Abstract
The transcription factor Pax4 plays an essential role in the development of insulin-producing β cells in pancreatic islets. Ectopic Pax4 expression not only promotes β cell survival but also induces α-to-β cell transdifferentiation. This dual functionality of Pax4 makes it an appealing therapeutic target for the treatment of insulin-deficient type 1 diabetes (T1D). In this study, we demonstrated that Pax4 gene delivery by an adenoviral vector, Ad5.Pax4, improved β cell function of mouse and human islets by promoting islet cell survival and α-to-β cell transdifferentiation, as assessed by multiple viability assays and lineage-tracing analysis. We then explored the therapeutic benefits of Pax4 gene delivery in the context of islet transplantation using T1D mouse models. Both mouse-to-mouse and human-to-mouse islet transplantation, via either kidney capsule or portal vein, were examined. In all settings, Ad5.Pax4-treated donor islets (mouse or human) showed substantially better therapeutic outcomes. These results suggest that Pax4 gene delivery into donor islets may be considered as an adjunct therapy for islet transplantation, which can either improve the therapeutic outcome of islet transplantation using the same amount of donor islets or allow the use of fewer donor islets to achieve normoglycemia.
Keywords: Pax4, islet transplantation, transdifferentiation, cell survival, diabetes
Introduction
Type 1 diabetes (T1D) is caused by the lack of insulin-producing β cells that leads to insulin deficiency and hyperglycemia. The ultimate treatment for T1D requires the ability to replace the lost β cells, not just the hormone insulin. Indeed, replenishing the lost β cells in T1D patients via islet transplantation has been remarkably successful in stabilizing glucose control, to a degree that is superior to intensive insulin therapy1–4. Specifically, glycemic control appears to be smoother and hypoglycemia less prevalent in the islet recipients, even though they might not have achieved insulin independence1,4,5. To date, islet transplantation via portal vein infusion is used in the clinic not only for selected T1D patients2,4,6 but also for preventing surgical diabetes after near-total or total pancreatectomy in the treatment of severe chronic pancreatitis. In fact, total pancreatectomy with islet auto-transplantation (TP-IAT), in which the islets are isolated from the patient’s pancreas and transplanted back via portal vein infusion, has become a standard practice for severe chronic pancreatitis7,8.
Despite these progresses, challenges remain. These include the following. (1) A limited supply of human islets. A large number of islets are required to achieve insulin-independence, but the number of pancreatic donors is limited. For TP-IAT, the amount of functional islets recovered from the pancreatitis patients is often not sufficient—a major reason for the inability to achieve 100% insulin-independence in all patients. (2) Inefficient islet survival and engraftment—only a fraction of the infused islets survive and engraft following islet transplantation via the portal vein9–13. These obstacles have significantly limited the use of islet transplantation for a broader patient range. Therefore, strategies promoting islet survival and β cell function are vital for enhancing the efficacy of islet transplantation.
The transcription factor Pax4 plays a critical role in the determination of β cell lineage during embryonic development and is important for β cell expansion and survival14–16. Genetic knockout of Pax4 from mice results in the absence of mature β cells, concomitant with a significant increase in the number of glucagon-producing α cells17,18. In contrast, genetic knock-in and lineage-tracing studies in mice have shown that ectopic Pax4 expression in α cells results in the restoration of functional β cells by converting α cells into β cells19,20. Other studies have shown that Pax4 promotes β cell survival and proliferation by regulating cell cycle proteins and maintaining endoplasmic reticulum (ER) integrity in response to stress21–24. In humans, Pax4 mutations are found to be associated with maturity-onset diabetes of the young and early onset of type 2 diabetes (T2D)25–32, confirming that Pax4 plays important role in maintaining β cell mass and β cell function in humans. The ability of Pax4 to induce α-to-β cell conversion and its ability to promote β cell survival make it an attractive therapeutic target for diabetes treatment33.
In this study, we explored the therapeutic benefits of Pax4 gene delivery in the context of islet transplantation. We examined the effects of Pax4 gene delivery into the primary mouse and human islets on islet cell survival and α-to-β cell conversion and evaluated the therapeutic outcome of islet transplantation with Ad5.Pax4-treated donor islets in various settings.
Materials and Methods
Animals
The α-yellow fluorescence protein (αYFP) mice (males and females) were generated and genotyped in-house as described34. Briefly, the glucagon-cre mice, B6.Cg-Tg(Gcg-cre)1Herr/Mmnc (Identification# 358-UNC, the Mutant Mouse Regional Resource Centers), were bred with Rosa26-YFP-LoxP mice (The Jackson Laboratory, Bar Harbor, ME, USA). The mice containing glucagon-cre and homozygous YFP genes designated as αYFP mice. Male NOD-SCID mice, 8 weeks old, were purchased from the Jackson Laboratory and used to establish diabetes models. A single-dose of streptozotocin (STZ) at 130 mg/kg body weight was injected into each mouse via intraperitoneal injection to induce diabetes as described previously35. All animal procedures were approved by the Institutional Animal Care and Use Committee at Tulane University.
Antibodies
The Guinea Pig anti-insulin antibody (#ab7842) was purchased from Abcam (Cambridge, MA, USA), the mouse anti-GFP antibody (#A11120) from Thermo Fisher Scientific (Waltham, MA, USA), the mouse antiglucagon antibody (#G2654), and the rabbit anti-Pax4 antibody (HPA006806) from Sigma-Aldrich (St. Louis, MO, USA). All of the secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA).
Cell Survival Assays
Three types of cell survival assays were used in this study: propidium iodide (PI) staining, adenosine triphosphate (ATP)-based cell viability assay, and apoptotic nucleosome-based cell death assay. All assays were performed at ∼48 h after Ad5.Pax4 or control treatments of isolated islets. For PI staining, the islets were incubated with freshly prepared fluorescein diacetate and PI solution, at a final concentration of 5 μg/ml each. After washing with phosphate-buffered saline (PBS), they were imaged under a fluorescence microscope (Nikon Instruments Inc. Melville, NY, USA). The ATP-based cell viability assay was performed using the CellTiter-Glo Luminescent Cell Viability Assay kit (Promega Corp, Fitchburg, WI, USA) according to the manufacturer’s protocol. The raw data are normalized with insulin content and then converted to relative cell viability in comparison to the untreated islet group. Apoptosis assay was performed using the Cell Death Detection ELISA kit (Roche Diagnostics Corporation, Indianapolis, IN, USA) according to the manufacturer’s instructions.
Glucose-Stimulated Insulin Secretion (GSIS) Assay
Mouse or human islets were cultured in 24-well plates, 8 wells per group, following Ad5.Pax4 or control treatments. 4 to 5 days later, GSIS assay was performed essentially as described36. Briefly, the islets were preincubated in Krebs-Ringer Bicarbonate (KRB) solutions containing 2.5 mM glucose (KRB-low) for 30 min at 37°C in a 5% carbon dioxide (CO2) humidified incubator. Then, the supernatants were replaced with either a fresh KRB-low solution (4 wells per group) or with KRB containing 16.5 mM glucose (KRB-high; 4 wells per group) and incubated for 1 h at 37°C. After centrifugation, the supernatants and islets were both collected. The islets were subsequently lysed in radioimmunoprecipitation assay (RIPA) buffer and used to determine insulin content. Insulin concentration in the supernatants and islet lysates was measured with ELISA kits from ALPCO Diagnostics (Salem, NH, USA). Insulin concentration in the supernatant (insulin secretion) was normalized by the insulin content of the corresponding sample. The data were expressed as mean ± SD.
Hormone Measurement
Hormones, including insulin, glucagon, and human C-peptide, were measured using corresponding ELISA kits from ALPCO. Specifically, mouse insulin was measured with the mouse insulin kit 80-INSMS-E01, human insulin with the kit 80-INSHU-E01.1, human C-peptide with the kit 80-CPTHU-E01.1, and glucagon with the kit 48-GLUHU-E01. All assays followed the manufacturer’s protocols. Note the glucagon kit can be used to measure both human and mouse glucagon according to the manufacturer’s instruction.
Islet Culture and Virus Infection
Freshly isolated human islets were obtained from the Integrated Islet Distribution Program (IIDP) and cultured in CMRL-1066 media supplemented with 0.1 g/L l-glutamine, 10% fetal bovine serum (FBS), 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and 0.2% sodium bicarbonate37. Mouse islets were isolated from αYFP mice following standard protocols and cultured in Roswell Park Memorial Institute (RPMI) media supplemented with 10% FBS and 10 mM HEPES. All islets were cultured at 37°C in a 5% CO2 humidified incubator, with media refreshing every other day. For adenoviral vector infection, the vectors were added to the culture media at a multiplicity of infection (MOI) of 250 viral particles per cell (VPs/cell), using an estimate of 2000 cells/islet equivalent quantity (IEQ). The media was refreshed after overnight culturing.
Immunofluorescence Staining
Immunostaining of paraffin-embedded tissue slices was performed as described previously38. To stain cultured islets, the day after adenoviral vector infection, the islets were treated with trypsin for 5 minutes and plated in 24-well plates. The trypsin treatment resulted in mild disruption of the islets, facilitating the attachment of islet cells to the culture plate. After 4 to 5 days in culture, the cells were fixed and processed for immunofluorescence staining as described36.
Islet Transplantation
T1D was induced in NOD-SCID mice (male, 10 to 12 weeks of age) 2 to 3 days prior to transplantation. Mouse blood glucose was monitored daily, and islet transplantation performed when blood glucose reaches >350 mg/dL, typically 2 to 3 days after STZ injection. For portal vein islet transplantation, each diabetic mouse was anesthetized by intraperitoneal (i.p.) injection of ketamine and xylazine and placed under a dissecting microscope. After the abdomen was opened, mouse islets (400 islets/recipient) or human islets (2000 IEQ/recipient) suspended in 200 µl PBS were injected into the portal vein. For islet transplantation via kidney capsule, mouse islets (200 islets/recipient mouse) or human islets (1000 IEQ/recipient mouse) were injected under the kidney capsule of each diabetic mouse. Briefly, each mouse under anesthesia was placed under a dissecting microscope. Its left kidney was externalized through a small incision, and a tiny hole was made in the renal capsule with a 271/2 G needle. Islets suspended in PBS were picked into a gel loading pipette tip (0.5 mm diameter), which was connected with sterilized BTPE-50 polyethylene tubing (0.58 mm diameter) on a 50 µl Hamilton syringe (Reno, NV, USA) and allowed them to settle. The tube was inserted through the hole beneath the kidney capsule, and islets were gently forced out from the tube into the kidney. After the abdominal wall was sutured, and the mouse awoke from anesthesia, the mice were proceeded for standard postoperative care and blood glucose monitoring.
Quantification of YFP+ Cells, Bihormonal Cells, and TUNEL+ β Cells
Following immunofluorescence staining, 20 to 30 islet images were taken for each set of experiments. The numbers of YFP+ Insulin+ (i.e., YFP+ β cells) cells, YFP+ cells (pre-existing α cells), and Insulin+ (total β) cells in each islet were manually counted. The percentage of YFP+ β cells versus the total YFP+ cells or versus total β cells was calculated for each islet and then averaged for the whole group. For bihormonal cells, the number of insulin+glucagon+ (bihormonal) cells, insulin+ cells, and glucagon+ cells were manually counted from >20 images in each group. The total α and β cell numbers in each image were calculated by adding α and β cell numbers, then subtracting the bihormonal cell number. The percentage of bihormonal cells (vs the total α and β cells) in each image was then calculated and averaged for each treatment group. For TUNEL+ cells, >30 images of islet grafts in the liver from each group were counted for Insulin+ (β) cells, glucagon+ (α) cells, TUNEL+ β cells, and TUNEL+ α cells. The percentage of TUNEL+ β cells was calculated for each mouse and averaged to obtain the data for each group. All data were expressed as mean ± SD.
Statistical Analysis
GraphPad Prism 8.3.0 software was used for all statistical analyses. When analyzing glucose levels among multiple groups over time, a two-way analysis of variance (ANOVA) was performed to determine the significance of their differences. For each data point, Student’s t-test was performed to determine the significance of differences between two groups, and one-way ANOVA was performed to compare three or more groups. P < 0.05 was considered statistically significant.
TUNEL Staining
TUNEL staining was performed using the In situ Cell Death Detection kit (TMR Red) from Roche Diagnostics (Indianapolis, IN, USA) following the manufacturer’s instruction. Briefly, the paraffin-embedded liver slices were deparaffinized, permeabilized with 0.25% Triton X-100 in PBS, blocked with bovine serum albumin blocking solution, and incubated with anti-insulin and anti-glucagon primary antibodies similarly as described previously for immunofluorescence staining38. Immediately before secondary antibodies incubation, the TUNEL reaction mixture was prepared as specified in the manufacturer’s protocol and used to dilute the fluorescence-conjugated secondary antibodies for insulin (green) and glucagon (blue). The mixture (100 μl) was then added onto each tissue slice and incubated for 1 h at room temperature. After washing and air drying, the slides were mounted and processed for fluorescence microscopy as described38.
Results
Pax4 Gene Delivery Induces α-to-β Cell Transdifferentiation in Primary Mouse Islets
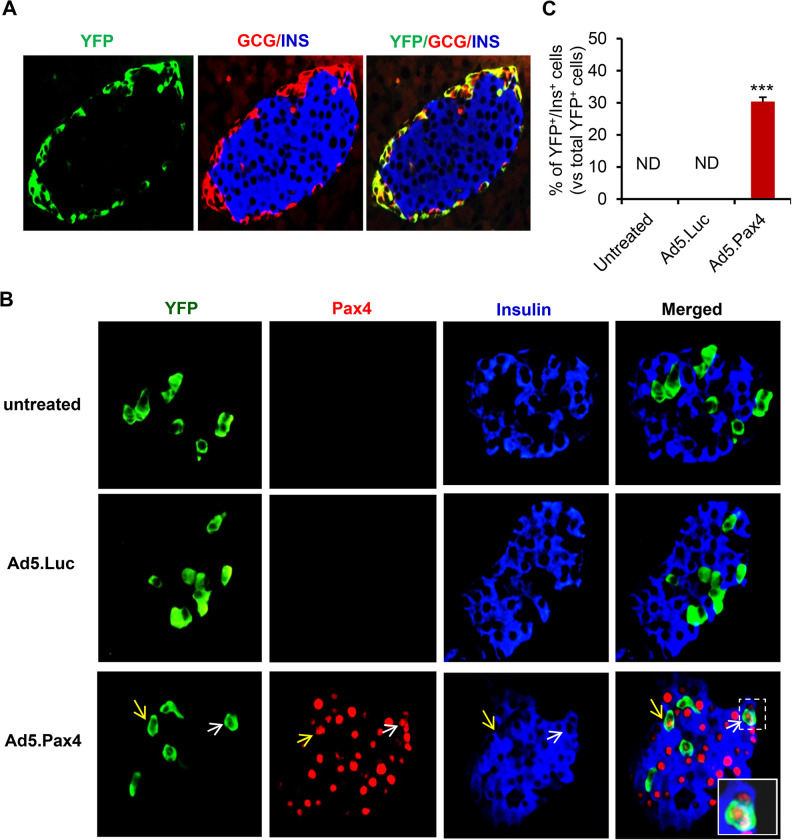
In our previous study, we developed a replication-deficient adenoviral vector-based Pax4 gene delivery vector, namely, Ad5.Pax4, in which human Pax4 cDNA is under the control of cytomegalovirus (CMV) promoter36. The vector induced α-to-β cell transdifferentiation in αTC1 cells and ameliorated hyperglycemia when injected directly into the pancreas of T1D mice via intrabile ductal injection36. In the current study, we investigated whether Pax4 gene delivery was able to induce α-to-β cell conversion in primary islets. To accomplish this, we performed lineage-tracing analysis using isolated islets from αYFP mice in which pancreatic α cells were labeled by enhanced yellow fluorescence protein (YFP)34. Immunofluorescence staining confirmed efficient and specific YFP-labeling of pre-existing α cells in the pancreas of αYFP mice (Fig. 1A). To examine whether ectopic Pax4 expression induces α-to-β cell conversion, freshly isolated αYFP islets were either untreated or treated with Ad5.Pax4 or control vector Ad5.Luc at MOI of 250 VPs/cell. Following overnight culture, the islets were slightly dissociated by trypsin treatment and then continued for in vitro culture for 4 to 5 days, with media refreshed every other day. The mild trypsin treatment facilitated islet cell attachment to the bottom of the culture plates, allowing subsequent immunofluorescence staining in situ. As shown in Fig. 1B, Pax4 was efficiently delivered into the islet cells and localized to the nuclei where a transcription factor is usually located. In addition, insulin expression was detected in many YFP+ cells in Ad5.Pax4-treated islets, but nearly undetectable in Ad5.Luc-treated or untreated islets, suggesting Pax4 induced the pre-existing α cells to express insulin. Quantification shows that Pax4 expression induced insulin expression in ∼30% of YFP+ cells (Fig. 1C). These data demonstrated that Pax4 expression induced α-to-β cell transdifferentiation in primary mouse islets.
Fig. 1.
Lineage tracing shows Pax4 gene delivery into primary mouse islets induces α-to-β cell transdifferentiation. (A) Immunofluorescence staining confirmed the YFP-labeling of pre-existing α cells in αYFP mice. The pancreatic slices of αYFP mice were triply stained for glucagon (red), insulin (blue), and YFP (green). YFP expression was detected in α cells and not in β cells. (B) Pax4 gene delivery induces α-to-β transdifferentiation in primary mouse islets. Freshly isolated islets from αYFP mice were untreated, treated with Ad5.Luc, or with Ad5.Pax4 at MOI of 250 viral particles/cell. Five days later, the cells were immunostained with antibodies against YFP (green), Pax4 (red), and insulin (blue). Arrows mark examples of insulin-producing cells that were pre-existing α cells. The inset shows an enlargement of one such cell. (C) Quantification showing the percentage of YFP+Ins+ cells versus total YFP+ cells. ***P < 0.001 compared with both control groups. MOI: multiplicity of infection; ND: not detectable; YFP: yellow fluorescence protein.
Pax4 Gene Delivery Promotes Islet Cell Survival in Primary Mouse Islets
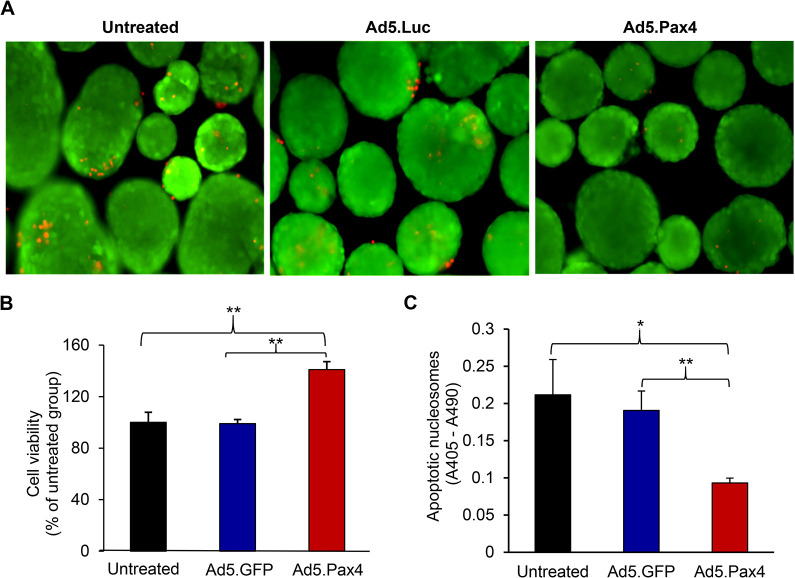
Previous studies have shown that Pax4 is essential for β cell survival and expansion15,21,23. We put Pax4 under the control of CMV promoter so that it can be expressed not only in α cells to induce α-to-β cell conversion but also in β cells to protect existing β cells, thus, maximizing the beneficial effects of Pax4 on β cell function. Here we examined whether Pax4 gene delivery into primary mouse islets was able to improve cell survival using three types of cell viability tests (Fig. 2). The PI staining showed that Ad5.Pax4-treated islets had fewer dead cells than the control groups (Fig. 2A). The quantitative ATP-based cell viability assays showed that Pax4 treatment significantly improved islet cell survival (Fig. 2B). In accordance, Ad5.Pax4-treated islets exhibited significantly less apoptosis than the control groups in the apoptotic nucleosome-based cell death assay (Fig. 2C). Note: all of these assays measured the total islet cell survival or apoptosis. Since β cells are the most abundant cells in the islets, these data support the beneficial role of Pax4 on β cell survival.
Fig. 2.
Ectopic Pax4 gene expression promotes islet cell survival in primary mouse islets. Freshly isolated mouse islets were untreated, treated with Ad5.GFP, or treated with Ad5.Pax4 at multiplicity of infection (MOI) of 250 viral particles/cell. Two days later, the islets were processed for cell viability and apoptosis assays. (A) Ad5.Pax4-treated islets appear to have fewer dead cells (red) than control groups as assessed by propidium iodide (PI) staining. (B) Adenosine triphosphate (ATP)-based cell viability assay showing Pax4 treatment significantly improved cell survival. The data were expressed as relative viability in comparison to the untreated islet group. (C) Apoptotic nucleosome-based assay showing Ad5.Pax4 treatment reduced the apoptosis of the islet cells. *P < 0.05; **P < 0.01.
Pax4 Gene Delivery Into Mouse Islets Improves the Therapeutic Efficacy of Islet Transplantation
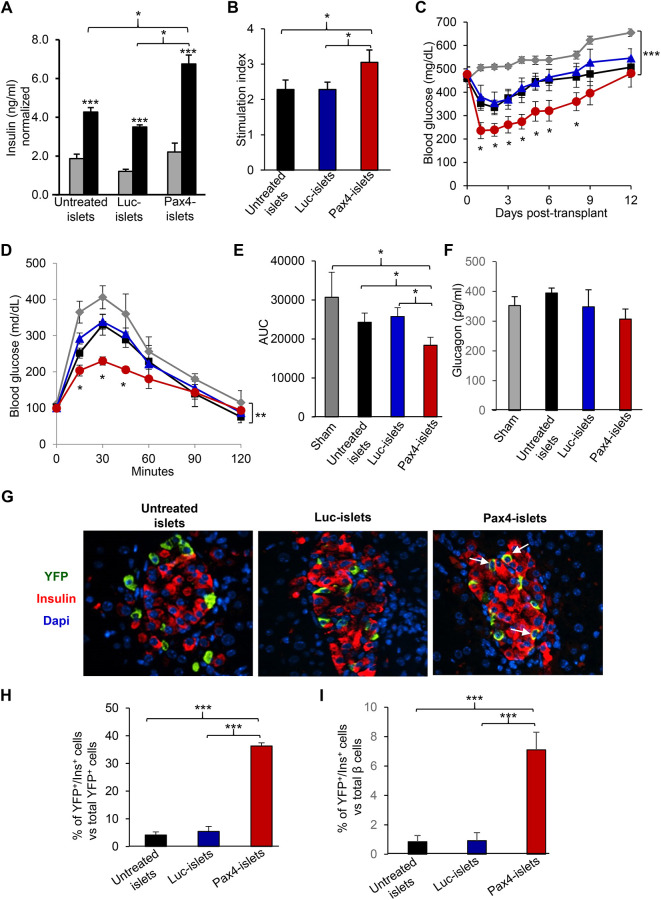
We hypothesized that the dual function of Pax4 made it an excellent target to improve β cell function of donor islets and to enhance the therapeutic efficacy of islet transplantation. To test the hypothesis, we first examined whether Pax4 gene delivery improved β cell function of donor mouse islets using GSIS assay (Fig. 3A). The Ad5.Pax4-treated islets showed substantially more insulin secretion in response to high glucose, with a significantly higher stimulation index than the control groups (Fig. 3A, B). We then performed islet transplantation studies in mouse-to-mouse allotransplantation settings via either kidney capsule (Supplemental Fig. S1) or portal vein injection (Fig. 3 C–G). In the experiments, freshly isolated islets from αYFP mice were treated with Ad5.Pax4 or control vectors and then transplanted into STZ-induced diabetic NOD-SCID mice. NOD-SCID mice were used to minimize immune rejection of the transplanted islets. All mice that were transplanted with islets showed better blood glucose control than the sham-transplanted group of mice, and mice receiving Ad5.Pax4-treated islets showed significantly better blood glucose control than those receiving untreated islets or control vector-treated islets (Fig. 3C; and Supplemental Fig. S1). Intraperitoneal glucose tolerance test (ipGTT), which was performed 1 week after islet transplantation, confirmed that Ad5.Pax4-treated islets improved the therapeutic outcome of islet transplantation compared with the control groups (Fig. 3D, E). In addition, we measured circulating glucagon levels of the mice at ∼2 weeks after transplantation (Fig. 3F) and did not detect significant differences among different groups. This is not surprising because the loss of α cells (due to α-to-β transdifferentiation) in transplanted Pax4-islets is not expected to cause glucagon deficiency because of the presence of α cells in the pancreas of the recipient mice. Also, it should be noted that in STZ-induced T1D models, glucagon levels are in general high compared with normal mice, which is largely contributable to the loss of paracrine insulin suppression of glucagon secretion. Of note, in normal mice, the nonfasting glucagon level is usually 100 to 150 pg/ml34.
Fig. 3.
Pax4 gene delivery into donor mouse islets improves β cell function and enhances the therapeutic efficacy of islet transplantation via portal vein injection. The freshly isolated islets from αYFP mice were untreated, treated with Ad5.Luc, or treated with Ad5.Pax4 at MOI of 250 viral particles/cell, and cultured in vitro for 2 to 3 days. In the meantime, NOD-SCID mice were rendered diabetic by a single dose of 130 mg/kg STZ, then transplanted with 400 islets/mouse (n = 7 to 8 mice per group). The sham transplanted group was included as a control. (A) GSIS assay showing Pax4 gene delivery improved β cell function. A small aliquot of treated islets (∼20 islets) from each group were subjected for GSIS assay in which 2.5 mM glucose was used as low glucose (gray bar) and 16.5 mM glucose (black bar) as high glucose. All data were normalized by insulin content. (B) SI of GSIS, which was calculated by dividing insulin concentrations at high glucose by that at low glucose for each group of islets. (C) Nonfasting BG of the diabetic mice in each group following islet transplantation. Gray: sham transplanted; black: transplanted with untreated islets; blue: transplanted with Ad5.Luc-treated islets (Luc-islets); red: transplanted with Ad5.Pax4-treated islets (Pax4-islets). (D) ipGTT of the recipient mice at one week after islet transplantation. Following overnight fasting (∼16 h), the mice were administered with 1 g glucose/kg bodyweight via intraperitoneal injection, and their BG concentrations were measured at various time points. The color codes for the groups are the same as in (C). (E) AUC of the ipGTT. (F) Nonfasting glucagon levels of the mice 2 weeks after islet transplantation. (G) Representative islet grafts in the liver of each group as identified by immunofluorescence staining. The liver slices were stained for YFP (green), insulin (red), and nuclei (blue). The arrows mark examples of YFP+Ins+ cells. (H) Quantification of YFP+Ins+ cells versus total YFP+ cells. (I) Percentage of YFP+Ins+ cells versus total β cells. *P < 0.05; **P < 0.01; ***P < 0.001. AUC: area under the curve; BG: blood glucose; GSIS: glucose-stimulated insulin secretion; ipGTT: intraperitoneal glucose tolerance test; MOI: multiplicity of infection; SI: stimulation index; STZ: streptozotocin; YFP: yellow fluorescence protein.
Furthermore, we examined islet grafts in the liver at the end of the transplantation study. Pax4 expression was hardly detectable in the islet grafts (data not shown), which is not unexpected because Ad5-mediated gene expression is transient, usually peaks at 2 to 3 days, and gradually reduces to undetectable levels after 2 weeks or so36. On the other hand, insulin, glucagon, and YFP were readily detected in the liver (Fig. 3G and data not shown). In agreement with the in vitro study, Ad5.Pax4-treated islet grafts in the liver had significantly more YFP+Ins+ cells than Ad5.Luc-treated and untreated groups (Fig. 3H), suggesting Pax4-induced α-to-β cell transdifferentiation persisted in vivo. Further quantification shows that the YFP+Ins+ cells constituted ∼7% of total insulin+ (β) cells in the Ad5.Pax4-treated islet grafts, which is substantially higher than the control groups (<1%) (Fig. 3I). In summary, these data demonstrated that Pax4 gene expression in donor mouse islets improved β cell function and enhanced the therapeutic benefits of islet transplantation in allotransplantation settings.
Effects of Pax4 Gene Delivery in Human Islets
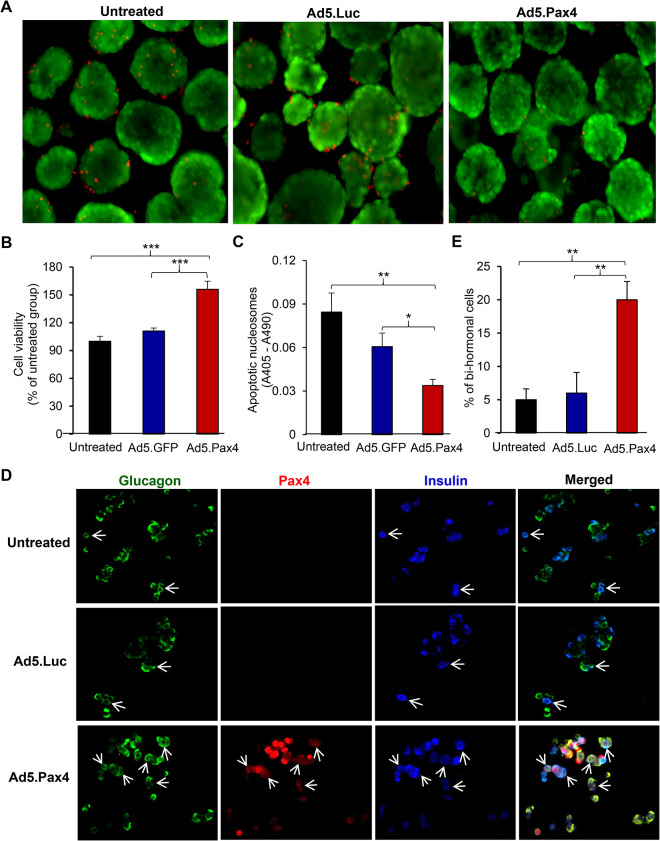
We next investigated the effects of Pax4 gene delivery on cell survival and α-to-β cell transdifferentiation in primary human islets. Freshly isolated human islets were either untreated or infected with Ad5.Pax4 or Ad5.Luc vectors at MOI of 250 VPs/cell. Two days later, islet cell survival was assessed. Similar to observed in primary mouse islets, Pax4 gene delivery significantly increased islet cell survival as assessed by PI staining (Fig. 4A), ATP-based cell viability test (Fig. 4B), and apoptotic nucleosome-based cell death assay (Fig. 4C). Since the pre-existing α cells in primary human islets were not labeled, we were unable to evaluate α-to-β cell transdifferentiation by lineage-tracing analysis. Nonetheless, we assessed potential α-to-β cell conversion by quantifying the number of glucagon+ insulin+ bihormonal cells because α-to-β conversion would go through a transition phase in which insulin expression is induced while glucagon not completely turned off or totally degraded. As shown in Fig. 4D, E, Ad5.Pax4-treated human islets had substantially more glucagon+ insulin+ bihormonal cells. It should be noted that primary islets undergo β-to-α cell dedifferentiation when cultured in vitro 39,40. This dedifferentiation process also leads to a transition phase involving glucagon+ insulin+ bihormonal cells, which may explain the presence of bihormonal cells in the control groups. Nonetheless, the significantly higher percentage of bihormonal cells (vs total α and β cells) in Ad5.Pax4-treated group supports that Pax4 induced α-to-β cell transitioning in primary human islets. Taken together, these data demonstrated that Pax4 gene delivery improved islet cell survival and promoted α-to-β cell transdifferentiation in primary human islets.
Fig. 4.
Pax4 gene delivery into human islets improves cell survival and promotes α-to-β cell transdifferentiation. Freshly obtained human islets were infected with Ad.Pax4 or control vectors at 250 viral particles/cell. Viability tests were performed 2 days after treatment (A–C). (A) PI staining shows fewer dead cells (red) in Ad5.Pax4-treated human islets. (B) ATP-based cell viability assay showing Pax4 gene delivery promoted islet cell survival in human islets. The data were expressed as relative viability in comparison to the untreated islet group. (C) Apoptotic nucleosome-based cell death assay showing Pax4 treatment reduced islet cell apoptosis in human islets. (D) Assessment of potential α-to-β cell conversion by quantifying bihormonal cells. One day after a viral infection, the human islets were slightly dissociated with trypsin treatment and continued to culture for 5 days before the cells were fixed for immunofluorescence staining of insulin (blue), glucagon (green), and Pax4 (red). Arrows mark examples of bihormonal (insulin+glucagon+) cells. (E) Quantification showing the percentage of bihormonal cells versus total α and β cells. *P < 0.05; **P < 0.01; ***P < 0.001. ATP: adenosine triphosphate; PI: propidium iodide.
Pax4 Gene Delivery Into Donor Human Islets Improves Islet Potency and Therapeutic Outcome as Assessed by In Vivo Human Islet Potency Test in T1D Mice
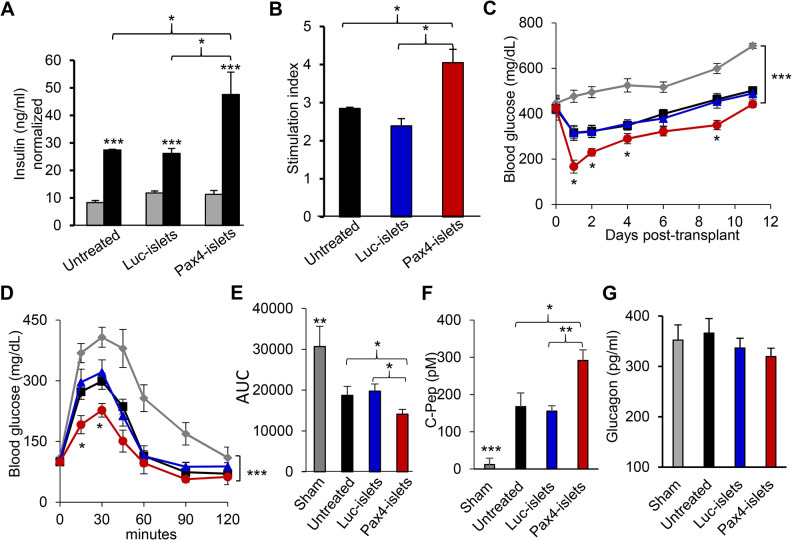
Furthermore, we examined whether Pax4 gene delivery improved human islet potency upon islet transplantation into diabetic NOD-SCID mice. Here we focus on portal vein transplantation because it resembles clinical islet transplantation (Fig. 5), but it should be noted that kidney capsule transplantation had a similar outcome (Supplemental Fig. S2). GSIS assay showed that Pax4 gene delivery significantly improved β cell function in human islets (Fig. 5A, B). For islet transplantation, a suboptimal islet dosage of 2000 IEQ/mouse was injected into the portal vein of each STZ-induced diabetic NOD-SCID mouse. The sham transplantation was included as a control. As shown in Fig. 5C, all diabetic mice transplanted with human islets exhibited lower nonfasting blood glucose than the sham-transplanted group. More importantly, the diabetic mice receiving Ad5.Pax4-treated human islets lowered their blood glucose more efficiently than mice receiving control islets, and they nearly normalized blood glucose (<250 mg/dL) within the first 2 days after islet transplantation, whereas the mice receiving the control islets did not. This suggests that Pax4 treatment of donor islets could allow the use of a suboptimal islet dosage to achieve normoglycemia. Note: the recipient mice were not able to maintain normoglycemia after 2 days, probably due to inefficient islet engraftment and/or immune rejection in this human-to-mouse transplantation setting.
Fig. 5.
Pax4 gene delivery improves the therapeutic outcome of human-to-mouse islet transplantation via the portal vein. Human islets were treated as described in Fig. 3 and injected into the portal vein of STZ-induced diabetic mice, at a dosage of 2000 IEQ/mouse, n = 7 to 8 mice per group. (A) GSIS assay showing Pax4 gene delivery improved β cell function in donor human islets. In the assay, 2.5 mM glucose was used as low (gray bar) and 16.5 mM glucose as high glucose concentrations (black bar). (B) Stimulation index of GSIS. (C) Nonfasting blood glucose levels of the recipient mice before and after islet transplantation via portal vein injection. Gray: sham transplanted; black: transplanted with untreated islets; blue: transplanted with Luc-islets; and red: transplanted with Pax4-islets. (D) ipGTT of the mice, which was performed 1 week after islet transplantation as described in Fig. 3D. The color codes are the same as in Fig. 5C. (E) AUC of the ipGTT. (F) Human C-peptide levels in the blood of recipient mice 2 weeks after transplantation. (G) Circulating glucagon levels in the mice 2 weeks after islet transplantation. *P < 0.05; **P < 0.01; ***P < 0.001. AUC: area under the curve; GSIS: glucose-stimulated insulin secretion; ipGTT: intraperitoneal glucose tolerance test; STZ: streptozotocin.
One week after transplantation, the mice were subjected to ipGTT following overnight fasting, and the Pax4 group of mice showed significantly better glucose tolerance than all the control groups (Fig. 5D, E). In addition, the Pax4 group of mice showed substantially higher concentrations of circulating human C-peptide than the control groups ∼2 weeks post-transplantation (Fig. 5F), suggesting there were more functional β cells in Ad5.Pax4-treated group than the control groups. On the other hand, circulating glucagon levels did not show significant differences among the treatment groups (Fig. 5G) suggesting Pax4 pretreatment of donor islets did not cause glucagon deficiency.
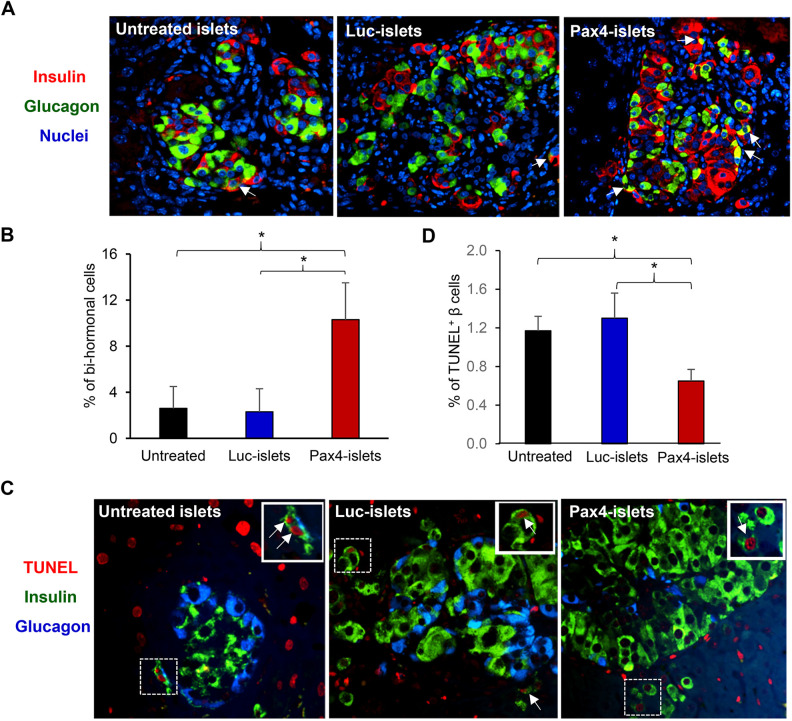
After the mice were sacrificed (2 weeks after transplantation), islet grafts in the liver were examined by immunofluorescence staining. Human islets were readily detected in the liver slices of each recipient mouse (Fig. 6A). There were significantly more bihormonal cells in Ad5.Pax4-treated group than control groups (Fig. 6B), supporting the presence of Pax4-induced α-to-β cell transdifferentiation. Furthermore, we assessed islet cell death in the liver slices using a TUNEL assay that detects apoptotic cells. TUNEL-positive β cells were detected in the grafts of each treatment group (Fig. 6C), and the Ad5.Pax4-treated islets showed significantly fewer apoptotic cells than the control groups (Fig. 6D). Interestingly, TUNEL-positive α cells were hardly detectable in all treatment groups—among ∼2000 α cells that were counted in each group, we only detected 1 to 2 TUNEL-positive α cells, suggesting α cell apoptosis in the islet grafts was rare at the time point (2 weeks after islet transplantation).
Fig. 6.
In situ assessment of islet grafts in the liver of recipient mice following human islets-to-mouse transplantation via the portal vein. (A) Immunofluorescence staining of insulin and glucagon showing islet grafts in the livers of the mice 2 weeks after transplantation. (B) Quantification of insulin+glucagon+ bihormonal cells in the islet grafts in the liver. (C) In situ cell death detection using TUNEL assay. The liver slices were co-stained for insulin (green), glucagon (blue), and TUNEL (red). The arrows mark examples of TUNEL+ β cells. The inset in each picture shows the enlargement of the marked area. (D) Quantification of TUNEL+ β cells in each treatment group. *P < 0.05.
We also examined Pax4 gene expression in the islet grafts at the end of the experiment using the liver slices. Pax4 expression was detectable but only in a few islet cells (Fig. S3), which is in agreement with the transient nature of Ad5-mediated gene expression. Interestingly, some islet grafts appeared to be under attack of host immune cells (characterized by the dense nuclei surrounding the islets) (Fig. S4). This explains why the recipient mice were not able to maintain normoglycemia in this in vivo human islet potency test despite the use of the immune-compromised NOD-SCID mice as the recipients. Nonetheless, these data demonstrated that Pax4 gene delivery into donor human islets improved islet cell function and enhanced the therapeutic efficacy of islet transplantation.
Discussion
In the current study, we explored the therapeutic benefits of Pax4 gene delivery in the context of islet transplantation. Our data show Ad5 vector efficiently delivered Pax4 into primary mouse and human islets and improved β cell function by inducing α-to-β cell transdifferentiation and promoting β cell survival. More importantly, Pax4 gene delivery into donor islets significantly improved the therapeutic outcome of islet transplantation via either kidney capsule or portal vein injection, in both mouse-to-mouse allotransplantation and in vivo human islet potency test. These results demonstrate that Pax4 gene delivery into donor islets is a valuable adjunct therapy for islet transplantation, which can either improve the therapeutic outcome of islet transplantation using the same amount of donor islets or allow the use of fewer donor islets to achieve normoglycemia.
Previous studies using transgenic mouse models have shown that ectopic Pax4 expression can improve β cell function by inducing α-to-β cell conversion19 and promoting β cell survival24. Nonetheless, long-term Pax4 expression has adverse effects. For instance, persistent Pax4 expression in α cells results in glucagon deficiency and islet hypertrophy because it induces continuous α-to-β cell conversion19,20. In addition, it has been shown that long-term Pax4 overexpression in β cells (>4 months) promotes β cell proliferation and reduces GSIS, which is accompanied by a decrease in MafA expression and increase in c-Myc and Cdk4 expression, a phenomenon associated with β cell dedifferentiation24. In contrast, short-term (1 month) Pax4 overexpression in β cells does not cause β cell proliferation or dedifferentiation; instead, it improves β cell function and protects the islets from stress-induced apoptosis24. Therefore, in order to take advantage of the beneficial effects of Pax4 for therapeutic purposes, its expression needs to be maintained at short term.
In our previous study, we have explored the adenovirus-mediated Pax4 gene therapy strategy by direct administration of Ad5.Pax4 into pancreases of T1D mouse models via intra-bile ductal injection. Although beneficial effects are observed, the gene delivery efficiency in vivo appears to be a major obstacle for a better outcome36. In this study, we thus explored Pax4 gene delivery as an adjunct therapy for islet transplantation. This strategy has several advantages. First, Ad5-mediated Pax4 gene delivery into isolated islets is highly efficient and thus maximizes the beneficial effects of Pax4 on β cell function. The dosage used in the study (250 VPs/cell) shows sufficient gene delivery and is well tolerated by primary islets, as evidenced when comparing the control Ad5.Luc-treated or Ad5.GFP-treated islets with untreated islets in all assays. Second, Ad5-mediated Pax4 gene expression is transient (∼2 weeks or so), but it is long enough for Pax4 to exert the protective effects because the most critical time for islet survival is the peri-transplantation period. Third, Ad5.Pax4 treatment of donor islets minimizes host immune responses against Ad5 vectors because the viral vectors are taken up by the islet cells, and thus not directly exposed to the host immune system in vivo. In addition, the vector is nonreplicative because the E1 region of Ad5, which is essential for initiating viral gene expression, is deleted. Therefore, viral gene expression is minimal in the transduced donor cells, which minimizes the possibility of eliciting an immune response against Ad5. Moreover, islet transplantation is performed in the presence of immunosuppression in the clinic, further diminishing Ad5-evoked immune responses.
Pax4 has been shown to reprogram α cells into β cells in genetic knock-in studies using in vivo lineage-tracing techniques19,20. In this study, we demonstrated that Pax4 delivered by the adenoviral vector, Ad5.Pax4, was able to induce α-to-β cell conversion in primary αYFP mouse islets using lineage-tracing techniques in vitro. One interesting question is whether the Pax4-induced α-to-β cell conversion is permanent. Early studies indicate that Pax4 acts as a short-term trigger for β-cell lineage determination19,20. Therefore, Pax4-induced lineage determination is expected to be permanent. Indeed, in the islet transplantation studies (Fig. 3 and Fig. S1), we were able to detect YFP+ β cells 2 weeks after islet transplantation when Pax4 expression became hardly detectable, supporting the concept that Pax4-induced α-to-β cell conversion is permanent.
In addition, our data show that Pax4 gene delivery into primary islets provides striking cytoprotective effects in in vitro culturing. This is in agreement with previous discoveries that Pax4 plays essential roles in β cell survival, especially in stressful conditions such as hyperglycemia and inflammation because Pax4 expression in mature β cells helps to preserve ER integrity15,22,24. In addition, Pax4 is upregulated in pancreatic β cells of high-fat diet-fed mice, and together with another transcription factor SREBP1c, mediates β cell compensatory responses upon metabolic stress23. It should be noted that in the current experimental setting, we were unable to distinguish the relative contributions of α-to-β cell conversion versus β cell survival to the improved β cell function and therapeutic benefits. Considering the small portion of α cells (15% to 20%) in mouse islets41 and the substantial improvement of β cell function, it is plausible that Pax4-mediated survival contributes more to the beneficial effects than transdifferentiation does in mouse islets. In human islets, because of higher α cell composition (∼40%)41, the contribution of Pax4-induced transdifferentiation may be more significant compared to that in mouse islets. On another note, Pax4 has also been shown to induce δ-to-β reprogramming42, which is beneficial for improving β cell function as well, and should have also been capitalized by the use of CMV promoter in our Ad5.Pax4 vector. In order to distinguish the contributions of each Pax4 function in our system, cell-specific Pax4 expression vectors are needed, which can be accomplished by using a glucagon promoter (for α cells), insulin promoter (for β cells), or somatostatin promoter (for δ cells). These specific contributions will be studied in the future.
Supplemental Material
Supplemental Material, Fig_S1_mouse_islet_kidney_capsule_transplant_revised-3 for Pax4 Gene Delivery Improves Islet Transplantation Efficacy by Promoting β Cell Survival and α-to-β Cell Transdifferentiation by Keshab R. Parajuli, Yanqing Zhang, Alexander M. Cao, Hongjun Wang, Vivian A. Fonseca and Hongju Wu in Cell Transplantation
Supplemental Material, Fig_S2_human-to-mouse_kidney_capsule_transplant_revised-3 for Pax4 Gene Delivery Improves Islet Transplantation Efficacy by Promoting β Cell Survival and α-to-β Cell Transdifferentiation by Keshab R. Parajuli, Yanqing Zhang, Alexander M. Cao, Hongjun Wang, Vivian A. Fonseca and Hongju Wu in Cell Transplantation
Supplemental Material, Fig_S3_Pax4_Ins_Gcg_in_liver_human_islets-3 for Pax4 Gene Delivery Improves Islet Transplantation Efficacy by Promoting β Cell Survival and α-to-β Cell Transdifferentiation by Keshab R. Parajuli, Yanqing Zhang, Alexander M. Cao, Hongjun Wang, Vivian A. Fonseca and Hongju Wu in Cell Transplantation
Supplemental Material, Fig_S4_human_islets_attacked_by_immune_cells-3 for Pax4 Gene Delivery Improves Islet Transplantation Efficacy by Promoting β Cell Survival and α-to-β Cell Transdifferentiation by Keshab R. Parajuli, Yanqing Zhang, Alexander M. Cao, Hongjun Wang, Vivian A. Fonseca and Hongju Wu in Cell Transplantation
Acknowledgments
We appreciate the excellent services from the core facilities in Tulane Hypertension and Renal Center of Excellence and Tulane Pathology Core Laboratory. We also would like to thank the Integrated Islet Distribution Program (IIDP), which was funded by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, 1UC4DK098085), for providing us with the human islets and for their outstanding services.
Footnotes
Author Contributions: KRP designed and conducted experiments, collected data, and performed data analysis. YZ and AMC conducted experiments and collected data. H Wang and VAF contributed to experimental design, data interpretation, and manuscript revision. H Wu designed the study, performed data analysis, and wrote the manuscript.
Ethical Approval: This study was approved by the Institutional Animal Care and Use Committee (IACUC) at Tulane University, New Orleans, LA, USA.
Statement of Human and Animal Rights: All of the experimental procedures involving animals were conducted in accordance with the Institutional Animal Care guidelines of Tulane University, and approved by Tulane IACUC.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this study was supported by NIDDK grant DK107412 (H.W.). YZ was partially supported by Susan Harling Robinson Fellowship in Diabetes research. H Wang was supported by NIDDK grant DK123094.
ORCID iDs: Keshab R. Parajuli  https://orcid.org/0000-0003-3799-6991
https://orcid.org/0000-0003-3799-6991
Hongjun Wang  https://orcid.org/0000-0001-7421-1917
https://orcid.org/0000-0001-7421-1917
Hongju Wu  https://orcid.org/0000-0003-0926-8655
https://orcid.org/0000-0003-0926-8655
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Ryan EA, Lakey JR, Paty BW, Imes S, Korbutt GS, Kneteman NM, Bigam D, Rajotte RV, Shapiro AM. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51(7):2148–2157. [DOI] [PubMed] [Google Scholar]
- 2. Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–1330. [DOI] [PubMed] [Google Scholar]
- 3. Shapiro AM, Ricordi C, Hering B. Edmonton’s islet success has indeed been replicated elsewhere. Lancet. 2003;362(9391):1242. [DOI] [PubMed] [Google Scholar]
- 4. Al-Adra DP, Gill RS, Imes S, O’Gorman D, Kin T, Axford SJ, Shi X, Senior PA, Shapiro AM. Single-donor islet transplantation and long-term insulin independence in select patients with type 1 diabetes mellitus. Transplantation. 2014;98(9):1007–1012. [DOI] [PubMed] [Google Scholar]
- 5. Bretzel RG, Brandhorst D, Brandhorst H, Eckhard M, Ernst W, Friemann S, Rau W, Weimar B, Rauber K, Hering BJ, Brendel MD. Improved survival of intraportal pancreatic islet cell allografts in patients with type-1 diabetes mellitus by refined peritransplant management. J Mol Med. 1999;77(1):140–143. [DOI] [PubMed] [Google Scholar]
- 6. Marathe CS, Drogemuller CJ, Marathe JA, Loudavaris T, Hawthorne WJ, O’Connell PJ, Radford T, Kay TW, Horowitz M, Coates PT, Torpy DJ. Islet cell transplantation in Australia: screening, remote transplantation, and incretin hormone secretion in insulin independent patients. Horm Metab Res. 2015;47(1):16–23. [DOI] [PubMed] [Google Scholar]
- 7. Kesseli SJ, Smith KA, Gardner TB. Total pancreatectomy with islet autologous transplantation: the cure for chronic pancreatitis? Clin Transl Gastroenterol. 2015;6(1):e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tai DS, Shen N, Szot GL, Posselt A, Feduska NJ, Habashy A, Clerkin B, Core E, Busuttil RW, Hines OJ, Reber HA, et al. Autologous islet transplantation with remote islet isolation after pancreas resection for chronic pancreatitis. JAMA Surg. 2015;150(2):118–124. [DOI] [PubMed] [Google Scholar]
- 9. Bottino R, Lemarchand P, Trucco M, Giannoukakis N. Gene- and cell-based therapeutics for type I diabetes mellitus. Gene Ther. 2003;10(10):875–889. [DOI] [PubMed] [Google Scholar]
- 10. Moberg L, Johansson H, Lukinius A, Berne C, Foss A, Kallen R, Ostraat O, Salmela K, Tibell A, Tufveson G, Elgue G, et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360(9350):2039–2045. [DOI] [PubMed] [Google Scholar]
- 11. Nanji SA, Shapiro AM. Advances in pancreatic islet transplantation in humans. Diabetes Obes Metab. 2006;8(1):15–25. [DOI] [PubMed] [Google Scholar]
- 12. Petrovsky N, Silva D, Schatz DA. Prospects for the prevention and reversal of type 1 diabetes mellitus. Drugs. 2002;62(18):2617–2635. [DOI] [PubMed] [Google Scholar]
- 13. Ricordi C, Lakey JR, Hering BJ. Challenges toward standardization of islet isolation technology. Transplant Proc. 2001;33(1–2):1709. [DOI] [PubMed] [Google Scholar]
- 14. Lorenzo PI, Juarez-Vicente F, Cobo-Vuilleumier N, Garcia-Dominguez M, Gauthier BR. The diabetes-linked transcription factor PAX4: from gene to functional consequences. Genes (Basel). 2017;8(3):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brun T, Gauthier BR. A focus on the role of Pax4 in mature pancreatic islet beta-cell expansion and survival in health and disease. J Mol Endocrinol. 2008;40(2):37–45. [DOI] [PubMed] [Google Scholar]
- 16. Lorenzo PI, Fuente-Martin E, Brun T, Cobo-Vuilleumier N, Jimenez-Moreno CM, Gomez IGH, Lopez Noriega L, Mellado-Gil JM, Martin-Montalvo A, Soria B, Gauthier BR. PAX4 defines an expandable beta-cell subpopulation in the adult pancreatic islet. Sci Rep. 2015;5:15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, Gruss P. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 2003;17(20):2591–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386(6623):399–402. [DOI] [PubMed] [Google Scholar]
- 19. Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138(3):449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al-Hasani K, Pfeifer A, Courtney M, Ben-Othman N, Gjernes E, Vieira A, Druelle N, Avolio F, Ravassard P, Leuckx G, Lacas-Gervais S, et al. Adult duct-lining cells can reprogram into beta-like cells able to counter repeated cycles of toxin-induced diabetes. Dev Cell. 2013;26(1):86–100. [DOI] [PubMed] [Google Scholar]
- 21. Brun T, Franklin I, St-Onge L, Biason-Lauber A, Schoenle EJ, Wollheim CB, Gauthier BR. The diabetes-linked transcription factor PAX4 promotes {beta}-cell proliferation and survival in rat and human islets. J Cell Biol. 2004;167(6):1123–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mellado-Gil JM, Jimenez-Moreno CM, Martin-Montalvo A, Alvarez-Mercado AI, Fuente-Martin E, Cobo-Vuilleumier N, Lorenzo PI, Bru-Tari E, Herrera-Gomez Ide G, Lopez-Noriega L, Perez-Florido J, et al. PAX4 preserves endoplasmic reticulum integrity preventing beta cell degeneration in a mouse model of type 1 diabetes mellitus. Diabetologia. 2016;59(4):755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee G, Jang H, Kim YY, Choe SS, Kong J, Hwang I, Park J, Im SS, Kim JB. SREBP1c-PAX4 axis mediates pancreatic beta-cell compensatory responses upon metabolic stress. Diabetes. 2019;68(1):81–94. [DOI] [PubMed] [Google Scholar]
- 24. Hu He KH, Lorenzo PI, Brun T, Jimenez Moreno CM, Aeberhard D, Vallejo Ortega J, Cornu M, Thorel F, Gjinovci A, Thorens B, Herrera PL, et al. In vivo conditional Pax4 overexpression in mature islet beta-cells prevents stress-induced hyperglycemia in mice. Diabetes. 2011;60(6):1705–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jo W, Endo M, Ishizu K, Nakamura A, Tajima T. A novel PAX4 mutation in a Japanese patient with maturity-onset diabetes of the young. Tohoku J Exp Med. 2011;223(2):113–118. [DOI] [PubMed] [Google Scholar]
- 26. Kooptiwut S, Plengvidhya N, Chukijrungroat T, Sujjitjoon J, Semprasert N, Furuta H, Yenchitsomanus PT. Defective PAX4 R192 H transcriptional repressor activities associated with maturity onset diabetes of the young and early onset-age of type 2 diabetes. J Diabetes Complications. 2012;26(4):343–347. [DOI] [PubMed] [Google Scholar]
- 27. Mauvais-Jarvis F, Smith SB, Le May C, Leal SM, Gautier JF, Molokhia M, Riveline JP, Rajan AS, Kevorkian JP, Zhang S, Vexiau P, et al. PAX4 gene variations predispose to ketosis-prone diabetes. Hum Mol Genet. 2004;13(24):3151–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Plengvidhya N, Kooptiwut S, Songtawee N, Doi A, Furuta H, Nishi M, Nanjo K, Tantibhedhyangkul W, Boonyasrisawat W, Yenchitsomanus PT, Doria A, et al. PAX4 mutations in Thais with maturity onset diabetes of the young. J Clin Endocrinol Metab. 2007;92(7):2821–2826. [DOI] [PubMed] [Google Scholar]
- 29. Shimajiri Y, Sanke T, Furuta H, Hanabusa T, Nakagawa T, Fujitani Y, Kajimoto Y, Takasu N, Nanjo K. A missense mutation of Pax4 gene (R121 W) is associated with type 2 diabetes in Japanese. Diabetes. 2001;50(12):2864–2869. [DOI] [PubMed] [Google Scholar]
- 30. Sujjitjoon J, Kooptiwut S, Chongjaroen N, Tangjittipokin W, Plengvidhya N, Yenchitsomanus PT. Aberrant mRNA splicing of paired box 4 (PAX4) IVS7-1G>A mutation causing maturity-onset diabetes of the young, type 9. Acta Diabetol. 2016;53(2):205–216. [DOI] [PubMed] [Google Scholar]
- 31. Chapla A, Mruthyunjaya MD, Asha HS, Varghese D, Varshney M, Vasan SK, Venkatesan P, Nair V, Mathai S, Paul TV, Thomas N. Maturity onset diabetes of the young in India - a distinctive mutation pattern identified through targeted next-generation sequencing. Clin Endocrinol (Oxf). 2015;82(4):533–542. [DOI] [PubMed] [Google Scholar]
- 32. Ming-Qiang Z, Yang-Li D, Ke H, Wei W, Jun-Fen F, Chao-Chun Z, Guan-Ping D. Maturity onset diabetes of the young (MODY) in Chinese children: genes and clinical phenotypes. J Pediatr Endocrinol Metab. 2019;32(7):759–765. [DOI] [PubMed] [Google Scholar]
- 33. Lorenzo PI, Cobo-Vuilleumier N, Gauthier BR. Therapeutic potential of pancreatic PAX4-regulated pathways in treating diabetes mellitus. Curr Opin Pharmacol. 2018;43:1–10. [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y, Parajuli KR, Fava GE, Gupta R, Xu W, Ngyuen LU, Zakaria AF, Fonseca VA, Wang H, Mauvais-Jarvis F, Sloop KW, et al. GLP-1 receptor in pancreatic alpha cells regulates glucagon secretion in a glucose-dependent bidirectional manner. Diabetes. 2019;68(1):34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bone RN, Icyuz M, Zhang Y, Zhang Y, Cui W, Wang H, Peng JB, Matthews QL, Siegal GP, Wu H. Gene transfer of active Akt1 by an infectivity-enhanced adenovirus impacts beta-cell survival and proliferation differentially in vitro and in vivo. Islets. 2012;4(6):366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Y, Fava GE, Wang H, Mauvais-Jarvis F, Fonseca VA, Wu H. PAX4 gene transfer induces alpha-to-beta cell phenotypic conversion and confers therapeutic benefits for diabetes treatment. Mol Ther. 2016;24(2):251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y, Wu M, Htun W, Dong EW, Mauvais-Jarvis F, Fonseca VA, Wu H. Differential effects of linagliptin on the function of human islets isolated from non-diabetic and diabetic donors. Sci Rep. 2017;7(1):7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Y, Zhang Y, Bone RN, Cui W, Peng JB, Siegal GP, Wang H, Wu H. Regeneration of pancreatic non-beta endocrine cells in adult mice following a single diabetes-inducing dose of streptozotocin. PLoS One. 2012;7(5):e36675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Negi S, Jetha A, Aikin R, Hasilo C, Sladek R, Paraskevas S. Analysis of beta-cell gene expression reveals inflammatory signaling and evidence of dedifferentiation following human islet isolation and culture. PLoS One. 2012;7(1):e30415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weinberg N, Ouziel-Yahalom L, Knoller S, Efrat S, Dor Y. Lineage tracing evidence for in vitro dedifferentiation but rare proliferation of mouse pancreatic beta-cells. Diabetes. 2007;56(5):1299–1304. [DOI] [PubMed] [Google Scholar]
- 41. Steiner DJ, Kim A, Miller K, Hara M. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets. 2010;2(3):135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Druelle N, Vieira A, Shabro A, Courtney M, Mondin M, Rekima S, Napolitano T, Silvano S, Navarro-Sanz S, Hadzic B, Avolio F, et al. Ectopic expression of Pax4 in pancreatic delta cells results in beta-like cell neogenesis. J Cell Biol. 2017;216(12):4299–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Fig_S1_mouse_islet_kidney_capsule_transplant_revised-3 for Pax4 Gene Delivery Improves Islet Transplantation Efficacy by Promoting β Cell Survival and α-to-β Cell Transdifferentiation by Keshab R. Parajuli, Yanqing Zhang, Alexander M. Cao, Hongjun Wang, Vivian A. Fonseca and Hongju Wu in Cell Transplantation
Supplemental Material, Fig_S2_human-to-mouse_kidney_capsule_transplant_revised-3 for Pax4 Gene Delivery Improves Islet Transplantation Efficacy by Promoting β Cell Survival and α-to-β Cell Transdifferentiation by Keshab R. Parajuli, Yanqing Zhang, Alexander M. Cao, Hongjun Wang, Vivian A. Fonseca and Hongju Wu in Cell Transplantation
Supplemental Material, Fig_S3_Pax4_Ins_Gcg_in_liver_human_islets-3 for Pax4 Gene Delivery Improves Islet Transplantation Efficacy by Promoting β Cell Survival and α-to-β Cell Transdifferentiation by Keshab R. Parajuli, Yanqing Zhang, Alexander M. Cao, Hongjun Wang, Vivian A. Fonseca and Hongju Wu in Cell Transplantation
Supplemental Material, Fig_S4_human_islets_attacked_by_immune_cells-3 for Pax4 Gene Delivery Improves Islet Transplantation Efficacy by Promoting β Cell Survival and α-to-β Cell Transdifferentiation by Keshab R. Parajuli, Yanqing Zhang, Alexander M. Cao, Hongjun Wang, Vivian A. Fonseca and Hongju Wu in Cell Transplantation