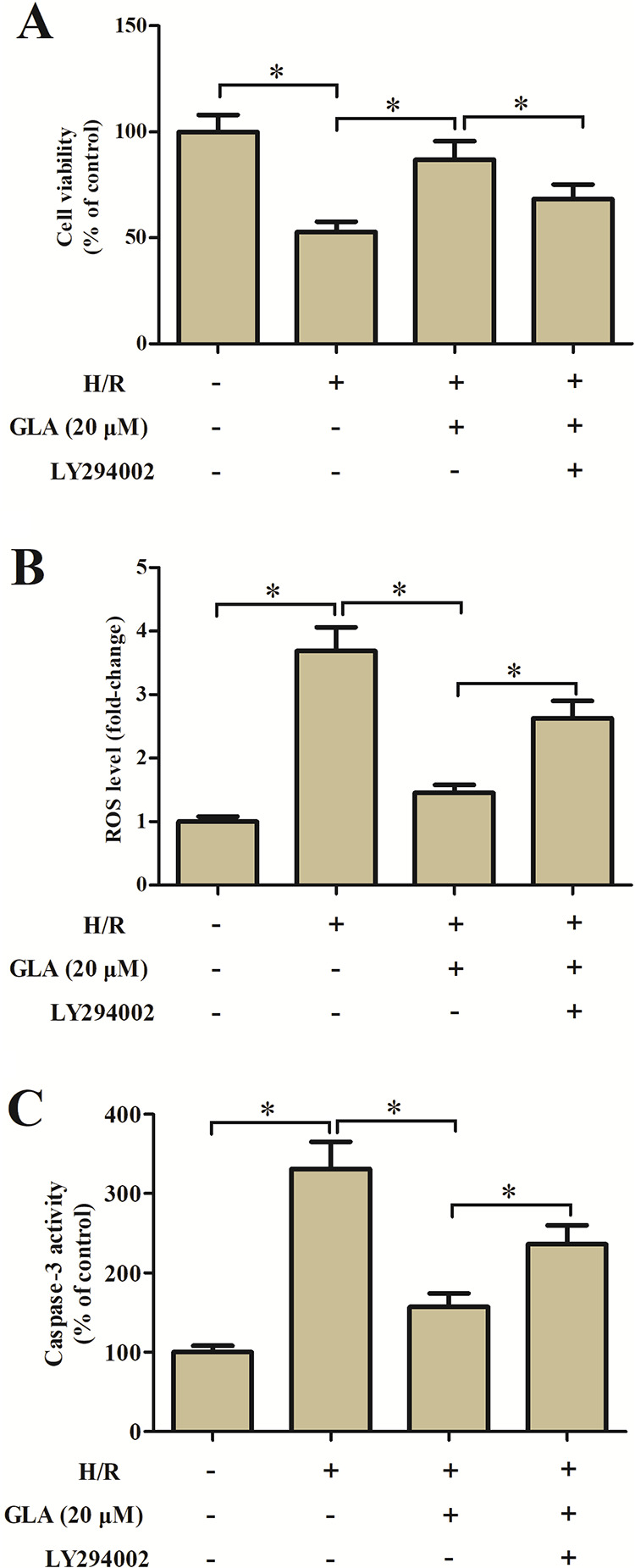
Effect of LY294002 on cell viability, oxidative stress, and apoptosis in H9c2 cells. H9c2 cells were preincubated with GLA (20 μM) and/or LY294002 (10 μM) for 2 h and then subjected to H/R stimulation. (A) MTT assay was performed to assess cell viability. (B) Flow cytometry was performed to evaluate ROS production. (C) Colorimetric method was used to determine the caspase-3 activity. *P < 0.05. GLA: glaucocalyxin A; H/R: hypoxia/reoxygenation; MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; ROS: reactive oxygen species.
Discussion
In the current study, the results indicated that GLA protected H9c2 cells from H/R-stimulated oxidative damage, as evidenced by increased cell viability, decreased oxidative stress, and apoptosis. The protective effect of GLA was proved to be associated with the activation of the Akt/Nrf2/HO-1 signaling pathway.
GLA is a biologically active ent-kauranoid diterpenoid isolated from Rabdosia japonica var. glaucocalyx. GLA has been found to possess protective effect on myocardial I/R injury in mice. GLA administration significantly reduces infarct size, improves left ventricular ejection fraction, and left ventricular fractional shortening in mice subjected to myocardial I/R. The protective effect of GLA myocardial I/R injury is proposed to be mediated by the attenuation of microvascular thrombosis15. Based on the mechanism investigations, increased production of ROS and ROS-mediated oxidative damage during the I/R process play crucial roles in the development of I/R injury. Dong et al.16 reported that pretreatment with GLA markedly suppresses transforming growth factor beta-1 (TGF-β1)-induced ROS level in hepatic stellate cells (HSCs), indicating that GLA may suppress the ROS production in response to TGF-β1 stimulation.
Thus, we aimed to explore the protective effect of GLA on ROS production and ROS-mediated oxidative damage during I/R injury. Our results showed that GLA treatment improved cell viability of H/R-stimulated H9c2 cells. GLA also suppressed the ROS production and elevated the activities of antioxidant enzymes, including SOD and GSH-Px. Additionally, GLA prevented H/R-stimulated cell apoptosis, as evidenced by increased bcl-2 expression and decreased bax expression and caspase-3 activity. These findings suggested that GLA protected H9c2 cells from H/R-stimulated oxidative damage. Our results demonstrated the mechanism underlying the protective effect of GLA on myocardial I/R injury, in addition to attenuation of microvascular thrombosis.
Nrf2 is known as an important transcription factor that plays an essential role in the cellular defense against inflammation and oxidative stress17. Under oxidative stress conditions, Nrf2 dissociates from Keap1 and translocates into the nucleus. The transcription factor then binds to the antioxidant response element and regulates the production of multiple antioxidative enzymes18–20. HO-1 is a well-known intracellular inducible phase II detoxifying enzyme that can be regulated byNrf221,22. Nrf2/HO-1 signaling pathway is involved in cellular defense against various oxidative-inducing agents22. Increasing evidence has proven that the Nrf2/HO-1 signaling pathway represents a promising therapeutic intervention in preventing and attenuating I/R injury23–25.
In addition to Nrf2 signaling, phosphatidylinositol 3-kinase (PI3K)/Akt is a key signaling pathway in governing the cellular defense system against oxidative injury26. Importantly, PI3K/Akt is upstream signaling of Nrf2 and associated with Nrf2 activation and HO-1 induction27,28. Indeed, the PI3K/Akt pathway has been observed to play a vital role in the Nrf2-mediated antioxidant response. Our results showed that GLA treatment significantly induced the expression levels of p-Akt, nuclear Nrf2, and HO-1 in H/R-stimulated H9c2 cells, implying that GLA induced the activation of Akt/Nrf2/HO-1 signaling pathway. Furthermore, inhibition of Akt signaling by LY294002 reserved the protective effects of GLA on H/R-stimulated oxidative injury in H9c2 cells. These findings suggested that the effects of GLA were mediated by Akt/Nrf2/HO-1 signaling pathway.
In conclusion, our findings demonstrated that GLA protects H9c2 cells against H/R-induced injury through the activation of the Akt/Nrf2/HO-1 pathway. Thus, GLA might be a promising therapeutic agent for the prevention and treatment of MI. Moreover, further in vivo investigations should be carried out in the future.
Footnotes
Ethical Approval: Ethical Approval is not applicable for this article.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Xiaoyan Dang  https://orcid.org/0000-0002-5626-8562
https://orcid.org/0000-0002-5626-8562
References
- 1. Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–1135. [DOI] [PubMed] [Google Scholar]
- 2. Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53(1):31–47. [DOI] [PubMed] [Google Scholar]
- 3. Sesso H. Maternal and paternal history of myocardial infarction and risk of cardiovascular disease in men and women. Circulation. 2001;104(4):393–398. [DOI] [PubMed] [Google Scholar]
- 4. Archacki S, Wang Q. Expression profiling of cardiovascular disease. Hum Genomics. 2004;1(5):355–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu C, Ren D, Wang X, Ha T, Liu L, Lee EJ, Hu J, Kalbfleisch J, Gao X, Kao R. Toll-like receptor 3 plays a role in myocardial infarction and ischemia/reperfusion injury. Biochimica Et Biophysica Acta. 2014;1842(1):22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Brit J Pharmacol. 2009;138(4):532–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bolli R. Oxygen-derived free radicals and myocardial reperfusion injury: an overview. Cardiovasc Drugs Ther. 1991;5(suppl 2):249–268. [DOI] [PubMed] [Google Scholar]
- 8. Zhou T, Chuang CC, Zuo L. Molecular characterization of reactive oxygen species in myocardial ischemia-reperfusion injury. Biomed Res Int. 2015;2015:864946-864954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalogeris T, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014;2:702–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xiang Z, Wu X, Liu X, Jin Y. Glaucocalyxin a: a review. Nat Prod Res. 2014;28(24):2221–2236. [DOI] [PubMed] [Google Scholar]
- 11. Jiang X, Zhang Z, Song C, Deng H, Yang R, Zhou L, Sun Y, Zhang Q. Glaucocalyxin a reverses EMT and TGF-beta1-induced EMT by inhibiting TGF-beta1/Smad2/3 signaling pathway in osteosarcoma. Chem Biol Interact. 2019;307:158–166. [DOI] [PubMed] [Google Scholar]
- 12. Yang F, Cao Y, Zhang J, You T, Zhu L. Glaucocalyxin a improves survival in bleomycin-induced pulmonary fibrosis in mice. Biochem Biophys Res Commun. 2017;482(1):147–153. [DOI] [PubMed] [Google Scholar]
- 13. Li W, Tang X, Yi W, Li Q, Ren L, Liu X, Chu C, Ozaki Y, Zhang J, Zhu L. Glaucocalyxin a inhibits platelet activation and thrombus formation preferentially via GPVI signaling pathway. PLoS One. 2013;8(12):e85120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Su Q, Zhang Y. Glaucocalyxin a attenuates angiotensin II-induced cardiac fibrosis in cardiac fibroblasts. Biochem Biophys Res Commun. 2018;503(3):1949–1954. [DOI] [PubMed] [Google Scholar]
- 15. Liu X, Xu D, Wang Y, Chen T, Wang Q, Zhang J, You T, Zhu L. Glaucocalyxin a ameliorates myocardial ischemia-reperfusion injury in mice by suppression of microvascular thrombosis. Med Sci Monit. 2016;22:3595–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dong Z, Gao Q, Guo H. Glaucocalyxin a attenuates the activation of hepatic stellate cells through the TGF-beta1/Smad signaling pathway. DNA Cell Biol. 2018;37(3):227–232. [DOI] [PubMed] [Google Scholar]
- 17. Hu L, Zhang Y, Miao W, Cheng T. Reactive oxygen species and Nrf2: functional and transcriptional regulators of hematopoiesis. Oxid Med Cell Longev. 2019;2019:5153268-5153278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao MY, Guo HM, Chen JM, Fujino M, Ito H, Takahashi K, Abe F, Nakajima M, Tanaka T, Wang JJ, Huang HL, et al. 5-aminolevulinic acid combined with sodium ferrous citrate ameliorates H2O2-induced cardiomyocyte hypertrophy via activation of the MAPK/Nrf2/HO-1 pathway. Am J Physiol Cell Physiol. 2015;308(8):C665–C672. [DOI] [PubMed] [Google Scholar]
- 19. Yan HY, Huang ZL, Bai QY, Sheng YC, Hao ZX, Wang ZT, Ji LL.Natural product andrographolide alleviated APAP-induced liver fibrosis by activating Nrf2 antioxidant pathway. Toxicology. 2018;396-397:1–12. [DOI] [PubMed] [Google Scholar]
- 20. Hosohata K. Role of oxidative stress in drug-induced kidney injury. Int J Mol Sci. 2016;17(11):1826–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alcaraz MJ, Ferrandiz ML. Relevance of Nrf2 and heme oxygenase-1 in articular diseases. Free Radic Biol Med. 2019;157:83–93. [DOI] [PubMed] [Google Scholar]
- 22. Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73(17):3221–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou C, Luo D, Xia W, Gu C, Lahm T, Xu X, Qiu Q, Zhang Z. Nuclear factor (Erythroid-Derived 2)-like 2 (Nrf2) contributes to the neuroprotective effects of histone deacetylase inhibitors in retinal ischemia-reperfusion injury. Neuroscience. 2019;418:25–36. [DOI] [PubMed] [Google Scholar]
- 24. Xu D, Chen L, Chen X, Wen Y, Yu C, Yao J, Wu H, Wang X, Xia Q, Kong X. The triterpenoid CDDO-imidazolide ameliorates mouse liver ischemia-reperfusion injury through activating the Nrf2/HO-1 pathway enhanced autophagy. Cell Death Dis. 2017;8(8):e2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu SH, Zhang YC. Effect of levocarnitine on cerebral ischemia-reperfusion rats via activating Nrf2/ARE signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(18):8168–8174. [DOI] [PubMed] [Google Scholar]
- 26. Gao Q. Oxidative stress and autophagy. Adv Exp Med Biol. 2019;1206:179–198. [DOI] [PubMed] [Google Scholar]
- 27. Zhuang Y, Wu H, Wang X, He J, He S, Yin Y. Resveratrol attenuates oxidative stress-induced intestinal barrier injury through PI3K/Akt-mediated Nrf2 signaling pathway. Oxid Med Cell Longev. 2019;2019:7591840-7591853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu Y, Zhang S, Xue J, Wei Z, Ao P, Shen B, Ding L. CGRP reduces apoptosis of DRG cells induced by high-glucose oxidative stress injury through PI3K/AKT induction of heme oxygenase-1 and Nrf-2 expression. Oxid Med Cell Longev. 2019;2019:2053149-2053157. [DOI] [PMC free article] [PubMed] [Google Scholar]