Abstract
Chimeric antigen receptor (CAR) T-cell immunotherapy still faces many challenges in the treatment of solid tumors, one of which is T-cell dysfunction or exhaustion. Immunomodulator lenalidomide may improve CAR T-cell function. In this study, the effects of lenalidomide on CAR T-cell functions (cytotoxicity, cytokine secretion, and cell proliferation) were investigated. Two different CAR T cells (CD133-specific CAR and HER2-specific CAR) were prepared, and the corresponding target cells including human glioma cell line U251 CD133-OE that overexpress CD133 and human breast cancer cell line MDA-MB-453 were used for functional assay. We found that lenalidomide promoted the killing of U251 CD133-OE by CD133-CAR T cells, the cytokine secretion, and the proliferation of CD133-CAR T cells. Lenalidomide also enhanced the cytotoxicity against MDA-MB-453 and the cytokine secretion of HER2-CAR T cells but did not affect their proliferation significantly. Furthermore, lenalidomide may regulate the function of CAR T cells by inducing the degradation of transcription factors Ikaros and Aiolos.
Keywords: chimeric antigen receptor, lenalidomide, immunotherapy, CD133, HER2
Introduction
Chimeric antigen receptor (CAR) T-cell immunotherapy is one of the hottest areas of tumor immunotherapy. Its working principle is to combine the tumor antigen recognition ability of CAR with the cytotoxicity of T cells. And its process mainly contains obtaining CAR T cells through gene engineering, activating and expanding them in vitro, and then injecting them back into cancer patients to kill tumor cells specifically. Classical CAR is composed of tumor antigen-binding, hinge, transmembrane, and intracellular signal regions. The extracellular antigen-binding region is usually a single-chain variable fragment derived from the monoclonal antibody, which specifically binds tumor antigens in a major histocompatibility complex (MHC)-independent manner. The intracellular signal region consists of signal domains that trigger T-cell activation. The first generation of CAR has only one CD3Ζ domain, the second generation contains the CD3Ζ domain and a co-stimulatory domain (CD28 or 4-1BB), and the third generation contains CD3Ζ domain and two co-stimulatory domains (CD28 + OX40 or CD28 + 4-1BB). The CAR gene can be introduced into cells through retroviral or transposon systems. Its unique structure confers T-cell tumor-specific cytotoxicity and antitumor activity in an MHC-independent manner1.
At present, CAR T-cell therapy has achieved outstanding efficacy in the treatment of hematological malignancies. However, for patients with solid tumors, it only induced very limited response. One of the critical factors is that CAR T cells become dysfunctional or exhausted in solid tumors2. Therefore, new strategies to improve CAR T-cell function against solid tumors are needed. Lenalidomide (CC-5013, Revlimid; Celgene Corporation, Warren, NJ, USA) is an oral immunomodulator that has been approved by the US Food and Drug Administration (FDA) for treatment of myelodysplastic syndromes and multiple myeloma (MM)3. And lenalidomide may also play a role in regulating CAR T-cell activity against hematological malignancies4–6. However, the effect of lenalidomide on CAR T-cell function against solid tumor cells has not been extensively studied.
In this study, two third-generation CAR (including two co-stimulatory molecules CD28 and 4-1BB) T cells, CD133-specific CAR T cells and HER2-specific CAR T cells, were prepared as effector cells. At the same time, two tumor cells, human glioma cell line overexpressing CD133 (U251 CD133-OE) and HER2-positive human breast cancer cell line (MDA-MB-453), were chosen as target cells corresponding to the two CAR T cells. Lenalidomide was used as an immunomodulator to investigate its effects on the functions of CD133-CAR T cells and HER2-CAR T cells. We demonstrated here that the combination of lenalidomide and CAR T cells had stronger antitumor effects than CAR T cells alone.
Materials and Methods
Reagents
Lenalidomide was purchased from MCE (HY-A0003, Monmouth Junction, NJ, USA), dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 mM, stored at −80°C, and used within 1 yr. TransAct was purchased from Miltenyi Biotec, Bergisch Gladbach, Germany (T Cell TransActTM, human: 130-111-160) and stored at 4°C.
Cells
Human glioma cell line U251, breast cancer cell line MDA-MB-453, and MDA-MB-468 were purchased from Cell Bank of Chinese Academy of Sciences (Shanghai, China). Wild-type U251 (U251 WT) was nucleofected with a transposon plasmid encoding human CD133 protein and a piggyBac transposase vector to get U251 CD133-OE. Then the firefly luciferase-expressing transposon plasmid and the piggyBac transposase vector were used to transfect U251 CD133-OE again to obtain tumor cell line U251 CD133-OE luc. Similarly, the tumor cell line U251 WT luc, MDA-MB-453 luc, and MDA-MB-468 luc were also obtained by nucleofection. Peripheral blood mononuclear cells (PBMCs) were bought from ALLCELLS (PB005F, Alameda, CA, USA). Following the previously described method, PBMCs were thawed and rest for 1.5 to 2 h, then 2 × 107 cells were resuspended in 100 µl of the buffer from the Human T cell Nucleofector® kit (VPA-1002, Lonza, Basel, Switzerland), and CD133-CAR or HER2-CAR piggyBac transposon plasmid and piggyBac transposase vector were delivered into T cells by nucleofection to obtain CD133-CAR T cells or HER2-CAR T cells7.
Cell Culture
AIM V™ (A3021002, ThermoFisher Scientific, Waltham, MA, USA) + 10% fetal bovine serum (FBS; 10099141C, ThermoFisher Scientific) and RPMI 1640 (10-040-CVR, CORNING, Corning, NY, USA) + 10% FBS + 1% P/S (penicillin-streptomycin, 15140122, ThermoFisher Scientific) were used to expand and culture CD133-CAR T cells, HER2-CAR T cells, and NT cells. The medium used for the culture of U251 CD133-OE luc and MDA-MB-453 luc cells was DMEM (10-017-CVR, CORNING) + 10% FBS + 1% P/S. The medium used for the coculture of CAR T cells and tumor cells was RPMI 1640 + 10% FBS + 1% P/S.
For CAR T cells preparation, nucleofected T cells were activated with Dynabeads™ Human T-Expander CD3/CD28 (11141D, ThermoFisher Scientific) at a ratio of 3 beads per cell on day 0, and 300 IU/ml interleukin 2 (IL-2) was added to the medium. On the 14th day, allogeneic PBMCs from five donors were irradiated at 40 Gy using an x-ray irradiator (RS2000PRO, Rad Source Technologies, Buford, GA, USA) and added to the culture of CAR T cells at a ratio of 10:1. For the second round of expansion, 300 IU/ml of IL-2 was added to the cell culture medium, and 50 ng/ml of anti-CD3 antibody (130-093-387, Miltenyi Biotec) was added as an activator. Two days after each stimulation, CAR T cells were washed and further cultured in CAR T-cell culture medium (AIM V™ + 10% FBS) with 300 IU/ml IL-2. A full or half-volume of the medium refresh was performed every 2 to 3 d and fresh IL-2 was supplemented. Seven days after the first stimulation, beads were removed, and T cells expressing CD133-CAR or HER2-CAR were selected with 0.5 μg/ml of puromycin. On day 24 (10 d after the second stimulation), transfected T cells were analyzed by flow cytometry. Nontransfected (NT) cells were obtained in the same way, but nucleofection and drug selection were avoided8. For the culture of tumor cells, medium exchange was performed every 2 to 3 d. Into the medium of U251 CD133-OE luc, 1 mg/ml G418 and 1 μg/ml puromycin were added, and 1 mg/ml G418 was added to the U251 WT luc, MDA-MB-453 luc, or MDA-MB-468 luc culture.
Cytotoxicity Assay
CD133-CAR T cells (or HER2-CAR T cells) were cocultured with U251 CD133-OE luc (or MDA-MB-453 luc) tumor cells in a 96-well plate according to different effectors: target ratios (104 tumor cells per well) in 200 µl medium. Lenalidomide (final concentrations of 10, 1, and 0.1 μM, respectively) and the same dilution of solvent DMSO (103×, 104×, and 105×) were added as different groups, and tumor cells were cultured separately as controls. After the appropriate time of coculture, the substrate d-Luciferin was added into the 96-well plate, and the emissive fluorescence was detected with a microplate reader. The killing efficiency of each group was calculated, and the groups with the same dilution factor were compared and analyzed. The comparisons between the lenalidomide group and DMSO group show the effects of lenalidomide at various concentrations on the cytotoxicity of CAR T cells.
Cytokine Secretion
CD133-CAR T cells (or HER2-CAR T cells) were cocultured with U251 CD133-OE (or MDA-MB-453) tumor cells at an effector-to-target ratio of 2:1, and the number of tumor cells was 105/well in a 96-well plate with a volume of 200 μl. Lenalidomide with a concentration of 10 μM, DMSO (103×) and T-cell culture medium at the same dilution were added. After 24 h, the supernatant in the culture was taken, and the cytokines IL-2, tumor necrosis factor alpha (TNFα), interferon gamma (IFNγ), and granulocyte-macrophage colony-stimulating factor (GM-CSF) were detected using AlphaLISA detection kits (IL-2, AL221C; TNFα, AL208C; IFNγ, AL217C; and GM-CSF, AL216C) from PerkinElmer, Waltham, MA, USA. Then the lenalidomide group and the DMSO group with the same dilution factor were compared to determine the effect of lenalidomide on cytokine secretion.
Cell Proliferation
U251 CD133-OE and MDA-MB-453 tumor cells were irradiated with 70 Gy. CD133-CAR T cells and HER2-CAR T cells were labeled with 0.5 μM CFSE (carboxyfluorescein diacetate succinimidyl ester, 65-0850-84, ThermoFisher Scientific) according to the instruction from the manufacturer. The two CAR T cells were cocultured with the corresponding irradiated target cells in 1 ml per well in a 24-well plate according to the effector-to-target ratio of 2:1. The CAR T-cell concentration was 1 × 106/ml. TransAct was added to the medium of NT cells at 1% to activate T cells, and the NT-cell concentration was 1 × 106/ml. Finally, different concentrations of lenalidomide (final concentrations of 10, 1, and 0.1 μM in the medium) and DMSO (103×, 104×, 105×) with same dilutions were added to each group. After 4 d, the CFSE signal in T cells was detected by flow cytometry. The lower CFSE signal indicates the faster proliferation. The difference between the lenalidomide group and the DMSO control group was analyzed. And then the effects of lenalidomide on the proliferation of two CAR T cells and NT cells were determined.
Western Blot
In 24-well plates, 2 × 106 CD133-CAR T, HER2-CAR T, or NT cells were cultured, 2 ml per well, and 1 μM, 10 μM lenalidomide or DMSO was added to the medium. After 48 h, the cells were collected, washed once with PBS, and lysed by ultrasound (5 s × 3 times). The proteins on the gel were transferred onto a polyvinylidene difluoride (PVDF) membrane using the semi-dry transfer method (15 V, 30 min), and blocked with 5% bovine serum albumin for 2 h. The PVDF membrane was incubated with anti-Aiolos (NBP2-24495SS, Novus, Littleton, CO, USA), anti-Ikaros (sc-398265, Santa Cruz Biotechnology, Santa Cruz, CA, USA), or anti-GAPDH (sc-32233, Santa Cruz Biotechnology) antibody at 4°C overnight, followed by peroxidase-conjugated AffiniPure Donkey Anti-Rabbit immunoglobulin G (IgG) (H + L) (711-035-152, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) or peroxidase-conjugated AffiniPure Donkey Anti-Mouse IgG (H + L) (715-035-150, Jackson ImmunoResearch Laboratories) for 1 h, and finally imaged in the Biorad Imager (Bio-Rad, Hercules, CA, USA).
Statistical Analysis
Excel was used to calculate the killing efficiency according to the formula: killing (%) = 100 × (1-fluorescence value of experimental group/fluorescence value of tumor cell alone group). FlowJo V-10 (BD Biosciences, Bedford, MA, USA) was used to determine the average number of cell division (Div. Index) in the cell proliferation experiment. Relative proliferation of T cells was calculated by following the formula: proliferation (%) = 100 × (Div. Index of experimental group/Div. Index of R-10 control group). Student’s t-test and one-way analysis of variance in Prism (GraphPad Software, San Diego, CA, USA) were used to analyze the significant difference in killing efficiency, cytokine secretion, and relative proliferation. The data were expressed as mean ± standard deviation. All experiments were repeated at least three times. In this study, P <0.05 was considered statistically significant (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
Results
Lenalidomide Enhances the Functions of CD133-CAR T Cells Efficiently
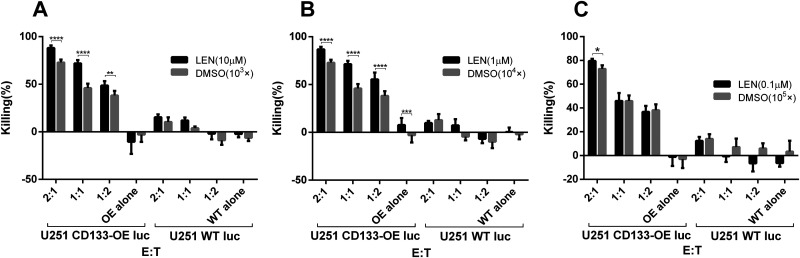
To investigate the effect of lenalidomide on the antitumor function of CD133-CAR T cells, the cytotoxicity of CD133-CAR T cells against tumor cells was first analyzed. CD133-CAR T cells were cocultured with glioma cell line U251 overexpressing firefly luciferase and CD133 (U251 CD133-OE luc) at different effector-to-target ratios. In the coculture, different concentrations (0.1, 1, and 10 μM) of lenalidomide were added, and the solvent of lenalidomide, DMSO, was used as the control. Three days later, the signal of bioluminescence produced by firefly luciferase in tumor cells was used to evaluate the viability of U251 CD133-OE luc tumor cells, and the killing efficiency was calculated. As shown in Fig. 1A, when the lenalidomide concentration was 10 μM, the killing increased as the effector-to-target ratio increased, and the killing efficiency was significantly improved in the lenalidomide group as compared with the control group, in which the same dilution factor of DMSO was added. When the lenalidomide concentration was 1 μM, the results were similar to those at 10 μM, as shown in Fig. 1B. When the lenalidomide concentration was 0.1 μM, the lenalidomide group was significantly different from the control group only when the effector-to-target ratio was the highest (2:1), and no effect of lenalidomide was observed in the case of the low effector-to-target ratio, as shown in Fig. 1C. These results indicated that lenalidomide with a high enough concentration could significantly promote the killing of U251 CD133-OE luc tumor cells by CD133-CAR T cells. Furthermore, lenalidomide would not influence the killing specificity of CD133-CAR T cells, as almost no killing of CD133-negative U251 WT luc was observed when lenalidomide was combined with CD133-CAR T cells.
Fig. 1.
Lenalidomide promotes the cytotoxicity of CD133-CAR T cells against U251 CD133-OE luc cells. CD133-CAR T cells were cocultured with U251 CD133-OE luc cells according to different effector:target ratios. Lenalidomide (final concentrations of 0.1, 1, 10 μM, respectively) or the same dilution fold of solvent DMSO (103×, 104×, 105×) were added into the medium. After 72 h, the fluorescence signal intensity in the coculture system was detected using a microplate reader, and the killing efficiency was calculated by the formula: killing efficiency (%) = 100 * (1 − fluorescence value of experimental group/fluorescence value of tumor cell alone group). Data are shown as the mean of killing efficiency ± standard deviation in three replicates.
CAR: chimeric antigen receptor; DMSO: dimethyl sulfoxide; E:T: effector:target ratio; WT: wild type.
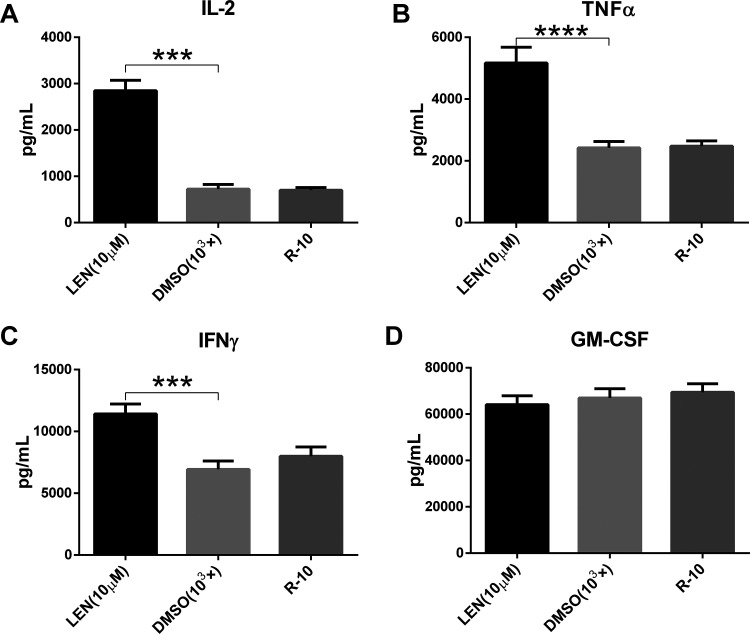
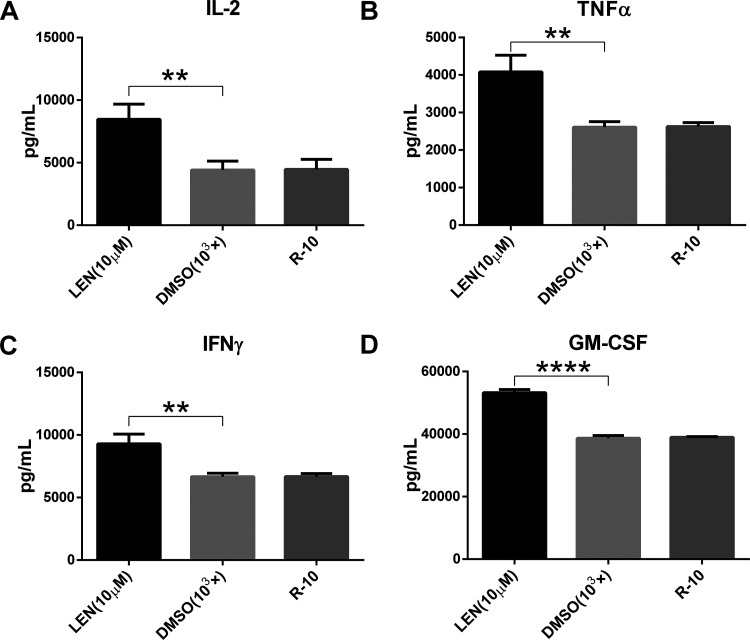
The effect of lenalidomide on cytokine secretion of CD133-CAR T cells was further studied. U251 CD133-OE cells were cocultured with CD133-CAR T cells, and lenalidomide was added to the medium at a final concentration of 10 μM. After 1 d, the amount of cytokine in the supernatant was measured using an AlphaLISA detection kit. As shown in Fig. 2A–D, there was no significant difference in the concentration of cytokines IL-2, TNFα, IFNγ, and GM-CSF between DMSO and T-cell medium (R-10) group. The secretion of IL-2, TNFα, and IFNγ was significantly increased in the lenalidomide (10 μM) group compared with DMSO group, as shown in Fig. 2A–C, but there was no significant difference in the secretion of GM-CSF between the two groups, as shown in Fig. 2D. This indicates that lenalidomide can promote the secretion of IL-2, TNFα, and IFNγ by CD133-CAR T cells, but does not affect the secretion of GM-CSF.
Fig. 2.
Lenalidomide enhances cytokine secretion by CD133-CAR T cell. To test the effect of lenalidomide on the cytokine secretion of CAR T cell, CD133-CAR T cells were cocultured with U251 CD133-OE tumor cells. There were three groups, and in different group lenalidomide (final concentration: 10 μM), DMSO (103×) at the same dilution or culture medium (R-10) was added. After 24 h, the secretion of the four cytokines IL-2, TNFα, IFNγ, and GM-CSF in the supernatant was detected using AlphaLISA detection kits.
CAR: chimeric antigen receptor; DMSO: dimethyl sulfoxide; GM-CSF: granulocyte-macrophage colony-stimulating factor; IFNγ: interferon gamma; IL-2: interleukin 2; LEN: lenalidomide; TNFα: tumor necrosis factor alpha.
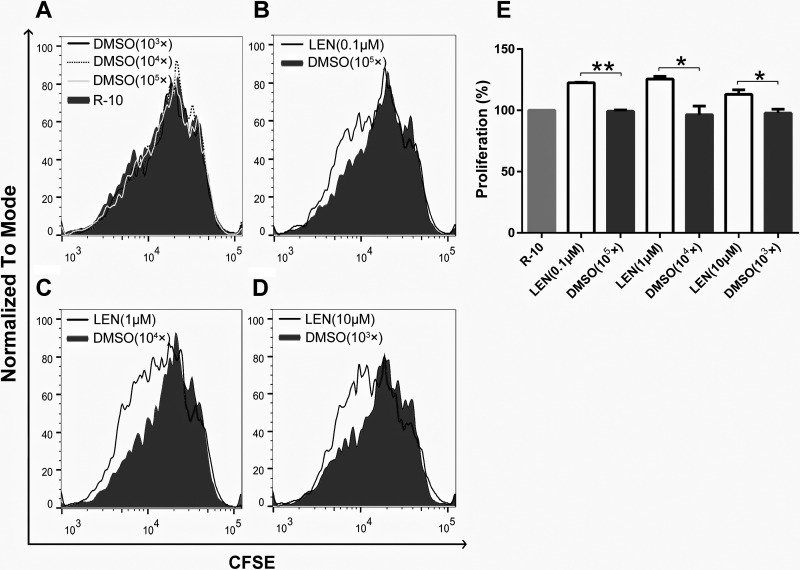
Next, the effect of lenalidomide on the proliferative capacity of CD133-CAR T cells was examined. The irradiated U251 CD133-OE cells were used to stimulate CFSE-labeled CD133-CAR T cells. The ratio of T cells to tumor cells was 2:1, and the final concentrations of lenalidomide tested in the culture medium were 0.1, 1, and 10 μM, respectively. After 4 d, the CFSE signal in CD133-CAR T cells was detected by flow cytometry, and the cell proliferation was evaluated. As shown in Fig. 3A, DMSO had little effect on the proliferation of CD133-CAR T cells. However, lenalidomide (0.1, 1, and 10 μM) was found to increase the proliferation of CD133-CAR T cells compared with the DMSO group with the same dilution factor, as shown in Fig. 3B–D.
Fig. 3.
Lenalidomide promotes CD133-CAR T-cell proliferation. To study the effect of lenalidomide on the proliferation of CAR T cells, CFSE-labeled CD133-CAR T cells were cocultured with irradiated U251 CD133-OE cells. At the same time, lenalidomide (final concentrations of 0.1, 1, and 10 μM, respectively), a corresponding dilution fold of the solvent DMSO (103×, 104×, 105×), and a medium control were added. After 96 h, the CFSE signal in the coculture system was detected by flow cytometry to evaluate the proliferation of CAR T cells. (A–D) The signal value of CFSE from each group was shown, and the smaller CFSE signal intensity indicates the faster cell proliferation. (E) The relative T-cell proliferation compared to the medium control group was summarized from three independent experiments.
CAR: chimeric antigen receptor; CFSE: carboxyfluorescein diacetate succinimidyl ester; DMSO: dimethyl sulfoxide.
Lenalidomide Could Also Regulate HER2-CAR T Cells
In the above experiments, it has been made clear from the three aspects of cytotoxicity, cytokine secretion, and cell proliferation that lenalidomide can enhance the function of CD133-CAR T cells. To further explore the role of lenalidomide in other CAR T cells, HER2-CAR T cells were prepared as effector cells.
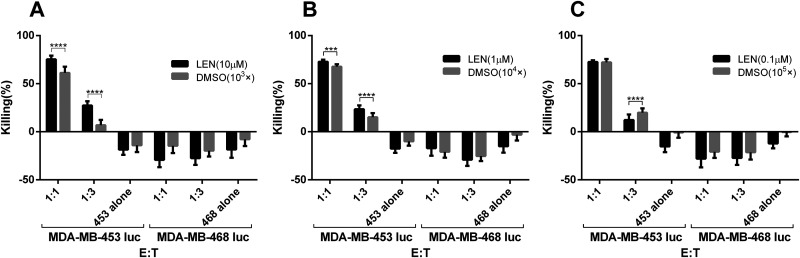
First, to investigate the effects of lenalidomide on the cytotoxicity of HER2-CAR T cells, they were cocultured with tumor cells MDA-MB-453 luc at different ratios (1:1, 1:3). Lenalidomide or DMSO with the same fold of dilution was added into the medium. The tumor signals were detected after 1 d. As shown in Fig. 4A–C, when the concentration of lenalidomide or DMSO is constant, the lysis efficiency of CAR T cells increased with the increase of the effector:target ratio. And in the case of the same effector:target ratio, the lysis efficiency of the lenalidomide group was significantly higher than the DMSO group with the same dilution factor (Fig. 4A and B), indicating that lenalidomide enhanced the killing of HER2-CAR T cells against tumor cells MDA-MB-453 luc. In addition, when HER2-negative MDA-MB-468 luc cells were used as targets, lenalidomide did not affect the function of HER2-CAR T cells.
Fig. 4.
Lenalidomide increases the cytotoxicity of HER2-CAR T cells against MDA-MB-453 luc tumor cells. HER2-CAR T cells were cocultured with MDA-MB-453 luc at different E:T. Lenalidomide was added with the final concentrations of 0.1, 1, and 10 μM, respectively. After 24 h, the fluorescence intensity in the coculture system was detected using a microplate reader, and then the killing efficiency was calculated.
CAR: chimeric antigen receptor; DMSO: dimethyl sulfoxide; E:T: effector:target ratio; LEN: lenalidomide.
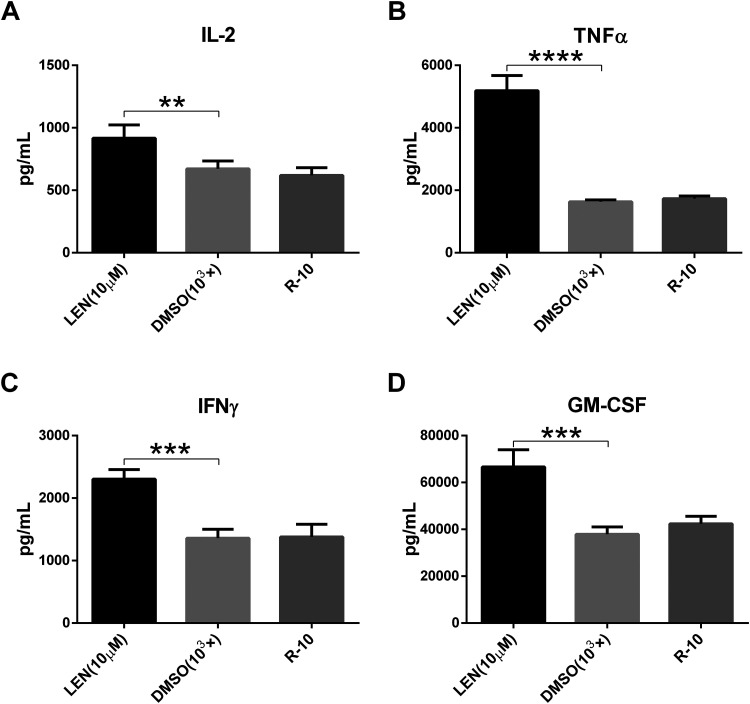
To know the effect of lenalidomide on the cytokine secretion of HER2-CAR T cells, the coculture was established at the effector:target ratio of 2:1 for 1 d. And 10 μM lenalidomide was tested. AlphaLISA kits were used to detect the cytokines secreted in the supernatant. The results showed that lenalidomide (10 μM) not only significantly promoted the secretion of cytokines IL-2, TNFα, and IFNγ by HER2-CAR T cells (Fig. 5A–C), but also the secretion of GM-CSF (Fig. 5D), which was not observed in the CD133-CAR T cell. Therefore, lenalidomide can promote the secretion of IL-2, TNFα, IFNγ, and GM-CSF by HER2-CAR T cells.
Fig. 5.
Lenalidomide can also enhance cytokine secretion of HER2-CAR T cells. MDA-MB-453 tumor cells and HER2-CAR T cells were cocultured according to the E:T of 2:1. And then 10 μM lenalidomide or solvent DMSO was added. After 24 h, the amount of cytokines secreted in the supernatant was detected. (A–D) Show the secretion of IL-2, TNFα, IFNγ, and GM-CSF, respectively.
CAR: chimeric antigen receptor; DMSO: dimethyl sulfoxide; E:T: effector:target ratio; GM-CSF: granulocyte-macrophage colony-stimulating factor; IFNγ: interferon gamma; IL-2: interleukin 2; LEN: lenalidomide; TNFα: tumor necrosis factor alpha.
Next, to investigate the effect of lenalidomide on the proliferation of HER2-CAR T cells, CFSE-labeled HER2-CAR T cells and irradiated tumor cells MDA-MB-453 were cocultured. Different concentrations of lenalidomide or DMSO were added. After 4 d, signals of CFSE in HER2-CAR T cells were detected. As shown in Fig. S1, neither DMSO nor lenalidomide could affect the proliferation of HER2-CAR T cells.
Lenalidomide Regulating the Function of T Cells Could Be CAR Independent
The above results show that lenalidomide has different regulatory effects on two different CAR T cells: CD133-CAR T cells and HER2-CAR T cells. However, in all experiments, CAR T cells are activated in the presence of tumor cells through CAR to recognize tumor antigen, thereby inducing antitumor functions. The above experiments cannot exclude the interference of target cells. To this end, NT control T cells were prepared, and TransAct (T Cell TransActTM, human) was used as the T-cell activator. TransAct would activate NT cells through CD3 and CD28 instead of the CAR activation. Then the effects of lenalidomide on cytokine secretion and cell proliferation of NT cells were examined.
First, the activated NT cells were divided into three groups, and lenalidomide was added to the first group at a final concentration of 10 μM. The second and third groups contained DMSO and T-cell culture medium. After 24 h, the supernatant was taken, and the cytokine was detected. Compared with the T-cell culture medium group, DMSO (103×) had almost no effect on the secretion of the cytokines IL-2, TNFα, IFNγ, and GM-CSF; but lenalidomide (10 μM) could significantly promote NT cell to secrete more IL-2, TNFα, IFNγ, and GM-CSF, as shown in Fig. 6A–D. This is similar to the results observed in HER2-CAR T cells.
Fig. 6.
Lenalidomide promotes cytokine secretion of NT cells. TransAct was used to activate NT cells, and lenalidomide (final concentration of 10 μM) or an equivalent diluted DMSO (103×) was added. After 24 h, the secretion of four cytokines IL-2, TNFα, IFNγ, and GM-CSF in the supernatant was detected, and the effects of lenalidomide and DMSO on cytokine secretion were compared and analyzed.
DMSO: dimethyl sulfoxide; GM-CSF: granulocyte-macrophage colony-stimulating factor; IFNγ: interferon gamma; IL-2: interleukin 2; LEN: lenalidomide; TNFα: tumor necrosis factor alpha.
CFSE-labeled NT cells were then used to study cell proliferation ability. And TransAct was added as an activator to NT-cell culture. After 4 d, the CFSE signal in NT cells was detected by flow cytometry. DMSO had almost no effect on the proliferation of NT cells, as shown in Fig. S2. And two concentrations of lenalidomide (0.1, 1 μM) showed inhibition of NT-cell proliferation.
Lenalidomide Did Not Influence Antigen Expression on Tumor Cells but Could Induce the Degradation of Ikaros and Aiolos in CAR T Cells
To investigate whether the enhanced functions of CAR T cells was caused by antigens or ligands on tumor cells, U251 CD133-OE or MDA-MB-453 was treated by lenalidomide. One day later, tumor antigens (CD133 or HER2) and the important immune checkpoint ligand PD-L1 on tumor cells were detected by flow cytometry. As shown in Fig. S3, the expression levels of CD133, HER2, or PD-L1 had not been changed by lenalidomide treatment compared with DMSO group.
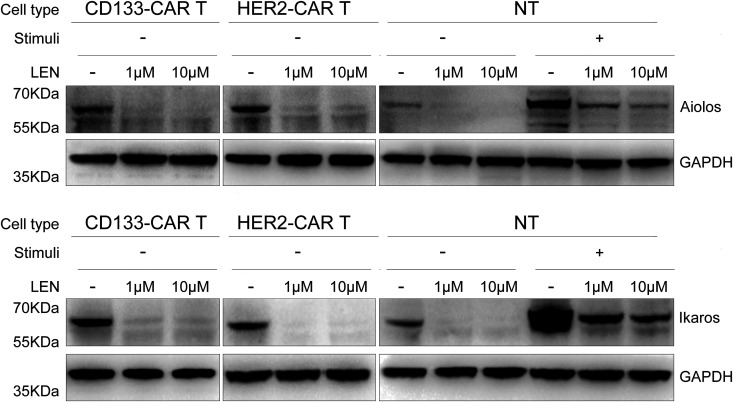
It is reported that lenalidomide can specifically mediate the connection between CRBN–DDB1 ubiquitin ligase complex and transcription factors Ikaros (encoded by IKZF-1) and Aiolos (encoded by IKZF-3), which will be ubiquitinated and degraded later, and then promote the secretion of IL-2 from T cells9,10. Therefore, we were wondering whether lenalidomide regulated the expression of Ikaros and Aiolos in CAR T cells we used in this study. CD133-CAR T cells, HER2-CAR T cells, or NT cells were treated by lenalidomide or DMSO for 48 h, then were lysed by ultrasound, and the expression levels of Ikaros and Aiolos were measured by western blot. We found that lenalidomide indeed induced the significant decrease of Ikaros and Aiolos expression in unstimulated CD133-CAR T cells, HER2-CAR T cells, or NT cells (Fig. 7).
Fig. 7.
Lenalidomide induced the degradation of Ikaros and Aiolos in T cells. CD133-CAR T cells, HER2-CAR T cells, or NT cells were treated with lenalidomide at 1 and 10 μM for 48 h. The cells were collected, lysed by ultrasound, and the expressions of Aiolos, Ikaros, and GAPDH were detected by western blot.
CAR: chimeric antigen receptor; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; LEN: lenalidomide; NT: nontransfected; stimuli: cells were activated by TransAct.
Discussion
In this study, we found that lenalidomide could regulate the function of T cells activated in different ways, including CD133-CAR T cells and HER2-CAR T cells activated by CAR signals, and NT T cells activated in a non-CAR way (TransAct). First, lenalidomide could promote CD133-CAR T cells killing U251 CD133-OE tumor cells, secreting IL-2, TNFα, IFNγ, and cell proliferation. Second, lenalidomide also promoted the cytotoxicity, and the secretion of IL-2, TNFα, IFNγ, and GM-CSF by HER2-CAR T cells, but had almost no effect on their proliferation. At last, lenalidomide could promote the secretion of cytokines IL-2, TNFα, IFNγ, and GM-CSF by NT cells, but significantly inhibit their proliferation. The mechanism of this difference needs further study.
Although the exact antitumor mechanism is not completely clear, extensive research has found that lenalidomide has multiple effects against tumors, including direct inhibition of tumor growth and indirect antitumor effects on the tumor microenvironment. Direct toxicity of lenalidomide against tumors includes inducing cancer suppressor genes11, causing actin polymerization, relocation of membrane proteins,12 and cytoskeleton regulation13, eventually leading to cell cycle arrest11,14,15 and tumor cell apoptosis. No direct effect of lenalidomide on tumor cells was observed in this study.
The impact on the tumor microenvironment mainly includes the following four aspects. First, lenalidomide changes the tumor microenvironment through anti-angiogenesis16,17. Second, lenalidomide promotes the recovery of immune synaptic defects, which has been observed in a variety of B-cell malignancies, such as chronic lymphocytic leukemia12,18 and follicular lymphoma19. Third, lenalidomide increases the cytotoxicity of natural killer cells, and then enhances antibody-dependent cell-mediated cytotoxicity11,20,21 and suppresses the function of regulatory T cells22. At last, lenalidomide has strong T-cell co-stimulation properties. Lenalidomide promotes T-cell proliferation and increases the secretion of IL-2 and IFNγ by activated T cells23,24, which is consistent with the phenomenon observed in this study.
Related studies on molecular mechanisms have found that Cereblon (encoded by the gene CRBN) is a target for lenalidomide in the treatment of MM and can mediate the co-stimulation effect of lenalidomide on T cells. Cereblon combines with DDB1 (damaged DNA binding protein 1), CUL4A (Cullin 4A), and ROC1 (Cullins 1 regulator) to form the E3 ubiquitin ligase complex (CRL4–CRBN). This complex uses ubiquitin to label specific proteins, which are then degraded25,26. Kronke et al. reported that lenalidomide could promote the CRBN–DDB1 ubiquitin ligase complex to selectively bind to the transcription factors Ikaros (encoded by IKZF-1) and Aiolos (encoded by IKZF-3) and induce their ubiquitination and degradation10. IKZF-1 and IKZF-3 are members of the Ikaros family, which includes IKZF1-5. They play an essential regulatory role in lymphocyte proliferation and development, and can selectively enhance or inhibit the transcription level of target genes. Ikaros and Aiolos are transcriptional regulators necessary for the development of B cells and T cells, and they can inhibit the production of IL-227.
DNA-binding domains in Ikaros and Aiolos can recognize the promoter region of the IL-2 gene. When the promoter site is occupied by Ikaros or Aiolos, epigenetic changes in the chromatin can be induced, which can silent the IL-2 gene expression28–30. Therefore, lenalidomide promotes the binding of the E3 ubiquitin ligase complex to Ikaros and Aiolos, which makes ubiquitination and degradation of them. This subsequently reduces the levels of Ikaros and Aiolos in the promoter of the IL-2 gene, which can restore the IL-2 gene transcription and promote IL-2 secretion9,10. The above mechanism may contribute to the enhanced functions of CAR T cells in our study, as we found that lenalidomide also caused the reduced expression of Ikaros and Aiolos in CAR T cells.
Currently, the direct relationship between the degradation of Ikaros and Aiolos and CAR T-cell function is still not clear. However, it has been reported that IL-2 directly regulates T-cell functions in many studies. Besides the well-known function of enhancing proliferation and survival of activated T cells, IL-2 binding to IL-2 receptor can induce the unregulated expression of cytolytic effector molecules such as granzyme and perforin, which is independent of the proliferation of T cells31. IL-2 also increases the secretion of IFNγ by T cells32,33. Moreover, IL-2 was approved for treatment of renal cell carcinoma in 1992 and metastatic melanoma in 1998 by US FDA34, and the combination of IL-2 and tumor-infiltrating lymphocytes35,36 or TCR-engineered T cells37,38 also showed objective responses in patients from several clinical trials. Therefore, it is possible that lenalidomide regulating CAR T-cell activity against tumor cells in our study is partially mediated by IL-2. The combination of IL-2/lenolidomide and CAR T cells may benefit patients with solid tumors.
At present, studies on lenalidomide as a single drug or in combination with other strategies to target multiple solid tumors have made some progress. As a single drug, the antitumor effect of lenalidomide was observed in a mouse model inoculated with colon cancer cells (CT26)39 or melanoma cell line B16-F1016. In the clinics, metastatic renal cell carcinoma patients have sustained stable disease (SD), reduced tumor volume, and even complete remission after taking lenalidomide40–42. And certain effects have also been observed in the treatment of advanced liver cancer43, non-small-cell lung cancer, epithelioid hemangioendothelioma44, recurrent ovarian cancer, and primary peritoneal cancer45.
The study of combining lenalidomide with other anticancer drugs to target multiple solid tumors has found that lenalidomide can restore the sensitivity of tumors to anticancer drugs. The combination of the two in in vivo and in vitro models shows a synergistic antitumor effect. For example, when lenalidomide was combined with cisplatin to target triple-negative breast cancer cell line MDA-MB-231, lenalidomide reduced the half-maximal inhibitory concentration (IC50) value of cisplatin. Compared with single drugs, their combined application significantly inhibits angiogenesis and induces apoptosis46. In another study, lenalidomide and 1,25-D3 (1α, 25-dihydroxyvitamin D3, the biologically active form of vitamins D) were also targeted to MDA-MB-231 tumor cells (with vitamin D resistance). The results showed that lenalidomide restored the vitamin D-resistant cell line MDA-MB-231 to be vitamin D sensitive, increased the expression of proapoptotic protein p53, inhibited the expression of antiapoptotic protein BCL-2, and induced apoptosis of MDA-MB-231 cells47. From another example, lenalidomide was combined with Docetaxel to treat prostate cancer using in vitro and in vivo models. Lenalidomide could significantly reduce the IC50 of Docetaxel, decrease the invasion of three prostate cancer cells (PC3, LNCaP, and DU145 cells), and inhibit anchorage-independent growth of PC3 cells. Compared with monotherapy or control group, the overall death and apoptosis of PC3 and DU145 cells treated with lenalidomide and Docetaxel were significantly increased, the tumor growth rate was reduced, and the median survival time of PC3-bearing mice was significantly extended48. In a clinical study, patients with stage III/IV melanoma were treated by combining lenalidomide with Darcabazine, then complete response in two patients, partial response in four patients, and SD effect in five patients were observed49. Other studies have reported SD for 33 mo when lenalidomide and gemcitabine were used in the treatment of metastatic pancreatic cancer50. Subsequent studies have shown that lenalidomide can reduce the IC50 of gemcitabine, enhance the sensitivity of pancreatic cell lines PANC-1, MIA-PaCa-2, and BxPC-3 to gemcitabine, and improve the therapeutic effect51. In our study, lenalidomide was combined with CAR T cells to target solid tumors, and the immune adjuvant properties of lenalidomide for CAR T cells were explored. Our results showed that lenalidomide could promote the cytotoxicity and cytokine secretion of both CD133-CAR T cells and HER2-CAR T cells, and enhance the proliferation of CD133-CAR T cells, reflecting the synergistic effects of the combination. To our knowledge, this is the first time to show the combination of lenalidomide and CAR T cells for targeting solid tumors. Subsequent experiments will be conducted on more CAR T cells targeting different types of solid tumors to expand the range of effective effects of lenalidomide. Furthermore, these in vitro findings may be transferred to a mouse model for in vivo functional study.
In summary, our study indicates that lenalidomide can regulate the functions of CD133-CAR T cells and HER2-CAR T cells to varying degrees, providing a new basis for lenalidomide to regulate CAR T cells’ function when targeting solid tumors. Small-molecule immunomodulators combined with CAR T-cell therapy may offer new hope for the treatment of solid tumors.
Supplemental Material
Supplemental Material, Fig._S1 for Lenalidomide Enhances CAR-T Cell Activity Against Solid Tumor Cells by Zhixiong Wang, Guomin Zhou, Na Risu, Jiayu Fu, Yan Zou, Jiaxing Tang, Long Li, Hui Liu, Qian Liu and Xuekai Zhu in Cell Transplantation
Supplemental Material, Fig._S2 for Lenalidomide Enhances CAR-T Cell Activity Against Solid Tumor Cells by Zhixiong Wang, Guomin Zhou, Na Risu, Jiayu Fu, Yan Zou, Jiaxing Tang, Long Li, Hui Liu, Qian Liu and Xuekai Zhu in Cell Transplantation
Supplemental Material, Fig._S3 for Lenalidomide Enhances CAR-T Cell Activity Against Solid Tumor Cells by Zhixiong Wang, Guomin Zhou, Na Risu, Jiayu Fu, Yan Zou, Jiaxing Tang, Long Li, Hui Liu, Qian Liu and Xuekai Zhu in Cell Transplantation
Acknowledgments
We would like to thank the HTS Platform in SIAIS for the technical assistance.
Footnotes
Ethical Approval: Ethical approval is not applicable for this article.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Key R&D Program (2019YFA0111001) of China.
ORCID iD: Xuekai Zhu  https://orcid.org/0000-0002-4297-8854
https://orcid.org/0000-0002-4297-8854
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3(4):388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang H, Ye ZL, Yuan ZG. New Strategies for the Treatment of Solid Tumors with CAR-T Cells. Int J Biol Sci. 2016;12(6):718–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zeldis JB, Knight R, Hussein M, Chopra R, Muller G. A review of the history, properties, and use of the immunomodulatory compound lenalidomide. Ann N Y Acad Sci. 2011;1222(1):76–82. [DOI] [PubMed] [Google Scholar]
- 4. Otahal P, Prukova D, Kral V, Fabry M, Vockova P, Lateckova L, Trneny M, Klener P. Lenalidomide enhances antitumor functions of chimeric antigen receptor modified T cells. Oncoimmunology. 2016;5(4): e1115940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang X, Walter M, Urak R, Weng L, Huynh C, Lim L, Wong CW, Chang WC, Thomas SH, Sanchez JF, Yang L, et al. Lenalidomide Enhances the Function of CS1 Chimeric Antigen Receptor-Redirected T Cells Against Multiple Myeloma. Clin Cancer Res. 2018;24(1):106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Works M, Soni N, Hauskins C, Sierra C, Baturevych A, Jones JC, Curtis W, Carlson P, Johnstone TG, Kugler D, Hause RJ, et al. Anti-B-cell Maturation Antigen Chimeric Antigen Receptor T cell Function against Multiple Myeloma Is Enhanced in the Presence of Lenalidomide. Mol Cancer Ther. 2019;18(12):2246–2257. [DOI] [PubMed] [Google Scholar]
- 7. Zhu X, Prasad S, Gaedicke S, Hettich M, Firat E, Niedermann G. Patient-derived glioblastoma stem cells are killed by CD133-specific CAR T cells but induce the T cell aging marker CD57. Oncotarget. 2015;6(1):171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hu B, Zou Y, Zhang L, Tang J, Niedermann G, Firat E, Huang X, Zhu X. Nucleofection with Plasmid DNA for CRISPR/Cas9-Mediated Inactivation of Programmed Cell Death Protein 1 in CD133-Specific CAR T Cells. Hum Gene Ther. 2019;30(4):446–458. [DOI] [PubMed] [Google Scholar]
- 9. Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, Ito T, Ando H, Waldman MF, Thakurta A, Klippel A, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol. 2014;164(6):811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, Svinkina T, Heckl D, Comer E, Li X, Ciarlo C, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343(6168):301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gandhi AK, Kang J, Capone L, Parton A, Wu L, Zhang LH, Mendy D, Lopez-Girona A, Tran T, Sapinoso L, Fang W, et al. Dexamethasone synergizes with lenalidomide to inhibit multiple myeloma tumor growth, but reduces lenalidomide-induced immunomodulation of T and NK cell function. Curr Cancer Drug Targets. 2010;10(2):155–167. [DOI] [PubMed] [Google Scholar]
- 12. Ramsay AG, Clear AJ, Fatah R, Gribben JG. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood. 2012;120(7):1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heise C, Carter T, Schafer P, Chopra R. Pleiotropic mechanisms of action of lenalidomide efficacy in del(5q) myelodysplastic syndromes. Expert Rev Anticancer Ther. 2010;10(10):1663–1672. [DOI] [PubMed] [Google Scholar]
- 14. Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4(4):314–322. [DOI] [PubMed] [Google Scholar]
- 15. Verhelle D, Corral LG, Wong K, Mueller JH, Moutouh-de Parseval L, Jensen-Pergakes K, Schafer PH, Chen R, Glezer E, Ferguson GD, Lopez-Girona A, et al. Lenalidomide and CC-4047 inhibit the proliferation of malignant B cells while expanding normal CD34+ progenitor cells. Cancer Res. 2007;67(2):746–755. [DOI] [PubMed] [Google Scholar]
- 16. Lu L, Payvandi F, Wu L, Zhang LH, Hariri RJ, Man HW, Chen RS, Muller GW, Hughes CC, Stirling DI, Schafer PH, et al. The anti-cancer drug lenalidomide inhibits angiogenesis and metastasis via multiple inhibitory effects on endothelial cell function in normoxic and hypoxic conditions. Microvasc Res. 2009;77(2):78–86. [DOI] [PubMed] [Google Scholar]
- 17. Dredge K, Horsfall R, Robinson SP, Zhang LH, Lu L, Tang Y, Shirley MA, Muller G, Schafer P, Stirling D, Dalgleish AG, et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro . Microvasc Res. 2005;69(1-2):56–63. [DOI] [PubMed] [Google Scholar]
- 18. Ramsay AG, Johnson AJ, Lee AM, Gorgun G, Le Dieu R, Blum W, Byrd JC, Gribben JG. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118(7):2427–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramsay AG, Clear AJ, Kelly G, Fatah R, Matthews J, Macdougall F, Lister TA, Lee AM, Calaminici M, Gribben JG. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood. 2009;114(21):4713–4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, Schafer P, Bartlett JB. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14(14):4650–4657. [DOI] [PubMed] [Google Scholar]
- 21. Wu L, Parton A, Lu L, Adams M, Schafer P, Bartlett JB. Lenalidomide enhances antibody-dependent cellular cytotoxicity of solid tumor cells in vitro: influence of host immune and tumor markers. Cancer Immunol Immunother. 2011;60(1):61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, Todryk S, Chen R, Muller G, Stirling D, Schafer P, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009;58(7):1033–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, Patterson RT, Stirling DI, Kaplan G. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163(1):380–386. [PubMed] [Google Scholar]
- 24. Schafer PH, Gandhi AK, Loveland MA, Chen RS, Man HW, Schnetkamp PP, Wolbring G, Govinda S, Corral LG, Payvandi F, Muller GW, et al. Enhancement of cytokine production and AP-1 transcriptional activity in T cells by thalidomide-related immunomodulatory drugs. J Pharmacol Exp Ther. 2003;305(3):1222–1232. [DOI] [PubMed] [Google Scholar]
- 25. Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, Kang J, Karasawa S, Carmel G, Jackson P, Abbasian M, Mahmoudi A, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26(11):2326–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu YX, Braggio E, Shi CX, Bruins LA, Schmidt JE, Van Wier S, Chang XB, Bjorklund CC, Fonseca R, Bergsagel PL, Orlowski RZ, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011;118(18):4771–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmitt C, Tonnelle C, Dalloul A, Chabannon C, Debre P, Rebollo A. Aiolos and Ikaros: regulators of lymphocyte development, homeostasis and lymphoproliferation. Apoptosis. 2002;7(3):277–284. [DOI] [PubMed] [Google Scholar]
- 28. Bandyopadhyay S, Dure M, Paroder M, Soto-Nieves N, Puga I, Macian F. Interleukin 2 gene transcription is regulated by Ikaros-induced changes in histone acetylation in anergic T cells. Blood. 2007;109(7):2878–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thomas RM, Chunder N, Chen C, Umetsu SE, Winandy S, Wells AD. Ikaros enforces the costimulatory requirement for IL2 gene expression and is required for anergy induction in CD4+ T lymphocytes. J Immunol. 2007;179(11):7305–7315. [DOI] [PubMed] [Google Scholar]
- 30. Quintana FJ, Jin H, Burns EJ, Nadeau M, Yeste A, Kumar D, Rangachari M, Zhu C, Xiao S, Seavitt J, Georgopoulos K, et al. Aiolos promotes TH17 differentiation by directly silencing Il2 expression. Nat Immunol. 2012;13(8):770–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Janas ML, Groves P, Kienzle N, Kelso A. IL-2 regulates perforin and granzyme gene expression in CD8+ T cells independently of its effects on survival and proliferation. J Immunol. 2005;175(12):8003–8010. [DOI] [PubMed] [Google Scholar]
- 32. Kasahara T, Hooks JJ, Dougherty SF, Oppenheim JJ. Interleukin 2-mediated immune interferon (IFN-gamma) production by human T cells and T cell subsets. J Immunol. 1983;130(4):1784–1789. [PubMed] [Google Scholar]
- 33. Reem GH, Yeh NH. Interleukin 2 regulates expression of its receptor and synthesis of gamma interferon by human T lymphocytes. Science. 1984;225(4660):429–430. [DOI] [PubMed] [Google Scholar]
- 34. Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192(12):5451–5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, Simpson C, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319(25):1676–1680. [DOI] [PubMed] [Google Scholar]
- 36. Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86(15):1159–1166. [DOI] [PubMed] [Google Scholar]
- 37. Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Robbins PF, Kassim SH, Tran TL, Crystal JS, Morgan RA, Feldman SA, Yang JC, Dudley ME, Wunderlich JR, Sherry RM, Kammula US, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. 2015;21(5):1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu WM, Henry JY, Meyer B, Bartlett JB, Dalgleish AG, Galustian C. Inhibition of metastatic potential in colorectal carcinoma in vivo and in vitro using immunomodulatory drugs (IMiDs). Br J Cancer. 2009;101(5):803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Choueiri TK, Dreicer R, Rini BI, Elson P, Garcia JA, Thakkar SG, Baz RC, Mekhail TM, Jinks HA, Bukowski RM. Phase II study of lenalidomide in patients with metastatic renal cell carcinoma. Cancer. 2006;107(11):2609–2616. [DOI] [PubMed] [Google Scholar]
- 41. Amato RJ, Hernandez-McClain J, Saxena S, Khan M. Lenalidomide therapy for metastatic renal cell carcinoma. Am J Clin Oncol. 2008;31(3):244–249. [DOI] [PubMed] [Google Scholar]
- 42. Patel PH, Kondagunta GV, Schwartz L, Ishill N, Bacik J, DeLuca J, Russo P, Motzer RJ. Phase II trial of lenalidomide in patients with metastatic renal cell carcinoma. Invest New Drugs. 2008;26(3):273–276. [DOI] [PubMed] [Google Scholar]
- 43. Semeraro M, Vacchelli E, Eggermont A, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: lenalidomide-based immunochemotherapy. Oncoimmunology. 2013;2(11): e26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miller AA, Case D, Harmon M, Savage P, Lesser G, Hurd D, Melin SA. Phase I study of lenalidomide in solid tumors. J Thorac Oncol. 2007;2(5):445–449. [DOI] [PubMed] [Google Scholar]
- 45. Zhang MM, Chan JK, Husain A, Guo HY, Teng NN. Safety and efficacy of lenalidomide (Revlimid) in recurrent ovarian and primary peritoneal carcinoma. Gynecol Oncol. 2007;105(1):194–198. [DOI] [PubMed] [Google Scholar]
- 46. Yin LL, Wen XM, Lai QH, Li J, Wang XW. Lenalidomide improvement of cisplatin antitumor efficacy on triple-negative breast cancer cells in vitro . Oncol Lett. 2018;15(5):6469–6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brosseau C, Colston K, Dalgleish AG, Galustian C. The immunomodulatory drug lenalidomide restores a vitamin D sensitive phenotype to the vitamin D resistant breast cancer cell line MDA-MB-231 through inhibition of BCL-2: potential for breast cancer therapeutics. Apoptosis. 2012;17(2):164–173. [DOI] [PubMed] [Google Scholar]
- 48. Henry JY, Lu L, Adams M, Meyer B, Bartlett JB, Dalgleish AG, Galustian C. Lenalidomide enhances the anti-prostate cancer activity of docetaxel in vitro and in vivo. Prostate. 2012;72(8):856–867. [DOI] [PubMed] [Google Scholar]
- 49. Hwu WJ, Knight RD, Patnana M, Bassett R, Papadopoulos NE, Kim KB, Hwu P, Bedikian A. Phase I safety study of lenalidomide and dacarbazine in patients with metastatic melanoma previously untreated with systemic chemotherapy. Melanoma Res. 2010;20(6):50–506. [DOI] [PubMed] [Google Scholar]
- 50. Liu WM, Nizar S, Dalgleish AG. Gemcitabine and lenalidomide combination in a patient with metastatic pancreatic cancer: a case study. Med Oncol. 2010;27(2):430–433. [DOI] [PubMed] [Google Scholar]
- 51. Fryer RA, Barlett B, Galustian C, Dalgleish AG. Mechanisms underlying gemcitabine resistance in pancreatic cancer and sensitisation by the iMiD lenalidomide. Anticancer Res. 2011;31(11):3747–3756. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Fig._S1 for Lenalidomide Enhances CAR-T Cell Activity Against Solid Tumor Cells by Zhixiong Wang, Guomin Zhou, Na Risu, Jiayu Fu, Yan Zou, Jiaxing Tang, Long Li, Hui Liu, Qian Liu and Xuekai Zhu in Cell Transplantation
Supplemental Material, Fig._S2 for Lenalidomide Enhances CAR-T Cell Activity Against Solid Tumor Cells by Zhixiong Wang, Guomin Zhou, Na Risu, Jiayu Fu, Yan Zou, Jiaxing Tang, Long Li, Hui Liu, Qian Liu and Xuekai Zhu in Cell Transplantation
Supplemental Material, Fig._S3 for Lenalidomide Enhances CAR-T Cell Activity Against Solid Tumor Cells by Zhixiong Wang, Guomin Zhou, Na Risu, Jiayu Fu, Yan Zou, Jiaxing Tang, Long Li, Hui Liu, Qian Liu and Xuekai Zhu in Cell Transplantation