Abstract
The αβ T-cell-depleted hematopoietic stem cell transplantation (HSCT) leads to lower relapse and better outcome, and may correlate strongly with expansion of donor-derived γδ T cells. γδ T cells play an important role in immune reconstitution and can exert a graft-versus-leukemia effect after HSCT. This review showed the recent literature on immune functions of γδ T cells after HSCT. The discrepancies between studies of γδ T cells in graft-versus-host disease may cause by its heterogeneous and various distinct subsets. And reconstitution of γδ T cells may play a potential immunoregulatory role in the infections after HSCT.
Keywords: γδ T cells, hematopoietic stem cell transplantation, immune function, graft-versus-host disease, infection
Introduction
Hematopoietic stem cell transplantation (HSCT) is an important treatment for hematological malignancies and is wildly used in leukemia for decades1,2. According to whether grafts are accepted to T cell depletion or not, HSCT is divided into T-cell-depleted HSCT and non-T-cell-depleted HSCT. T-cell-depleted HSCT leads to favorable overall survival (OS) and disease-free survival and deduction of graft-versus-host disease (GVHD), while non-T-cell-depleted HSCT leads to higher risks of GVHD3,4. αβ T cells were depleted in T-cell-depleted HSCT for purposes of lower relapse and better outcome5, and αβ T-cell-depleted HSCT showed that event free and OS correlated strongly with expansion of donor-derived γδ T cells6. γδ T cells might play an important role in immune reconstitution (IR) and could exert a graft-versus-leukemia effect after HSCT6–11. Moreover, higher numbers of γδ T cells might improve clinical outcome following HSCT12. In this report, we review the recent literature on immune functions of γδ T cells after HSCT.
General Characteristics of γδ T Cells
According to the surface receptors, T cells can be divided into different subsets. Based on the difference of T cell receptor (TCR), T cells can be divided into αβ T cells and γδ T cells. A large amount of T cells are αβ T cells, while γδ T cells account for a small proportion, approximately 1.2%–15.4%13. Human γδ T cells can be recognized by the expression of TCR Vδ and TCR Vγ, and TCR Vδ1, Vδ2, Vδ3, Vγ2, Vγ3, Vγ4, Vγ5, Vγ8, Vγ9, and Vγ11 are the most commonly used gene fragments for rearrangement of δ and γ chains14. In human peripheral blood, a large amount of γδ T cells express TCR Vδ2 chain paired with Vγ9 chain, and γδ T− cells expressing TCR Vδ1 or Vδ3 chain can be paired with various Vγ chains15,16. Most of γδ T cells in lymphoid organs and peripheral tissue like skin, lung, and intestine usually express TCR Vδ1 or Vδ3 chain, but not Vδ2. As we all known, γδ T cells are CD3+ lymphocytes, and most of them neither express CD4 nor CD8, but a small percentage of cells are CD8+ γδ T cells17.
T cells can be divided into cytotoxic T cells, helper T cells, memory T cells, and regulatory T cells (Tregs) according to their different functions. Nowadays, the research of the functions and effects of different subsets of γδ T cells and their application have been a heated topic. Antitumor activities can be exerted by γδ T cells to fight against solid tumors and hematological malignancies via in vitro and in vivo mechanisms like cytotoxicity effect. γδ T cells can effectively kill tumor and directly identify target molecules expressed by leukemic cells in a major histocompatibility complex (MHC) independent manner18. In addition, UL16 binding proteins (ULBP) 4 on tumor cells can be matched with γδ T cells, and the overexpression of ULBP-1 and ULBP-4 will cause the cytotoxicity of Vγ9+ Vδ2+ T cells19. Various cytokines such as IFN-γ and TNF-α can be produced by activated γδ T cells and play a significant role in potent cytotoxic activity. However, Iwasaki et al. found that the expression of programmed cell death-1 (PD-1) on γδ T cells may diminish the levels of cytokines production and cytotoxicity against programmed cell death ligand-1 tumor cells20. And they also reported that the inhibitory effect of PD-1 in γδ T cells may partially overcome by TCR triggering. Moreover, through the inflammatory CCR2/CCL2 chemokine pathway, the γδ T cells that are tumor infiltrating can be collected into the tumor bed1.
Previous studies suggested that Tregs might affect the clinical outcome of HSCT, and Tregs counter-regulation might promote allograft tolerance21. Distinct subsets of γδ T cells, especially γδ Tregs, can also play an important role in HSCT7. We found that γδ Tregs expressed the novel immune checkpoint receptors T cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain, CD226, and DNAX accessory molecule-1 (DNAM-1) in aute myeloid leukemia (AML) patients and the expressions were associated with clinical outcomes22,23. γδ Tregs were also found to inhibit the proliferation of CD4+CD25− T cells in patients with multiple myeloma24. And in healthy donors, Casetti et al. initially discovered Foxp3+ rich Vδ2 T cells, which were stimulated by transforming growth factor-beta 1 (TGF-β1)/IL-15, suppress the proliferation of peripheral blood mononuclear cells (PBMC)25. What’s more, another study also suggested that γδ Tregs from human PBMC could facilitate the induction and immunosuppressive function by decitabine combined with zoledronate (ZOL)/IL-2/IL-15-TGF-β126.
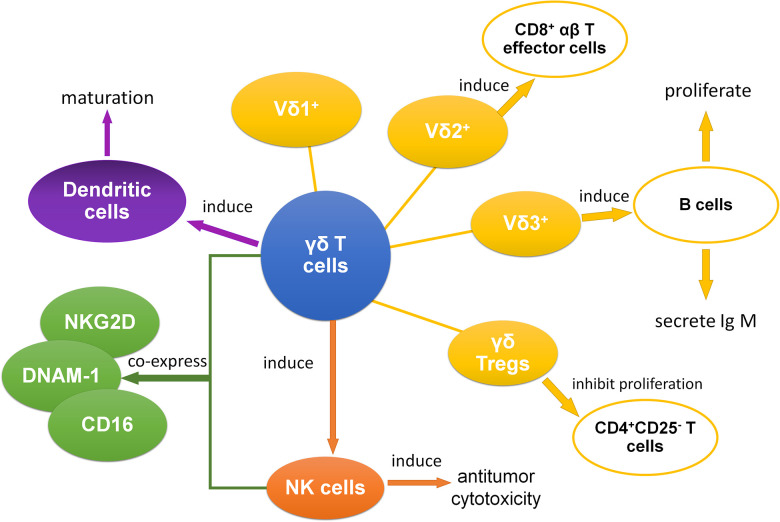
Meanwhile, γδ T cells have the ability to effectively induce the CD8+ T cells to proliferate and kill target cells27. And γδ T cells can induce the antitumor cytotoxicity of other cells, especially natural killer (NK) cells28. Additionally, subsets of γδ T cells coexpress some receptors of NK cells like natural killer group 2D (NKG2D), DNAM-1, and CD1613,29,30. Moreover, NKG2D that is expressed on Vδ1 T cells can be combined with MHC class I chain-related A (MICA), and this combination between MICA and NKG2D was stronger than the one between MICA and TCR31. As for B cells, B cells can be induced to proliferate as well as secrete IgM by Vδ3 T cells32. In another research about dendritic cells, it suggests that γδ T cells might be able to promote the maturation of dendritic cells33. In conclusion, through antigen presentation, inducing other immune cells, and many other pathways, γδ T cells play an essential role in interacting with other immune cells (Fig. 1).
Figure 1.
The interaction between γδ T cells and other immune cells. γδ T cells mainly show interactive relation with other immune cells, which include αβ T cells, B cells, NK cells, dendritic cells, and CD4+CD25− T cells.14,26,28–34 NK: natural killer.
With complexity of cell surface expressed molecules and ability inducing antitumor cytotoxicity of other immune cells, γδ T cells play a crucial role in HSCT. A study established that quick reconstitution of the γδ T cell repertoire after allogeneic HSCT (allo-HSCT), which might help improve OS, retained the overall complexity and proportion of public clonotypes34. Increased γδ T cells also have an advantage in improving leukemia-free survival and OS of the patients with acute leukemia after allo-HSCT, which is expected to reduce the recurrence rate considerably and contribute to better effect6. γδ T cells also have effect on the tolerance of immunotransplantation. Our previous studies showed that granulocyte colony-stimulating factor (G-CSF) could induce immune tolerance and it might associate with clonality of TCRs on γδ T cells and the increase of γδ Tregs in grafts7,35. We demonstrated that γδ Tregs had certain cytotoxic effects on tumor cells after G-CSF treatment7. They also found that the proportion of CD27+ Vδ1 Tregs in graft was negatively associated with acute GVHD (aGVHD)7. In addition, in liver transplantation, Vδ1 T cells with a unique clone in graft were reported which could recognize a specific ligand and contributed to the establishment of tolerance by suppressing rejection36. Therefore, γδ T cells might play a vital role in immune tolerance of transplantation and be beneficial to outcome of HSCT.
IR of γδ T Cells After HSCT
IR is one of the key factors for the success of allo-HSCT and depends on transplant modality, graft treatment, pretreatment, and immunosuppression after transplantation37. γδ T cells as effective cells to kill tumors can reconstitute early after transplantation34. Donor source and virus reactivation might associate with the reconstitution of γδ T cells and their subsets. It has been shown that in recipients of umbilical cord blood-HCT (UCB-HCT), Vδ2 T cells were almost undetectable9. However, Vδ2 T cells have a bimodal response in recipients of HLA-matched sibling or unrelated donor allo-HCT (MSD/MUD-HCT), about 30% individuals have higher Vδ2 repertoires, and others have lower Vδ2 repertoires9. Another study on HLA-haploidentical HSCT (haplo-HSCT) also showed that homeostatic donor γδ T cell content was associated with the early γδ T cell recovery following HSCT38. Similarly, Perko et al. suggested that donor source affected the IR of γδ T cells10. Patients who underwent matched related donor or haplo-HSCT had a significant difference in the reconstitution of γδ T cells compared with matched unrelated donor patients, whereas there was no difference between patient characteristics of age, gender, disease, GVHD prophylaxis, and the recovered γδ T cells10. Furthermore, they also found that CD3+, CD4+, and CD8+ T cells also affected the recovery of γδ T cells10. In other side, in cytomegalovirus (CMV) reactivation, Vδ1 T cells were increased in both MSD/MUD-HCT and UCB-HCT, but the difference was statistically significant only in recipients of UCB-HCT9. Day 30 Vδ1 recovery inversely associated with CMV reactivation in HSCT recipients38. And Vδ2 T cells recovery had the negative correlation with Epstein-Barr virus (EBV) reactivation after HSCT39. Indeed, more research is required to investigate the IR of γδ T cells after transplantation with a view to develop better application in HSCT.
The Roles of γδ T Cells in GVHD
GVHD is one of the most serious complications of allo-HSCT and is a major obstacle to improve outcomes of patients after transplantation. GVHD is divided into aGVHD and chronic GVHD (cGVHD) according to the time and type of organ involvement. The mechanism of aGVHD is immunocompetent T cells from the donor could recognize host alloantigens and interact with host antigen-presenting cells, then lead to donor T-cell activation and expansion, and cytotoxic effect40,41. The aGVHD often implicates skin, liver, and gastrointestinal tract, and combined with the involvement of these three organ systems could give a clinical stage, ranging from 0 to IV42. Unlike aGVHD, the mechanism of cGVHD is more complex and involves more organs. It involves three-phase model: early inflammatory response and tissue injury (phase 1), dysregulated B cell and T cell immunity and chronic inflammation (phase 2), and activation of the profibrotic pathway (phase 3)40,43,44. Based on National Institutes of Health consensus criteria and the number and severity of affected organs or sites, cGVHD is divided into three grades: mild, moderate, and severe45. With the depth understanding of the mechanism of GVHD, it was found that T cells might play an important role in GVHD. In this regard, the role of T cell subsets on GVHD has been extensively investigated. Several researchers reported that αβ T cells were considered to relate to the pathogenesis of GVHD, whereas γδ T cells receptor did not lead to GVHD and had strong antileukemia and antivirus activities5,46,47. Furthermore, γδ T cells were reported to play a protective role in GVHD, a heavy obstacle to the effectiveness of HSCT, as the cause of less risk of aGVHD in patients with higher γδ T cell concentration8. γδ T cells have potential good effect on GVHD prevention; however, the exact role of γδ T cells in GVHD remains an open question.
In mice models, some research showed that donor γδ T cells were involved in GVHD pathogenesis48,49. However, other studies demonstrated that these cells were not associated with the development of GVHD50,51. Similar to mice, in human, γδ T cells also play different roles in GVHD (Table 1). Pabst et al. suggested that donor γδ TCR-expressing CD3+ cells counted above the median were related to the cumulative risk of aGVHD II-IV, and γδ T cells in the grafts of patients in GVHD class II to IV were much higher than in the grafts of patients in GVHD class 0 to I52. Noteworthy, in their study γδ T cells in human were from unrelated donors and examined as a single-cell population without analyzed the effect of different subsets of γδ T cells. Another study that divided patients with GVHD into limited cGVHD group, extensive cGVHD group, and non-cGVHD group found that the percent of γδ T cells in non-cGVHD group significantly increased compared with other groups, and increasing number of γδ Treg was also reported to be a preferential strategy for controlling cGVHD following HSCT53.
Table 1.
| Regions | Phenotype of γδ T cells | In aGVHD | In cGVHD |
|---|---|---|---|
| In recipient | Total γδ T cells | Negative correlation | No relation |
| Vδ1 T cells | Negative correlation | Negative correlation | |
| Vδ2 T cells | Negative correlation | Negative correlation | |
| In grafts | Total γδ T cells | Positive correlation | No relation |
| CD27+Vδ1 Treg | Negative correlation | Unknown | |
| CD3+ γδ T cells | Positive correlation | Unknown |
aGVHD: acute graft-versus-host disease; cGVHD: chronic graft-versus-host disease; GVHD: graft-versus-host disease.
Recently, Arruda et al. used a high-throughput analysis of TCR Vγ repertoire in AML patients after accepted grafts, which suggested that TCR gamma locus (TRG) clonal distribution were not associated with occurrence or absence of aGVHD54. However, our previous study suggested that grafts with higher proportion of CD27+ Vδ1 Tregs was associated with a lower incidence of aGVHD in G-CSF-mobilized allogeneic peripheral blood stem cell transplantation recipients7. Similar to our study, a prospective, clinical study from Europe recently suggested that patients with high concentrations of total γδ T cells had lower incidence of aGVHD but did not affect the development of cGVHD after transplantation8.
The discrepancies between studies of γδ T cells in GVHD may cause by its heterogeneous and various distinct subsets, and using G-CSF might influence the distribution, expression levels, and clonality of γδ T cell repertoire. Furthermore, the origin of the graft and the heterogeneity of patient characteristics also can infect the clinical outcome. Consequently, it needs further work to evaluate the detailed phenotype of γδ T cells after transplantation, which may provide a great prospect for the treatment of GVHD.
γδ T Cells and Virus Reactivation After Allo-HSCT
γδ T Cells and CMV Reactivation
CMV infection is a common complication following allo-HSCT. Some previous studies reported that CMV reactivation increased transplant-related mortality (TRM)55,56. However, others studies demonstrated that CMV reactivation had no significant influence on the OS of patients after allo-HSCT57. Moreover, CMV reactivation might decrease the relapse rate of AML patients who accept HSCT58. IR following HSCT might be the key of these differences59.
CMV reactivation could occur in receptors who accepted the CMV+ grafts from donor or in immunocompromised patients like following HSCT60,61. γδ T cells reconstituted rapidly by 30–60 days following transplantation and had promoting influence to IR34,55. During CMV reactivation after early HSCT, proliferations of Vδ2 negative T cells but not Vδ2 positive T cells were observed in peripheral blood62,63. This result was similar to the finding of Raven et al. who found expansions of heterogeneous Vδ1, Vδ3, and unconventional Vδ2 clones during CMV reactivation after 25–60 days of HSCT34. Other studies found that Vδ1 T cells recovery was correlated with CMV reactivation in HSCT recipients by using multiplex PCR system to sequence TRG and TCR delta locus (TRD) CDR3 regions or flow cytometry analysis9,38. Several CMV-related γδ T cell clones proliferation in grafts from CMV+ donors were also observed54. Therefore, reconstitution of γδ T cells might be involved in the outcome of CMV reactivation after transplantation.
γδ T Cells and EBV Reactivation
EBV infection is another complication about virus reactivation following allo-HSCT. EBV reactivation was related with post-transplantation lympho-proliferation disorder and led to higher TRM and lower OS64,65. Vγ9 Vδ2 T cells, which were expanded by pamidronate, with engagement of Fas and TRAIL, were demonstrated to kill EBV-transformed autologous lymphoblastoid B cell lines66. Liu et al. found that recovered Vδ2 T cells were inversely related with EBV reactivation after HSCT and had cytotoxic on EBV-infected cells, while immunosuppressants play a negative roll on the anti-EBV capacity of Vδ2 T cells39,67. Djaoud et al. also found that Vγ9 Vδ2 T cells were the major innate immunity against EBV and could be expanded after EBV infection68. Moreover, their recent study reported that basing on the response to EBV, γδ T cells can be defined to the strong immunity group with large population, which expressing Vγ9 JγP and could produce activated effector cells, and the weak one with small population, which expressing Vγ9 Jγ269.
EBV can be found in the skin or mucosa and may be associated with mucositis70. γδ T cells, with distinct subsets, as important innate immune cells for human, present in both blood and tissues and play multiple roles in mucosal inflammation71. Though one case report was shown that γδ T cells were found out in peripheral blood but not skin or lung during EBV-associated lymphoproliferative disease72. We suggest that γδ T cells might play some certain roles in tissue immunity during EBV-related infection. Regrettably, previous studies about γδ T cells IR after HSCT mainly reported total γδ T cells or Vδ2 T cells in peripheral blood during EBV infection. More studies need to be investigated to find out the effect of distinct γδ T cells IR in tissues during EBV reactivation following HSCT.
γδ T Cells and Other Infection After Allo-HSCT
Hepatitis B Virus
The high risk of hepatitis B virus (HBV) reactivation is associated with GVHD after HSCT and will make negative impact on post-transplant prognosis73,74. Currently, some studies reflect the relation between HBV inflection and γδ cells. It shows that in chronic hepatitis B (CHB), Vδ2 T cells decrease in patients’ peripheral blood and relate to less IFN-γ production and cytotoxic activity75. In a mouse model of acute HBV infection, liver γδ T cells and IFN-β production increased during the early stages of HBV infection, but there were no much changes in peripheral γδ T cells76. And IFN-α can enhance the cytotoxic function of peripheral γδ T cells in CHB77. γδ T cells have a potential role in the treatments of HBV infection and the disease it possibly lead to, and it can be more studied in the future.
Human Immunodeficiency Virus
Human immunodeficiency virus (HIV) is a lymphotropic virus, which will lead to acquired and innate immune suppression. Recent study suggested the potential function of allo-HSCT lead to sustained, anti-retroviral-free HIV-1 remission78,79. But it is unclear which mechanism might contribute to the HIV remission. HIV infection will cause to the normal ratio of Vδ2:Vδ1 cells by Vδ1 T cells increase and Vδ2 T cells depletion80,81. The dysfunction of Vδ2 T cells was probably caused by skewing toward a terminal effector memory phenotype82. And abnormal expression of γδ T cells can be a potential surrogate marker of acquired immunodeficiency syndrome progression83.
Fungus and Bacteria
Gram-negative bacteria, then followed by gram-positive bacteria and fungus are the main pathogens of bloodstream infection after HSCT84. Aspergillus and Candida albicans are common pathogens of invasive fungal diseases following HSCT85,86. γδ T cells reconstitution in early HSCT may play a potential immunoregulatory role in bloodstream infection. Vδ1 T cells could respond with proliferation and IFN-γ/IL-17 production to Candida albicans in vitro, whereas Vδ2 T cells could proliferate and produce IFN-γ/IL-17 in response to mycobacteria87. In addition, in response to Aspergillus fumigatus antigens, Vγ9Vδ2 T cell clones reactive by phosphate antigens were found to produce tumor necrosis factor in healthy individuals88. Similar results were found that Aspergillus fumigatus antigens could induce the expansion of γδ T cells indirectly89.
γδ T Cells and Relapse After HSCT
Relapse is a common cause of mortality and treatment failure after HSCT (Table 2). Arruda et al. found that patients with higher γδ T cells present less relapse during IR after HSCT12. Moreover previous study has shown higher cumulative incidence of death from relapse in patients with low γδ T cells and Vδ2 T cells8. It seems that γδ T cells can be a predict marker of relapse after HSCT. Measurable residual disease, also known as minimal residual disease (MRD), was reported as a predictor for relapse following HSCT90. MRD monitoring by using TRD and TRG rearrangement was demonstrated as a useful predictor for the risk of relapse in T-ALL patients with chemotherapy 91. Furthermore, Galimberti et al. found that MRD eradication did significantly affect TCR γ/δ clones profiles in patients with multiple myeloma follow allogeneic non-myeloablative transplantation92. In a case report, Nomura et al. suggested that MRD, which assessed by PCR assay for TRD in the bone marrow, was useful for the prediction of relapse following bone marrow transplantation93. More studies need to be investigated to find out the relationship between γδ T cells and MRD, which may explain the mechanism that γδ T cells improved relapse following HSCT.
Table 2.
| Phenotype of T cells | Risk of relapse | Risk of death from relapse | OS | RFS |
|---|---|---|---|---|
| Total γδ T cells | Negative correlation | Negative correlation | Positive correlation | Positive correlation |
| Vδ1 T cells | Negative correlation | Negative correlation | Positive correlation | Positive correlation |
| Vδ2 T cells | Negative correlation | Negative correlation | Positive correlation | Positive correlation |
| CD3+γδ T cells | Unknown | Negative correlation | Negative correlation | Negative correlation |
| CD4+ T cells | Unknown | Negative correlation | Negative correlation | Negative correlation |
HCST: hematopoietic stem cell transplantation; OS: overall survival; RFS: relapse-free survival.
γδ T Cells and Immunotherapy
More and more studies showed that γδ T cells have antitumor activity and play an unique role in immunosurveillance, and can be used as a tool for immunotherapy. Ex vivo antigen-driven generating large numbers of autologous Vδ2 T cells and adoptive reinfusion is possible to be a viable strategy for immunotherapy-based intervention94. Furthermore, ZOL/IL-2 have significant effect on improving the cytotoxic effect of γδ T expanded in vivo 95. Phase I clinical trials on γδ T cells, including both expanded in vitro and stimulated in vivo, purpose on adoptive transfer, were completed96,97. A few clinical trials (ClinicalTrials. gov Identifier: NCT03533816, NCT03862833) about using γδ T cells for transplantation improvement are ongoing, but the results of these clinical studies have not been reported. Now HSCT following chimeric antigen receptor (CAR)-T therapy were reported favorable outcomes like higher OS and lower relapsed rate98,99. CAR-γδ T cells therapy was a novel promote immunotherapy for antitumor100 and CAR-γδ T cells may be a potential immunological treatment to improve clinical outcomes of HSCT.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (grant number 81770150), Natural Science Foundation of Guangdong Province (grant number 2020A1515010817), the Guangzhou Science and Technology Project (grant number 201804010425), Medical Scientific Research Foundation of Guangdong Province (grant numbers A2018565 and A2017198), and College Students’ Scientific and Technological Innovation (grant number 202010559081).
ORCID iDs: Wanyi Ye  https://orcid.org/0000-0002-2369-4581
https://orcid.org/0000-0002-2369-4581
References
- 1. Lanca T, Costa MF, Goncalves-Sousa N, Rei M, Grosso AR, Penido C, Silva-Santos B. Protective role of the inflammatory CCR2/CCL2 chemokine pathway through recruitment of type 1 cytotoxic gammadelta T lymphocytes to tumor beds. J Immunol. 2013;190(12):6673–6680. [DOI] [PubMed] [Google Scholar]
- 2. Kunacheewa C, Ungprasert P, Phikulsod P, Issaragrisil S, Owattanapanich W. Comparative efficacy and clinical outcomes of haploidentical stem cell transplantation to other stem sources for treatment in acute myeloid leukemia and myelodysplastic syndrome patients: a systematic review and meta-analysis. Cell Transplant. 2020;29:963689720904965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Montoro J, Ceberio I, Hilden P, Maloy MA, Barker J, Castro-Malaspina H, Dahi P, Koehne G, Perales MA, Ponce D, Sauter C, et al. Ex vivo t cell-depleted hematopoietic stem cell transplantation for adult patients with acute myelogenous leukemia in first and second remission: long-term disease-free survival with a significantly reduced risk of graft-versus-host disease. Biol Blood Marrow Transplant. 2020;26(2):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uhm J, Hamad N, Shin EM, Michelis FV, Shanavas M, Gupta V, Kuruvilla J, Lipton JH, Messner HA, Seftel M, Kim DD. Incidence, risk factors, and long-term outcomes of sclerotic graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20(11):1751–1757. [DOI] [PubMed] [Google Scholar]
- 5. Abdelhakim H, Abdel-Azim H, Saad A. Role of alphabeta T cell depletion in prevention of graft versus host disease. Biomedicines. 2017;5(3):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Godder KT, Henslee-Downey PJ, Mehta J, Park BS, Chiang KY, Abhyankar S, Lamb LS. Long term disease-free survival in acute leukemia patients recovering with increased gammadelta T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant. 2007;39(12):751–757. [DOI] [PubMed] [Google Scholar]
- 7. Xuan L, Wu X, Qiu D, Gao L, Liu H, Fan Z, Huang F, Jin Z, Sun J, Li Y, Liu Q. Regulatory gammadelta T cells induced by G-CSF participate in acute graft-versus-host disease regulation in G-CSF-mobilized allogeneic peripheral blood stem cell transplantation. J Transl Med. 2018;16(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Minculescu L, Marquart HV, Ryder LP, Andersen NS, Schjoedt I, Friis LS, Kornblit BT, Petersen SL, Haastrup E, Fischer-Nielsen A, Reekie J, et al. Improved overall survival, relapse-free-survival, and less graft-vs.-host-disease in patients with high immune reconstitution of TCR gamma delta cells 2 months after allogeneic stem cell transplantation. Front Immunol. 2019;10:1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Witte MA, Sarhan D, Davis Z, Felices M, Vallera DA, Hinderlie P, Curtsinger J, Cooley S, Wagner J, Kuball J, Miller JS. Early reconstitution of NK and gammadelta T cells and its implication for the design of post-transplant immunotherapy. Biol Blood Marrow Transplant. 2018;24(6):1152–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perko R, Kang G, Sunkara A, Leung W, Thomas PG, Dallas MH. Gamma delta T cell reconstitution is associated with fewer infections and improved event-free survival after hematopoietic stem cell transplantation for pediatric leukemia. Biol Blood Marrow Transplant. 2015;21(1):130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamb LS, Henslee-Downey PJ, Parrish RS, Godder K, Thompson J, Lee C, Gee AP. Rapid communication: increased frequency of TCRγδ+ T cells in disease-free survivors following T cell-depleted, partially mismatched, related donor bone marrow transplantation for leukemia. J Hematother. 1996;5(5):503–509. [DOI] [PubMed] [Google Scholar]
- 12. Arruda LCM, Gaballa A, Uhlin M. Impact of gammadelta T cells on clinical outcome of hematopoietic stem cell transplantation: systematic review and meta-analysis. Blood Adv. 2019;3(21):3436–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fonseca S, Pereira V, Lau C, Teixeira MDA, Bini-Antunes M, Lima M. Human peripheral blood gamma delta t cells: report on a series of healthy caucasian portuguese adults and comprehensive review of the literature. Cells. 2020;9(3):729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adams EJ, Gu S, Luoma AM. Human gamma delta T cells: evolution and ligand recognition. Cell Immunol. 2015;296(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thedrez A, Sabourin C, Gertner J, Devilder MC, Allain-Maillet S, Fournie JJ, Scotet E, Bonneville M. Self/non-self discrimination by human gammadelta T cells: simple solutions for a complex issue? Immunol Rev. 2007;215:123–135. [DOI] [PubMed] [Google Scholar]
- 16. Zhao Y, Niu C, Cui J. Gamma-delta (gammadelta) T cells: friend or foe in cancer development? J Transl Med. 2018;16(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhagat G, Naiyer AJ, Shah JG, Harper J, Jabri B, Wang TC, Green PH, Manavalan JS. Small intestinal CD8+TCRgammadelta+NKG2A+ intraepithelial lymphocytes have attributes of regulatory cells in patients with celiac disease. J Clin Invest. 2008;118(1):281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gertner-Dardenne J, Castellano R, Mamessier E, Garbit S, Kochbati E, Etienne A, Charbonnier A, Collette Y, Vey N, Olive D. Human Vgamma9Vdelta2 T cells specifically recognize and kill acute myeloid leukemic blasts. J Immunol. 2012;188(9):4701–4708. [DOI] [PubMed] [Google Scholar]
- 19. Kong Y, Cao W, Xi X, Ma C, Cui L, He W. The NKG2D ligand ULBP4 binds to TCRgamma9/delta2 and induces cytotoxicity to tumor cells through both TCRgammadelta and NKG2D. Blood. 2009;114(2):310–317. [DOI] [PubMed] [Google Scholar]
- 20. Iwasaki M, Tanaka Y, Kobayashi H, Murata-Hirai K, Miyabe H, Sugie T, Toi M, Minato N. Expression and function of PD-1 in human gammadelta T cells that recognize phosphoantigens. Eur J Immunol. 2011;41(2):345–355. [DOI] [PubMed] [Google Scholar]
- 21. Jaiswal SR, Bhakuni P, Joy A, Murli N, Rajoreya A, Chakrabarti A, Chakrabarti S. Higher CD45RA(+) regulatory t cells in the graft improves outcome in younger patients undergoing T cell-replete haploidentical transplantation: where donor age matters. Biol Blood Marrow Transplant. 2018;24(10):2025–2033. [DOI] [PubMed] [Google Scholar]
- 22. Jin Z, Ye W, Lan T, Zhao Y, Liu X, Chen J, Lai J, Chen S, Zhong X, Wu X. Characteristic of TIGIT and DNAM-1 expression on Foxp3+ gammadelta T cells in AML patients. Biomed Res Int. 2020;2020:4612952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jin Z, Lan T, Zhao Y, Du J, Chen J, Lai J, Xu L, Chen S, Zhong X, Wu X, Li Y. Higher TIGIT(+)CD226(-) gammadelta T cells in patients with acute myeloid leukemia. Immunol Invest. 2020:1–11. [DOI] [PubMed] [Google Scholar]
- 24. Ma Y, Lei H, Tan J, Xuan L, Wu X, Liu Q. Characterization of gammadelta regulatory T cells from peripheral blood in patients with multiple myeloma. Biochem Biophys Res Commun. 2016;480(4):594–601. [DOI] [PubMed] [Google Scholar]
- 25. Casetti R, Agrati C, Wallace M, Sacchi A, Martini F, Martino A, Rinaldi A, Malkovsky M. Cutting edge: TGF-beta1 and IL-15 Induce FOXP3+ gammadelta regulatory T cells in the presence of antigen stimulation. J Immunol. 2009;183(6):3574–3577. [DOI] [PubMed] [Google Scholar]
- 26. Hu Y, Cui Q, Gu Y, Sheng L, Wu K, Shi J, Tan Y, Fu H, Liu L, Fu S, Yu X, et al. Decitabine facilitates the generation and immunosuppressive function of regulatory gammadeltaT cells derived from human peripheral blood mononuclear cells. Leukemia. 2013;27(7):1580–1585. [DOI] [PubMed] [Google Scholar]
- 27. Brandes M, Willimann K, Bioley G, Levy N, Eberl M, Luo M, Tampe R, Levy F, Romero P, Moser B. Cross-presenting human gammadelta T cells induce robust CD8+ alphabeta T cell responses. Proc Natl Acad Sci U S A. 2009;106(7):2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maniar A, Zhang X, Lin W, Gastman BR, Pauza CD, Strome SE, Chapoval AI. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood. 2010;116(10):1726–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poggi A, Zancolli M, Boero S, Catellani S, Musso A, Zocchi MR. Differential survival of gammadeltaT cells, alphabetaT cells and NK cells upon engagement of NKG2D by NKG2DL-expressing leukemic cells. Int J Cancer. 2011;129(2):387–396. [DOI] [PubMed] [Google Scholar]
- 30. Toutirais O, Cabillic F, Le Friec G, Salot S, Loyer P, Le Gallo M, Desille M, de La Pintiere CT, Daniel P, Bouet F, Catros V. DNAX accessory molecule-1 (CD226) promotes human hepatocellular carcinoma cell lysis by Vgamma9Vdelta2 T cells. Eur J Immunol. 2009;39(5):1361–1368. [DOI] [PubMed] [Google Scholar]
- 31. Xu B, Pizarro JC, Holmes MA, McBeth C, Groh V, Spies T, Strong RK. Crystal structure of a gammadelta T-cell receptor specific for the human MHC class I homolog MICA. Proc Natl Acad Sci U S A. 2011;108(6):2414–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petrasca A, Melo AM, Breen EP, Doherty DG. Human Vdelta3(+) gammadelta T cells induce maturation and IgM secretion by B cells. Immunol Lett. 2018;196:126–134. [DOI] [PubMed] [Google Scholar]
- 33. Fang H, Welte T, Zheng X, Chang GJ, Holbrook MR, Soong L, Wang T. gammadelta T cells promote the maturation of dendritic cells during West Nile virus infection. FEMS Immunol Med Microbiol. 2010;59(1):71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ravens S, Schultze-Florey C, Raha S, Sandrock I, Drenker M, Oberdorfer L, Reinhardt A, Ravens I, Beck M, Geffers R, von Kaisenberg C, et al. Human gammadelta T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat Immunol. 2017;18(4):393–401. [DOI] [PubMed] [Google Scholar]
- 35. Xuan L, Wu X, Zhang Y, Fan Z, Ling Y, Huang F, Zhang F, Zhai X, Liu Q. Granulocyte colony-stimulating factor affects the distribution and clonality of TRGV and TRDV repertoire of T cells and graft-versus-host disease. J Transl Med. 2011;9:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao X, Li Y, Ohe H, Nafady-Hego H, Uemoto S, Bishop GA, Koshiba T. Intragraft Vdelta1 gammadelta T cells with a unique T-cell receptor are closely associated with pediatric semiallogeneic liver transplant tolerance. Transplantation. 2013;95(1):192–202. [DOI] [PubMed] [Google Scholar]
- 37. Bosch M, Khan FM, Storek J. Immune reconstitution after hematopoietic cell transplantation. Curr Opin Hematol. 2012;19(4):324–335. [DOI] [PubMed] [Google Scholar]
- 38. Bian Z, Xu LP, Fu Q, Huo M, Liu L, Zhao X, Huang XJ, Liu J. Homeostatic gammadelta T Cell Contents Are Preserved by Granulocyte Colony-Stimulating Factor Priming and Correlate with the Early Recovery of gammadelta T Cell Subsets after Haploidentical Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2018;24(2):252–259. [DOI] [PubMed] [Google Scholar]
- 39. Liu J, Bian Z, Wang X, Xu LP, Fu Q, Wang C, Chang YJ, Wang Y, Zhang XH, Jiang Z, Huang XJ. Inverse correlation of Vdelta2(+) T-cell recovery with EBV reactivation after haematopoietic stem cell transplantation. Br J Haematol. 2018;180(2):276–285. [DOI] [PubMed] [Google Scholar]
- 40. Ramachandran V, Kolli SS, Strowd LC. Review of graft-versus-host disease. Dermatol Clin. 2019;37(4):569–582. [DOI] [PubMed] [Google Scholar]
- 41. Zeiser R, Blazar BR. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med. 2017;377(26):2565–2579. [DOI] [PubMed] [Google Scholar]
- 42. Vogelsang GB, Lee L, Bensen-Kennedy DM. Pathogenesis and treatment of graft-versus-host disease after bone marrow transplant. Annu Rev Med. 2003;54:29–52. [DOI] [PubMed] [Google Scholar]
- 43. Jin H, Ni X, Deng R, Song Q, Young J, Cassady K, Zhang M, Forman S, Martin PJ, Liu Q, Zeng D. Antibodies from donor B cells perpetuate cutaneous chronic graft-versus-host disease in mice. Blood. 2016;127(18):2249–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jin H, Yang K, Zhang H, Chen Y, Qi H, Fan Z, Huang F, Xuan L, Lin R, Zhao K, Liu Q. Expansion of circulating extrafollicular helper T-like cells in patients with chronic graft-versus-host disease. J Autoimmun. 2019;100:95–104. [DOI] [PubMed] [Google Scholar]
- 45. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS, Kerr H., et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: i. the 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21(3):389–401.E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Radestad E, Sundin M, Torlen J, Thunberg S, Onfelt B, Ljungman P, Watz E, Mattsson J, Uhlin M. Individualization of hematopoietic stem cell transplantation using alpha/beta t-cell depletion. Front Immunol. 2019;10:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Handgretinger R, Schilbach K. The potential role of gammadelta T cells after allogeneic HCT for leukemia. Blood. 2018;131(10):1063–1072. [DOI] [PubMed] [Google Scholar]
- 48. Maeda Y, Reddy P, Lowler KP, Liu C, Bishop DK, Ferrara JL. Critical role of host gammadelta T cells in experimental acute graft-versus-host disease. Blood. 2005;106(2):749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ellison CA, MacDonald GC, Rector ES, Gartner JG, Gamma delta T. Cells in the pathobiology of murine acute graft-versus-host disease. evidence that gamma delta T cells mediate natural killer-like cytotoxicity in the host and that elimination of these cells from donors significantly reduces mortality. J Immunol. 1995;155(9):4189–4198. [PubMed] [Google Scholar]
- 50. Anderson BE, McNiff JM, Matte C, Athanasiadis I, Shlomchik WD, Shlomchik MJ. Recipient CD4+ T cells that survive irradiation regulate chronic graft-versus-host disease. Blood. 2004;104(5):1565–1573. [DOI] [PubMed] [Google Scholar]
- 51. Drobyski WR, Majewski D, Hanson G. Graft-facilitating doses of ex vivo activated gammadelta T cells do not cause lethal murine graft-vs.-host disease. Biol Blood Marrow Transplant. 1999;5(4):222–230. [DOI] [PubMed] [Google Scholar]
- 52. Pabst C, Schirutschke H, Ehninger G, Bornhauser M, Platzbecker U. The graft content of donor T cells expressing gamma delta TCR+ and CD4+foxp3+ predicts the risk of acute graft versus host disease after transplantation of allogeneic peripheral blood stem cells from unrelated donors. Clin Cancer Res. 2007;13(10):2916–2922. [DOI] [PubMed] [Google Scholar]
- 53. Hu Y, Cui Q, Ye Y, Luo Y, Tan Y, Shi J, Huang H. Reduction of Foxp3+ T cell subsets involved in incidence of chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Hematol Oncol. 2017;35(1):118–124. [DOI] [PubMed] [Google Scholar]
- 54. Arruda LCM, Gaballa A, Uhlin M. Graft gammadelta TCR Sequencing identifies public clonotypes associated with hematopoietic stem cell transplantation efficacy in acute myeloid leukemia patients and unravels cytomegalovirus impact on repertoire distribution. J Immunol. 2019;202(6):1859–1870. [DOI] [PubMed] [Google Scholar]
- 55. Teira P, Battiwalla M, Ramanathan M, Barrett AJ, Ahn KW, Chen M, Green JS, Saad A, Antin JH, Savani BN, Lazarus HM., et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127(20):2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schmidt-Hieber M, Tridello G, Ljungman P, Mikulska M, Knelange N, Blaise D, Socie G, Volin L, Blijlevens N, Fegueux N, Yakoub-Agha I., et al. The prognostic impact of the cytomegalovirus serostatus in patients with chronic hematological malignancies after allogeneic hematopoietic stem cell transplantation: a report from the Infectious Diseases Working Party of EBMT. Ann Hematol. 2019;98(7):1755–1763. [DOI] [PubMed] [Google Scholar]
- 57. Lin HC, Han SM, Hwang WL, Chou CW, Chang KH, Shi ZY, Jerry Teng CL. Cytomegalovirus infection and treatment in allogeneic hematopoietic stem cell transplantation: a retrospective study from a single institution in an endemic area. Turk J Haematol. 2017;34(2):159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nakamura R, Gendzekhadze K, Palmer J, Tsai NC, Mokhtari S, Forman SJ, Zaia JA, Senitzer D, Marcucci G, Stein A. Influence of donor KIR genotypes on reduced relapse risk in acute myelogenous leukemia after hematopoietic stem cell transplantation in patients with CMV reactivation. Leuk Res. 2019;87:106230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Park J, Lim SH, Kim SH, Yun J, Kim CK, Lee SC, Won JH, Hong DS, Park SK. Is immunological recovery clinically relevant at 100 days after allogeneic transplantation?. Korean J Intern Med. 2020;35(4):957–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schmidt-Hieber M, Labopin M, Beelen D, Volin L, Ehninger G, Finke J, Socie G, Schwerdtfeger R, Kroger N, Ganser A, Niederwieser D., et al. CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013;122(19):3359–3364. [DOI] [PubMed] [Google Scholar]
- 61. Forte E, Zhang Z, Thorp EB, Hummel M. Cytomegalovirus latency and reactivation: an intricate interplay with the host immune response. Front Cell Infect Microbiol. 2020;10:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Knight A, Madrigal AJ, Grace S, Sivakumaran J, Kottaridis P, Mackinnon S, Travers PJ, Lowdell MW. The role of Vdelta2-negative gammadelta T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood. 2010;116(12):2164–2172. [DOI] [PubMed] [Google Scholar]
- 63. Scheper W, van Dorp S, Kersting S, Pietersma F, Lindemans C, Hol S, Heijhuurs S, Sebestyen Z, Grunder C, Marcu-Malina V, Marchant A, et al. gammadeltaT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia. 2013;27(6):1328–1338. [DOI] [PubMed] [Google Scholar]
- 64. Luo XY, Mo XD, Xu LP, Zhang XH, Wang Y, Liu KY, Chang YJ, Zhao XY, Huang XJ. A retrospective analysis on anti-CD20 antibody-treated epstein-barr virus-related posttransplantation lymphoproliferative disorder following ATG-based haploidentical T-replete hematopoietic stem cell transplantation. Ann Hematol. 2020. [DOI] [PubMed] [Google Scholar]
- 65. Zhou L, Gao ZY, Lu DP. Incidence, risk factors, and clinical outcomes associated with Epstein-Barr virus-DNAemia and Epstein-Barr virus-associated disease in patients after haploidentical allogeneic stem cell transplantation: a single-center study. Clin Transplant. 2020;34(6):e13856. [DOI] [PubMed] [Google Scholar]
- 66. Xiang Z, Liu Y, Zheng J, Liu M, Lv A, Gao Y, Hu H, Lam KT, Chan GC, Yang Y, Chen H., et al. Targeted activation of human Vgamma9Vdelta2-T cells controls epstein-barr virus-induced B cell lymphoproliferative disease. Cancer Cell. 2014;26(4):565–576. [DOI] [PubMed] [Google Scholar]
- 67. Liu J, Gao H, Xu LP, Mo XD, Liu R, Liang S, Wu N, Wang M, Wang Z, Chang YJ, Wang Y., et al. Immunosuppressant indulges EBV reactivation and related lymphoproliferative disease by inhibiting Vdelta2(+) T cells activities after hematopoietic transplantation for blood malignancies. J Immunother Cancer. 2020;8(1):e000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Djaoud Z, Guethlein LA, Horowitz A, Azzi T, Nemat-Gorgani N, Olive D, Nadal D, Norman PJ, Munz C, Parham P. Two alternate strategies for innate immunity to epstein-barr virus: one using NK cells and the other NK cells and gammadelta T cells. J Exp Med. 2017;214(6):1827–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Djaoud Z, Parham P. Dimorphism in the TCRgamma-chain repertoire defines 2 types of human immunity to Epstein-Barr virus. Blood Adv. 2020;4(7):1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Prieto-Torres L, Erana I, Gil-Redondo R, et al. The spectrum of EBV-positive mucocutaneous Ulcer: a study of 9 cases. Am J Surg Pathol. 2019;43(2):201–210. [DOI] [PubMed] [Google Scholar]
- 71. Yang Q, Li C, Wang W, Zheng R, Huang X, Deng H, Jin P, Tan K, Yan Y, Wang D. Infiltration pattern of gammadelta T cells and its association with local inflammatory response in the nasal mucosa of patients with allergic rhinitis. Int Forum Allergy Rhinol. 2019;9(11):1318–1326. [DOI] [PubMed] [Google Scholar]
- 72. Hoshino T, Tatsuno K, Shimauchi T, Okada S, Ito T, Ono T, Ohshima K, Tokura Y. Epstein-Barr virus-associated T-cell lymphoproliferative disorder affecting skin and lung in an elderly patient. J Dermatol. 2014;41(9):837–840. [DOI] [PubMed] [Google Scholar]
- 73. Hilgendorf I, Loebermann M, Borchert K, Junghanss C, Freund M, Schmitt M. Tenofovir for treatment of hepatitis B virus reactivation in patients with chronic GVHD. Bone Marrow Transplant. 2011;46(9):1274–1275. [DOI] [PubMed] [Google Scholar]
- 74. Hammond SP, Borchelt AM, Ukomadu C, Ho VT, Baden LR, Marty FM. Hepatitis B virus reactivation following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15(9):1049–1059. [DOI] [PubMed] [Google Scholar]
- 75. Chen M, Zhang D, Zhen W, Shi Q, Liu Y, Ling N, Peng M, Tang K, Hu P, Hu H, Ren H. Characteristics of circulating T cell receptor gamma-delta T cells from individuals chronically infected with hepatitis B virus (HBV): an association between V(delta)2 subtype and chronic HBV infection. J Infect Dis. 2008;198(11):1643–1650. [DOI] [PubMed] [Google Scholar]
- 76. Chang L, Wang L, Ling N, Peng H, Chen M. Increase in liver gammadelta T cells with concurrent augmentation of IFN-beta production during the early stages of a mouse model of acute experimental hepatitis B virus infection. Exp Ther Med. 2020;19(1):67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen M, Hu P, Ling N, Peng H, Lei Y, Hu H, Zhang D, Ren H. Enhanced functions of peripheral gammadelta T cells in chronic hepatitis B infection during interferon alpha treatment in vivo and in vitro. PLoS One. 2015;10(3):e0120086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Henrich TJ, Hanhauser E, Marty FM, Sirignano MN, Keating S, Lee TH, Robles YP, Davis BT, Li JZ, Heisey A, Hill AL., et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med. 2014;161(5):319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gupta RK, Abdul-Jawad S, McCoy LE, Mok HP, Peppa D, Salgado M, Martinez-Picado J, Nijhuis M, Wensing AMJ, Lee H, Grant P., et al. HIV-1 remission following CCR5Delta32/Delta32 haematopoietic stem-cell transplantation. Nature. 2019;568(7751):244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li H, Chaudhry S, Poonia B, Shao Y, Pauza CD. Depletion and dysfunction of Vgamma2Vdelta2 T cells in HIV disease: mechanisms, impacts and therapeutic implications. Cell Mol Immunol. 2013;10(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pauza CD, Poonia B, Li H, Cairo C, Chaudhry S., Gammadelta T. Cells in HIV disease: past, present, and future. Front Immunol. 2014;5:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Li Z, Jiao Y, Hu Y, Cui L, Chen D, Wu H, Zhang J, He W. Distortion of memory Vdelta2 gammadelta T cells contributes to immune dysfunction in chronic HIV infection. Cell Mol Immunol. 2015;12(5):604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li Z, Li W, Li N, Jiao Y, Chen D, Cui L, Hu Y, Wu H, He W. gammadelta T cells are involved in acute HIV infection and associated with AIDS progression. PLoS One. 2014;9(9):e106064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wang L, Wang Y, Fan X, Tang W, Hu J. Prevalence of resistant gram-negative bacilli in bloodstream infection in febrile neutropenia patients undergoing hematopoietic stem cell transplantation: a single center retrospective cohort study. Medicine (Baltimore). 2015;94(45):e1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Al-Bader N, Sheppard DC. Aspergillosis and stem cell transplantation: an overview of experimental pathogenesis studies. Virulence. 2016;7(8):950–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bartlett AW, Cann MP, Yeoh DK, Bernard A, Ryan AL, Blyth CC, Kotecha RS, McMullan BJ, Moore AS, Haeusler GM, Clark JE. Epidemiology of invasive fungal infections in immunocompromised children; an Australian national 10-year review. Pediatr Blood Cancer. 2019;66(4):e27564. [DOI] [PubMed] [Google Scholar]
- 87. Fenoglio D, Poggi A, Catellani S, Battaglia F, Ferrera A, Setti M, Murdaca G, Zocchi MR. Vdelta1 T lymphocytes producing IFN-gamma and IL-17 are expanded in HIV-1-infected patients and respond to Candida albicans. Blood. 2009;113(26):6611–6618. [DOI] [PubMed] [Google Scholar]
- 88. Hebart H, Bollinger C, Fisch P, Sarfati J, Meisner C, Baur M, Loeffler J, Monod M, Latge JP, Einsele H. Analysis of T-cell responses to Aspergillus fumigatus antigens in healthy individuals and patients with hematologic malignancies. Blood. 2002;100(13):4521–4528. [DOI] [PubMed] [Google Scholar]
- 89. Ramadan G. In vitro expansion of human gammadelta and CD56(+) T-cells by Aspergillus-antigen loaded fast dendritic cells in the presence of exogenous interleukin-12. Immunopharmacol Immunotoxicol. 2012;34(2):309–316. [DOI] [PubMed] [Google Scholar]
- 90. Chang YJ, Wang Y, Xu LP, Zhang XH, Chen H, Chen YH, Wang FR, Wei H, Sun YQ, Yan CH, Tang FF., et al. Haploidentical donor is preferred over matched sibling donor for pre-transplantation MRD positive ALL: a phase 3 genetically randomized study. J Hematol Oncol. 2020;13(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Toubai T, Tanaka J, Ota S, Fukuhara T, Hashino S, Kondo T, Kasai M, Kakinoki Y, Masauzi N, Morioka M, Kawamura T., et al. Minimal residual disease (MRD) monitoring using rearrangement of T-cell receptor and immunoglobulin H gene in the treatment of adult acute lymphoblastic leukemia patients. Am J Hematol. 2005;80(3):181–187. [DOI] [PubMed] [Google Scholar]
- 92. Galimberti S, Benedetti E, Morabito F, Petrini I, Battolla B, Papineschi F, Fazzi R, Ciabatti E, Martino M, Cuzzola M, Console G., et al. Different gamma/delta T clones sustain GVM and GVH effects in multiple myeloma patients after non-myeloablative transplantation. Leuk Res. 2006;30(5):529–535. [DOI] [PubMed] [Google Scholar]
- 93. Nomura K, Okamoto T, Nakao M, Ueda K, Akano Y, Fujita Y, Kobayashi M, Yokota S, Horiike S, Nishida K, Kusuzaki K., et al. Multiple bone lesions after allogeneic bone marrow transplantation in a patient with relapsed adult acute lymphoblastic leukemia: minimal residual disease analysis may predict extramedullary relapse. Leuk Lymphoma. 2001;42(6):1305–1308. [DOI] [PubMed] [Google Scholar]
- 94. Juno JA, Eriksson EM. gammadelta T-cell responses during HIV infection and antiretroviral therapy. Clin Transl Immunology. 2019;8(7):e01069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Poonia B, Pauza CD. Gamma delta T cells from HIV+ donors can be expanded in vitro by zoledronate/interleukin-2 to become cytotoxic effectors for antibody-dependent cellular cytotoxicity. Cytotherapy. 2012;14(2):173–781. [DOI] [PubMed] [Google Scholar]
- 96. Aoki T, Matsushita H, Hoshikawa M, Hasegawa K, Kokudo N, Kakimi K. Adjuvant combination therapy with gemcitabine and autologous gammadelta T-cell transfer in patients with curatively resected pancreatic cancer. Cytotherapy. 2017;19(4):473–485. [DOI] [PubMed] [Google Scholar]
- 97. Pressey JG, Adams J, Harkins L, Kelly D, You Z, Lamb LS., Jr In vivo expansion and activation of gammadelta T cells as immunotherapy for refractory neuroblastoma: a phase 1 study. Medicine (Baltimore). 2016;95(39):e4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fabrizio VA, Kernan NA, Boulad F, Cancio M, Allen J, Higman M, Margossian SP, Mauguen A, Prockop S, Scaradavou A, Shah N., et al. Low toxicity and favorable overall survival in relapsed/refractory B-ALL following CAR T cells and CD34-selected T-cell depleted allogeneic hematopoietic cell transplant. Bone Marrow Transplant. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhao H, Wei J, Wei G, Luo Y, Shi J, Cui Q, Zhao M, Liang A, Zhang Q, Yang J, Li X., et al. Pre-transplant MRD negativity predicts favorable outcomes of CAR-T therapy followed by haploidentical HSCT for relapsed/refractory acute lymphoblastic leukemia: a multi-center retrospective study. J Hematol Oncol. 2020;13(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Capsomidis A, Benthall G, Van Acker HH, Fisher J, Kramer AM, Abeln Z, Majani Y, Gileadi T, Wallace R, Gustafsson K, Flutter B., et al. Chimeric antigen receptor-engineered human gamma delta t cells: enhanced cytotoxicity with retention of cross presentation. Mol Ther. 2018;26(2):354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]