Abstract
Post-transplant cyclophosphamide (PTCy) has been explored in several types of stem cell transplantations (SCTs) and it proved highly effective in controlling graft-versus-host disease (GvHD) without aggravating relapsed disease. However, PTCy alone has resulted in inferior outcomes in matched sibling donor (MSD) employing peripheral blood (PB) SCTs. We hypothesized that adding thymoglobulin to PTCy would be able to control GvHD effectively. We retrospectively compared the use of standard GvHD prophylaxis encompassing a combination of PTCy and thymoglobulin (ATG) in patients with myeloid malignancies in a myeloablative conditioning MSD PBSCT. Forty-two patients underwent PBSCT using either methotrexate and cyclosporine (MTX/CSA, 21 patients) or PTCy and ATG (21 patients) as a GvHD prophylaxis. With median follow-ups of 71 months, the 1-year GvHD-free, relapse-free survival rates and chronic GvHD-free survival rate of the standard and PTCy/ATG groups were similar: 24% versus 37% (P = 0.251) and 29% versus 43% (P = 0.095), respectively. When focusing on chronic GvHD we observed that 17/35 patients (48.6%) suffered from this, 5/18 (27.8%) treated with MTX/CSA had extensive chronic GvHD, but 0/17 PTCy/ATG did. Twenty-one patients required additional GvHD treatment; 7/21 in the PTCy/ATG received only corticosteroid, while 8/14 MTX/CSA required at least 2 drugs. The 5-year overall survival rates were 52% and 52% (P = 0.859), and the 5-year disease-free survival rates were 52% and 52% (P = 0.862) for the MTX/CSA and PTCy/ATG groups, respectively. We conclude that PTCy in combination with ATG without immunosuppression of a calcineurin inhibitor can effectively control GvHD.
Keywords: post-transplant Cy, MSD transplantation, calcineurin-free GvHD prophylaxis, acute myeloid leukemia
Introduction
Graft-versus-host disease (GvHD) prophylaxis remains a challenge in allogeneic hematopoietic stem cell transplantation (HSCTs) in hematological malignancies. Although current standard GvHD prophylaxis with a calcineurin inhibitor (CNI) and short-course methotrexate (MTX) are standard of care, CNI delayed immune reconstitution, and increased chronic GvHD after discontinuation of CNI. The latter problem has, however, been addressed by the introduction of post-transplant cyclophosphamide (PTCy)1–4, which acts by eliminating proliferating host-to-graft and graft-to-host alloreactive T lymphocytes while preserving the regulatory T cell, which control the GvHD and promote graft–host tolerance after allogeneic HSCTs5,6. However, severe GvHD has not been totally eliminated, and the addition of low-dose anti-thymocyte globulin, which targets both T cells, B cells, natural killer cells, macrophage, and dendritic cells, also has been able to further reduce the GvHD rate without increasing the relapse rate in acute leukemia patients7.
Hitherto, PTCy has been used as a prophylaxis for both acute and chronic GvHD after bone marrow transplantations (BMTs) from matched donors3,8. Disappointingly, in the setting of peripheral blood stem cell transplantation (PBSCT), PTCy alone resulted in inferior outcomes, due to a high incidence of severe acute GvHD9,10. Given that PBSCs are easier to procure than bone marrow and are now most frequently utilized for matched sibling transplants, we hypothesized that adding anti-thymocyte globulin to PTCy will be able to better control GvHD in PBSCs allogeneic HSCTs and improve survival. To address this, we conducted a study to assess the use of a CNI-free GvHD prophylaxis regimen combining PTCy and thymoglobulin (ATG) (PTCy/ATG) in matched-sibling donor PBSCTs with myeloablative conditioning. We retrospectively compared this to the standard GvHD prophylaxis with short-course methotrexate and cyclosporine (MTX/CSA).
Materials and Methods
Study Design
Patients with a diagnosis of a myeloid neoplasm (acute myeloid leukemia in first complete remission, myelodysplastic syndrome, and chronic myelogenous leukemia), who underwent PBSCT during the period from January 1, 2010 to December 31, 2013, were included. The prophylaxis regimen was applied according to the year of transplantation. Standard GvHD prophylaxis was applied to patients treated in the year 2010 or 2011, while patients who underwent PBSCT during 2011 to 2012 used PTCy/ATG as prophylaxis regimen. The outcomes were evaluated on March 1, 2019.
The primary endpoint was the GvHD-free, relapse-free survival (GRFS). The secondary endpoints were the incidences of grades 3 to 4 acute GvHD, and chronic GvHD, chronic GvHD-free survival (CRFS), disease-free survival (DFS), overall survival (OS), and nonrelapse mortality (NRM) rates. GRFS was defined as the absence of grade III to IV acute GVHD, systemic therapy–requiring chronic GVHD, relapse, or death. CRFS is defined as the absence of systemic therapy–requiring chronic GVHD, relapse, or death. DFS was defined as survival without relapsed disease from the first date of the PBSCT to the date that patients had either a relapse or died from any causes. This study was approved by the Ethics Review Committee of the Faculty of Medicine Siriraj Hospital, Mahidol University 283/2560 (EC4).
Patients
Forty-two consecutive patients were included. Inclusion criteria were Eastern Cooperative Oncology Group performance status <2, liver function test results <2 times upper normal limit, a creatinine level <2 mg/dl, and a left ventricular ejection fraction i50%. Patients with acute myeloid leukemia who could achieve morphologic and cytogenetics complete remission would undergo HSCT in our center.
Conditioning Regimen and GvHD Prophylaxis
A myeloablative conditioning, comprising busulfan 3.2 mg/kg intravenously (IV) on D−7 to D−4 and cyclophosphamide 50 mg/kg IV on D−3 to D−2, was used.
The standard GvHD prophylaxis included CSA 3 mg IV for 1 h every 12 h, starting on D−1 followed by a short-course MTX (15 mg/m2 IV on D+1, and 10 mg/m2 on D+3, D+6, and D+11)11. The CSA trough level was kept at 200 to 400 ng/ml for 3 months, then the dose was taped until stop within 6 months. The PTCy/ATG regimen consisted of ATG 2.5 mg/kg IV/day from D−3 to D−2, followed by cyclophosphamide 50 mg/m2 IV on D+3 and D+4.
Supportive Treatment
All patients received phenytoin 300 mg per day on D−7 to D−3 to prevent busulfan toxicity and filgrastim 5 µg per kg since D+1 and D+5 until ANC engraftment in MTX/CSA and PTCy/ATG groups, respectively. For infectious prophylaxis, ciprofloxacin 500 mg every 12 h until neutrophil engraftment and acyclovir either 400 or 800 mg every 12 h were used in those recipients or donors who were positive for herpes simplex and varicella zoster virus, respectively, since the starting of conditioning regimen until 1 year after ceasing immunosuppression. Those patients who did not have any history of invasive fungal infection received fluconazole 400 mg per day, while those with a history of invasive fungal infection were given voriconazole starting from the date of conditioning regimen to the date of neutrophil engraftment.
GvHD Evaluation
Acute GvHD was graded by the Mount Sinai Acute GVHD International Consortium12. Chronic GvHD was divided into limited and extensive stages, according to the revised Seattle Classification13. For chronic GvHD, we included all patients who survived after D+100 of the transplantation.
Engraftment
Neutrophil engraftment was defined as the first of 3 consecutive days of the absolute neutrophil count being ≥0.5 × 103/µl. Platelet engraftment was defined as the first of 7 days in which the platelet count exceeded 20 × 103/µl without transfusion.
Statistical Analysis
Descriptive statistics were used for the cumulative incidences of GvHD, complications, and all variables. Comparisons between each GvHD prophylaxis group and the quantitative factors utilized the Mann–Whitney or unpaired t-test, while the qualitative factors employed the chi-square test.
Time-to-event parameters (comprising the GRFS, CRFS, OS, NRM, and DFS rates) used the Kaplan–Meier method, and comparisons between groups of patients were made by log-rank test.
Results
Patient Characteristics
Patients’ characteristics are depicted in Table 1. The median follow-up time was 71 (range: 0.6 to 108.3) months, which was 81.3 (range 0.57 to 108.33) months for the standard MTX/CSA group, and was 69.6 (range 1.93 to 92.70) months for the PTCy/ATG group (P = 0.004).
Table 1.
Patients’ Characteristics.
| MTX–CSA | ATG–PTCY | P-value | |
|---|---|---|---|
| Number | 21 | 21 | |
| Sex male | 12 (57.1%) | 10 (47.6%) | 0.537 |
| Age | 36.2 (22-52) | 45.47 (17-49) | 0.030 |
| Disease | |||
| AML | 12 (57.1%) | 17 (81.0%) | 0.181 |
| MDS | 1 (4.8%) | 1 (4.8%) | 1.0 |
| CMLCP | 8 (38.1%) | 3 (14.3%) | 0.306 |
| Donor sex | |||
| F => M | 6 (28.6%) | 5 (23.8%) | 0.726 |
| Number of prior chemotherapies | |||
| ≥2 cycles | 10 (47.6%) | 17 (81.0%) | 0.024 |
| CMV status | 1.0 | ||
| Patient positive | 21 (100%) | 20 (95.2%) | |
| Donor positive | 21 (100%) | 21 (100%) | |
| Stem cell dose | |||
| Median CD 34+ | 5.27 (1.5 10.5) | 4.73 (2.12 10.8) | 0.448 |
| ≥3 × 106 | 18 (85.7%) | 18 (85.7%) | 1.0 |
ATG: thymoglobulin; AML: acute myeloid leukemia; CD: cluster of differentiation; CML: chronic myeloid leukemia; CMV: cytomegalovirus; CSA: cyclosporine; F => M: female donor and male recipient; MDS: myelodysplastic syndrome; MTX: methotrexate; PTCY: post-transplant cyclophosphamide.
Of the 42 patients, 40 (95%) achieved neutrophil engraftment. The median times to neutrophil and platelet engraftment for the study cohort were 13 (range: 10 to 20) and 20 (range: 16 to 73) days, respectively. The median time to neutrophil engraftment for the MTX/CSA group was 14 (range: 10 to 22) days, compared with 13 (range: 12 to 15) days for the PTCy/ATG group (P = 0.52). The median times to platelet engraftment were 23 (range: 16 to 73) and 19.5 (range: 17 to 46) days for the standard GvHD prophylaxis and PTCy/ATG groups, respectively (P = 0.204). These data show that both regimens have comparable successful engraftment.
Graft-Versus-Host Disease
The total incidence of grades 1 to 4 acute GvHD in all cohorts stood at 28.6% (12/42). The incidence breakdown for selected subgroups (grades 2 to 4 and 3 to 4) is detailed in Table 2. Both cohorts have comparably all grades of acute GvHD.
Table 2.
Acute GvHD and Chronic GvHD, Divided According to GvHD Prophylaxis Group.
| MTX–CSA | ATG–PTCY | P-value | |
|---|---|---|---|
| Acute GvHD | |||
| Grades 2-4 | 5/21 (23.8%) | 4/21 (19.0%) | 1.0 |
| Grades 3-4 | 4/21 (19.0%) | 2/21 (9.5%) | 0.39 |
| Chronic GvHD | |||
| Limited | 9/18 (50.0%) | 3/17 (17.6%) | 0.04 |
| Extensive | 5/18 (27.8%) | 0/17 | <0.0001 |
ATG: thymoglobulin; CSA: cyclosporine A; GVHD: graft-versus-host disease; MTX: methotrexate; PTCY: post-transplant cyclophosphamide.
A total of 7 patients passed away within 100 days of their transplantations (3 in the MTX/CSA group and 4 in the PTCy/ATG group). Out of the 35 patients surviving for over 100 days, 17 (48.6%) had chronic GvHD. Five cases in the standard GvHD prophylaxis group had extensive chronic GvHD, compared with none in the PTCy/ATG group (P < 0.001; Table 2).
After PBSCT, 21 out of 42 (50%) patients required immunosuppressive therapy (IST) for GvHD treatment; 14/21 (66.7%) patients for the MTX/CSA group and 7/21 (33.3%) patients for the PTCy/ATG group (P = 0.03). While all 7 PTCy/ATG patients received only corticosteroid for GvHD treatment, 8/14 patients in the standard MTX/CSA group required at least 2 regimens of IST. For example, one case with MDS in MTX/CSA cohort experienced grade 3 acute GvHD proceeded by chronic GvHD (myositis). He was treated with four-drug combination including prednisolone, CSA, chloroquine, and azathioprine. Unfortunately, he passed away from respiratory failure.
The median duration of the IST use was 8.1 (range: 0.7 to 75.9) months, comprising 25.3 (range: 0.7 to 75.9) months for the MTX/CSA group and 7.0 (range: 0.9 to 19.1) months for the PTCy/ATG group (P = 0.025).
Survival Outcomes
GRFS rate and CRFS
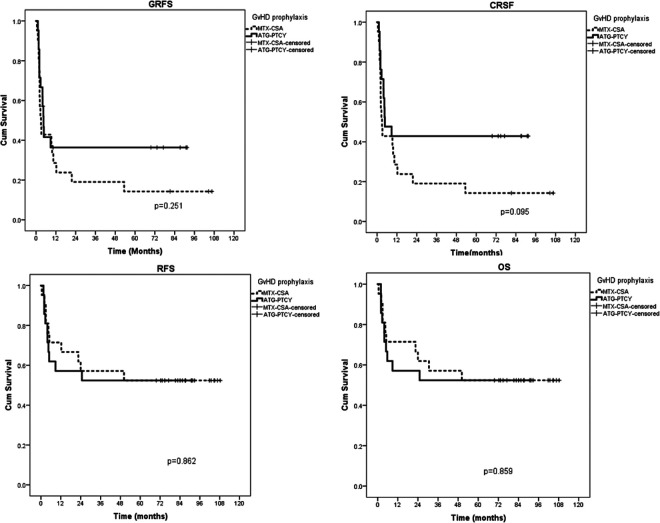
With a median follow-up of 71 (range, 0.6 to 108.3) months, the 1-year GRFS rates of the standard and PTCy/ATG groups were 24% and 37%, respectively. The PTCy/ATG groups had higher 1-year GRFS compared to the standard group but there was no statistical difference (P = 0.251). The trend is similar for CRFS rate, with 1-year CRFS rates of 29% and 43% for the standard and PTCy/ATG groups (P = 0.095), respectively. The GRFS and CRFS are shown in Fig. 1 and Table 3. Importantly, ≥2 years after transplantation, no PTCy/ATG patients relapsed or died, whereas one case in the MTX/CSA arm passed away from severe chronic GvHD at 4.2 years.
Figure 1.
GRFS, CRFS, RFS, and OS rates.ATG: thymoglobulin; cGVHD: chronic graft-versus-host disease; CRFS: cGvHD-free, relapse-free survival; CSA: cyclosporine; GRFS: GvHD-free, relapse-free survival; MTX: methotrexate; OS: overall survival; PTCY: post-transplant cyclophosphamide; RFS: relapse-free survival.
Table 3.
GRFS, CRFS, DFS, and OS Rates.
| GvHD prophylaxis | Number of patients having events | Median GRFS (months) | GRFS rate at 1 year | GRFS rate at 3 years | GRFS rate at 5 years | GRFS rate at 7 years | P-value |
|---|---|---|---|---|---|---|---|
| MTX/CSA, n = 21 | 18 | 3.8 | 24 | 19 | 14 | 14 | 0.251 |
| ATG/PTCY, n = 21 | 13 | 4.8 | 37 | 37 | 37 | 37 | |
| All patients | 31 | 4.6 | 32 | 27 | 24 | 24 | |
| GvHD prophylaxis | Number of patients having events |
Median CRFS (months) | CRFS rate at 1 year |
CRFS rate at 3 years |
CRFS rate at 5 years |
CRFS rate at 7 years |
P-value |
| MTX/CSA, n = 21 | 18 | 3.8 | 29 | 19 | 14 | 14 | 0.095 |
| ATG/PTCY, n = 21 | 12 | 5.7 | 43 | 43 | 43 | 43 | |
| All patients | 30 | 5.1 | 36 | 31 | 29 | 29 | |
| GvHD prophylaxis | Number of patients having events |
Median DFS (months) | DFS rate at 1 year |
DFS rate at 3 years |
DFS rate at 5 years |
DFS rate at 7 years |
P-value |
| MTX/CSA, n = 21 | 10 | NR | 71 | 57 | 52 | 52 | 0.862 |
| ATG/PTCY, n = 21 | 10 | NR | 57 | 52 | 52 | 52 | |
| All patients | 20 | NR | 64 | 55 | 52 | 52 | |
| GvHD prophylaxis | Number of patients having events |
Median OS (months) | OS rate at 1 year |
OS rate at 3 years |
OS rate at 5 years |
OS rate at 7 years |
P-value |
| MTX/CSA, n =2 1 | 10 | NR | 71 | 57 | 52 | 52 | 0.859 |
| ATG/PTCY, n = 21 | 10 | NR | 57 | 52 | 52 | 52 | |
| All patients | 20 | NR | 64 | 55 | 52 | 52 |
ATG: thymoglobulin; CRFS: chronic GvHD-relapse-free survival; CSA: cyclosporine; DFS: disease-free survival; GRFS: GvHD-relapse-free survival; GvHD: graft-versus-host disease; MTX: methotrexate; NR: not reached—the median time to events was undefined since the survival curve did not cross 50% survival; n: number; OS: overall survival; PTCy: post-transplant cyclophosphamide.
NRM, relapse rate, progression-free survival, and OS rates
The 100-day NRM rate was 16.6% (7/42), comprising 14.3% (3/21) for the MTX/CSA group and 19.0% (4/21) for the PTCy/ATG group. Fourteen cases died without disease relapse within 2 years (eight in the MTX/CSA group and six in the PTCy/ATG group). The 2-year NRM rates were 38% and 28% for the MTX/CSA and PTCy/ATG groups, respectively (P = 0.769). The causes of death by group are detailed in Table 4. Most common causes of death in PTCy/ATG were disease relapse and bacterial infection, whereas the most common cause of death in MTX/CSA was bacterial sepsis. Five cases had relapsed disease, 4 (19%) cases in PTCy/ATG and 1 (4.8%) case in MTX/CSA group (P = 0.3), at the median time of 8.8 (range 1.57 to 24.73) months.
Table 4.
Multivariate Analysis for GRFS, CRFS, PFS, and OS Rates.
| Variables | GRFS | CRFS (recalculated) | PFS | OS | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Grades 3-4 acute GvHD | 7.4 (2.4-22.3) | <0.001 | 2.8 (1.03-7.7) | 0.043 | - | - | - | - |
| Creatinine >2 | 2.6 (1.1-6.4) | 0.033 | 3 (1.2-7.4) | 0.015 | 5.1 (2-13.2) | 0.001 | 4.4 (1.7-11.8) | 0.003 |
CRFS: chronic GvHD-relapse-free survival; GRFS: GvHD-relapse-free survival; GvHD: graft-versus-host disease; HR: hazard ratio; OS: overall survival; PFS: progression-free survival.
Both groups had the same 5- and 7-year DFS and OS rate (52%), with P-values =0.862 and 0.859 for DFS and OS, respectively. The GRFS, CRFS, DFS, and OS rates are detailed in Fig. 1 and Table 3. Two years and beyond after their transplantations in PTCy/ATG cohort, no patients relapsed or died, whereas one case in MTX/CSA arm passed away from severe chronic GvHD at 4.2 years.
We added the age of patient and donor, patient’s gender, donor’s gender (female to male vs others), creatinine level, and stem cell dose to perform uni- and multivariate analyses for GRFS, CRFS, PFS, and OS (Table 4). Grade 3 to 4 aGvHD and creatinine >2 were the independent variables associated with GRFS and CRFS. However, the only independent factor predicting PFS and OS was creatinine >2.
Complications
Table 5 shows that the MTX/CSA group had a higher incidence of complications except for cytomegalovirus (CMV) reactivation. Nine (42.9%) patients in the PTCy/ATG group had CMV reactivation, compared with 6 (28.6%) patients in the MTX/CSA group (P = 0.334). Although members of the PTCy/ATG group received about twice as much cyclophosphamide as patients in the standard GvHD prophylaxis group, the incidences of hemorrhagic cystitis in each group were comparable.
Table 5.
Complications and Causes of Death.
| MTX–CSA | ATG–PTCY | P-value | |
|---|---|---|---|
| Complications | |||
| VOD | 3/21 (14.3%) | 0/21 | 0.232 |
| Cr rising >2 | 5/21 (23.8%) | 2/21 (9.5%) | 0.22 |
| Fungal infection | 2/21 (9.5%) | 2/21 (9.5%) | 1 |
| Hemorrhagic cystitis | 3/21 (14.3%) | 2/21 (9.5%) | 1 |
| CMV reactivation | 6/21 (28.6%) | 9/21 (42.9%) | 0.334 |
| Causes of death | |||
| CMV infection | 0/10 | 2/10 (20.0%) | 0.136 |
| Disease relapse | 1/10 (10%) | 4/10 (40.0%) | 0.121 |
| Bacterial infection | 7/10 (70%) | 4/10 (40.0%) | 0.178 |
| VOD | 1/10 (10%) | 0/10 | 0.305 |
| GvHD | 1/10 (10%) | 0/10 | 0.305 |
| Total | 10 cases | 10 cases |
ATG: thymoglobulin; CMV: cytomegalovirus; CSA: cyclosporine: GvHD: graft-versus-host disease; MTX: methotrexate; PTCY: post-transplant cyclophosphamide; VOD: veno-occlusive d.
Discussion
We have reported the use of a combination of PTCy with ATG 5 mg/kg as a GvHD prophylaxis for matched-sibling donor PBSCTs. Our study showed a trend toward better GRFS and CRFS rates for the PTCy/ATG group than the standard prophylaxis regimen group, but comparable DFS and OS rates. The overall incidences of grades 2 to 4 and grades 3 to 4 acute GvHD of this regimen were lower than those for the standard MTX and CSA regimen (19% vs 23% for grades 2 to 4 acute GvHD and 9.5% vs 19% for grades 3 to 4 acute GvHD), although there was no statistically significant difference. However, only 3/17 patients in the PTCy group had chronic GvHD with limited disease, and none had extensive disease. By contrast, 14/18 patients (77.8%) in the standard group developed chronic GvHD, and nearly half of those 14 had extensive disease. This strikingly lower incidence of chronic GvHD in the PTCy/ATG group, without any immunosuppressive drugs for GvHD prophylaxis afterwards, underlines the effectiveness of cyclophosphamide that eradicates alloreactive T-cells and ATG that targets on several immune cells including T cells, B cells, natural killer cells, macrophage, and dendritic cells7,14.
PTCy was first introduced for haploidentical transplantations and showed a low incidence of both acute and chronic GvHD, a low rate of graft rejection, and better NRM and OS rates1–4. The use of PTCy alone as a GvHD prophylaxis for matched sibling donor (MSD) BMTs was favorable to both acute and chronic GvHD8,14. These two studies indicate that PTCy can be safe and effective as a single-agent prophylaxis for both acute and chronic GvHD after an MSD BMT. However, the sole use of PTCy is not sufficient with PBSCTs either after a reduced-intensity conditioning or a myeloablative conditioning regimen9,10
Previous studies have shown that adding a low dose of ATG is effective in reducing GvHD without increasing the relapse rate of MSD and matched-unrelated donor HSCTs for hematologic malignancies. In the ATG group, the incidences of grades 2 to 4 and grades 3 to 4 acute GvHD were significantly diminished (27% vs 55% and 14% vs 28%, respectively). Extensive chronic GvHD was also significantly reduced (28% vs 46%)15,16. Prior studies of MSD PBSCTs in patients with hematologic malignancies using PTCy as a GvHD prophylaxis indicate that other drugs such as bortezomib, sirolimus, and tacrolimus-mycophenolate mofetil need to be combined with PTCy in order to reduce the incidence of GvHD17–20.
The use of PTCy and low-dose ATG as a GvHD prophylaxis in MSD PBSCTs showed more favorable outcomes. As to acute GvHD, although there was no statistically significant difference in the incidence compared with control, acute GvHD was less severe, easier to treat with the steroid alone, whereas half of the patients in the control group required at least two immunosuppressive agents with a significantly longer duration (25.3 vs 7.0 months, P = 0.025), ultimately leading to infection and mortality (NRM 38% vs 28% at the 2-year mark, P = 0.769).
PTCy/ATG has also been associated with more CMV reactions than the MTX/CSA group (42.9% vs 28.1%), but without statistical significance. That result was most likely due to the effects of the ATG. We then monitored the CMV viral load weekly, and patients were empirically treated with ganciclovir when the CMV viral load was >600 copies/ml. All CMV infections were able to be controlled without clinical symptoms.
There was a trend toward a higher relapse rate in the PTCy/ATG group than in the MTX/CSA group (19% vs 4.8%; P = 0.3). This may be due to a reduction of the graft versus leukemia effect in those patients administered PTCy/ATG. However, the relapse rate in PTCy/ATG group was comparable to the reported studies21. In addition, the lower relapse rate in MTX/CSA group might be obscured by other causes of death in this arm. Nevertheless, recently published data of Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation showed that the use of ATG-based regimen did not produce higher relapse rate in AML’s first complete remission undergoing Allo-HSCT with measurable residual disease negative, otherwise the significant lower acute GvHD and extensive chronic GvHD led to significant better leukemic-free survival and OS22.
This is a retrospective study with limited included cases in each arm. Moreover, the chronic GvHD grading using modified Seattle criteria and several prognostic markers and outcomes such as molecular profiling, measuring the residual disease, and Epstein-barr virus monitoring are not available. The prospective comparison in the larger study is required to determine the efficacy of this GvHD prophylaxis regimen. Moreover, we lack the data on immune reconstitution of this combination (PTCy/ATG). Previous study reported the early immune reconstitution in the PTCy than ATG containing GvHD prophylaxis regimens without increasing the relapse rate23. We plan to explore the immune reconstitution of this regimen in the prospective study.
In conclusion, PTCy/ATG can be effectively used to prevent GvHD in MSD PBSCTs for hematological malignancies, with a statistically significantly lower incidence of chronic GvHD.
Acknowledgments
We would like to thank all the patients participating in this study and all laboratory workers, nurses, and doctors who have assisted in the treatment in the stem cell transplantation.
Author Note: This manuscript was presented in conjunction with the presentation at the 2019 Transplantation & Cellular Therapy Meetings of ASBMT and CIBMTR, Houston, USA, 20 to 24 February, 2019.
Availability of Data and Material: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' Contributions: All authors designed the study and collected the data. CK performed the statistical analysis, drafted the manuscript, and revised the final manuscript. CJ collected the data. WO revised the manuscript. SI supervised the project and made critical revisions to the manuscript. All authors read and approved the final manuscript.
Consent for Publication: A copy of the consent document is available for review by the Editor-in-Chief.
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the Ethics Review Committee of the Faculty of Medicine Siriraj Hospital, Mahidol University 283/2560 (EC4).
Statement of Informed Consent: All patients was informed consent.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Chutima Kunacheewa  https://orcid.org/0000-0002-3429-5826
https://orcid.org/0000-0002-3429-5826
Weerapat Owattanapanish  https://orcid.org/0000-0002-1262-2005
https://orcid.org/0000-0002-1262-2005
References
- 1. Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA, Piantadosi S, Kaup M, Ambinder RF, Huff CA, et al. Hla-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, Devine SM, Wingard JR, Aljitawi OS, Cutler CS, Jagasia MH, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially hla-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kasamon YL, Luznik L, Leffell MS, Kowalski J, Tsai HL, Bolanos-Meade J, Morris LE, Crilley PA, O’Donnell PV, Rossiter N, Huff CA, et al. Nonmyeloablative hla-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: Effect of hla disparity on outcome. Biol Blood Marrow Transplant 2010;16(4):482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Munchel A, Kesserwan C, Symons HJ, Luznik L, Kasamon YL, Jones RJ, Fuchs EJ. Nonmyeloablative, hla-haploidentical bone marrow transplantation with high dose, post-transplantation cyclophosphamide. Pediatr Rep. 2011;3 Suppl 2(Suppl 2):e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luznik L, Fuchs EJ. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res 2010;47(1-3):65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to hla-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol. 2016;13(1):10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baron F, Mohty M, Blaise D, Socie G, Labopin M, Esteve J, Ciceri F, Giebel S, Gorin NC, Savani BN, Schmid C, et al. Anti-thymocyte globulin as graft-versus-host disease prevention in the setting of allogeneic peripheral blood stem cell transplantation: a review from the acute leukemia working party of the european society for blood and marrow transplantation. Haematologica. 2017;102(2):224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kanakry CG, O’Donnell PV, Furlong T, de Lima MJ, Wei W, Medeot M, Mielcarek M, Champlin RE, Jones RJ, Thall PF, Andersson BS, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32(31):3497–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bradstock KF, Bilmon I, Kwan J, Micklethwaite K, Blyth E, Deren S, Bayley A, Gebski V, Gottlieb D. Single-agent high-dose cyclophosphamide for graft-versus-host disease prophylaxis in human leukocyte antigen-matched reduced-intensity peripheral blood stem cell transplantation results in an unacceptably high rate of severe acute graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(5):941–944. [DOI] [PubMed] [Google Scholar]
- 10. Holtick U, Chemnitz JM, Shimabukuro-Vornhagen A, Theurich S, Chakupurakal G, Krause A, Fiedler A, Luznik L, Hellmich M, Wolf D, Hallek M, et al. Octet-cy: a phase ii study to investigate the efficacy of post-transplant cyclophosphamide as sole graft-versus-host prophylaxis after allogeneic peripheral blood stem cell transplantation. Eur J Haematol. 2016;96(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. von Bueltzingsloewen A, Belanger R, Perreault C, Bonny Y, Roy DC, Lalonde Y, Boileau J, Kassis J, Lavallee R, Lacombe M, et al. Acute graft-versus-host disease prophylaxis with methotrexate and cyclosporine after busulfan and cyclophosphamide in patients with hematologic malignancies. Blood. 1993;81(3):849–855. [PubMed] [Google Scholar]
- 12. Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, Chanswangphuwana C, Efebera YA, Holler E, Litzow M, Ordemann R, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the mount sinai acute gvhd international consortium. Biol Blood Marrow Transplant. 2016;22(1):4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9(4):215–233. [DOI] [PubMed] [Google Scholar]
- 14. Luznik L, Bolanos-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R, Huff CA, Borrello I, Matsui W, Powell JD, Kasamon Y, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Devillier R, Labopin M, Chevallier P, Ledoux M-P, Socié G, Huynh A, Bourhis J-H, Cahn J-Y, Roth-Guepin G, Mufti G, Desmier D, et al. Impact of antithymocyte globulin doses in reduced intensity conditioning before allogeneic transplantation from matched sibling donor for patients with acute myeloid leukemia: a report from the acute leukemia working party of european group of bone marrow transplantation. Bone Marrow Transplantation. 2018;53(4):431–437. [DOI] [PubMed] [Google Scholar]
- 16. Duléry R, Mohty M, Duhamel A, Robin M, Beguin Y, Michallet M, Vigouroux S, Lioure B, Garnier A, El Cheikh J, Bulabois CE, et al. Antithymocyte globulin before allogeneic stem cell transplantation for progressive myelodysplastic syndrome: a study from the french society of bone marrow transplantation and cellular therapy. Biology of Blood and Marrow Transplantation. 2014;20(5):646–654. [DOI] [PubMed] [Google Scholar]
- 17. Solomon SR, Sanacore M, Zhang X, Brown S, Holland K, Morris LE, Bashey A. Calcineurin inhibitor--free graft-versus-host disease prophylaxis with post-transplantation cyclophosphamide and brief-course sirolimus following reduced-intensity peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(11):1828–1834. [DOI] [PubMed] [Google Scholar]
- 18. Carnevale-Schianca F, Caravelli D, Gallo S, Coha V, D’Ambrosio L, Vassallo E, Fizzotti M, Nesi F, Gioeni L, Berger M, Polo A, et al. Post-transplant cyclophosphamide and tacrolimus-mycophenolate mofetil combination prevents graft-versus-host disease in allogeneic peripheral blood hematopoietic cell transplantation from hla-matched donors. Biol Blood Marrow Transplant. 2017;23(3):459–466. [DOI] [PubMed] [Google Scholar]
- 19. Al-Homsi AS, Cole K, Bogema M, Duffner U, Williams S, Mageed A. Short course of post-transplantation cyclophosphamide and bortezomib for graft-versus-host disease prevention after allogeneic peripheral blood stem cell transplantation is feasible and yields favorable results: a phase i study. Biol Blood Marrow Transplant. 2015;21(7):1315–1320. [DOI] [PubMed] [Google Scholar]
- 20. Al-Homsi AS, Cole K, Muilenburg M, Goodyke A, Abidi M, Duffner U, Williams S, Parker J, Abdel-Mageed A. Calcineurin and mtor inhibitor–free post-transplantation cyclophosphamide and bortezomib combination for graft-versus-host disease prevention after peripheral blood allogeneic hematopoietic stem cell transplantation: a phase i/ii study. Biology of Blood and Marrow Transplantation. 2017;23(10):1651–1657. [DOI] [PubMed] [Google Scholar]
- 21. Mawad R, Lionberger JM, Pagel JM. Strategies to reduce relapse after allogeneic hematopoietic cell transplantation in acute myeloid leukemia. Curr Hematol Malig Rep. 2013;8(2):132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nagler A, Labopin M, Socié G, Huynh A, Itälä-Remes M, Deconinck E, Yakoub-Agha I, Cahn J-Y, Bourhis JH, Labussière-Wallet H, Chantepie S, et al. The role of anti-thymocyte globulin (atg) in patients with aml transplanted in cr1 from sibling and unrelated donors with or without measurable residual disease (mrd) at the time of allogeneic stem cell transplantation: A study on behalf of the acute leukemia working party (alwp) of the european society for blood and marrow transplantation (ebmt). Biology of Blood and Marrow Transplantation. 2019; 25(3):S48–S49. [Google Scholar]
- 23. Retiere C, Willem C, Guillaume T, Vie H, Gautreau-Rolland L, Scotet E, Saulquin X, Gagne K, Bene MC, Imbert BM, Clemenceau B, et al. Impact on early outcomes and immune reconstitution of high-dose post-transplant cyclophosphamide vs anti-thymocyte globulin after reduced intensity conditioning peripheral blood stem cell allogeneic transplantation. Oncotarget. 2018;9(14):11451–11464. [DOI] [PMC free article] [PubMed] [Google Scholar]