Abstract
Osteoarthritis (OA) is a major cause of disability worldwide with increasing age. Knee OA (KOA) is the most prevalent type of OA. Recently, it is considered that KOA is a whole joint disease, including articular cartilage, subchondral bone, synovium, ligaments, joint capsules, and muscles around the joint. Exosomes in knee joint are mainly secreted by articular chondrocytes and synoviocytes. They participate in cell and tissue cross-talk by carrying a complex cargo of proteins, lipids, nucleic acids, etc. Under normal conditions, exosomes maintain the microenvironmental homeostasis of the joint cavity. Under pathological conditions, the composition and function of exosomes changes, which in turn, disrupts the balance of anabolism and catabolism of articular chondrocyte and facilitates inflammatory responses, thus accelerating KOA progression. As a regenerative medicine, mesenchymal stem cells (MSCs) are promised to facilitate repair of degenerated cartilage and decelerate OA process. The therapeutic function of MSC mainly depends on MSC-derived exosomes, which can restore the homeostasis of the articular microenvironment. In the future, the specific mechanism of exosomes for OA treatment needs further elucidation, and the treatment effect of exosomes for long-term and/or severe OA needs further exploration.
Keywords: osteoarthritis, exosomes, articular, microenvironment
Introduction
Osteoarthritis (OA) is a chronic musculoskeletal degenerative disease, which accompanies the symptoms of joint swelling, pain, and stiffness. The major risk factor includes ageing, prior joint trauma, gender, mechanical stress, and genetics. The complicated pathogenesis of OA is mainly characterized by articular cartilage degeneration, synovial inflammation, subchondral bone deterioration, osteophyte formation, ligament degeneration, and hypertrophy of the joint capsule1,2. OA has become more common with ageing and the estimated number of patients has reached 250 million worldwide3. This disease seriously affects the quality of patient life, and the prevention and treatment of OA have led to an extreme financial burden to individuals and society3,4. Nowadays, Knee OA (KOA) is the most prevalent type of OA disease in society.
As the vital component of extracellular vesicles (EVs), exosomes play important and complicated roles in the process and treatment of KOA. Increasing evidence has demonstrated that exosomes are involved in a variety of pathological mechanism, including extracellular matrix (ECM) degradation, inflammation, regulation of angiogenesis, and antigen presenting5,6. Recently, exosomes in the research of pathogenesis and treatment on KOA has got more and more attention. Exosomes have dual roles in maintaining the microenvironmental homeostasis of the joint cavity. In this review, we discuss the current research of exosomes in the biological, pathological, and therapeutic actions of knee joint.
Articular Microenvironment and KOA
With the progress of research, KOA is not only a single disease of articular cartilage degeneration but an organ-related disease affecting whole joint tissues, including articular cartilage, subchondral bone, synovium, meniscus, ligaments, joint capsules, and periarticular muscles7–9. Although meniscus dysfunction may be present in asymptomatic knees or in knees with no evidence of OA, meniscal degeneration is one of the most vital risk factors for KOA, and partial meniscectomy is the most common type of orthopedic surgery for inducing KOA animals and promotes KOA progression including cartilage damage and synovitis9–13. In the process of KOA, pathological changes mainly include cartilage degeneration, synovial inflammation, subchondral sclerosis, and osteophyte formation14. During the early stages of KOA, the modified superficial layer of articular cartilage gradually develops to affect the deep cartilage15. At the same time, the disequilibrium of anabolism and catabolism of the chondrocyte ECM disrupts the articular cartilage16. Further, these pathological changes stimulate synovitis and synovial hyperplasia, resulting in increased release of inflammatory factors from synovial cells, which further damages the cartilage17. Without any intervention, such changes will gradually deteriorate the joint functionality and promote disease progression18,19. The pathology of subchondral bone deterioration involves in the functional imbalance of osteoblasts and osteoclasts20. Osteophyte is caused by intrachondral osteogenesis according to the induction of inflammatory factors in the joints and their abnormal activity at the site21.
The homeostasis of the articular microenvironment in KOA is destroyed and accompanied by increased TNF-α, IL-1β, IL-6, MMP-13, etc22,23. The tissues in the joint are in concordance with articular microenvironment. Therefore, KOA is a disease in which the articular microenvironment is dysregulated or even disordered. Cells in vivo secrete EVs into the local environment to affect the microenvironment24. Such EVs are classified into three groups: exosomes, microvesicles, and apoptotic bodies; among them, exosomes and cellular microvesicles are secreted by living cells24,25. The microvesicles are derived from outward budding of the plasma membrane, while exosomes are produced through the inward invagination of the endosomal membrane pathway. Consequently, a specific subset of endosomal proteins has been identified in exosomes25. Exosomes are found in bodily fluids including blood, urine, cerebrospinal liquid, breast milk, and saliva26,27. In the case of KOA, variation in the composition of exosomes helps to understand the disease mechanism, and the effect of specific exosomes on the articular microenvironment provides new insights for the treatment of disease.
Exosomes
The diameter of exosomes ranges from 40 to 160 nm (average 100 nm) enclosed by a phospholipid bilayer. Exosomes contain large amounts of protein, lipid, DNA, RNA, and amino acid metabolite28. They are first produced by endocytosis of the cell membrane and undergo further processing before being released from parent cells via membrane fusion29. After their release, they communicate with neighboring cells or distant cells through endocrine and paracrine pathways. Exosomes can selectively target recipient cells either through direct fusion with the plasma membrane, or by interacting with receptors, or through endocytosis and fusion with endosomes30. Therefore, exosomes can be employed as important signal messengers and carriers to transmit signals to the corresponding target cells, then change their physiological functions and status. Also, exosomes can be used as vehicles for cell excretion to eliminate unused or harmful RNA and proteins.
It was reported that a variety of exosomes from heart cells were involved in angiogenesis, cell migration, proliferation, apoptosis, hypertrophy, and regeneration. Nevertheless, the number and content of exosomes in circulation are different among cells from various sources and in alternate internal environment, such as hypoxia31. Similarly, in articular tissues, exosomes derived from different cells are involved in various pathological mechanisms, including ECM metabolism and regulation of inflammation7,32,33. At present, the function of exosomes is limited to preclinical studies and there is no report of clinical case for OA treatment.
The separation of exosomes with high purity is critical to understanding their action mechanisms and for their further application in preclinical and clinical treatment. Currently, the methods of isolation exosomes include ultracentrifugation divided into differential ultracentrifugation and density gradient ultracentrifugation, size-based ultrafiltration, size-exclusion chromatography, polymer precipitation, immunoaffinity purification, and microfluidics-based isolation techniques24,34,35. Each method has its advantage and disadvantage, adapts to different situations. For example, ultracentrifugation is a conventional method suitable for large sample volumes, but not for clinical samples35,36. These methods need to be further improved to promote the application of exosomes. Different methods of exosome separation have no significant differences in exosome characteristics and therapeutic effects for OA treatment in preclinical research37,38.
Exosomes Derived From Articular Chondrocytes and KOA
The lack of blood and lymphatic system in the cartilage tissue makes it difficult to self-repair after injury. Instead, exosomes derived from normal chondrocyte help in maintaining the stability of chondrocytes and their surrounding microenvironment. Chen et al. found that exosomes derived from normal chondrocytes promote cartilage formation by regulating chondrocyte precursor cells. While the chondrocyte exosomes were added to tissue engineering scaffold, they promoted the formation of regenerated cartilage through the TGF-β/SMAD signaling pathway, where SOX-9 and COL-II were stably expressed39. The study of Liu et al. showed that exosomes derived from articular chondrocytes protected chondrocytes from destruction by downregulating inflammatory factors and promoted the synthesis of aggrecan and COL-II of ECM, and the exosomes could promote the chondrogenic differentiation of C3H10T1/2 cells40. Mao et al. studied the miRNA expression profiling assays in exosomes and found that the expression of miR-95-5p in normal human chondrocyte exosomes was higher than that of KOA patients. Further study showed that miR-95-5p directly targeted HDAC2/8, regulated cartilage development and homeostasis, leading to the elevated expression of AcH3, aggrecan, and Col2A1. Exosomes derived from primary chondrocytes overexpressing miR-95-5p promoted chondrogenic differentiation of mesenchymal stem cells (MSCs), cartilage development, and increased ECM expression in chondrocytes41. The exosomes produced by chondrocytes act on not only near chondrocytes and chondrocyte precursor cells through the paracrine pathway but also other type cells from other tissues in the articular microenvironment. Zheng et al. found that proteins belonged to mitochondrion were enriched in immune system processes in the exosomes derived from primary articular chondrocytes of C57BL/6 mice compared with those induced by IL-1ß. They validated that exosomes derived from normal chondrocytes eliminated the mitochondrial dysfunction of chondrocytes induced by IL-1ß. Through intra-articular injection, they found that exosomes from normal chondrocytes could repair damaged mitochondria in the chondrocytes from KOA mice induced by anterior cruciate ligament transection surgery in both knee joints. The exosomes promoted the differentiation of synovial macrophages from M1 into M2 type, thereby effectively treating KOA and delaying KOA progression42.
Exosomes derived from articular chondrocytes of patients with KOA inhibited ECM formation of chondrocyte by enhancing the release of MMP-1 and MMP-1343. Liu et al. found that exosomes derived from chondrocytes precursor cells induced by IL-1β promoted the chondrocyte catabolism and inhibited the cartilage formation40. Mao reported that exosomes derived from chondrocytes transfected with an antisense inhibitor of miR-95-5p prevented chondrogenic differentiation and reduced cartilage matrix synthesis by enhancing the expression of HDAC2/841. Exosomes derived from KOA articular chondrocytes also affect other surrounding tissues and cells in articular cavity. Ni et al. showed that the expression of miR-449a-5p was increased in exosomes derived from KOA chondrocytes. They demonstrated that miR-449a-5p inhibited autophagy of synovial macrophages and increased the release of IL-1β by suppressing the expression of ATG4B, which in turn, increased inflammation in KOA and promoted KOA44. Besides, it was reported the osteoarthritic chondrocytes showed increasing levels of Cx43 within their exosomes compared to those isolated from healthy donors. Cx43-positive exosomes released by osteoarthritic chondrocytes were involved in the spread of cellular senescence, inflammatory factors, and responsible for the progression of OA among cartilage, synovium, and subchondral bone45. Therefore, with the changes in the articular microenvironment of KOA patients, the composition of exosomes derived from articular chondrocyte was changed, thereby destroying the cartilage homeostasis and aggravating the OA progression. However, the changes in the specific composition and corresponding mechanisms of exosome need further exploration (summarized in Table 1).
Table 1.
The Variation and Function of Exosomes Derived From the Articular Microenvironment of Normal and Osteoarthritic Conditions.
| Origin | Comparison | Compositional variation | Function | Mechanism | Reference |
|---|---|---|---|---|---|
| AC | NC versus OA patients | miR-95-5p | Promote chondrogenic differentiation and cartilage matrix synthesis in vitro | Target HDAC2/8 | 29 |
| AC | Normal CC versus CC treated with IL-1ß | Proteins belonged to mitochondrion involved in immune system processes | Restore mitochondrial dysfunction in CC in vitro and in vivo; prevent OA process and polarize macrophage to M2 in vivo | NM | 30 |
| SFB | IL-1β stimulated SFB compared with normal SFB | miR-518-3d, 623, 1537, 532-3p, 1306-3p, 548b-5p, etc. | Inhibits ECM production in AC in vitro | NM | 35 |
| SFB | NC versus OA patients | LncRNA H19 | Promote chondrocyte proliferation and migration and inhibit matrix degradation in vitro | Target miR-106b-5p/TIMP2 axis | |
| SF | Late-stage OA patients versus early-stage OA patients | IL1β, IL2, IL4, IL5, IL6, IL-13, IL17, TNF-α, IFN-γ (cytokines)CCL2, CCL3, CCL15, CXCL8, CXCL9, CXCL12 (chemokines) | Activate PBMC chemotaxis and inhibit chondrocyte proliferation in vitro; predicted to recruit inflammatory cells and promote joint degeneration in vivo | NM | 39 |
| SF | NC versus OA patients (female and male, respectively) | miR-24-3p, 23a-3p, 26a-5p, 4487, 6821-5p, 4508, 4532, 7107-5p, etc. (female specific); miR-6878-3p, 5090, 4749-5p, etc. (male specific) | Predicted that female OA-specific miRNAs are estrogen responsive and target TLR signaling pathways in vivo | NM | 40 |
| SF | OA patients versus NC | miR-200c | NM | NM | 41 |
| SF | Different stages of OA patients | lncRNA PCGEM1 | A biomarker from the early-stage OA to the late-stage OA in patients | Targeted miR-770 as a sponge | 42,43 |
AC: articular chondrocytes; CC: chondrocytes; ECM: extracellular matrix; HUVEC: human umbilical vein endothelial cells; NC: normal controls; NM: not mentioned; OA: osteoarthritis; PBMC: peripheral blood mononuclear cell; SF: synovial fluid; SFB: synovial fibroblasts.
Exosomes Derived From Synoviocytes and KOA
Synoviocytes mainly referred to as synovial fibroblasts (SFB) and synovial macrophages, and SFB is the main cell type in synovium tissues. Under normal conditions, exosomes derived from synoviocytes were released into articular microenvironment and maintain the homeostasis; nevertheless, under osteoarthritic conditions, exosomes derived from synoviocytes cause the imbalance of anabolism and catabolism of chondrocyte in articular cartilage, and promote the progression of KOA46. Treatment with exosomes from IL-1β stimulated SFB compared to non-stimulated SFB, significantly increased the expression of MMP13 and ADAMTS5, and decreased the expression of COL2A1 and ACAN in the articular chondrocytes. Besides, the proteoglycan release was increased from cartilage explants, and migration and tube formation activity were significantly higher in human umbilical vein endothelial cells. The result suggested that exosomes promoted KOA progression in the inflammatory microenvironment. The NanoString analysis showed that levels of 50 miRNAs were differentially expressed in exosomes from IL-1β stimulated SFB compared to non-stimulated SFB47 (Table 1). Tan et al. investigated the cellular processes of human chondrocytes regulated by FBS-derived exosomes and found the exosomal lncRNA H19-mediated regulation of the miR-106b-5p/TIMP2 axis48. In the cases related to KOA, M1 type macrophages can present antigens, activate Th1 responses, and promote inflammation. On the contrary, M2 type macrophages have anti-inflammatory effects and promote cartilage repair by inhibiting inflammation49. To date, the effect of macrophage-derived exosomes on OA has not been reported.
The Role and Diagnostic Value of Exosomes in Synovial Fluid in KOA
With the progress of exosome research, the role of exosomes in synovial fluid is being gradually recognized, especially in cartilage homeostasis of KOA50. Exosomes in the synovial fluid of healthy joints derive from cells in surrounding tissues, mainly including articular cartilage and synovium, and act as valuable cargoes to exchange information between cells. However, with the progression of KOA, the amount and content of exosomes in synovial fluids change drastically and result in adverse effects. Domenis et al. found that exosomes derived from KOA synovial fluid can significantly stimulate M1 macrophages to release IL-1β, chemokines (CCL8, CCL15, CCL20, and CXCL1), and metalloproteinases (MMP12 and MMP7), thus exacerbating inflammation reaction and cartilage degeneration51. Gao et al. reported that exosomes from end-stage KOA patients had a higher level of cytokines and chemokines in comparison to profiles of free cytokines and chemokines in soluble synovial fluids. Some specific cytokines and chemokines were elevated in the exosomes of the end-stage patients with KOA compared to the early-stage group (Table 1). Exosomes derived from synovial fluids of end-stage patients recruited inflammatory cells and inhibited cartilage proliferation, thus promoting joint degeneration. This study provided a new perspective for understanding the changes in the internal environment of KOA52. Microarray analysis of miRNA from exosomes derived from synovial fluids of KOA patients and healthy controls showed that exosomal miRNA content was altered in KOA patients and the changes were gender specific. Bioinformatics analysis revealed that female-specific miRNAs in the synovial fluid of exosomes were sensitive to estrogen and participated in Toll-like receptor signaling pathways. Further functional experiments showed that the expression of anabolic genes was decreased, while the expression of catabolic and inflammatory genes was elevated in articular chondrocytes treated with KOA synovial fluid exosomes. Therefore, miRNAs in the synovial fluid exosomes of female patients might be associated with a high incidence of KOA53. The differently expressed cytokines or miRNAs may serve as a potential diagnostic marker for tracking the development and progression of OA52–54 (Table 1). Zhao et al. firstly analyzed the diagnostic value of the differential components of exosomes in joint fluids. They found that lncRNA PCGEM1 was differentially expressed between the early and late stages of the OA group and the area value under the receiver operating characteristic curve was 0.879, which implied that lncRNA PCGEM1 was a powerful indicator in distinguishing early OA from late-stage OA55. Before this study, Kang et al. had reported that lncRNA PCGEM1 could act as a sponge lncRNA that targeted miR-770 and stimulated the proliferation of osteoarthritic synoviocytes56 (Table 1).
Exosomes Derived From MSCs and OA Treatment
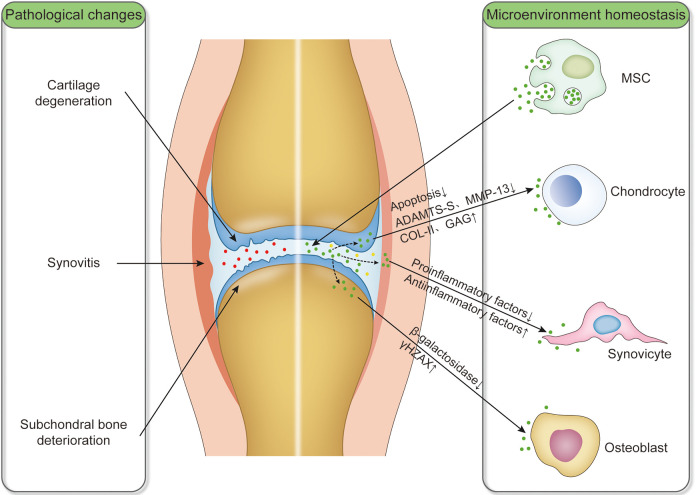
Both preclinical and clinical trials have shown that the application of MSC had great potential in the treatment of OA. MSC not only plays a role in free radical scavenging, immune regulation, and cell proliferation but also in tissue homeostasis2,57. Many pieces of evidence showed that the therapeutic function of MSC mainly depended on their paracrine mechanism and the MSC-derived exosomes played a crucial role in promoting its therapeutic effects58–62. Therefore, MSC-derived exosomes from various sources had been reported to maintain the homeostasis of articular microenvironment during the treatment of OA (Fig. 1).
Figure 1.
Schematic diagram of intra-articular injection of exosomes derived from MSC that act on articular chondrocytes, synoviocytes, osteoblasts, and maintain articular microenvironment homeostasis. The pathological changes of OA involved in cartilage degeneration, synovitis, and subchondral bone deterioration. Exosomes derived from MSC alter the component contents of synovial fluid. Red dots: exosomes in synovial fluid of OA; green dots: exosomes derived from MSC; yellow dots: exosomes of altered composition in OA after intra-articular injection of exosomes derived from MSC; down arrows: downregulation; and up arrows: upregulation. MSC: mesenchymal stem cell; OA: osteoarthritis.
The preclinical therapy of exosomes derived from MSCs on OA and osteochondral defects is mainly applied to mouse and rat OA animals. Exosomes derived from MSCs can inhibit inflammation and apoptosis of chondrocytes, downregulated oxidative stress and senescence of osteoblasts, protect cartilage and bone from degradation, ameliorate gait abnormality, promote cartilage regeneration, and impede OA progression37,38,58–61,63–69. The therapeutic effects and molecular mechanisms of exosomes derived from MSCs for the treatment of OA and osteochondral defects in vivo and in vitro are summarized in Table 2. Exosomes targeted articular chondrocytes by regulating p38/ERK/Akt, miR-92a-3p/WNT5A, TGF-ß1/miR-135b/Sp1, lncRNA-KLF3-AS1/miR-206/GIT1 axis, and autophagy-related mTOR signaling pathway, which leads to increased synthesis of COL-II and proteoglycans and reduced production of ADAMTS-5 and MMP-13, thereby inhibiting the apoptosis of target chondrocytes and remodeling the stability of cartilage for KOA therapies37,63–66,69 (Table 2). In cardiovascular disease, exosomes derived from MSCs target macrophages by regulating miR-182 and activating S1P/SK1/S1PR1 signaling to initiate their switch from the M1 to M2 phenotype, thereby reducing the release of inflammatory factors and exerting anti-inflammatory effects70–72. Although no studies report about MSC-derived exosomes acting on synovial macrophages in the articular cavity, it is accepted that the exosomes play an important role in regulating macrophage polarization. Exosomes derived from MSCs also target osteoblasts by regulating β-galactosidase involved in aging, to increase the accumulation of γH2AX, thereby reducing the release of inflammatory mediators for the treatment of KOA67.
Table 2.
Therapeutic Effects and Molecular Mechanisms of Exosomes Derived From MSCs for the treatment of OA and Osteochondral Defects Treatment In Vivo and In Vitro.
| Origin | Isolation methods | Subjects | Therapeutic effects and molecular mechanisms | References |
|---|---|---|---|---|
| Human MSCs (HuES9) | HPLC fractionation | In vivo: rat osteochondral defects created on the trochlear grooves of the distal femurs. In vitro: NM | In vivo: Exosome-treated defects showed enhanced gross appearance and improved histological scores than the contralateral PBS-treated defects. In vitro: NM | 59 |
| Human MSCs (E1-MYC 16.3) | Ultrafiltration | In vivo: rat osteochondral defects created on the trochlear grooves of the distal femurs. In vitro: PRAC |
In vivo: exosomes increased cellular proliferation and infiltration, enhanced matrix synthesis and a regenerative immune phenotype In vitro: CD73 in exosomes activated AKT and ERK signaling pathway |
58 |
| Human SMSCs | Ultrafiltration | In vivo: KOA rats induced by ACLT and MMT. In vitro: PHC | In vivo: SMSC-140-Exos prevented OA better than SMSC-Exos. In vitro: SMSC-140-Exos enhanced the proliferation and migration of ACs without damaging ECM secretion via RalA | 61 |
| Human MSCs | Density gradient ultracentrifugation | In vivo: KOA rats induced by type II collagenase. In vitro: PRAC treated with IL-1β | In vivo: MSCs exosomes promoted cartilage repair better than those knocking down lncRNA-KLF3-AS1. In vivo: lncRNA KLF3-AS1 enriched in exosomes promoted cell proliferation and suppressed apoptosis | 63 |
| Rat MSC | Extraction kit | In vivo: KOA rats induced by medial collateral ligament and MMT. In vitro: rat chondrocyte line (C5.18 cell) | In vivo: exosomes treated with TGF-β1 promoted cartilage repair better and miR-135b inhibitor inhibited the therapeutic effects. In vitro: TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through miR-135b enriched in MSC exosomes | 64 |
| Human ESC-MSCs | Differential ultracentrifugation | In vivo: KOA mice induced by DMM. In vitro: PMACs treated with IL-1β | In vivo: protected cartilage and bone from degradation. In vitro: exerted similar chondroprotective and anti-inflammatory | 60 |
| Murine BMSCs | Differential ultracentrifugation | In vivo: KOA mice induced by type II collagenase. In vitro: PMAC treated with IL-1β | In vivo: impeded cartilage destruction and OA process. In vitro: maintained chondrocyte phenotype by increasing Col2a1 synthesis and decreasing ADAMTS5 expression | 38 |
| Human BMSCs | Differential ultracentrifugation | In vivo: KOA mice induced by type II collagenase. In vitro: human MSCs, normal and OA PHC | In vivo: MSC-92a-Exos inhibited the progression of early OA and prevented articular cartilage damage better than MSC-Exos. In vitro: MSC-92a-Exos promoted chondrocyte proliferation and matrix genes expression better, and targeted WNT5A expression | 65 |
| Human IPFP MSCs | ExoQuick™ (EQ) reagent kit (SBI) and ultrafiltration | In vivo: KOA mice induced by DMM in right knees. In vitro: PHC treated with IL-1β | In vivo: protected AC from damage and ameliorated gait abnormality; miR-100-5p in exosomes targeted mTOR pathway. In vitro: inhibited cell apoptosis, enhanced matrix synthesis, and enhanced autophagy level partially via mTOR inhibition | 37 |
| Human MSCs | Density gradient ultracentrifugation | In vivo: KOA mice induced by type II collagenase. In vitro: PMAC treated with IL-1β, KOA articular chondrocytes | In vivo: NM. In vitro: increased Col2a1 and aggrecan, and decreased MMP13 and RUX2 expression in KOA chondrocytes; attenuated apoptosis in KOA articular chondrocyte; LncRNA-KLF3-AS1 in exosomes targeted miR-206/GIT1 axis | 66 |
| Human ADSCs | Differential ultracentrifugation | In vivo: NM. In vitro: osteoblasts of osteoarthritic patients | In vivo: NM. In vitro: anti-inflammation, and downregulation oxidative stress and senescence in osteoblasts | 67 |
| Human BMSCs | Differential ultracentrifugation | In vivo: NM. In vitro: PMAC treated with TNF-α | In vivo: NM. In vitro: inhibited adverse effects of inflammation on cartilage homeostasis and promoted cartilage regeneration | 68 |
| Rabbit BMSCs | Ultrafiltration | In vivo: NM. In vitro: primary rabbit chondrocytes | In vivo: NM. In vitro: inhibited MS-induced apoptosis of chondrocytes via p38, ERK, and Akt pathways | 69 |
AC: articular cartilage; ACLT: anterior cruciate ligament transection; ADSCs: adipose-derived MSCs; DMM: destabilization of the medial meniscus; ECM: extracellular matrix; HPLC: high-performance liquid chromatography; IPFP: infrapatellar fat pad MSCs; KOA: knee OA; MMT: medial meniscus transection; MS: mitochondrial dysfunction; MSC: mesenchymal stem cells; MSC-92a-Exos: exosomes derived from BMSCs overexpressing miR-92a-3p; NM: not mentioned; OA: osteoarthritis; PHC: primary human chondrocytes; PMAC: primary mouse articular chondrocytes; PRAC: primary rat articular chondrocytes; SMSCs: synovial MSCs; SMSC-140-Exos: exosomes derived from SMSCs overexpressing miR-140-5p.
Therefore, exosomes derived from MSCs showed efficient therapeutic effects on OA and osteochondral defects as similar to MSCs. There are several advantages of exosomes derived from MSCs compared with MSCs for the treatment. Firstly, exosomes can be stored for 2 years at −80°C without loss of biological activity, facilitating the use as an off-the-shelf regenerative medicine73. On the contrary, the storage and transportation of viable MSCs are relatively tricky for clinical application. Secondly, the use of exosomes is safer than cell-based therapy. The viable transplanted MSCs cannot be removed in the event of adverse activity, while exosomes are not permanent and can be easily stopped during the therapeutic process. In addition, the secreted exosomes derived from genetically modified MSCs with viral vectors are small and relatively safer. Thirdly, the quality and quantity of exosomes are accessibly controlled. Exosomes can be produced at a specific growth or differentiated time point of MSCs under controlled conditions, avoiding poor viability or senescence stage following multiple passages of cells. Further, exosomes can be characterized and quantified by nanoparticle tracking analysis, dynamic light scattering, morphological analysis, and protein analysis, facilitating developing therapeutic standards24,25.
However, there are limitations in translating exosomes as regenerative medicine for clinical practice. Firstly, none isolated method can ultimately retain the nature of exosomes. Secondly, it is difficult to yield sufficient quantities of exosomes for animal studies and human clinical trials74. It was reported that 1 l of MSC-conditioned media from a total of about 60 million MSCs yields about 1–2 mg (protein content) EVs, sufficient for experimentation in only a few mice75. Nonetheless, compared with direct cell transplantation, exosomes derived from MSCs provide a much simpler, safer, more practical, and more easily controlled solution for KOA treatment.
In short, MSC-derived exosomes can act on intra-articular cells by regulating the anabolism and catabolism of cartilage matrix, thereby improving the inflammatory environment in the joint, and then remodeling the joint homeostasis for the treatment of KOA. In the future, exosomes can also be used as a drug-delivery vehicle or as compound exosomes in tissue engineering scaffolds for OA therapies.
Conclusion and Perspective
KOA is a common joint disease, and at present, the treatment of KOA mainly depends on the drug and surgery treatment. Nevertheless, these treatments still face many problems, such as drug side effects, surgical complications, and financial burden. The steady state of the microenvironment in the joint cavity is crucial to the occurrence and progression of KOA. Exosomes derived from joint tissues are the key factors for microenvironmental homeostasis. Exosomes in normal joints maintain the balance of articular microenvironment; nevertheless, exosomes in OA joints disrupt the balance and further aggravate the disease. Injecting exosomes derived from MSCs into the joint cavity will change the molecular composition and improve the microenvironment of the joint cavity. The specific trophic factors released from exosomes could administrate anti-inflammation and immunoregulation, restore homeostasis of ECM, and promote cartilage regeneration, so that the exosomes repair articular cartilage, ameliorate the symptoms of KOA, and delay the progress of the disease. Although great breakthroughs have been achieved in the research based on exosomal treatment for KOA, the specific mechanism for the treatment still needs to be further elucidated. For example, the study of exosomes involved in the pathological processes of subchondral bone sclerosis and osteophyte formation is not clear. Also, exosomal treatment has good results on early-stage KOA treatment in the short term, and the effect on long-term and severe KOA needs further observation and exploration.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (No. 81772410) and the Natural Science Foundation of Shaanxi Province (No. 2018JM7039, No. 2018JM7081).
ORCID iD: Rui Zhang  https://orcid.org/0000-0002-8664-6915
https://orcid.org/0000-0002-8664-6915
References
- 1. Nelson AE. Osteoarthritis year in review 2017: clinical. Osteoarthritis Cartilage. 2018;26(3):319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang R, Ma J, Han J, Zhang W, Ma J. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am J Transl Res. 2019;11(10):6275–6289. [PMC free article] [PubMed] [Google Scholar]
- 3. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. [DOI] [PubMed] [Google Scholar]
- 4. Puig-Junoy J, Ruiz Zamora A. Socio-economic costs of osteoarthritis: a systematic review of cost-of-illness studies. Semin Arthritis Rheum. 2015;44(5):531–541. [DOI] [PubMed] [Google Scholar]
- 5. Cosenza S, Ruiz M, Maumus M, Jorgensen C, Noel D. Pathogenic or therapeutic extracellular vesicles in rheumatic diseases: role of mesenchymal stem cell-derived vesicles. Int J Mol Sci. 2017;18(4):889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toh WS, Lai RC, Hui JHP, Lim SK. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56–64. [DOI] [PubMed] [Google Scholar]
- 7. Brandt KD, Radin EL, Dieppe PA, van de Putte L. Yet more evidence that osteoarthritis is not a cartilage disease. Ann Rheum Dis. 2006;65(10):1261–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, Goldring SR, Jones G, Teichtahl AJ, Pelletier JP. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. [DOI] [PubMed] [Google Scholar]
- 9. Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodeo SA, Monibi F, Dehghani B, Maher S. Biological and mechanical predictors of meniscus function: basic science to clinical translation. J Orthop Res. 2020;38(5):937–945. [DOI] [PubMed] [Google Scholar]
- 11. Englund M, Roemer FW, Hayashi D, Crema MD, Guermazi A. Meniscus pathology, osteoarthritis and the treatment controversy. Nat Rev Rheumatol. 2012;8(7):412–419. [DOI] [PubMed] [Google Scholar]
- 12. Tsujii A, Nakamura N, Horibe S. Age-related changes in the knee meniscus. Knee. 2017;24(6):1262–1270. [DOI] [PubMed] [Google Scholar]
- 13. Katz JN, Martin SD. Meniscus--friend or foe: epidemiologic observations and surgical implications. Arthritis Rheum. 2009;60(3):633–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365(9463):965–973. [DOI] [PubMed] [Google Scholar]
- 15. Nasiri N, Hosseini S, Alini M, Khademhosseini A, Baghaban Eslaminejad M. Targeted cell delivery for articular cartilage regeneration and osteoarthritis treatment. Drug Discov Today. 2019;24(11):2212–2224. [DOI] [PubMed] [Google Scholar]
- 16. Guilak F, Nims RJ, Dicks A, Wu CL, Meulenbelt I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol. 2018;71-72:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Manferdini C, Paolella F, Gabusi E, Silvestri Y, Gambari L, Cattini L, Filardo G, Fleury-Cappellesso S, Lisignoli G. From osteoarthritic synovium to synovial-derived cells characterization: synovial macrophages are key effector cells. Arthritis Res Ther. 2016;18:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berenbaum F, Eymard F, Houard X. Osteoarthritis, inflammation and obesity. Curr Opin Rheumatol. 2013;25(1):114–118. [DOI] [PubMed] [Google Scholar]
- 19. Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6(11):625–635. [DOI] [PubMed] [Google Scholar]
- 20. Zhang L, Hu H, Tian F, Song H, Zhang Y. Enhancement of subchondral bone quality by alendronate administration for the reduction of cartilage degeneration in the early phase of experimental osteoarthritis. Clin Exp Med. 2011;11(4):235–243. [DOI] [PubMed] [Google Scholar]
- 21. Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, Carr AJ. Osteoarthritis. Lancet. 2015;386(9991):376–387. [DOI] [PubMed] [Google Scholar]
- 22. Rezus E, Cardoneanu A, Burlui A, Luca A, Codreanu C, Tamba BI, Stanciu GD, Dima N, Badescu C, Rezus C. The Link between inflammaging and degenerative joint diseases. Int J Mol Sci. 2019;20(3):614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van den Bosch MHJ. Inflammation in osteoarthritis: is it time to dampen the alarm(in) in this debilitating disease? Clin Exp Immunol. 2019;195(2):153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Colao IL, Corteling R, Bracewell D, Wall I. Manufacturing exosomes: a promising therapeutic platform. Trends Mol Med. 2018;24(3):242–256. [DOI] [PubMed] [Google Scholar]
- 25. Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. 2018;118(4):1917–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deatherage BL, Cookson BT. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun. 2012;80(6):1948–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kalluri R, LeBleu VS The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Willms E, Cabanas C, Mager I, Wood MJA, Vader P. Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol. 2018;9:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Asghar S, Litherland GJ, Lockhart JC, Goodyear CS, Crilly A. Exosomes in intercellular communication and implications for osteoarthritis. Rheumatology (Oxford). 2020;59(1):57–68. [DOI] [PubMed] [Google Scholar]
- 31. Xu MY, Ye ZS, Song XT, Huang RC. Differences in the cargos and functions of exosomes derived from six cardiac cell types: a systematic review. Stem Cell Res Ther. 2019;10(1):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Esa A, Connolly KD, Williams R, Archer CW. Extracellular vesicles in the synovial joint: is there a role in the pathophysiology of osteoarthritis? Malays Orthop J. 2019;13(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu X, Wang Y, Xiao Y, Crawford R, Mao X, Prasadam I. Extracellular vesicles: potential role in osteoarthritis regenerative medicine. J Orthop Translat. 2020;21:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peterson MF, Otoc N, Sethi JK, Gupta A, Antes TJ. Integrated systems for exosome investigation. Methods. 2015;87:31–45. [DOI] [PubMed] [Google Scholar]
- 35. Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8(4):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, Larcher LM, Chen S, Liu N, Zhao Q, Tran PHL, et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10(8):3684–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu J, Kuang L, Chen C, Yang J, Zeng WN, Li T, Chen H, Huang S, Fu Z, Li J, Liu R, et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019;206:87–100. [DOI] [PubMed] [Google Scholar]
- 38. Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noel D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7(1):16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen Y, Xue K, Zhang X, Zheng Z, Liu K. Exosomes derived from mature chondrocytes facilitate subcutaneous stable ectopic chondrogenesis of cartilage progenitor cells. Stem Cell Res Ther. 2018;9(1):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu X, Shortt C, Zhang F, Bater MQ, Cowman MK, Kirsch T. Extracellular vesicles released from articular chondrocytes play a major role in cell-cell communication. J Orthop Res. 2020;38(4):731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mao G, Hu S, Zhang Z, Wu P, Zhao X, Lin R, Liao W, Kang Y. Exosomal miR-95-5p regulates chondrogenesis and cartilage degradation via histone deacetylase 2/8. J Cell Mol Med. 2018;22(11):5354–5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zheng L, Wang Y, Qiu P, Xia C, Fang Y, Mei S, Fang C, Shi Y, Wu K, Chen Z, Fan S, et al. Primary chondrocyte exosomes mediate osteoarthritis progression by regulating mitochondrion and immune reactivity. Nanomedicine (Lond). 2019;14(24):3193–3212. [DOI] [PubMed] [Google Scholar]
- 43. Jeon OH, Wilson DR, Clement CC, Rathod S, Cherry C, Powell B, Lee Z, Khalil AM, Green JJ, Campisi J, Santambrogio L, et al. Senescence cell-associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight. 2019;4(7):e125019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ni Z, Kuang L, Chen H, Xie Y, Zhang B, Ouyang J, Wu J, Zhou S, Chen L, Su N, Tan Q, et al. The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1beta production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis. 2019;10(7):522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Varela-Eirín M V-VA, Guitian-Caamano A, Bravo-Lopez S.B, Paíno C, Fonseca E, Kandouz M, Aasen T, Tabernero A, Blanco A, Caeiro J.R, Mayan M. Connexin43-positive exosomes from osteoarthritic chondrocytes spread senescence and inflammatory mediators to nearby synovial and bone cells. Osteoarthr Cartilage. 2019:27(Supplement 1):S91. [Google Scholar]
- 46. Asghar S LG, Meek D, Cole J, Lockhart J, Goodyear C.S, Crilly A. Differential effects of exosomes derived from separate osteoarthritis synovial compartments: implications for erosive cartilage disease Osteoarthritis and cartilage. Osteoarthr Cartilage. 2019:27(Supplement 1):S192. [Google Scholar]
- 47. Kato T, Miyaki S, Ishitobi H, Nakamura Y, Nakasa T, Lotz MK, Ochi M. Exosomes from IL-1beta stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res Ther. 2014;16(4):R163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tan F, Wang D, Yuan Z. The Fibroblast-like synoviocyte derived exosomal long non-coding RNA H19 alleviates osteoarthritis progression through the miR-106b-5p/TIMP2 axis. Inflammation. 2020;43(4):1498–1509. [DOI] [PubMed] [Google Scholar]
- 49. Chen Y, Jiang W, Yong H, He M, Yang Y, Deng Z, Li Y. Macrophages in osteoarthritis: pathophysiology and therapeutics. Am J Transl Res. 2020;12(1):261–268. [PMC free article] [PubMed] [Google Scholar]
- 50. Miyaki S, Lotz MK. Extracellular vesicles in cartilage homeostasis and osteoarthritis. Curr Opin Rheumatol. 2018;30(1):129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Domenis R, Zanutel R, Caponnetto F, Toffoletto B, Cifu A, Pistis C, Di Benedetto P, Causero A, Pozzi M, Bassini F, Fabris M, et al. Characterization of the proinflammatory profile of synovial fluid-derived exosomes of patients with osteoarthritis. Mediators Inflamm. 2017;2017:4814987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gao K, Zhu W, Li H, Ma D, Liu W, Yu W, Wang L, Cao Y, Jiang Y. Association between cytokines and exosomes in synovial fluid of individuals with knee osteoarthritis. Mod Rheumatol. 2020;30(4):758–764. [DOI] [PubMed] [Google Scholar]
- 53. Kolhe R, Hunter M, Liu S, Jadeja RN, Pundkar C, Mondal AK, Mendhe B, Drewry M, Rojiani MV, Liu Y, Isales CM, et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci Rep. 2017;7(1):2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Withrow J, Murphy C, Liu Y, Hunter M, Fulzele S, Hamrick MW. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther. 2016;18(1):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhao Y, Xu J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int Orthop. 2018;42(12):2865–2872. [DOI] [PubMed] [Google Scholar]
- 56. Kang Y, Song J, Kim D, Ahn C, Park S, Chun CH, Jin EJ. PCGEM1 stimulates proliferation of osteoarthritic synoviocytes by acting as a sponge for miR-770. J Orthop Res. 2016;34(3):412–418. [DOI] [PubMed] [Google Scholar]
- 57. Vizoso FJ, Eiro N, Costa L, Esparza P, Landin M, Diaz-Rodriguez P, Schneider J, Perez-Fernandez R. Mesenchymal stem cells in homeostasis and systemic diseases: hypothesis, evidences, and therapeutic opportunities. Int J Mol Sci. 2019;20(15):3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. [DOI] [PubMed] [Google Scholar]
- 59. Zhang S, Chu WC, Lai RC, Lim SK, Hui JH, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24(12):2135–2140. [DOI] [PubMed] [Google Scholar]
- 60. Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B, Zhou J, Heng BC, Zou XH, Ouyang H, Liu H. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017;8(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fang W, Vangsness CT., Jr Implications of anti-inflammatory nature of exosomes in knee arthritis. Cartilage. 2020;1947603520904766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu Y, Zou R, Wang Z, Wen C, Zhang F, Lin F. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem J. 2018;475(22):3629–3638. [DOI] [PubMed] [Google Scholar]
- 64. Wang R, Xu B, Xu H. TGF-beta1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle. 2018;17(24):2756–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mao G, Zhang Z, Hu S, Zhang Z, Chang Z, Huang Z, Liao W, Kang Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther. 2018;9(1):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu Y, Lin L, Zou R, Wen C, Wang Z, Lin F. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle. 2018;17(21-22):2411–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tofino-Vian M, Guillen MI, Perez Del Caz MD, Castejon MA, Alcaraz MJ. Extracellular vesicles from adipose-derived mesenchymal stem cells downregulate senescence features in osteoarthritic osteoblasts. Oxid Med Cell Longev. 2017;2017:7197598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vonk LA, van Dooremalen SFJ, Liv N, Klumperman J, Coffer PJ, Saris DBF, Lorenowicz MJ. Mesenchymal Stromal/stem Cell-derived Extracellular Vesicles Promote Human Cartilage Regeneration In Vitro. Theranostics. 2018;8(4):906–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Qi H, Liu DP, Xiao DW, Tian DC, Su YW, Jin SF. Exosomes derived from mesenchymal stem cells inhibit mitochondrial dysfunction-induced apoptosis of chondrocytes via p38, ERK, and Akt pathways. In Vitro Cell Dev Biol Anim. 2019;55(3):203–210. [DOI] [PubMed] [Google Scholar]
- 70. Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, Gao L, Xie J, Xu B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res. 2019;115(7):1205–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Deng S, Zhou X, Ge Z, Song Y, Wang H, Liu X, Zhang D. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int J Biochem Cell Biol. 2019;114:105564. [DOI] [PubMed] [Google Scholar]
- 72. Lo Sicco C, Reverberi D, Balbi C, Ulivi V, Principi E, Pascucci L, Becherini P, Bosco MC, Varesio L, Franzin C, Pozzobon M, et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Transl Med. 2017;6(3):1018–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820(7):940–948. [DOI] [PubMed] [Google Scholar]
- 74. Katsuda T, Kosaka N, Takeshita F, Ochiya T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics. 2013;13(10-11):1637–1653. [DOI] [PubMed] [Google Scholar]
- 75. Riazifar M, Pone EJ, Lotvall J, Zhao W. Stem cell extracellular vesicles: extended messages of regeneration. Annu Rev Pharmacol Toxicol. 2017;57:125–154. [DOI] [PMC free article] [PubMed] [Google Scholar]