Abstract
Allogeneic blood transfusion (ABT) is associated with transfusion-related immune modulation (TRIM) and subsequent poorer patient outcomes including perioperative infection, multiple organ failure, and mortality. The precise mechanism(s) underlying TRIM remain largely unknown. During intraoperative cell salvage (ICS) a patient’s own (autologous) blood is collected, anticoagulated, processed, and reinfused. One impediment to understanding the influence of the immune system on transfusion-related adverse outcomes has been the inability to characterize immune profile changes induced by blood transfusion, including ICS. Dendritic cells and monocytes play a central role in regulation of immune responses, and dysfunction may contribute to adverse outcomes. During a prospective observational study (n = 19), an in vitro model was used to assess dendritic cell and monocyte immune responses and the overall immune response following ABT or ICS exposure. Exposure to both ABT and ICS suppressed dendritic cell and monocyte function. This suppression was, however, significantly less marked following ICS. ICS presented an improved immune competence. This assessment of immune competence through the study of intracellular cytokine production, co-stimulatory and adhesion molecules expressed on dendritic cells and monocytes, and modulation of the overall leukocyte response may predict a reduction of adverse outcomes ( i.e., infection) following ICS.
Keywords: intraoperative cell savage, transfusion, immune modulation
Introduction
Blood transfusion during major surgery is essential and lifesaving. Even though the safety of allogeneic blood transfusion (ABT) has improved, significant risks remain1. Many adverse outcomes related to blood transfusion appear to have immune-mediated processes as a key element of their pathogenesis. The term “Transfusion related immune modulation” (TRIM) has been used to describe immune suppression and subsequent adverse outcomes following ABT such as perioperative infection, cancer recurrence, myocardial infarction, and stroke2–8. ABT is implicated as an independent risk factor in the etiology of TRIM, considering clinical evidence, basic science, and animal model data4,9.
One of the challenges to understanding the potential influence of the immune system upon adverse outcomes related to transfusion has been the inability to characterize immune profile changes induced by blood transfusion, including intraoperative cell salvage (ICS). Many laboratory-based studies have been conducted to understand TRIM and a number of mechanisms have been postulated4. In 2009, Hellings and Blajchman categorized potential mechanisms into three groups: (1) cell-associated, (2) bioactive soluble factors, or (3) microchimerism4. In theory, the infusion of foreign antigens in both soluble and cellular forms during transfusion may cause the development of immune modulation (immune suppression)10. Considering the reinfusion of foreign allogeneic leukocytes as a postulated mechanism for TRIM, leukodepletion of allogeneic red blood cell products was introduced. The incidence of infection following ABT, however, still increased11. Leukodepletion by itself may therefore not be the solution. Despite postulated mechanisms and extensive research, no clear solution to TRIM has been found.
Dendritic cell and monocytes are important to ensure the activation and regulation of numerous immune processes12,13. The functional impairment or deactivation of these important immune cells to produce inflammatory cytokines and express or activate co-stimulatory molecules (e.g., human leukocyte antigen DR phenotype [HLA-DR]) in response to bacterial stimulation (e.g., lipopolysaccharide [LPS]) is characterized as immunoparalysis13,14. Immunoparalysis predicts the inability to resist infectious insult and a subsequent increased risk to develop infection-related adverse outcomes14.
The immunological differences between autologous (own blood) and allogeneic (foreign) transfusion may be important in the search to clarify the exact mechanism of TRIM. Preoperative autologous donation and ICS involve the collection, processing, and reinfusion of a patient’s own red blood cells. These techniques reduce the requirement for ABT and TRIM-related adverse outcomes7,15–17. In addition, ICS blood is also fresh, that is, not stored but instead collected and infused at the time of surgery and, considering the immune-suppressive effects of storage on monocyte function (i.e., production ability of tumor necrosis factor α [TNF-α])9,18, may provide additional immunological benefit.
Through an in vitro model, comparing ABT and ICS exposure, we assessed modulation of dendritic cell- and monocyte-specific cytokine production and expression of activation markers as well as the overall leukocyte response. Even though adverse outcomes following surgery and transfusion are multifactorial, we confirmed that ICS may improve immune competence and therefore subsequently reduce infectious complications.
Materials and Methods
Patient Recruitment
Elective orthopedic cases with potential for significant blood loss, booked to receive ICS, were consented and recruited (n = 20). Ethics approval was obtained from Royal Brisbane and Women’s Hospital (RBWH [HREC/17/QRBW/685]) and University of Queensland (2018000297).
Blood Collection and Preparation
Blood samples (10 ml each into ethylenediaminetetraacetic acid phlebotomy tubes [Becton Dickinson (BD), Oxford, England]) were collected from the patient preoperatively and from ICS product (processed and filtered) (Fig. 1). Regarding the 20 patients recruited, 1 procedure was cancelled, and in 6 cases insufficient blood loss occurred to enable cell salvage collection. ICS blood samples were therefore available for full analysis from 13 patients. Relevant procedures for the 13 patients in this study included ORIF pelvis (n = 3), spine fusion (n = 8), and complex total hip replacement (n = 2). ICS samples were transported immediately following surgery to the Australian Red Cross Lifeblood (Kelvin Grove) (couriered at room temperature).
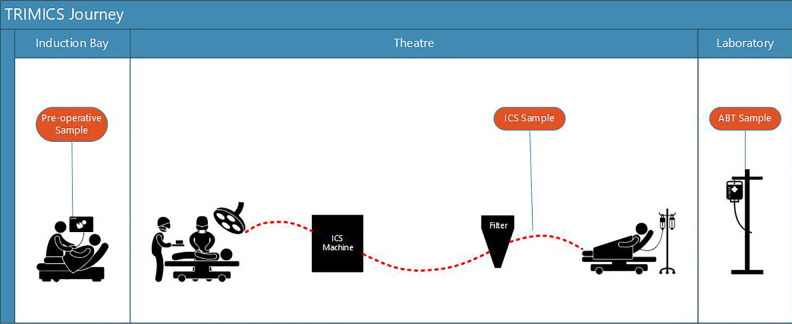
Figure 1.
TRIMICS patient journey: preoperative sample, ICS sample, and ABT sample.
ABT: allogeneic blood transfusion; ICS: intraoperative cell salvage.
Leukodepleted packed red blood cell (PRBC) units were obtained from Australian Red Cross Lifeblood. Whole blood units (450 ± 45 ml) were collected into top-and-bottom bags containing citrate phosphate dextrose (66.5 ml; Macopharma, Mouvaux, Nord, France) and processed within 24 h of collection according to standard Lifeblood protocols based on the Council of Europe Guidelines for the preparation, use, and quality assurance of blood components (Council of Europe 2008). Briefly, whole-blood units were centrifuged (3,640 × g, 10 min, 22°C) and RBCs were separated into the bottom bag containing 105 ml of saline, adenine, glucose, and mannitol storage solution using a MacoPress component extractor (Macopharma). PRBC units (n = 10, group O+, ≥220 ml RBC volume; ≤1.0 × 106 leukocytes/unit, 0.5 to 0.7 l/l hematocrit) were stored at 2 to 6°C and used in the in vitro transfusion assay within 21 days (routine storage of PRBC is 42 days)19.
Anesthesia and ICS
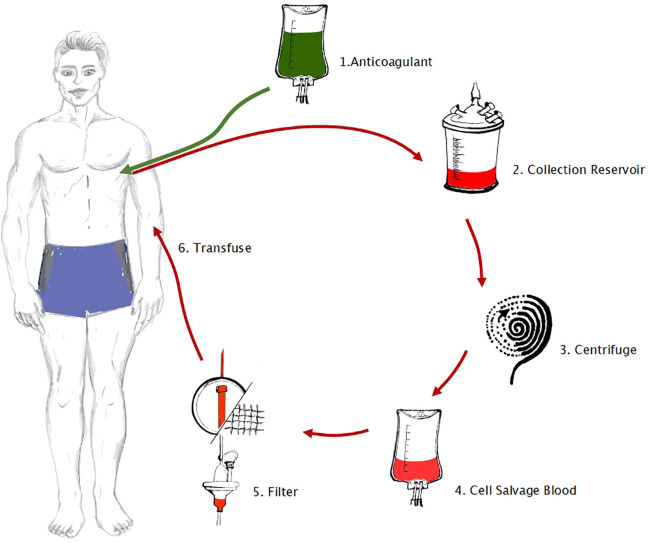
Standard anesthetic induction included propofol, fentanyl ± midazolam, muscle relaxant (rocuronium/suxamethonium/cisatracurium/vecuronium), and analgesia (±ketamine/±lignocaine/±oxycodone/± local anesthetic field block/±regional or epidural block). The XTRA autotransfusion system (ATS; LivaNova) was used according to a standard operating procedure for orthopedic cases. During the ICS technique blood was aspirated and anticoagulated from the surgical bleeding site, collected in a cardiotomy reservoir, and processed through centrifuge (Fig. 2). Processing removes unwanted aspects of shed blood, that is, debris, clot, free hemoglobin, fat globules, and so on7.
Figure 2.
The intraoperative cell savage technique: (1) anticoagulation (30,000 IU heparin sodium in 1,000 ml 0.9% NaCl), and aspiration from the surgical site (using a double-lumen suction tube to ensure anticoagulation via one lumen before aspiration occurs through the second lumen), (2) collection, (3) processing through centrifuge, (4) collection of processed salvaged blood, (5) filtering, and (6) transfusion.
The same ICS equipment, standardized for orthopedic surgery at the RBWH, was used for all 13 study cases: The Sorin Xtra ATS, relevant consumables (LivaNova), and optimal (manufacturer default settings) wash program (POPT ± PFAT). This wash cycle ensures a high level of lipid removal, considered important during orthopedic surgery (98.50% ± 0.9% to ensure Therapeutic Goods Administration [TGA] approval in Australia)20. The Xtra system includes an autotransfusion device and standard specified disposables for collection and processing of salvaged blood: a blood collection reservoir (40 µm filter), the Xtra Bowl set (225 ml), a 10-l waste bag, and 1-l primary red blood cell collection bag. ICS blood is immediately available for reinfusion and is not stored. The ICS product achieved during standard processing provides red blood cells suspended in saline and >98% removal of other cells (platelets, leukocytes), free hemoglobin, plasma, heparin, clotting factors, and unwanted debris21. By using the same default program for all studied cases, the same cycle time, bowl, similar cycle speed, and volumes, the same relative hematocrit would be achieved. A leukodepletion filter was used for all cases (Haemonetics LipiGuard SB filter [Pall]), except in one case where an Imugard III filter was used, due to surgery for malignancy. Suction pressure is kept at or below 150 mmHg. If higher suction pressure is used, this is done via a second suction catheter and the collected volume discarded. ICS product manufacturing occurred following the same standardized (default) methods across all study cases, to ensure similar removal of residual platelets, leukocytes, and free hemoglobin, as per TGA requirements.
Assessment of Immune Competence Using an In Vitro Whole-Blood Transfusion Model
A previously established in vitro whole-blood transfusion model22 was used to compare (a) monocyte- and dendritic cell-specific intracellular cytokine production, (b) monocyte- and dendritic cell-specific activation and adhesion markers, and (c) overall leukocyte response (from culture supernatant) following exposure to the patients’ own ICS or ABT (ABO-compatible allogeneic blood). The in vitro assay is based on 25% replacement volume, modeling a 2 to 3 packed red cell unit transfusion. LPS (1 µg ml−1; Sigma) was added in parallel to model a bacterial infection. The patients’ preoperative sample was incubated 1:1 v/v with RPMI (Roswell Park Memorial Institute) 1640 media (containing 2 mm l-glutamine; Gibco by Life Technologies, Australia) in 24-well plate tissue culture plates (Costar, Corning Life Sciences, Sigma, Australia) for a total of 5.5 h (37°C, 5% CO2). Wells were set up as follows: (i) preoperative patient blood, (ii) preoperative patient blood + patient’s own ICS (25% volume replacement), (iii) preoperative patient blood + ABT (25% volume replacement), (iv) preoperative patient blood + LPS, (v) preoperative patient blood + patient’s own ICS (25% volume replacement) + LPS, and (vi) preoperative patient blood + ABT (25% volume replacement) + LPS. The total volume was 1.5 ml/well with “25% replacement volume” modeled as 375 µl blood component (ABT or ICS) + 375 µl RPMI + 750 µl patient whole blood. Control wells without blood component contained 750 µl RPMI + 750 µl patient whole blood. Duplicate plates were set up with the addition of GolgiPlug (containing brefeldin-A; 1 µg ml−1; BD Biosciences) to one plate for the last 4.5 h to facilitate the detection of cytokines intracellularly.
Assessment of Dendritic Cell- and Monocyte-Specific Cytokine Production
Following 5.5 h total incubation, wells were harvested from the plate containing GolgiPlug and cells were stained with fluorescently labeled monoclonal antibodies (15 min, 22°C) to identify dendritic cell and monocyte populations (FITC Lin Cocktail, CD34-FITC, CD45-PerCP, HLA-DR-V450, CD14-V500, CD11c-APC, CD3-APCH7). Cells were then washed, red blood cells lysed (1 × FACS lyse, 10 min, 22°C), remaining leukocytes permeabilized (1 × FACS Perm 2, 10 min, 22°C) and stained for 30 min (22°C) with a panel of phycoerythrin-conjugated monoclonal antibodies (interleukin 6 [IL-6], interleukin-8 [IL-8], interleukin-10 [IL-10], interleukin-12 [IL-12], interleukin-1 alfa [IL-1α], TNF-α, macrophage inflammatory protein-1 alfa [MIP-1α], macrophage inflammatory protein-1 beta [MIP-1β], monocyte chemoattractant protein-1 [MCP-1], inducible protein-10 [IP-10]). Cells were washed and resuspended in 1 × cell fixative for assessment of intracellular cytokines by flow cytometry. All antibodies and flow cytometry reagents from BD Biosciences, Australia. Median fluorescent intensity (MFI) was used to analyze changes in cytokines and chemokines in gated dendritic cell (Lin−, HLADR+, CD11c+) and monocyte (CD14+) populations.
Assessment of Dendritic Cell- and Monocyte-Specific Activation and Adhesion Markers
Following 5.5 h incubation, wells were harvested from the plate without GolgiPlug. Well contents were centrifuged and supernatants removed and stored at −80°C for later assessment of overall leukocyte response (see below). Cell pellets were stained with fluorescently labeled monoclonal antibodies (15 min, 22°C) to identify dendritic cell and monocyte populations (FITC Lin Cocktail, CD34-FITC, CD45-PerCP, HLA-DR-V450, CD14-V500, CD11c-APC, CD3-APCH7). Cells were then washed, red blood cells lysed (1 × FACS lyse, 10 min, 22°C) and remaining leukocytes stained for 30 min (22°C) with a panel of PE-conjugated monoclonal antibodies to detect activation and adhesion molecules (CD9, CD38, CD40, CD80, CD83, CD86). Cells were washed and resuspended in 1 × cell fixative for assessment of intracellular cytokines by flow cytometry. MFI was used to analyze changes in activation and adhesion markers in gated DC (Lin−, HLADR+, CD11c+) and monocyte (CD14+) populations.
Assessment of Overall Leukocyte Response Using Cytometric Bead Array
Cytometric bead array (CBA) is a flow cytometry-based immunoassay that allows the quantification of multiple analytes simultaneously. CBA was used to measure the overall leukocyte response from the culture supernatant of the in vitro assay, according to the manufacturer instructions (BD Biosciences) with slight modification. Briefly, 25 µl of culture supernatant and capture beads were mixed and incubated (1 h, 22°C), followed by the addition of matched PE detection reagent for 2 h (22°C). Beads were then washed with assay wash buffer, centrifuged (200 × g, 5 min) and resuspended in wash buffer for flow cytometric analysis. The panel of inflammatory mediators quantified were interleukin-4 (IL-4), IL-6, IL-8, IL-10, IL-12, interleukin-1 alfa (IL-1 α), interleukin-1 beta (IL-1β), TNF-α, MIP-1α, MCP-1, IP-10, interferon alfa (IFN-α), and interferon gamma (IFN-γ). Unknown sample concentrations were interpolated from standard curves ran in parallel.
Flow Cytometry
MFI was used to assess changes in monocyte and dendritic cell activation and adhesion molecules and production of cytokines. A three-laser FACSCanto II flow cytometer was used for all acquisition. FACSDiva was used to analyze MFI of intracellular cytokines and surface molecules. FCAP Array was used to analyze CBA. All flow cytometry equipment and analysis software were from BD Biosciences.
Statistical Analysis
A repeated-measure one-way analysis of variance (ANOVA) with Tukey’s post hoc test was used for analyses of dendritic cell and monocyte cytokine production, co-stimulatory and surface markers, and the overall inflammatory response. Responses were compared across the three groups: (1) preoperative sample, (2) preoperative sample + ABT, (3) preoperative sample + ICS (ANOVA P < 0.05 as statistically significant, Tukey’s post hoc test indicated as *P < 0.05, **P < 0.01, ***P < 0.001, GraphPad Prism 7, GraphPad software). Sample size was calculated for a continuous outcome with two independent groups based on a 5% difference between groups, three standard deviations, and 90% power. Based on these parameters a minimum sample size of 7.56 was required.
Trial Registration
Australian and New Zealand Clinical Trials Registry (ACTRN12618001459213, registered 30/8/2018)23.
Results
General Demographics
The study group consisted of 60% male (n = 12) and 40% female (n = 8). The average age was 51.05 years on the first day of surgery.
ICS Improved Dendritic Cell Immune Competence
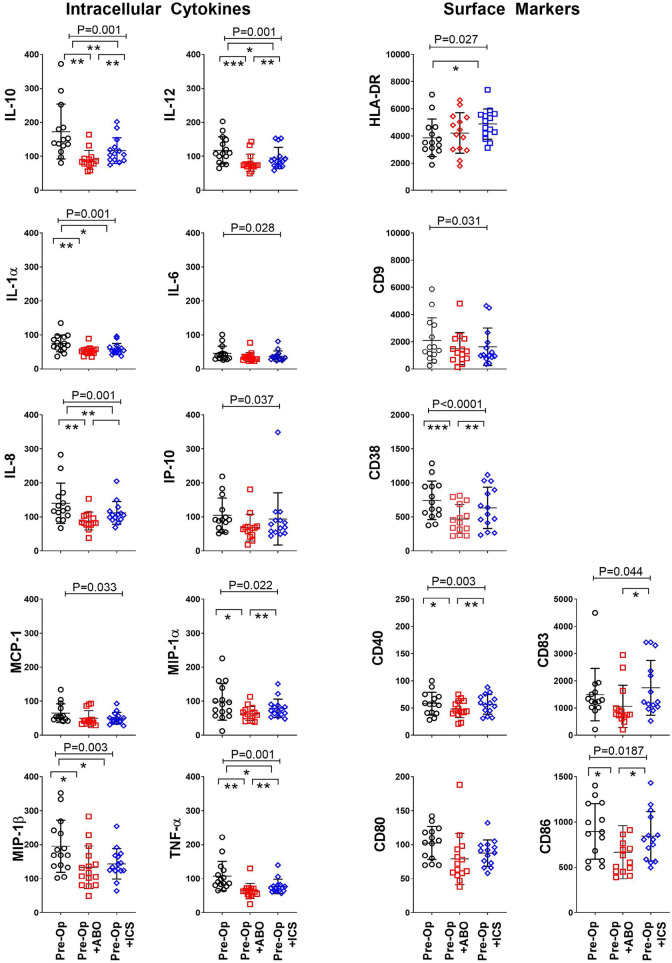
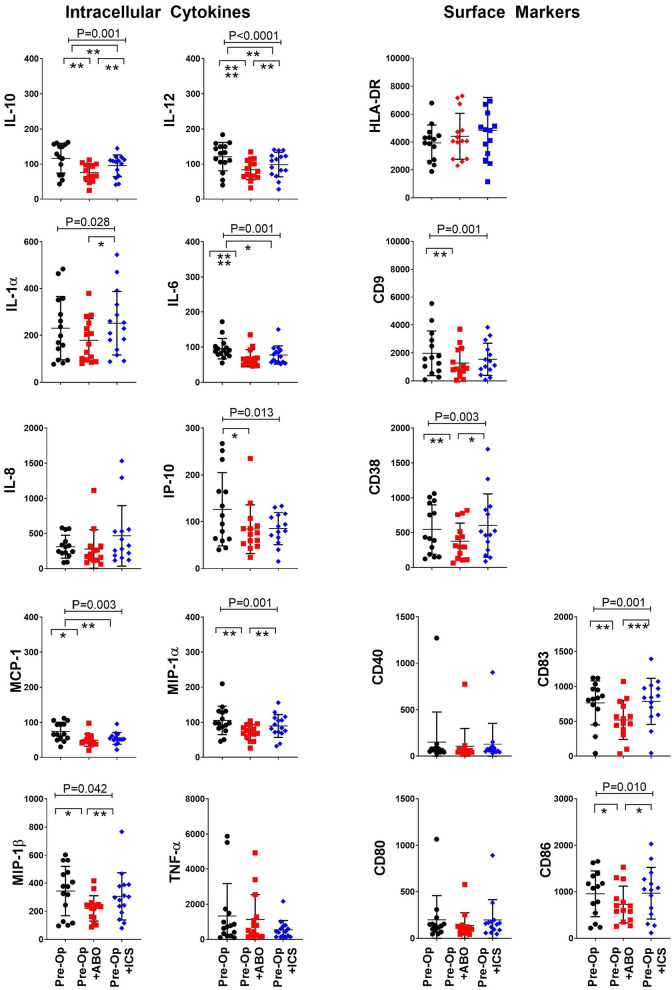
Dendritic cells are important regulatory immune cells at the interface of the innate and adaptive immune system. The ability of dendritic cells to produce cytokines and express activation and co-stimulatory markers confirms immune competence24. Dendritic cell immune profile was assessed following exposure of the patient’s preoperative blood to ABT or their own ICS. Exposure to either ABT or ICS was associated with suppression of dendritic cell IL-10 (P < 0.001), IL-12 (P < 0.001), IL-1α (P < 0.001), IL-6 (P = 0.028), IL-8 (P < 0.001), IP-10 (P = 0.037), MCP-1 (P = 0.033), MIP-1α (P = 0.022), MIP-1b (P = 0.003), and TNF-α (P < 0.001) (Fig. 3). Suppression of dendritic cell IL-10, IL-12, IL-8, MIP-1α, and TNF-α was ameliorated by ICS compared to ABT ([P < 0.05], Tukey’s post hoc test) (Fig. 3). Immune suppression is indicated when dendritic cell co-stimulatory and adhesion molecule expression is impaired25. Exposure to ABT and ICS increased expression of HLA-DR (P = 0.027) but reduced expression of dendritic cell adhesion CD9 (P = 0.031), CD38 (P < 0.001) and activation CD40 (P = 0.003), CD83 (P = 0.044), and CD86 (P = 0.019) molecules (Fig. 3). The expressions of CD38, CD40, CD83, and CD86 were significantly improved following exposure to ICS compared to ABT (P < 0.05, Tukey’s post hoc test) (Fig. 3). Together, the improved expression of important dendritic cell surface activation and co-stimulatory markers combined with improved cytokine production provides evidence that transfusion with ICS instead of ABT is associated with improved immune competence.
Figure 3.
ICS reduced transfusion-related immune modulation of dendritic cells. Dendritic cell intracellular cytokine production and expression of surface activation and adhesion molecules following exposure to standard allogenic blood or autologous ICS. X axis: in vitro “transfusion” conditions, preoperative sample (Pre-Op, open black circles), preoperative sample + ABO-compatible allogeneic blood (Pre-Op + ABO, open red squares), and preoperative sample + their own cell salvage blood (Pre-Op + ICS, open blue diamonds). Y axis: median fluorescent intensity of indicated cytokine or surface marker in gated mDC (Lin−, HLADR+, CD11c+). Analysis of variance indicated by bar and P-value with Tukey’s post-test indicated by *P < 0.05, **P < 0.01, ***P < 0.001.
HLA-DR: human leukocyte antigen DR phenotype; ICS: intraoperative cell salvage; IL: interleukin; MCP-1: monocyte chemoattractant protein-1; MIP-1α: macrophage inflammatory protein-1 alfa; TNF-α: tumor necrosis factor alfa.
ICS Improved Monocyte Immune Competence
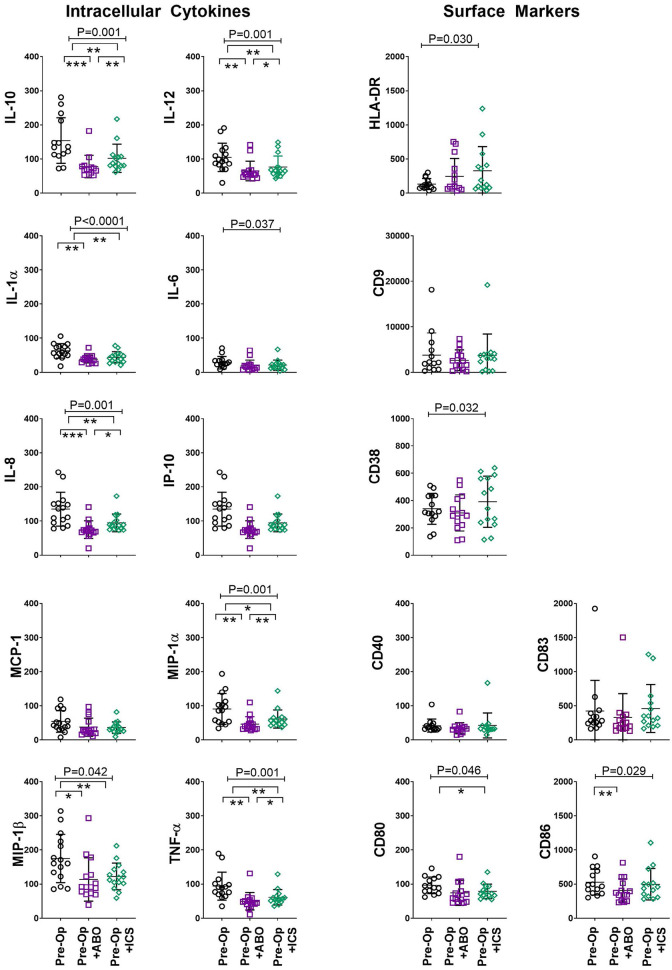
Monocytes are critical for pathogen recognition and clearance. Reduced monocyte function is associated with increased risk to develop infection-related adverse outcomes13. In our study, exposure to ABT and ICS was associated with suppression of monocyte IL-10 (P < 0.001), IL-12 (P < 0.001), IL-1α (P < 0.001), IL-6 (P = 0.037), IL-8 (P < 0.001), MIP-1α (P = 0.001), MIP-1β (P = 0.003), and TNF-α (P < 0.001) production. Compared to ABT, ICS improved monocyte production of IL-10, IL-12, IL-8, MIP-1α, and TNF-α was evident (Tukey’s post hoc test, P < 0.05, Fig. 4).
Figure 4.
ICS reduced transfusion-related immune modulation of monocytes. Monocyte intracellular cytokine production and expression of surface activation and adhesion molecules following exposure to standard allogenic blood or autologous ICS. X axis: in vitro “transfusion” conditions, preoperative sample (Pre-Op, open black circles), preoperative sample + ABO-compatible allogeneic blood (Pre-Op + ABO, open purple squares), and preoperative sample + their own cell salvage blood (Pre-Op + ICS, open green diamonds). Y axis: median fluorescent intensity of indicated cytokine or surface marker in gated monocytes (CD14+). Analysis of variance indicated by bar and P-value with Tukey’s post-test indicated by *P < 0.05, **P < 0.01, ***P < 0.001.
HLA-DR: human leukocyte antigen DR phenotype; ICS: intraoperative cell salvage; IL: interleukin; MCP-1: monocyte chemoattractant protein-1; MIP-1α: macrophage inflammatory protein-1 alfa; TNF-α: tumor necrosis factor alfa.
Assessment of monocyte HLA-DR expression is important to determine monocyte function in numerous clinical settings26. Exposure to ABT and ICS was associated with increased expression of HLA-DR on monocytes (P = 0.030, Fig. 4). However, monocyte expression of other important adhesion molecule CD38 (P = 0.032) and activation molecules (CD80 [P = 0.046], CD86 [P = 0.030]) were suppressed following exposure to ABT and ICS (Fig. 4). These results demonstrate that assessment of HLA-DR alone does not confirm monocyte function, but also the assessment of a panel of activation markers as well as cytokine production may be required.
ICS Reduced Dendritic Cell Immune Paralysis Associated With ABT
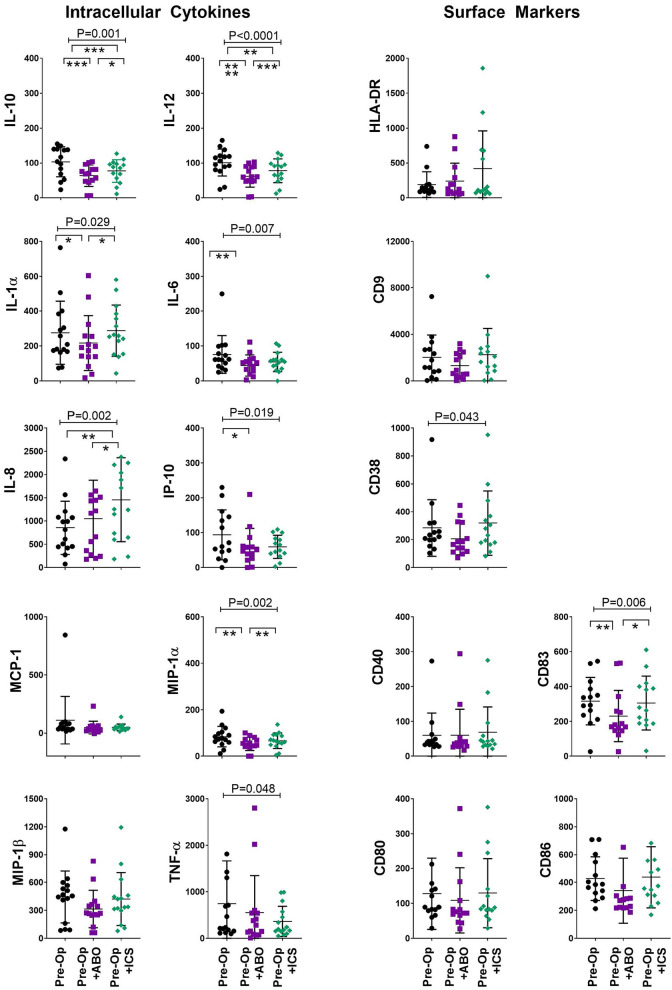
The ability of myeloid dendritic cells to produce intracellular cytokines in response to LPS exposure is another measure of immune competence22. We added LPS to in vitro cultures to assess immune competence following exposure to ABT and ICS alternatively, to model bacterial infection. Impaired production of dendritic cell IL-10 (P < 0.001), IL-12 (P < 0.0001), IL-1α (P = 0.028), IL-6 (P = 0.001), IP-10 (P = 0.013), MCP-1 (P = 0.003), MIP-1α, and MIP-1β (P = 0.042) was seen following exposure to ABT and ICS (Fig. 5). However, the transfusion-associated suppression was significantly improved considering production of IL-10, IL-12, IL-1α, MIP-1α, and MIP-1β following ICS compared to ABT (P < 0.05, Tukey’s post hoc test, Fig. 5).
Figure 5.
ICS reduced transfusion-related immune modulation of dendritic cells in a model of bacterial infection. Dendritic cell intracellular cytokine production and expression of surface activation and adhesion molecules following exposure to standard allogenic blood or autologous ICS. X axis: in vitro “transfusion” conditions, preoperative sample (Pre-Op, closed black circles), preoperative sample + ABO-compatible allogeneic blood (Pre-Op + ABO, closed red squares), and preoperative sample + their own cell salvage blood (Pre-Op + ICS, closed blue diamonds). Y axis: median fluorescent intensity of indicated cytokine or surface marker in gated dendritic cells (Lin−, HLADR+, CD11c+). Analysis of variance indicated by bar and P-value with Tukey’s post-test indicated by *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
HLA-DR: human leukocyte antigen DR phenotype; ICS: intraoperative cell salvage; IL: interleukin; MCP-1: monocyte chemoattractant protein-1; MIP-1α: macrophage inflammatory protein-1 alfa; TNF-α: tumor necrosis factor alfa.
In addition to production of proinflammatory cytokines such as TNF-α and IL-12, dendritic cell maturation is associated with upregulation of CD80 and CD86 expression27. The inability of dendritic cells to respond with an adequate expression of co-stimulatory and adhesion molecules when exposed to LPS suggests immune paralysis13. Surprisingly, we found exposure to ABT and ICS had no significant impact on expression of HLA-DR in our model of bacterial infection. However, exposure to ABT and ICS was associated with reduced expression of CD9 (P = 0.001), CD38 (P = 0.003), CD83 (P = 0.001), and CD86 (P = 0.10) in the infection model (Fig. 5). Even though this reduction occurred following both ABT and ICS, exposure to ICS demonstrated an improved immune response. In the bacterial infection model, dendritic cell expression of CD38, CD83, and CD86 was comparable between the preoperative and ICS groups (P < 0.05, Tukey’s post hoc test, Fig. 5). In combination the improved expression of important surface activation molecules and inflammatory response in the presence of ICS provide evidence that immune paralysis associated with transfusion of ABT can be overcome by using ICS.
ICS Reduced Monocyte Immune Paralysis Associated With ABT
The capacity of monocytes to produce intracellular cytokines in response to endotoxin exposure is important to ensure an adequate defense against infection13. In our model of bacterial infection, exposure to ABT and ICS impaired monocyte production of IL-10 (P = 0.001), IL-12 (P < 0.001), IL-1α (P = 0.029), IL-6 (P = 0.007), IP-10 (P = 0.019), MIP-1α (P = 0.002), and TNF-α (P = 0.048, Fig. 6). These results suggest that exposure to ABT and ICS negatively impacts on the patient’s ability to respond against infection. However, we provide evidence that ICS improves the capacity to respond to bacterial infection considering that suppression of monocyte IL-10, IL-12, IL-1α, and MIP-1α was ameliorated by ICS (P < 0.05, Tukey’s post hoc test, Fig. 6).
Figure 6.
ICS reduced transfusion-related immune modulation of monocytes in a model of bacterial infection. Monocyte intracellular cytokine production and expression of surface activation and adhesion molecules following exposure to standard allogenic blood or autologous ICS. X axis: in vitro “transfusion” conditions, preoperative sample (Pre-Op, closed black circles), preoperative sample + ABO-compatible allogeneic blood (Pre-Op + ABO, closed purple squares), and preoperative sample + their own cell salvage blood (Pre-Op + ICS, closed green diamonds). Y axis: median fluorescent intensity of indicated cytokine or surface marker in gated monocytes (CD14+). Analysis of variance indicated by bar and P-value with Tukey’s post-test indicated by *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
HLA-DR: human leukocyte antigen DR phenotype; ICS: intraoperative cell salvage; IL: interleukin; MCP-1: monocyte chemoattractant protein-1; MIP-1α: macrophage inflammatory protein-1 alfa; TNF-α: tumor necrosis factor alfa.
The inability of monocytes to respond with an adequate expression of co-stimulatory and adhesion molecules when exposed to LPS suggests immune paralysis26. As observed for dendritic cells, we found exposure to ABT and ICS had no significant impact on monocyte expression of HLA-DR in our model of bacterial infection. Expression of monocyte CD38 and CD83 was reduced following exposure to ABT and ICS (Fig. 6). Compared to ABT, exposure to ICS was associated with improved expression of CD83 (P < 0.05, Tukey’s post hoc test, Fig. 6). The improved capacity of monocytes to generate an inflammatory response and upregulate cell activation markers following ICS compared to ABT in our bacterial model demonstrates a reduction in immune paralysis following ICS.
ICS Had Minimal Impact on the Overall Leukocyte Response
The overall leukocyte response from ex vivo cultured blood was used to assess cytokine response from all cell subsets. Exposure to ABT and ICS was associated with increased levels of IP-10 (P < 0.0001) and IFN-γ (P = 0.049, Fig. 7). For all cytokines measured, there was no difference in the overall response following exposure to ABT versus ICS. Exposure to ABT and ICS, in the LPS model, was associated with reduced expression of IL-1α (P = 0.0146), TNF-α (P = 0.021), IL-1β (P = 0.043), and increased expression of IL-8 (P = 0.001), MCP-1 (P = 0.0451), and IL-10 (P = 0.058). In the presence of LPS, ICS was associated with increased IL-8 expression but decreased IL-1α expression compared to ABT. For the remaining cytokines there was little difference in expression following exposure to ABT or ICS. IL-4, IL-12, and IFN-α were at the limits of detection.
Figure 7.
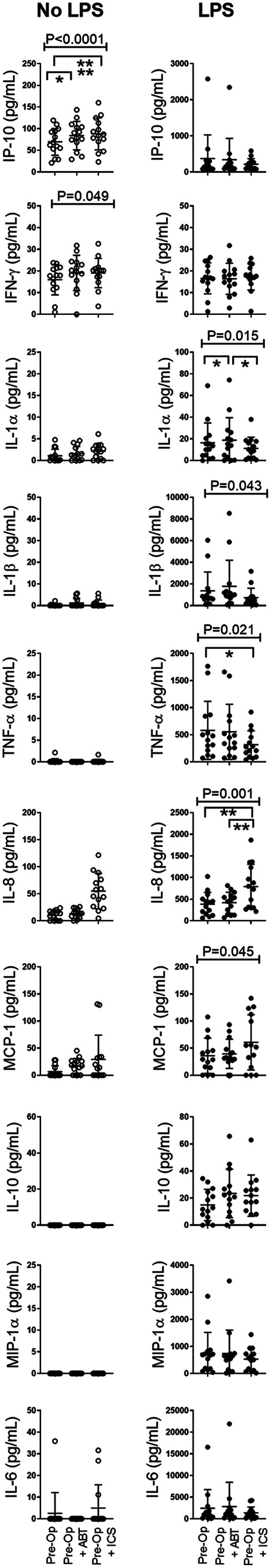
ICS had minimal impact on overall leukocyte inflammatory response. Inflammatory markers were quantified from supernatants of in vitro model. X axis: in vitro “transfusion” conditions, preoperative sample (Pre-Op), preoperative sample + ABO-compatible allogeneic blood (Pre-Op + ABO), and preoperative sample + their own cell salvage blood (Pre-Op + ICS). Left panel: no LPS data (open black circles). Right panel: +LPS (bacterial infection model, closed black circles). Y axis: level of indicated cytokine (pg/ml). Analysis of variance indicated by bar and P-value with Tukey’s post-test indicated by *P < 0.05, **P < 0.01, ***P < 0.001.
ICS: intraoperative cell salvage; IFN-γ: interferon gamma; IL: interleukin; LPS: lipopolysaccharide; MCP-1: monocyte chemoattractant protein-1; MIP-1α: macrophage inflammatory protein-1 alfa; TNF-α: tumor necrosis factor alfa.
Discussion
ICS is a safe autologous alternative to ABT15. Considering that the immune consequences of “foreign” blood transfusion are postulated as a mechanism of TRIM4, this novel study characterizes immune modulation following ABT and ICS. Our results suggest there is potential to reduce immune modulation (suppression) by using ICS. Dendritic cells play a unique and specialized role positioned between the innate and adaptive immune responses12. The normal protective human immune response depends on the ability of dendritic cells to produce cytokines28. The functional impairment of cytokine production following surgery, trauma, or transfusion represents the downregulation of the immune system and may lead to increased susceptibility to infection and worsened outcomes29,30. The results from this study confirmed that intracellular dendritic cell production of IL-12, IL-1α, IL-6, IL-8, IP-10, MCP-1, MIP-1α, MIP-1β, TNF-α, and IL-10 was modulated by ABT and ICS. Following ICS, compared to ABT, an improved immune competence was evident as reflected by the increased production of these cytokines. The observed modulation of IL-6 and IL-1α production from dendritic cells confirmed an improved response following ICS. IL-8 is produced in response to inflammatory stimuli and plays a role in chemotactic and stimulatory activity within the immune system31. In our study, improved dendritic cell production of IL-8, IP-10, MCP-1, MIP-1α, and MIP-1β in the ICS group suggests an improved chemotactic ability. IL-10, as regulatory mediator, holds important pro-inflammatory and anti-inflammatory functions and ensures the activation of various immune pathways14. In our study, dendritic cell production of IL-10 was reduced in the ABT group but relatively improved in the ICS group, suggesting improved immune mediation following ICS. Dendritic cells are the first producers of IL-12 in response to pathogens during infections and regulates T-lymphocyte and natural killer cell responses and the production of IFN-γ32. The impaired ability to produce IL-12 (an essential central mediator between the innate and adaptive immune responses) increases a patient’s susceptibility to develop postoperative sepsis33. Our study identified a significant increase in the dendritic and monocyte production of IL-12, following ICS compared to ABT. In our study, dendritic cell TNF-α production (important to ensure T-lymphocyte, macrophage, and proinflammatory response in sepsis)30,34 was reduced following ABT and comparably increased following ICS. Co-stimulatory and adhesion molecule expression on dendritic cells triggers intracellular signals responsible for the regulation of antigen presentation12,35. In this study, the improved ability of dendritic cells to express CD9, CD38, CD40, CD83, and CD86 following ICS compared to ABT was confirmed. Dendritic cell HLA-DR expression was increased following both ABT and ICS, with the most significant improvement following ICS.
Changes to dendritic cell response following LPS stimulation may represent the downregulation of the immune system29. The increased dendritic cell IL-6, IL-1α, and IL-10 production following ICS suggests improved pro-inflammatory systemic activation and regulation, compared to ABT. ABT exposure reduced dendritic cell IL-12 production, and significantly improved following ICS. In addition, improved chemotactic ability of dendritic cells (i.e., superior production of MIP-1β) was seen following ICS versus ABT. Dendritic cell maturation leads to release of TNF-α, followed by surface remodeling and increased CD80 and CD86 co-stimulatory molecule expression, to generate a functional T-lymphocyte response30. When dendritic cells are activated by LPS, co-stimulatory molecules and HLA-DR are upregulated30,36. Downregulation of HLA-DR early postoperatively is associated with a significant increased risk of sepsis (odds ratio 2.9)37 and increased mortality33,38. The expression of dendritic cell co-stimulatory and adhesion molecules ensures activation and regulation of various pathways within the adaptive immune response39. In our study, expression of dendritic cell CD9, CD38, and CD83 improved following ICS, suggesting improved activation, cell migration, antigen presentation, and T-lymphocyte regulation.
Pro-inflammatory and anti-inflammatory monocyte responses are essential to ensure immune homeostasis following surgery14. This study results confirmed that intracellular monocyte production of IL-6, IL-1α, TNF-α, IL-10, IL-12, IL-8, MIP-1α, and MIP-1β was modulated by ABT and ICS. The monocyte production ability of IL-6, TNF-α, and Il-1α following ICS suggested improved pro-inflammatory competency. IL-10 is produced by circulating monocytes, and other sources, for example, other macrophages, lymphocytes in the liver, lung, kidney, myocardium, and brain14. Il-10 is important as a potent inhibitor of pro-inflammatory cytokines. In our study, monocyte Il-10 production was reduced flowing ABT but relatively improved following ICS. Our study identified a significant increase in the monocyte IL-12 and IL-8 production, following ICS versus ABT. An improved monocyte production of MIP-1α and MIP-1β (essential chemokines)22 and expression of HLA-DR, CD38, CD80, and CD86 followed ICS, suggesting superior activation and regulation.
The increased monocyte IL-6 and IL-1α production following LPS stimulation suggests improved pro-inflammatory systemic activation30 evident following ICS versus ABT. Impaired monocyte HLA-DR expression and inability to produce TNF-α in response to LPS exposure is referred to as immunoparalysis13,14 and leads to a susceptibility to develop postoperative infection40, worse outcomes, and increased mortality13,25,26. During our infection model, reduced TNF-α production followed ABT and ICS. IL-10 production was reduced following ABT and relatively improved following ICS, confirming an improved regulatory ability following ICS. In addition, the improved expression of monocyte CD83 seen following ICS may predict an associated postoperative adverse outcome (infection-related) reduction.
Many laboratory-based TRIM studies assessed changes in cytokine levels in patient plasma or supernatants from in vitro models14,41. Although our study focused on specific dendritic cell and monocyte responses, to facilitate comparison with previous studies, we also assessed changes in overall leukocyte response following exposure to ABT and ICS using an in vitro model. Our panel focused on inflammatory markers associated with early-phase immune responses. In the absence of LPS, minimal modulation of the overall immune response occurred. Exposure to ABT and ICS resulted in increased IP-10 and decreased IFN-γ, with no difference in response dependent on ABS or ICS. The impaired production of TNF-α and IL-1β that occurs after in vitro LPS stimulation is associated with worse outcomes in patients with sepsis13,42. In line with previous publications13, in vitro LPS stimulation concurrent with ABT exposure was associated with suppression of IL-1α, TNF-α, and IL-1β and increased production of IL-8, MCP-1, and IL-10. For the majority of cytokines studied (during overall leukocyte production) there was no difference in response to LPS following exposure to ICS versus ABT; however, ICS was associated with increased production of chemokine IL-8 and reduced production of pro-inflammatory cytokines IL-1α and TNF-α. A reduction in overall TNF-α production capacity is associated with increased infection-related postoperative complications, Staphylococcus aureus co-infection, and longer intensive care unit stay41,43–45. Understanding differences in specific immune pathways leading to differences in expression of TNF-α, IL-1α, and IL-8 is important and worthy of further study. This trio of cytokines are produced in large quantities by neutrophils—the most numerous leukocyte in peripheral blood. Therefore, the results of this study suggest that specific analysis of neutrophil responses would be warranted to further understand mechanisms associated with immune competence following transfusion with ABT and ICS. It is important to note that assessing supernatants from ex vivo stimulation of peripheral blood cells is not truly representative of the complexity of the biology associated with changes in cytokine levels in patient plasma.
Conclusion
This study provides evidence of a different immune profile and improved immune competence following transfusion of ICS versus ABT. Despite the clinical evidence of the association between transfusion and immune modulation, the precise mechanism(s) remain largely undefined. TRIM is likely multifactorial and many adverse outcomes may be a consequence of other confounding factors such as complex surgery and/or patient comorbidities. As there is no consensus on the definition of TRIM and in the absence of specific diagnostic tests to define it, the importance of TRIM is subject of ongoing debate. The in vitro evidence provided in this study is essential to support the benefits of ICS as an alternative to ABT. Rather than proving the presence of TRIM, we provide evidence that the use of ICS as an alternative to ABT may improve immune competence and subsequently reduce infection-related adverse outcomes. In addition, this evidence may provide a method of testing to use while studying the link between immune consequences seen in clinical and in vitro studies. The results support our hypothesis that adverse outcomes may be reduced by using ICS instead of ABT, due to improved immune competence following transfusion. Considering >800,000 red blood cell units are transfused in Australia per year46, the value of ICS as an alternative to ABT to improve immune competence during surgery may be substantial.
Key Messages
Dendritic cell and monocyte immune competence is improved following ICS compared to ABT.
Considering the complexity of the immune system, it is important to study specific immune cell responses in vitro in addition to plasma levels of cytokines to clarify immunological changes that occur during TRIM.
Acknowledgments
Royal Brisbane and Women’s Hospital intraoperative cell savage group and research nurses. Australian governments fund Australian Red Cross Lifeblood to provide blood, blood products, and services to the Australian community.
Trial Registration
ACTRN12618001459213. Registered 30/8/2018. http://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=374841
Ethical Approval: Ethical approval was obtained from Royal Brisbane and Women’s Hospital Human Research Ethics Committee (chair Allison Sutherland, RBWH (HREC/17/QRBW/685)) and University of Queensland.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the Royal Brisbane and Women’s Hospital Human Research Ethics Committee (chair Allison Sutherland) (RBWH [HREC/17/QRBW/685]); and University of Queensland Office of Research Ethics, University if Queensland (chair Chris Rose’Meyer [2018000297]) approved protocols.
Statement of Informed Consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Study recruitment conducted within the Royal Brisbane and Women’s hospital (RBWH), Herston, Brisbane, Queensland, Australia. Grant funding received (details in the support statement). Sample analysis at Australian Red Cross Lifeblood, Herston, Brisbane, Queensland, Australia (details in the support statement).
This study was conducted within the Royal Brisbane and Women’s hospital (RBWH, Herston, Brisbane, Queensland, Australia). Patient recruitment and sample collection were supported by the intraoperative cell savage group, the research nursing staff, and staff specialist anesthetists within the anesthetic department at the RBWH through funding received from the grants mentioned below.
Sample analysis occurred at the Australian Red Cross Blood Service (Herston, Brisbane, Queensland, Australia), who supported the equipment, facilities, and staff, funded in part by the grant below and in part in kind.
The author(s) disclosed receipt of the following financial support for the research: PhD scholarship grant support (grant number PSc01) for Dr Michelle Roets from the Australian National Blood Authority (Lyneham, Australian Capital Territory, Australia) and administered through the University of Queensland (St Lucia, Brisbane, Queensland, Australia); and PhD scholarship and project grant funding (grant number 18/023) for Dr Michelle Roets from the Australian and New Zealand College of Anaesthetists (Melbourne, Victoria, Australia) and administered through the Royal Brisbane and Women’s Hospital and foundation (Herston, Brisbane, Queensland, Australia).
ORCID iDs: Michelle Roets  https://orcid.org/0000-0002-0882-9636
https://orcid.org/0000-0002-0882-9636
Jaisil Eldo Jos Punnasseril  https://orcid.org/0000-0001-6748-0959
https://orcid.org/0000-0001-6748-0959
References
- 1. National Blood Authority (NBA). Australian Haemovigilence Report [Internet]. 2016. In: Australian Government 2016, ed Volume Accessed June 10, 2020 https://www.blood.gov.au/document/australian-haemovigilance-report-2016-pdf2016.
- 2. Leahy MF, Hofmann A, Towler S, Trentino KM, Burrows SA, Swain SG, Hamdorf J, Gallagher T, Koay A, Geelhoed GC, Farmer SL. Improved outcomes and reduced costs associated with a health-system–wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017;57(6):1347–1358. [DOI] [PubMed] [Google Scholar]
- 3. Surgenor SD, Kramer RS, Olmstead EM, Ross CS, Sellke FW, Likosky DS, Marrin CA, Helm RE, Leavitt BJ, Morton JR, Charlesworth DC, et al. The association of perioperative red blood cell transfusions and decreased long-term survival after cardiac surgery. Anesth Analg. 2009;108(6):1741–1746. [DOI] [PubMed] [Google Scholar]
- 4. Hellings S, Blajchman MA. Transfusion-related immunosuppression. Anaesth. Intensive Care Med. 2009;10(5):231–234. [Google Scholar]
- 5. Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21(6):327–348. [DOI] [PubMed] [Google Scholar]
- 6. Taylor RW, O’Brien J, Trottier SJ, Manganaro L, Cytron M, Lesko MF, Arnzen K, Cappadoro C, Fu M, Plisco MS, Sadaka FG, et al. Red blood cell transfusions and nosocomial infections in critically ill patients. Crit Care Med. 2006;34(9):2302–2308; quiz 2309. [DOI] [PubMed] [Google Scholar]
- 7. Meybohm P, Choorapoikayil S, Wessels A, Herrmann E, Zacharowski K, Spahn DR. Washed cell salvage in surgical patients: a review and meta-analysis of prospective randomized trials under PRISMA. Medicine (Baltimore). 2016;95(31):e4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Australian Red Cross Blood Service. Transfusion Related Immune Modulation [Internet]. In: National Blood Authority ed 2017. Accessed June 10, 2020 https://transfusion.com.au/adverse_transfusion_reactions/trim2017.
- 9. Muszynski JA, Spinella PC, Cholette JM, Acker JP, Hall MW, Juffermans NP, Kelly DP, Blumberg N, Nicol K, Liedel J. Transfusion-related immunomodulation: review of the literature and implications for pediatric critical illness. Transfusion. 2017;57(1):195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vamvakas EC. Deleterious clinical effects of allogeneic blood transfusion–related immunomodulationfact or fiction? an update through 2005. Pathol Patt Rev. 2006;126(suppl_1): S71–S85. [Google Scholar]
- 11. Innerhofer P, Klingler A, Klimmer C, Fries D, Nussbaumer W. Risk for postoperative infection after transfusion of white blood cell-filtered allogeneic or autologous blood components in orthopedic patients undergoing primary arthroplasty. Transfusion. 2005;45(1):103–110. [DOI] [PubMed] [Google Scholar]
- 12. Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30(1):1–22. [DOI] [PubMed] [Google Scholar]
- 13. Poehlmann H, Schefold JC, Zuckermann-Becker H, Volk HD, Meisel C. Phenotype changes and impaired function of dendritic cell subsets in patients with sepsis: a prospective observational analysis. Crit care. 2009;13(4):R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allen LM, Hoschtitzky AJ, Peters JM, Elliott JM, Goldman JA, James JI, Klein JN. Interleukin-10 and its role in clinical immunoparalysis following pediatric cardiac surgery. Crit Care Med. 2006;34(10):2658–2665. [DOI] [PubMed] [Google Scholar]
- 15. Carless PA, Henry DA, Moxey AJ, O’Connell D, Brown T, Fergusson DA. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2010;(4):Cd001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shander A, Hofmann A, Gombotz H, Theusinger OM, Spahn DR. Estimating the cost of blood: past, present, and future directions. Best Pract Res Clin Anaesthesiol. 2007;21(2):271–289. [DOI] [PubMed] [Google Scholar]
- 17. Heiss MM, Fasol-Merten K, Allgayer H, Strohlein MA, Tarabichi A, Wallner S, Eissner HI, Jauch KW, Schildberg FW. Influence of autologous blood transfusion on natural killer and lymphokine-activated killer cell activities in cancer surgery. Vox Sang. 1997;73(4):237–245. [DOI] [PubMed] [Google Scholar]
- 18. Biedler AE, Schneider SO, Seyfert U, Rensing H, Grenner S, Girndt M, Bauer I, Bauer M. Impact of alloantigens and storage-associated factors on stimulated cytokine response in an in vitro model of blood transfusion. Anesthesiology. 2002;97(5):1102–1109. [DOI] [PubMed] [Google Scholar]
- 19. Council of Europe. Guide to the Preparation, Use and Quality Assurance of Blood Components. 14th ed Council of Europe Publishing; 2008. [Google Scholar]
- 20. Seyfried TF, Gruber M, Breu A, Aumeier C, Zech N, Hansen E. Fat removal during cell salvage: an optimized program for a discontinuous autotransfusion device. Transfusion. 2016;56(1):153–159. [DOI] [PubMed] [Google Scholar]
- 21. Ashworth A, Klein A. Cell salvage as part of a blood conservation strategy in anaesthesia. Br J Anaesth. 2010;105(4):401–416. [DOI] [PubMed] [Google Scholar]
- 22. Perros AJ, Christensen A-M, Flower RL, Dean MM. Soluble mediators in platelet concentrates modulate dendritic cell inflammatory responses in an experimental model of transfusion. J Interferon Cytokine Res. 2015;35(10):821–830. [DOI] [PubMed] [Google Scholar]
- 23. Roets M. Does transfusion-related immune modulation occur following intraoperative cell salvage: A pilot study [Internet]. ANZCTR trials registry 2018. Accessed June 10, 2020 http://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=3748412018.
- 24. Perros A, Esguerra-Lallen A, Rooks K, Chong F, Engkilde-Pedersen S, Faddy H, Hewlett E, Naidoo R, Tung J, Fraser J, Tesar P, et al. Coronary artery bypass grafting is associated with immunoparalysis of monocytes and dendritic cells. J Cell Mol Med. 2020;24(8):4791–4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Monneret G, Lepape A, Voirin N, Bohé J, Venet F, Debard A-L, Thizy H, Bienvenu J, Gueyffier F, Vanhems P. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006;32(8):1175–1183. [DOI] [PubMed] [Google Scholar]
- 26. Kim OY, Monsel A, Bertrand M, Coriat P, Cavaillon J-M, Adib-Conquy M. Differential down-regulation of HLA-DR on monocyte subpopulations during systemic inflammation. Crit care. 2010;14(2):R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roghanian A. Dendritic Cells [Internet]. BSI, University of Southampton Faculty of Medicine; Accessed June 10, 2020 https://www.immunology.org/public-information/bitesized-immunology/cells/dendritic-cells2019. [Google Scholar]
- 28. Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med. 2001;163(2):316–321. [DOI] [PubMed] [Google Scholar]
- 29. Volk H-D. Immunodepression in the surgical patient and increased susceptibility to infection. Crit Care. 2002;6(4):279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fujii S-I, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199(12):1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marie C, Roman-Roman S, Rawadi G. Involvement of mitogen-activated protein kinase pathways in interleukin-8 production by human monocytes and polymorphonuclear cells stimulated with lipopolysaccharide or Mycoplasma fermentans membrane lipoproteins. Infect Immun. 1999;67(2):688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Giorgio T. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3(2):133–146. [DOI] [PubMed] [Google Scholar]
- 33. Hensler T, Heidecke CD, Hecker H, Heeg K, Bartels H, Zantl N, Wagner H, Siewert JR, Holzmann B. Increased susceptibility to postoperative sepsis in patients with impaired monocyte IL-12 production. J Immunol. 1998;161(5):2655–2659. [PubMed] [Google Scholar]
- 34. Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets--an updated view. Mediators Inflamm. 2013;2013:165974–165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6(6):476–483. [DOI] [PubMed] [Google Scholar]
- 36. Morel AS, Coulton G, Londei M. Regulation of major histocompatibility complex class II synthesis by interleukin-10. Immunology. 2002;106(2):229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Allen LM, Peters JM, Goldman JA, Elliott JM, James JI, Callard JR, Klein JN. Early postoperative monocyte deactivation predicts systemic inflammation and prolonged stay in pediatric cardiac intensive care. Crit Care Med. 2002;30(5):1140–1145. [DOI] [PubMed] [Google Scholar]
- 38. Majetschak M, Flach R, Kreuzfelder E, Jennissen V, Heukamp T, Neudeck F, Schmit-Neuerburg KP, Obertacke U, Schade FU. The extent of traumatic damage determines a graded depression of the endotoxin responsiveness of peripheral blood mononuclear cells from patients with blunt injuries. Crit Care Med. 1999;27(2):313–318. [DOI] [PubMed] [Google Scholar]
- 39. Shipkova M, Wieland E. Surface markers of lymphocyte activation and markers of cell proliferation. Clin Chim Acta. 2012;413(17-18):1338–1349. [DOI] [PubMed] [Google Scholar]
- 40. Wakefield CH, Carey PD, Foulds S, Monson JRT, Guillou PJ. Changes in major histocompatibility complex class II expression in monocytes and T cells of patients developing infection after surgery. Br J Surg. 1993;80(2):205–209. [DOI] [PubMed] [Google Scholar]
- 41. Ploder M, Pelinka L, Schmuckenschlager C, Wessner B, Ankersmit HJ, Fuerst W, Redl H, Roth E, Spittler A. Lipopolysaccharide-induced tumor necrosis factor α production and not monocyte human leukocyte antigen-DR expression is correlated with survival in septic trauma patients. Shock. 2006;25(2):129–134. [DOI] [PubMed] [Google Scholar]
- 42. Blackwell TS, Christman JW. Sepsis and cytokines: current status. Br J Anaesth. 1996;77(1):110–117. [DOI] [PubMed] [Google Scholar]
- 43. Hall WM, Geyer MS, Guo KC-Y, Panoskaltsis-Mortari GA, Jouvet GP, Ferdinands GJ, Shay GD, Nateri GJ, Greathouse GK, Sullivan GR, Tran T, et al. Innate immune function and mortality in critically ill children with influenza: a multicenter study*. Crit Care Med. 2013;41(1):224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Belge K-U, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. The proinflammatory CD14+ CD16+ DR++ monocytes are a major source of TNF. J Immunol. 2002;168(7):3536–3542. [DOI] [PubMed] [Google Scholar]
- 45. Strohmeyer JC, Blume C, Meisel C, Doecke WD, Hummel M, Hoeflich C, Thiele K, Unbehaun A, Hetzer R, Volk HD. Standardized immune monitoring for the prediction of infections after cardiopulmonary bypass surgery in risk patients. Cytometry B Clin Cytom. 2003;53(1):54–62. [DOI] [PubMed] [Google Scholar]
- 46. National Blood Authority. Annual Report 2017-2018 [Internet]. Australian government; 2019. Accessed June 10, 2020 https://www.blood.gov.au/pubs/1718report/sites/default/files/publication/nba-annual-report-2017-18.pdf2019.