Abstract
Islet transplantation has emerged as a promising treatment for type 1 diabetes mellitus. Liraglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, protects beta cells after islet transplantation by improving glycemic control through several mechanisms. In this study, we compared the effects of local pretreatment and systemic treatment with liraglutide on islet transplantation in a diabetic mouse model. Streptozotocin (STZ)-induced diabetic C57BL/6 mice were transplanted with syngeneic islets under the kidney capsule. Isolated islets were either locally treated with liraglutide before transplantation or mice were treated systemically by intraperitoneal injection after islet transplantation. Local pretreatment of islets with liraglutide was more effective in increasing body weight, decreasing hemoglobin A1c levels, and lowering blood glucose levels in STZ-diabetic mice transplanted with islets. Local pretreatment was also more effective in increasing insulin secretion and islet survival in STZ-diabetic mice. Histological analysis of the transplantation site revealed fewer apoptotic cells following local pretreatment compared with systemic injection of liraglutide. These findings indicate that liraglutide administered once locally before transplantation might have superior effects on islet preservation than systemic administration.
Keywords: type 1 diabetes, pancreatic islet transplantation, liraglutide, local or systemic treatment
Introduction
Type 1 diabetes mellitus (T1DM) is caused by the autoimmune-mediated destruction of insulin-producing pancreatic beta (β)-cells, which leads to insulin deficiency and finally the development of hyperglycemia1. Insulin injection has been clinically used to treat T1DM; however, it can cause hypoglycemic side effects and does not completely prevent chronic complications, such as kidney failure, coronary heart disease, retinopathy, and neuropathy2.
Islet transplantation has emerged as a promising treatment for T1DM. It is a safe and relatively less invasive method that can normalize glycometabolic control3,4. However, the lack of pancreatic islet donors limits the successful use of allogenic islet transplantation as a clinical therapy for T1DM. Xenogeneic islets5, stem cell-derived insulin-producing cells6,7, and three-dimensionally assembled artificial islets8,9 have been investigated as sources of islets for transplantation; however, they are still far from clinical application. Unfortunately, even though immunosuppression can be effectively achieved in diabetic patients, many transplanted islets are lost in the first 10 to 14 days after transplantation10,11. Loss of transplanted syngeneic islets soon after transplantation has also been observed in an experimental animal model, indicating the sensitivity of islet transplants to early damage12. Treatment with preserving and supportive agents in the early stages following transplantation may improve the function and survival of transplanted islets, and increase metabolic control in T1DM13.
Glucagon-like peptide-1 (GLP-1) is an incretin hormone secreted from intestinal L cells. It stimulates insulin secretion in β-cells and suppresses glucagon secretion in α-cells14,15. GLP-1 also protects β-cells from apoptosis and induces β-cell proliferation16. Analogs of GLP-1, such as exendin-4 or liraglutide, have beneficial effects on islet transplantation. Liraglutide is a long-acting GLP-1 analog. Daily subcutaneous injections of liraglutide from the early stage of transplantation can improve the success of islet transplantation in diabetic animal models17,18. Recent studies by Langlois et al. demonstrated that culturing islets with liraglutide for 24 h prior to transplantation resulted in improved glycemic control, which was attributed to anti-inflammatory and antioxidant activities17,19. In addition, islets co-encapsulated with microspheres containing exenatide (extendin-4) showed improved islet viability and function through local exenatide delivery20. Although GLP-1 analogs have a beneficial effect on islet transplantation outcomes, the effects may vary depending on the treatment conditions.
In this study, we compared the effects of systemic and local administration of liraglutide on islet transplantation in a streptozotocin (STZ)-induced diabetic mouse model.
Materials and Methods
Animals
Eight-week-old male C57BL/6J and C57BL/6-Tg(Ins2-luc/EGFP/TK)300Kauf/J (Ins2; stock #012943) mice were purchased from Orient Bio Inc. (Seongnam, Gyeonggi-do, Korea) and the Jackson Laboratory (Bar Harbor, ME, USA), respectively. Mice were housed under specific pathogen-free conditions and maintained under a 12 h/12 h light/dark cycle at the animal facility of Lee Gil Ya Cancer and Diabetes Institute (CACU, Gachon University, Incheon, Korea). All mice were fed a normal chow diet ad libitum, with free access to drinking water at all times. All experiments were performed in accordance with the “Guidelines for Animal for Users” (LCDI-2016-0035, LCDI-2018-0101). Diabetes was induced by a single intraperitoneal (i.p.) injection of 180 mg/kg STZ (Sigma-Aldrich, St. Louis, MO, USA). Blood was subsequently obtained from the tail, and blood glucose levels were measured using a glucometer (OneTouch Ultra, LifeScan). Mice with stable blood glucose levels above 300 mg/dl after STZ treatment were used as recipients for transplantation.
Islet Isolation
Islets from C57BL/6 J or Ins2 mice were isolated by intraductal liberase digestion (Liberase, 0.3 mg/ml; Roche, Indianapolis, IN, USA) and purified by Ficoll gradient centrifugation (Sigma-Aldrich), as previously described21. Briefly, after injection of liberase into the bile duct, the swollen pancreas was excised and incubated for 10 min at 37 °C. The islets were then separated by Ficoll gradient centrifugation at 2000×g for 10 min at 4 °C. Islets were collected from the second and fourth layers and washed with Hank’s balanced salt solution (HBSS; 114 mmol/L sodium chloride, 4.7 mmol/L potassium chloride, 1.2 mmol/L monopotassium phosphate, 1.16 mmol/L magnesium sulphate, 20 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 2.5 mmol/L calcium chloride, 25 mmol/L sodium bicarbonate, and 0.2% bovine serum albumin). The isolated islets were cultured overnight in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, Grand Island, NY, USA) containing 5.5 mM glucose and supplemented with 10% fetal bovine serum, 2-mercaptoethanol (50 μM), penicillin (100 U/ml), and streptomycin (100 μg/ml). Size-matched healthy islets were handpicked under a stereomicroscope.
Insulin Secretion Assay
To examine glucose-stimulated insulin secretion in vitro, 10 isolated islets per well were plated in 24-well plates and incubated with HBSS for 2 h followed by stimulation with 3 or 17 mM glucose. Insulin secreted into the solution was measured22. To examine serum insulin levels, mice were fasted for 4 h, and blood samples were collected 7 and 35 days after islet transplantation. To measure glucose-stimulated insulin secretion in vivo, blood samples were collected from the mouse tail tip 30 min after the i.p. injection of glucose (2 g/kg). The blood was centrifuged at 3000×g for 20 min at 4 °C, and the serum was collected. The insulin released into the HBSS or serum was quantified using an insulin ELISA kit (Alpco Diagnostics, Salem, NH, USA) according to the manufacturer’s instructions and was normalized to the amount of protein.
Islet Transplantation
Diabetic mice were randomly divided into five groups (Fig. 1A): sham-operated group (n = 6, sham), 300 islets-transplanted group (n = 6, I300), 200 islets-transplanted group (n = 8, I200), 200 islets with local liraglutide group (n = 8, I200-local), and 200 islets with systemic liraglutide (n = 8, I200-systemic). As previously described23, 200 islets were considered the marginal islet mass for normal blood glucose restoration. Islet transplantation was performed as previously described24. Purified islets were cultured overnight in RPMI 1640 medium and suspended in phosphate-buffered saline (PBS). For local treatment with liraglutide, islets were incubated with liraglutide (0.5 μg/10 µl PBS; VICTOZA, Novo Nordisk, Denmark) for 10 min. Islets were then transplanted under the capsule of the right kidney using a 1-ml syringe connected to a single lumen polyethylene tube (inner diameter, 0.58 mm; outer diameter, 0.96 mm). For systemic treatment, mice were injected intraperitoneally with liraglutide (0.5 μg/200 µl PBS/mouse) after islet transplantation. Age-matched and sex-matched nondiabetic normal C57BL/6 J mice were used as controls (n = 5, Control). Nonfasting blood glucose levels and body weights were measured regularly.
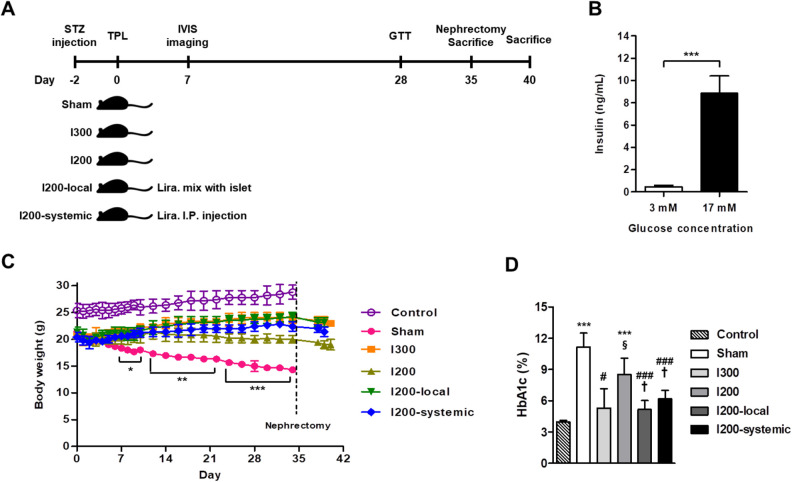
Figure 1.
Local pretreatment of islets with liraglutide is more effective in increasing body weight and decreasing HbA1c levels in STZ-diabetic mice transplanted with islets. STZ-induced diabetic mice were randomly divided into five groups: sham-operated group (n = 6, Sham), 300 islets-transplanted group (n = 6, I300), 200 islets-transplanted group (n = 8, I200), 200 islets with local liraglutide (n = 8, I200-local), and 200 islets with systemic liraglutide (n = 8, I200-systemic). Age-matched and sex-matched C57BL/6 J mice were used as a nondiabetic control group (n = 5, control). (A) The time frame of the experimental design. (B) Insulin secretion of isolated islets was measured by glucose-stimulated insulin secretion assays. The insulin concentration in the culture medium was measured using a commercial insulin ELISA kit as detailed in the Materials and Methods section. The data are presented as mean ± SD. ***P < 0.005. (C) Body weight changes were monitored for 40 days. Diabetes was induced in 10-week-old C57BL6 mice by injection of STZ (180 mg/kg). The pretransplant blood glucose levels were >400 mg/dl and isolated islets were transplanted under the mouse kidney capsule. The normalized mice underwent nephrectomy 35 days after transplantation. The data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.005 versus sham. (D) HbA1c levels in whole blood were measured on day 35 following transplantation. The data are presented as mean ± SD. ***P < 0.005 versus control; # P < 0.05, ### P < 0.005 versus sham; § P < 0.05 versus I300; † P < 0.05 versus I200. HbA1c: glycated hemoglobin; STZ: streptozotocin.
Intraperitoneal Glucose Tolerance Test (IPGTT)
IPGTT was performed in the different groups of mice 28 days after transplantation as well as in the sham group. Following an overnight fast, mice were injected intraperitoneally with glucose (2 g/kg body weight). A glucometer was used to measure glucose in the tail vein blood at 0, 15, 30, 60, 90, and 120 min after glucose injection.
Analysis of Hemoglobin A1c (HbA1c)
Mice were fasted for 4 h, and blood samples were collected 35 days after transplantation. HbA1c levels from whole blood were measured using a DCA 200 Vantage Analyzer (Siemens Healthcare Diagnostics, Tarrytown, NY, USA) and DCA Systems HbA1c Reagent Kit (Siemens Healthcare Diagnostics).
In Vivo Bioluminescence Imaging
For in vivo assessment of islet survival, islets isolated from Ins2-luc transgenic mice were used for transplantation. Bioluminescent images were obtained 7 days after transplantation. The mice were anesthetized by gas anesthesia with 3% isoflurane (Hana Pharm Co. Ltd., Hwaseong, Korea) and injected intraperitoneally with 150 mg/kg d-luciferin (15 mg/ml in PBS; Sigma-Aldrich). After 15 min, the mice were scanned using a Caliper-IVIS CCD device (Caliper Life Sciences, Hopkinton, MA, USA). Imaging signals were quantified as maximum photons per second per square centimeter per steradian (p/s/cm2/sr).
Histological Analysis
Tissues were fixed in 10% neutral buffered formalin and embedded in paraffin. Tissue sections (4 to 6 µm thick) were stained with hematoxylin and eosin and examined using a light microscope.
Apoptosis Assay
Islet-containing kidney tissues were embedded in optimum cutting temperature (OCT) compound (Tissue-Tek, CA, USA), and beta-cell apoptosis was examined in cryosections (5-µm thick) using an in situ cell death detection kit (Roche Applied Science, Indianapolis, IN, USA) according to the manufacturer’s protocol. Briefly, the tissue sections were permeabilized with 1% proteinase K for 15 min, rinsed with PBS, incubated with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) reagents for 1 h at 37 °C, and washed in PBS for 5 min. Then, tissues were stained with an anti-insulin antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA), a fluorescein isothiocyanate-conjugated secondary antibody, and 4′-6-diamidino-2-phenylindole (Life Technologies, Carlsbad, CA, USA). Images were captured using a laser scanning confocal microscope (Carl Zeiss).
Statistical Analyses
All data are presented as the mean ± SD. Statistical analyses were performed using an unpaired Student’s t-test for comparison between two groups or one-way analysis of variance with Tukey’s multiple comparison test for multiple groups. Statistical analyses were performed using the GraphPad Prism version 5 software (GraphPad Software Inc, La Jolla, CA, USA). A P value < 0.05 was considered statistically significant.
Results
Local Pretreatment of Islets With Liraglutide is More Effective for Increase in Body Weight and Decrease in HbA1c Levels in STZ-Diabetic Mice Transplanted With Islets
In our preliminary experiments, we measured the blood glucose levels for 1 to 2 weeks in STZ-induced diabetic mice transplanted with different number of islets and found that most of the recipients (approximately 70% to 80%), transplanted with 300 islets, reached normoglycemia. Therefore, to compare the effects of local pretreatment and systemic treatment with liraglutide on islet graft survival, we transplanted a marginal number of islets (200 islet equivalents [IEQ]), which did not show normoglycemia in the recipients, into STZ-induced diabetic mice. The six study groups comprised the control, sham, I300, I200, I200-local, and I200-systemic groups (Fig. 1A). Before transplantation, the quality of isolated islets was checked by measuring glucose-stimulated insulin secretion. Insulin secretion of isolated islets normally increased in response to high glucose (17 mM; Fig. 1B). After islet transplantation, body weights and blood glucose levels were monitored for 5 weeks. As expected, the body weight of all diabetic mice was significantly reduced after STZ treatment compared with that in the nondiabetic control group (Fig. 1C). The body weight of the sham group decreased steadily, while that of the I200 group remained unchanged, that of the I200-systemic group increased slightly, and that of the I200-local group increased steadily, similar to the I300 group (Fig. 1C). The average body weights for the final 2 weeks after islet transplantation were 23.67 ± 0.84 g for I300, 23.52 ± 1.01 g for I200-local, 22.16 ± 0.82 g for I200-systemic, 20.36 ± 0.96 g for I200, and 15.14 ± 0.57 g for the sham group, and 28.08 ± 0.91 g for the non-diabetic control group. At day 35 after islet transplantation, HbA1c levels in the I300, I200-local, and I200-systemic groups were also significantly reduced compared with the I200 group, similar to that in the nondiabetic control group (Fig. 1D). The HbA1c levels in the I200-local pretreatment group also appeared to be lower than that in the I200-systemic group, although they were not significantly different (Fig. 1D).
Local Pretreatment of Islets With Liraglutide is More Effective in Lowering Blood Glucose Levels in STZ-Diabetic Mice Transplanted with Islets
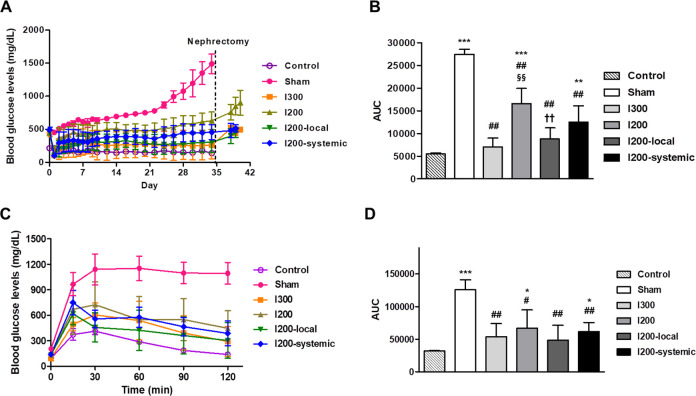
Corresponding with the body weight changes, blood glucose levels in the sham group continuously increased. After islet transplantation, the average blood glucose levels in the I300 and I200 local groups appeared to be higher than those in the nondiabetic control group, although not significant, while the blood glucose levels in the I200-systemic and I200 groups indicated mild and severe hyperglycemia, respectively (Fig. 2A). The average blood glucose levels for the final 2 weeks after islet transplantation were 257.05 ± 210.91 mg/dl for I300, 284.80 ± 144.14 mg/dl for I200-local, 421.63 ± 128.99 mg/dl for I200-systemic, 561.57 ± 125.28 mg/dl for I200, and 1107.14 ± 106.97 mg/dl for the sham group. The average blood glucose level in the nondiabetic control group was 147.71 ± 13.36 mg/dl (Fig. 2A). The area under the curve (AUC) for blood glucose levels was significantly reduced in all the islet transplanted groups compared with that in the sham group (Fig. 2B). The AUC of the I300 and I200-local groups was significantly decreased compared with that of the I200 group, but not the I200-systemic group, and showed comparable levels to that of the non-diabetic control group (Fig. 2B). After nephrectomy, hyperglycemia was observed in all recipient mice, indicating that the transplanted islets were responsible for lowering blood glucose levels. To examine the effect of the transplanted islets on the control of blood glucose levels in islet transplanted mice, IPGTT was performed 28 days after transplantation. Blood glucose levels peaked at 15 and 30 min after the glucose injection and significantly decreased at all time points in all islet transplantation groups compared with that in the sham control group. The I200-local pretreatment group showed the lowest blood glucose levels at all time points after glucose injection among the groups with transplantation. In addition, the AUC for blood glucose levels was also more substantially decreased in the I200-local group, which was comparable to that in the nondiabetic control group (Fig. 2C, D). These results suggest that a single, local pretreatment of islets with liraglutide was more effective in lowering blood glucose levels than in STZ-diabetic mice transplanted with islets systemic treatment with liraglutide.
Figure 2.
Local pretreatment of islets with liraglutide is more effective for lowering blood glucose levels in STZ-diabetic mice transplanted with islets. STZ-induced diabetic mice were randomly divided into five groups: sham-operated group (n = 6, Sham), 300 islets-transplanted group (n = 6, I300), 200 islets-transplanted group (n = 8, I200), 200 islets with local liraglutide (n = 8, I200-local), and 200 islets with systemic liraglutide (n = 8, I200-systemic). The normalized mice underwent nephrectomy 35 days after transplantation. Age-matched and sex-matched C57BL/6 J mice were used as a nondiabetic control group (n = 5, control). (A) Nonfasting blood glucose levels were monitored for 40 days. (B) The AUC for blood glucose levels was measured up to 35 days prior to nephrectomy. (C) The glucose tolerance test was performed 28 days after islet transplantation. (D) AUC for blood glucose levels was measured during glucose tolerance testing. The data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.005 versus control; # P < 0.05, ## P < 0.01 versus sham; §§ P < 0.01 versus I300; †† P < 0.01 versus I200. AUC: area under the curve; STZ: streptozotocin.
Local Pretreatment of Islets With Liraglutide More Effectively Increases Insulin Secretion than Systemic Injection of Liraglutide in STZ-Diabetic Mice Transplanted With Islets
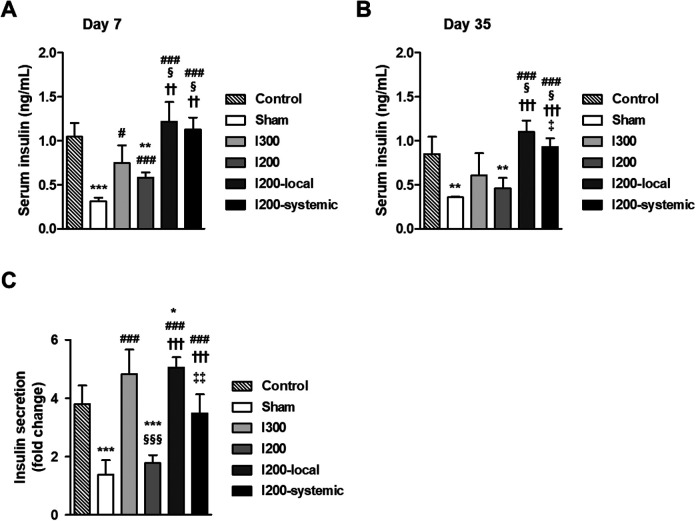
Blood glucose levels were more effectively decreased in mice transplanted with islets pretreated with liraglutide than in mice systemically injected with liraglutide. Therefore, we further checked whether there was a difference in serum insulin levels. Serum insulin levels on days 7 and 35 after transplantation were significantly increased in all islet transplanted groups compared with those in the sham group (Fig. 3A, B). The serum insulin levels of the I300, I200-local, and I200-systemic groups were significantly increased compared with that of the I200 group and showed comparable levels to that of the nondiabetic control group (Fig. 3A, B). In particular, the serum insulin levels of the I200-local and I200-systemic groups were significantly higher than those of the I300 group (Fig. 3A, B). There was no significant difference between the I200-local and I200-systemic groups on day 7 after islet transplantation. On day 35 after islet transplantation, however, the insulin levels in the I200-local group were significantly higher than those in the I200-systemic group (Fig. 3B). To examine glucose-stimulated insulin secretion in vivo, serum insulin levels were measured at 0 min and 30 min after glucose injection on day 28 after islet transplantation. Glucose-stimulated insulin secretion was significantly increased in the I300, I200-local, and I200-systemic groups compared with that in the sham group (Fig. 3C). In addition, the fold increase in insulin secretion in the I300, I200-local, and I200-systemic groups was significantly higher than that in the I200 group. In particular, the fold change in secreted insulin was significantly increased in the I200-local group compared with that in the I200-systemic group (Fig. 3C). These results suggest that single, local pretreatment with liraglutide was more effective for the preservation of transplanted islet function.
Figure 3.
Serum insulin level and glucose-stimulated insulin secretion are higher in STZ-diabetic mice transplanted with islets locally pretreated with liraglutide than systemically injected with liraglutide. STZ-induced diabetic mice were randomly divided into five groups: sham-operated group (n = 6, Sham), 300 islets-transplanted group (n = 6, I300), 200 islets-transplanted group (n = 8, I200), 200 islets with local liraglutide (n = 8, I200-local), and 200 islets with systemic liraglutide (n = 8, I200-systemic). Age-matched and sex-matched C57BL/6 J mice were used as a nondiabetic control group (n = 5, control). (A and B) Blood samples were collected from the tails of experimental mice on days 7 and 35 after islet transplantation. Serum insulin was measured using a commercial insulin ELISA kit on day 7 (A) and day 35 (B). (C) Glucose-stimulated insulin secretion was measured 28 days after islet transplantation. Blood samples were collected at 0 and 30 min after glucose injection. Fold change in insulin secretion was expressed as a ratio of insulin levels at 30 min and those at 0 min after glucose injection. The data are presented as mean ± SD, n = 5 to 8/group. *P < 0.05, **P < 0.01, ***P < 0.005 versus control; # P < 0.05, ### P < 0.005 versus sham; § P < 0.05, §§§ P < 0.005 versus I300; †† P < 0.01, ††† P < 0.005 versus I200; ‡ P < 0.05, ‡‡ P < 0.01 versus I200-local. STZ: streptozotocin.
Local Pretreatment of Islets With Liraglutide is More Effective on Islet Survival than Systemic Injection of Liraglutide in STZ-Diabetic Mice Transplanted With Islets
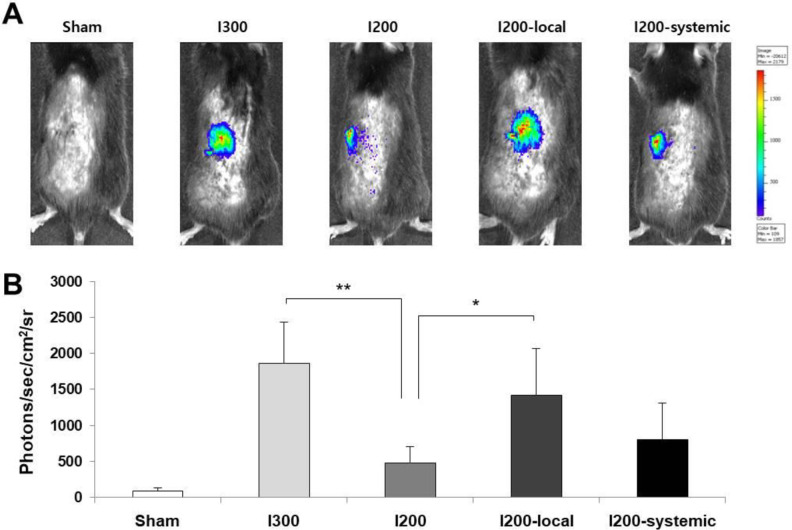
Changes in the mass of transplanted islets in the experimental groups were assessed by in vivo imaging. Islets were isolated from transgenic mice expressing luciferase under the control of the mouse insulin promoter25 and used for transplantation. Bioluminescence imaging on 7 days after transplantation revealed luciferase activity at the sites of transplanted islets. Transplanted islets in the I200-local pretreatment and I300 groups showed significantly higher luciferase activity than those in the I200 group (Fig. 4A, B). The luciferase activity in the I200-local pretreatment group appeared to be higher than that in the I200-systemic group (Fig. 4B). These results suggest that local pretreatment with liraglutide more effectively preserved islet mass than systemic treatment.
Figure 4.
Local pretreatment of islets with liraglutide is more effective for islet survival than systemic injection of liraglutide in streptozotocin-diabetic mice transplanted with islets. (A) Representative bioluminescence images on 7 days after transplantation of Ins2-luc islets. (B) Bioluminescence intensity was measured for each mouse from (A). The data are presented as mean ± SD, n = 3/group, *P < 0.05, **P < 0.01 versus I200.
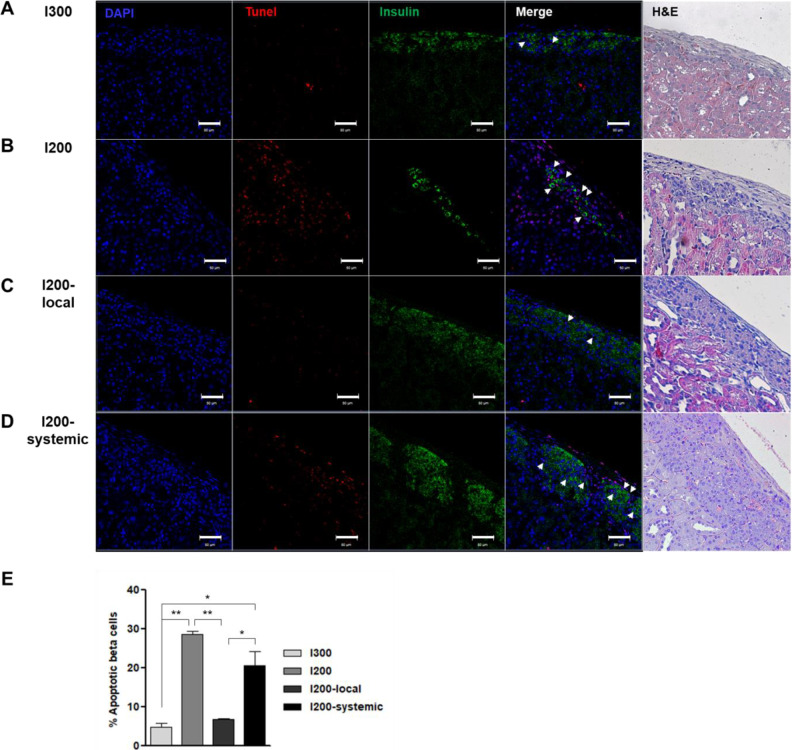
Local Pretreatment of Islets With Liraglutide Results in Less Apoptosis Than Systemic Injection of Liraglutide in STZ-Diabetic Mice Transplanted With Islets
To evaluate the apoptotic β-cells in transplanted islets in STZ-diabetic mice, kidney sections containing islets were stained with an anti-insulin antibody, and a TUNEL assay was performed. Insulin expression was observed in the islets of kidney subcapsules on day 35 after transplantation. Unlike the low expression in the islets of the I200 group, the islets of the I200-local pretreatment or I200-systemic groups highly expressed insulin, similar to the islets of the I300 group (Fig. 5). Many TUNEL-positive cells were observed in the I200 and I200-systemic groups. However, TUNEL-positive cells were rarely detected in the I200-local pretreatment group (Fig. 5). When we analyzed TUNEL+Insulin+ apoptotic beta cells, the ratio of TUNEL+Insulin+ cells among insulin+ cells was 4.6%, 28.6%, 6.6%, and 20.5% in the I300, I200, I200-local, and I200-systemic groups, respectively. TUNEL+Insulin+ apoptotic beta cells were significantly reduced in the I200-local group compared with the I200 and I200-systemic groups (Fig. 5E). These results suggest that local pretreatment with liraglutide more effectively protected the transplanted islets.
Figure 5.
Apoptosis is reduced by local pretreatment of islets with liraglutide as compared to systemic injection of liraglutide in STZ-diabetic mice transplanted with islet. (A–D) TUNEL (red), insulin (green), 4′-6-diamidino-2-phenylindole (blue) and hematoxylin/eosin (H&E) staining of kidney section. Representative images are shown (A) I300, (B) I200, (C) I200-local, and (D) I200-systemic groups on 35 days after islet transplantation (Scale bars, 10 μm). White arrows indicate TUNEL+insulin+ cells. (E) Quantification of TUNEL+insulin+ apoptotic beta cells in the transplanted islets. Percentile of apoptotic β cells was calculated as the number of TUNEL+insulin+ cells divided by the number of insulin+ cells in islet grafts; n =3 mice/group. The data are presented as mean ± SD, *P < 0.05, **P < 0.01. STZ: streptozotocin; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling.
Discussion
Pancreatic islet transplantation is considered a useful therapeutic approach for T1DM; however, it is hindered by the loss of most of the transplanted islets in the early stage of transplantation26. Various approaches have been explored to improve the efficacy and survival of transplanted islets, including the application of supplements prior to transplantation27,28, surface modification29,30, encapsulation31, and the use of delivery vehicles, such as liposomal clodronate in Matrigel and curcumin-loaded polymeric microspheres32,33. GLP-1 receptor agonists, including exendin-4 and liraglutide, enhance islet transplantation efficiency by stimulating insulin secretion, upregulating insulin gene transcription, regulating β-cell proliferation and survival, and modulating immune reaction34.
Early treatment with liraglutide after islet transplantation can protect transplanted islets by enhancing angiogenic19, anti-inflammatory17, antioxidative17, and antiapoptotic effects35. Pretreatment of islets with liraglutide prior to transplantation protected the islets from damage17,36. It has also been reported that multiple injections of liraglutide after transplantation or injection starting 2 days before the transplantation, followed by continuous injection after transplantation increased transplantation efficiency17,35,36. However, the transplantation efficacy may vary depending on the strategy used for treatment with liraglutide during islet transplantation.
In this study, we compared the effects of local pretreatment and systemic treatment with liraglutide on islet transplantation in STZ-induced diabetic mice. For systemic treatment, we injected liraglutide just after transplantation, considering the maximum effect of liraglutide on the transplanted islets. We used a marginal mass of islets for transplantation and found that local pretreatment with liraglutide more effectively improved glycemic control compared with systemic treatment. The benefit of local pretreatment seemed to be mainly attributed to increased graft survival and insulin secretion as a result of the reduced apoptosis of islet β-cells. The islet marginal mass model has been used to evaluate islet function, survival, or engraftment by comparing simple conditions such as islet size37 or transplant site38 in syngeneic transplantations36. Similar to previous studies37,38, our study using 200 IEQ as a marginal mass did not completely regulate blood glucose levels, and hyperglycemia was delayed compared with the sham group. However, as shown in Fig. 1, treatment with a single dose of liraglutide, either locally or systemically, maintained blood glucose at a significantly lower level than in the I200 group. In particular, local treatment with liraglutide was more effective in regulating blood glucose levels than systemic treatment. The direct action of liraglutide on the transplanted islets may increase islet survival and insulin secretion. In addition to blood glucose levels, insulin levels and HbA1c levels are used as indicators of glycemic control in islet transplants39. These levels were also more effectively improved by local pretreatment with liraglutide compared with systemic treatment, indicating that local liraglutide treatment may effectively protect the transplanted islets and maintain islet survival and function.
Transplanted islets from transgenic mice expressing luciferase under the control of the insulin promoter, including the Ins2 mice used in this study, can be evaluated for graft survival and efficacy through noninvasive monitoring of islet grafts in living recipients25,40,41. Our results showed that the luciferase activity in islet transplants was correlated with the blood glucose levels of the islet transplanted groups and was significantly higher in the I200-local pretreatment group than in the I200-systemic treatment group. In addition, the condition of transplanted islet cells is typically assessed by measuring apoptosis via the TUNEL assay18,35,42. Similar to previous reports35, we observed many TUNEL+Insulin+ cells in the marginal mass islet transplantation group. However, TUNEL+Insulin+ cells were significantly reduced in the I200-local pretreatment group compared to that in the I200 and I200-systemic treatment groups. These results indicate that local pretreatment with liraglutide may effectively protect islet cells and maintain islet function and glycemic control.
In summary, topical treatment with liraglutide prior to transplantation may help restore normoglycemia following the transplantation of a small number of islets. These findings could be useful for improved treatment of diabetes, given the shortage of islet donors for transplantation.
Acknowledgments
We thank the Center of Animal Care and Use (Lee Gil Ya Cancer and Diabetes Institute, Korea) for providing animal care.
Footnotes
Author Contributions: KWK, BK, and HJ conceived and designed the study. SL, DK, KMK, and PPK performed the experiments. DK and OKL performed the statistical analysis of the data. SL, DK, and HJ wrote the manuscript, and OKL and HJ critically revised the manuscript. All authors approved the final version of the manuscript.
Ethical Approval: This study was approved by the Institutional Animal Care and Use Committee at Gachon University, Korea (LCDI-2016-0035, LCDI-2018-0101).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the Institutional Animal Care and Use Committee at Gachon University (LCDI-2016-0035, LCDI-2018-0101) approved protocols.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C1135).
ORCID iD: Hee-Sook Jun  https://orcid.org/0000-0002-1166-4932
https://orcid.org/0000-0002-1166-4932
References
- 1. Simmons KM, Michels AW. Type 1 diabetes: a predictable disease. World J Diabetes. 2015;6(3):380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ben Nasr M, Vergani A, Avruch J, Liu L, Kefaloyianni E, D’Addio F, Tezza S, Corradi D, Bassi R, Valderrama-Vasquez A, Usuelli V, et al. Co-transplantation of autologous MSCs delays islet allograft rejection and generates a local immunoprivileged site. Acta Diabetol. 2015;52(5):917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am J Transplant. 2008;8(10):1990–1997. [DOI] [PubMed] [Google Scholar]
- 4. Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol. 2017;13(5):268–277. [DOI] [PubMed] [Google Scholar]
- 5. Ellis CE, Korbutt GS. Justifying clinical trials for porcine islet xenotransplantation. Xenotransplantation. 2015;22(5):336–344. [DOI] [PubMed] [Google Scholar]
- 6. Godfrey KJ, Mathew B, Bulman JC, Shah O, Clement S, Gallicano GI. Stem cell-based treatments for Type 1 diabetes mellitus: bone marrow, embryonic, hepatic, pancreatic and induced pluripotent stem cells. Diabet Med. 2012;29(1):14–23. [DOI] [PubMed] [Google Scholar]
- 7. Kanafi MM, Rajeshwari YB, Gupta S, Dadheech N, Nair PD, Gupta PK, Bhonde RR. Transplantation of islet-like cell clusters derived from human dental pulp stem cells restores normoglycemia in diabetic mice. Cytotherapy. 2013;15(10):1228–1236. [DOI] [PubMed] [Google Scholar]
- 8. Jo YH, Jang IJ, Nemeno JG, Lee S, Kim BY, Nam BM, Yang W, Lee KM, Kim H, Takebe T, Kim YS, et al. Artificial islets from hybrid spheroids of three pancreatic cell lines. Transplant Proc. 2014;46(4):1156–1160. [DOI] [PubMed] [Google Scholar]
- 9. Lebreton F, Lavallard V, Bellofatto K, Bonnet R, Wassmer CH, Perez L, Kalandadze V, Follenzi A, Boulvain M, Kerr-Conte J, Goodman DJ, et al. Insulin-producing organoids engineered from islet and amniotic epithelial cells to treat diabetes. Nat Commun. 2019;10(1):4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Evgenov NV, Medarova Z, Pratt J, Pantazopoulos P, Leyting S, Bonner-Weir S, Moore A. In vivo imaging of immune rejection in transplanted pancreatic islets. Diabetes. 2006;55(9):2419–2428. [DOI] [PubMed] [Google Scholar]
- 11. Makhlouf L, Kishimoto K, Smith RN, Abdi R, Koulmanda M, Winn HJ, Auchincloss H, Jr, Sayegh MH. The role of autoimmunity in islet allograft destruction: major histocompatibility complex class II matching is necessary for autoimmune destruction of allogeneic islet transplants after T-cell costimulatory blockade. Diabetes. 2002;51(11):3202–3210. [DOI] [PubMed] [Google Scholar]
- 12. Huang X, Moore DJ, Ketchum RJ, Nunemaker CS, Kovatchev B, McCall AL, Brayman KL. Resolving the conundrum of islet transplantation by linking metabolic dysregulation, inflammation, and immune regulation. Endocr Rev. 2008;29(5):603–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Emamaullee JA, Merani S, Toso C, Kin T, Al-Saif F, Truong W, Pawlick R, Davis J, Edgar R, Lock J, Bonner-Weir S, et al. Porcine marginal mass islet autografts resist metabolic failure over time and are enhanced by early treatment with liraglutide. Endocrinology. 2009;150(5):2145–2152. [DOI] [PubMed] [Google Scholar]
- 14. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439. [DOI] [PubMed] [Google Scholar]
- 15. Ramracheya R, Chapman C, Chibalina M, Dou H, Miranda C, Gonzalez A, Moritoh Y, Shigeto M, Zhang Q, Braun M, Clark A, et al. GLP-1 suppresses glucagon secretion in human pancreatic alpha-cells by inhibition of P/Q-type Ca(2+) channels. Physiol Rep. 2018;6(17):e13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther. 2007;113(3):546–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Langlois A, Dal S, Vivot K, Mura C, Seyfritz E, Bietiger W, Dollinger C, Peronet C, Maillard E, Pinget M, Jeandidier N, et al. Improvement of islet graft function using liraglutide is correlated with its anti-inflammatory properties. Br J Pharmacol. 2016;173(24):3443–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Toso C, McCall M, Emamaullee J, Merani S, Davis J, Edgar R, Pawlick R, Kin T, Knudsen LB, Shapiro AM. Liraglutide, a long-acting human glucagon-like peptide 1 analogue, improves human islet survival in culture. Transpl Int. 2010;23(3):259–265. [DOI] [PubMed] [Google Scholar]
- 19. Langlois A, Mura C, Bietiger W, Seyfritz E, Dollinger C, Peronet C, Maillard E, Pinget M, Jeandidier N, Sigrist S. In vitro and in vivo investigation of the angiogenic effects of liraglutide during islet transplantation. PLoS One. 2016;11(3):e0147068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lew B, Kim IY, Choi H, Kim KK. Sustained exenatide delivery via intracapsular microspheres for improved survival and function of microencapsulated porcine islets. Drug Deliv Transl Res. 2018;8(3):857–862. [DOI] [PubMed] [Google Scholar]
- 21. Linetsky E, Bottino R, Lehmann R, Alejandro R, Inverardi L, Ricordi C. Improved human islet isolation using a new enzyme blend, liberase. Diabetes. 1997;46(7):1120–1123. [DOI] [PubMed] [Google Scholar]
- 22. Bleich D, Chen S, Zipser B, Sun D, Funk CD, Nadler JL. Resistance to type 1 diabetes induction in 12-lipoxygenase knockout mice. J Clin Invest. 1999;103(10):1431–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sakata N, Tan A, Chan N, Obenaus A, Mace J, Peverini R, Sowers L, Chinnock R, Hathout E. Efficacy comparison between intraportal and subcapsular islet transplants in a murine diabetic model. Transplant Proc. 2009;41(1):346–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dao LT, Park EY, Lim SM, Choi YS, Jung HS, Jun HS. Transplantation of insulin-producing cells differentiated from human periosteum-derived progenitor cells ameliorate hyperglycemia in diabetic mice. Transplantation. 2014;98(10):1040–1047. [DOI] [PubMed] [Google Scholar]
- 25. Lu Y, Dang H, Middleton B, Zhang Z, Washburn L, Campbell-Thompson M, Atkinson MA, Gambhir SS, Tian J, Kaufman DL. Bioluminescent monitoring of islet graft survival after transplantation. Mol Ther. 2004;9(3):428–435. [DOI] [PubMed] [Google Scholar]
- 26. Ahearn AJ, Parekh JR, Posselt AM. Islet transplantation for type 1 diabetes: where are we now?. Expert Rev Clin Immunol. 2015;11(1):59–68. [DOI] [PubMed] [Google Scholar]
- 27. Kaviani M, Keshtkar S, Azarpira N, Aghdaei MH, Geramizadeh B, Karimi MH, Shamsaeefar A, Motazedian N, Nikeghbalian S, Al-Abdullah IH, Ghahremani MH. Cytoprotective effects of olesoxime on isolated human pancreatic islets in order to attenuate apoptotic pathway. Biomed Pharmacother. 2019;112:108674. [DOI] [PubMed] [Google Scholar]
- 28. Keshtkar S, Kaviani M, Jabbarpour Z, Geramizadeh B, Motevaseli E, Nikeghbalian S, Shamsaeefar A, Motazedian N, Al-Abdullah IH, Ghahremani MH, Azarpira N. Protective effect of nobiletin on isolated human islets survival and function against hypoxia and oxidative stress-induced apoptosis. Sci Rep. 2019;9(1):11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jeong JH, Yook S, Hwang JW, Jung MJ, Moon HT, Lee DY, Byun Y. Synergistic effect of surface modification with poly(ethylene glycol) and immunosuppressants on repetitive pancreatic islet transplantation into antecedently sensitized rat. Transplant Proc. 2013;45(2):585–590. [DOI] [PubMed] [Google Scholar]
- 30. Jung YS, Jeong JH, Yook S, Im BH, Seo J, Hong SW, Park JB, Yang VC, Lee DY, Byun Y. Surface modification of pancreatic islets using heparin-DOPA conjugate and anti-CD154 mAb for the prolonged survival of intrahepatic transplanted islets in a xenograft model. Biomaterials. 2012;33(1):295–303. [DOI] [PubMed] [Google Scholar]
- 31. de Vos P, Marchetti P. Encapsulation of pancreatic islets for transplantation in diabetes: the untouchable islets. Trends Mol Med. 2002;8(8):363–366. [DOI] [PubMed] [Google Scholar]
- 32. Haque MR, Lee DY, Ahn CH, Jeong JH, Byun Y. Local co-delivery of pancreatic islets and liposomal clodronate using injectable hydrogel to prevent acute immune reactions in a type 1 diabetes. Pharm Res. 2014;31(9):2453–2462. [DOI] [PubMed] [Google Scholar]
- 33. Pathak S, Regmi S, Gupta B, Poudel BK, Pham TT, Kim JR, Park PH, Yong CS, Kim JO, Bae YK, Kim SK, et al. Hybrid congregation of islet single cells and curcumin-loaded polymeric microspheres as an interventional strategy to overcome apoptosis associated with pancreatic islets transplantation. ACS Appl Mater Interfaces. 2016;8(39):25702–25713. [DOI] [PubMed] [Google Scholar]
- 34. Wang Y, Qi M, McGarrigle JJ, Rady B, Davis ME, Vaca P, Oberholzer J. Use of glucagon-like peptide-1 agonists to improve islet graft performance. Curr Diab Rep. 2013;13(5):723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Merani S, Truong W, Emamaullee JA, Toso C, Knudsen LB, Shapiro AM. Liraglutide, a long-acting human glucagon-like peptide 1 analog, improves glucose homeostasis in marginal mass islet transplantation in mice. Endocrinology. 2008;149(9):4322–4328. [DOI] [PubMed] [Google Scholar]
- 36. Abdulreda MH, Rodriguez-Diaz R, Caicedo A, Berggren PO. Liraglutide compromises pancreatic beta cell function in a humanized mouse model. Cell Metab. 2016;23(3):541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pepper AR, Pawlick R, Gala-Lopez B, MacGillivary A, Mazzuca DM, White DJ, Toleikis PM, Shapiro AM. Diabetes is reversed in a murine model by marginal mass syngeneic islet transplantation using a subcutaneous cell pouch device. Transplantation. 2015;99(11):2294–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zorzi D, Phan T, Sequi M, Lin Y, Freeman DH, Cicalese L, Rastellini C. Impact of islet size on pancreatic islet transplantation and potential interventions to improve outcome. Cell Transplant. 2015;24(1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meloche RM. Transplantation for the treatment of type 1 diabetes. World J Gastroenterol. 2007;13(47):6347–6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim D, Jun HS. In vivo imaging of transplanted pancreatic islets. Front Endocrinol (Lausanne). 2017;8:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Virostko J, Chen Z, Fowler M, Poffenberger G, Powers AC, Jansen ED. Factors influencing quantification of in vivo bioluminescence imaging: application to assessment of pancreatic islet transplants. Mol Imaging. 2004;3(4):333–342. [DOI] [PubMed] [Google Scholar]
- 42. Todorov I, Nair I, Avakian-Mansoorian A, Rawson J, Omori K, Ito T, Valiente L, Iglesias-Meza I, Orr C, Shiang KD, Ferreri K, et al. Quantitative assessment of beta-cell apoptosis and cell composition of isolated, undisrupted human islets by laser scanning cytometry. Transplantation. 2010;90(8):836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]