Abstract
Transplantation of liver organoids has been investigated as a treatment alternative to liver transplantation for chronic liver disease. Transportal approach can be considered as a method of delivering organoids to the liver. It is important to set the allowable organoid amount and verify translocation by intraportal transplantation. We first examined the transplantation tolerance and translocation of porcine fetal liver-derived allogeneic organoids using piglets. Fetal liver-derived organoids generated from the Kusabira Orange-transduced pig were transplanted to the 10-day-old piglet liver through the left branch of the portal vein. All recipients survived without any observable adverse events. In contrast, both local and main portal pressures increased transiently during transplantation. In necropsy samples, Kusabira Orange-positive donor cells were detected primarily in the target lobe of the liver and partly in other areas, including the lungs and brain. As we confirmed the transplantation allowance by porcine fetal liver-derived organoids, we performed intraportal transplantation of human-induced pluripotent stem cell (iPSC)-derived liver organoid, which we plan to use in clinical trials, and portal pressure and translocation were investigated. Human iPSC-derived liver organoids were transplanted into the same 10-day-old piglet. Portal hypertension and translocation of human iPSC-derived liver organoids to the lungs were observed in one of two transplanted animals. Translocation occurred in the piglet in which patent ductus venosus (PDV) was observed. Therefore, a 28-day-old piglet capable of surgically ligating PDV was used, and after the PDV was ligated, human iPSC-derived liver organoids with the amount of which is scheduled in clinical trials were transplanted. This procedure inhibited the translocation of human iPSC-derived liver organoids to extrahepatic sites without no portal hypertension. In conclusion, human iPSC-derived liver organoids can be safely transplanted through the portal vein. Ligation of the ductus venosus prior to transplantation was effective in inhibiting extrahepatic translocation in newborns and infants.
Keywords: stem cell therapy, organoid, translocation, portal vein, ductus venosus
Introduction
Through recent developments, it has become possible to grow stem cells, such as embryonic stem cells, induced pluripotent stem cells (iPSCs), and fetal cells1, as mini-organs, termed organoids, in culture. Organoids have been successfully generated for various organs, including the liver, which develops from a condensed tissue mass before blood perfusion. This condensed tissue mass comprises mesenchymal cells, undifferentiated vascular endothelial cells, and endodermal cells. By imitating this early event in organogenesis, we developed a culture method, in a dish, for transplantable liver organoids from mesenchymal cells, endothelial cells, and endodermal progenitors differentiated from human iPSCs2–4.
Although organoid transplantation is a promising strategy for the treatment of liver disease, the most alarming potential consequence is vascular embolism because the graft is larger than a single cell, such as a hepatocyte or a mesenchymal stem cell. Among the different forms of vascular embolism, portal vein embolism is the most serious but is also the most likely complication. In previous studies, to avoid portal vein embolism, organoids were disaggregated into a single-cell suspension before transplantation into the liver5–7. However, the clinical feasibility of organoid disaggregation is still unknown, and methods of disaggregation may result in another concern: extrahepatic distribution of donor cells.
Thus far, two studies have reported successful organoid transplantation through the portal vein. Nicolas et al. transplanted hepatocyte spheroids through the portal vein; however, large numbers of cells were found in other organs, such as the spleen, stomach, and intestines. Unfortunately, those authors did not discuss the translocation of the organoids to the lungs, heart, or brain, which should always be carefully examined8. We recently reported that human iPSC-derived liver organoid transplantation via the portal vein does not allow translocation of organoids outside the liver, whereas, in one experiment, a single-cell component of the liver organoid translocated to the lung in an adult mouse. The diameter of a human iPSC-derived organoid is 130 µm9.
It is important to determine a practical and effective method for functional organoid transplantation to the liver. In the present study, we investigated the safety of human iPSC-derived liver organoid transplantation in newborns and infants by using infantile piglets.
Materials and Methods
Animals
Transgenic pigs carrying the red fluorescent protein humanized Kusabira Orange (hKO) gene, which was originally cloned from the coral stone Fungia concinna,10 were used as donors of fetal allogeneic liver organoids. The pigs were maintained and bred at an institutional animal facility, and fetuses were obtained at day 30 of gestation. Infantile pigs (Hybrid of Landrace/Large White × Duroc, 9 to 10 days old; body weight, 1.9 to 2.5 kg, Table 1) purchased from Tokyo Laboratory Animals Co. Ltd. (Tokyo, Japan) were used as sham animals. The same pigs were used as recipients of 1.0 × 105/kg, 3.0 × 105/kg, and 9.0 × 105/kg fetal liver-derived transplanted organoids and 1.0 × 105/kg human iPSC-derived transplanted liver organoids. For human iPSC-derived liver organoid transplantation with patent ductus venosus (PDV) ligation, 28-day-old infantile pigs (Large White × Duroc strain; body weight, 6.0 to 6.8 kg, Table 1) were purchased from Tokyo Laboratory Animals Co. Ltd. and used as recipients due to the presence of PDV. Piglets are listed in Table 1.
Table 1.
Summary of Experiments.
| Groups | Type of organoids | Diameter (μm) mean ± SD | No. of transplanted organoids | Recipient pigsa sex/age/body weight (kg) | Sampling time after Tx | Immunosuppressantsb |
|---|---|---|---|---|---|---|
| Fetal liver organoid transplantation without PDV ligation | ||||||
| Shamc | No | No | 0/body | ♂/9 days/2.0 | 7 days | tac + MMF |
| Sham | No | No | 0/body | ♂/9 days/2.0 | 7 days | tac + MMF |
| Sham | No | No | 0/body | ♂/9 days/2.5 | 7 days | tac + MMF |
| Low dose | ED30 fetal liver derived | 92.7 ± 15.7 | 2.1 × 105/body | ♀/10 days/2.1 | 7 days | tac + MMF |
| Low dose | ED30 fetal liver derived | 82.6 ± 11.7 | 2.2 × 105/body | ♀/10 days/2.2 | 7 days | tac + MMF |
| Low dose | ED30 fetal liver derived | 87.5 ± 15.0 | 2.4 × 105/body | ♂/10 days/2.4 | 7 days | tac + MMF |
| Medium dose | ED30 fetal liver derived | 105.3 ± 22.4 | 6.15 × 105/body | ♀/10 days/2.1 | 7 days | tac + MMF |
| Medium dose | ED30 fetal liver derived | 86.3 ± 13.1 | 6.0 × 105/body | ♂/9 days/2.0 | 7 days | tac + MMF |
| Medium dose | ED30 fetal liver derived | 87.5 ± 15.0 | 7.05 × 105/body | ♂/10 days/2.4 | 7 days | tac + MMF |
| High dose | ED30 fetal liver derived | 105.3 ± 22.4 | 1.89 × 106/body | ♂/10 days/2.1 | 7 days | tac + MMF |
| High dose | ED30 fetal liver derived | 89.1 ± 15.1 | 1.71 × 106/body | ♂/10 days/1.9 | 7 days | tac + MMF |
| Human iPSC liver organoid transplantation without PDV ligation | ||||||
| Low dose | Human iPSC derived | 133.6 ± 22.6 | 2.1 × 105/body | ♂/10 days/2.1 | 5 min | − |
| Low dose | Human iPSC derived | 143.4 ± 20.5 | 2.15 × 105/body | ♂/10 days/2.2 | 5 min | − |
| Human iPSC liver organoid transplantation with PDV ligation | ||||||
| Assumed clinical dose | Human iPSC derived | 156.4 ± 23.2 | 2.38 × 105/body | ♂/28 days/6.8 | 5 min | − |
| Assumed clinical dose | Human iPSC derived | 123.0 ± 14.8 | 2.1 × 105/body | ♂/28 days/6.0 | 5 min | − |
| Assumed clinical dose | Human iPSC derived | 129.8 ± 16.0 | 2.1 × 105/body | ♂/28 days/6.0 | 5 min | − |
a All recipient pigs were LWD (hybrid of Landrace/Large White × Duroc) strain.
b tac (1.0 mg/day, orally), MMF (250 mg/day, orally).
c Pigs in the sham group were treated with vehicles only (saline containing heparin and Elaspol).
ED30: embryonic day 30; iPSC: induced pluripotent stem cell; MMF: mycophenolate mofetil; PDV: patent ductus venosus; tac: tacrolimus; Tx: transplantation.
Low dose: 1.0 × 105/kg; Medium dose: 3.0 × 105/kg; High dose: 9.0 × 105/kg; Assumed clinical dose: 3.5 × 104/kg.
Anesthesia and Analgesia
All surgical procedures, including the Caesarian section and organoid transplantation, were performed according to standard veterinary medicine protocols. Pigs were sedated by intramuscular injection of 0.5 mg/kg mafoprazine mesylate (Mafropane; DS Pharma Animal Health Co., Ltd., Osaka, Japan) followed by an intravenous injection of 1.5 mg/kg sodium thiopental (Ravonal; NIPRO ES Pharma). After intubation, inhalation anesthesia (2.5% to 3.0% isoflurane [FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan]) was initiated with ventilation. As an analgesic, 0.3 mg/kg of butorphanol tartrate (Vetorphale; Meiji Seika Pharma Co., Ltd., Japan) was administered intramuscularly before and after the operation.
Isolation of Fetal Liver Cells and Formation of Allogeneic Liver Organoids
Fetal cell donors were obtained by the Caesarian section of pregnant hKO pigs at 30 days of gestation. Unfractionated fetal liver cells were isolated as previously described9. Harvested fetal liver cells were suspended in 10 ml of William’s E Medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with the following: 10% fetal bovine serum (MP Biomedicals, LLC, Irvine, CA, USA), 2 mM l-glutamine (Thermo Fisher Scientific), 1% penicillin/streptomycin (Thermo Fisher Scientific), 10 mM nicotinamide (Sigma-Aldrich, St. Louis, MO, USA), 50 μM 2-ME (β-ME; Sigma-Aldrich), 260 mM l-ascorbic acid 2-phosphate sesquimagnesium salt hydrate (Sigma-Aldrich), 5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Dojindo Laboratories, Kumamoto, Japan), and 1 μg/ml human recombinant insulin expressed in yeast (FUJIFILM Wako Pure Chemical Corporation). Each liver organoid contained approximately 1000 fetal liver cells.
Formation of Human iPSC-Derived Liver Organoids
A human iPSC line (Ff-I01s04) was maintained on Laminin 511 E8-fragment-coated (iMatrix-511, kindly provided by Nippi, Incorporated, Tokyo, Japan) dishes in StemFit AK02 N culture medium (Ajinomoto, Tokyo, Japan). Then, hepatic endodermal cells, endothelial cells, and mesenchymal cells were generated, as previously described11, which was followed by the generation of human liver organoids. To generate the organoids, 900 cells/microwell at a ratio of 10:7:1 (human iPSC-hepatic endoderm cells:iPSC-endothelial cells:iPSC-mesenchymal cells) were resuspended in a mixture of endothelial cell growth medium and hepatocyte culture medium (Cambrex, Baltimore, MD, USA). The medium contained dexamethasone (0.1 mM; Sigma-Aldrich), oncostatin M (10 ng/ml; R&D Systems, Minneapolis, MN, USA), and components from a SingleQuots kit (Lonza Walkersville, Inc., Walkersville, MD, USA). Cells were then plated on a six-well Elplasia platform (co-developed by Kuraray, Tokyo, Japan). hKO knockin reporters under the expression of adeno-associated virus integration site 1 were used for live imaging analysis in vivo. After human iPSC liver organoids were cultivated for 2 days, 1.0 × 105/kg liver organoids were transplanted into 10-day-old infantile piglets without PDV ligation and 3.5 × 104/kg liver organoids were transplanted into 28-day-old infantile piglets with PDV ligation, respectively.
Surgical Procedure and Sampling
For allogeneic fetal liver organoid transplantation, 10-day-old piglets were prepared. Under general anesthesia as described above, a 20-G catheter was introduced into the external jugular vein through which daily blood samples were obtained. Then, the abdomen was incised at the midline, and the main trunk of the portal and umbilical veins was exposed. Then, an 18-G double-lumen catheter (Covidien, Dublin, Ireland) was inserted through the umbilical vein down to the left portal branch, which is the inflow vessel to the organoid transplantation target area. The target area of organoid transplantation was confirmed by injection of indocyanine green (ICG; DIAGNOGREEN, Daiichi Sankyo, Tokyo, Japan). During transplantation, one lumen of the catheter was connected to a transducer for portal vein pressure monitoring. The fetal liver-derived organoids were suspended in 20 ml of normal saline supplemented with 10 mg/kg of Elaspol (Ono Pharmaceutical Co. Ltd., Osaka, Japan) and 5 U/ml of heparin sodium (Mochida Pharmaceutical Co. Ltd., Tokyo, Japan). The liver organoid injection rate was 1 ml/min, with a 2-min interval between every injection. Before and after the transplantation procedure and at the time of sacrifice (1 week after transplantation), the portal blood pressure was measured by insertion of a 21-G indwelling needle (Terumo, Tokyo, Japan) into the main trunk of the portal vein. For immunosuppression, 1 mg/kg of tacrolimus (Prograf; Astellas Pharma Tech Co., Ltd., Toyama, Japan) was injected intramuscularly prior to the transplantation, while 1.0 mg of tacrolimus hydrate (Graceptor; Astellas Pharma Inc., Tokyo, Japan)) and 250 mg of mycophenolate mofetil (CellCept; Chugai Pharmaceutical Co. Ltd., Tokyo, Japan) were administered orally once a day for 1 week. The drug trough concentrations were calculated using the blood obtained at the time of sacrifice. In addition, recipients were administered antibiotics (enrofloxacin [Baytril; Bayer]) intramuscularly until postoperative day 3 and were fed artificial milk via a feeding tube for 1 week. The recipients were monitored carefully for 1 week by daily observation of physical condition and body weight. Blood samples were obtained from the jugular catheter for blood chemistry analysis. One week later, the recipients were anesthetized, as described above, for macroscopic observation, portal vein pressure measurement, and organ harvest.
Next, we transplanted 1.0 × 105/kg human iPSC-derived liver organoids into two 10-day-old piglets using a protocol similar to that for fetal liver-derived liver organoids. The human iPSC-derived liver organoids were suspended in 20 mL normal saline with heparin sodium and Elaspol. The liver organoid injection rate was 1 ml/min, with a 2-min interval between each injection. Five minutes after transplantation, the recipients were anesthetized so that macroscopic observation, portal pressure measurement, and organ harvest could be performed. Left portal vein pressure during transplantation, portal main trunk pressure before and after transplantation, KO fluorescence images of the liver and lungs, and immunostained images of KO animals were assessed. An Alu sequence PCR analysis was performed only in case 2, in which translocation of organoids was found in the lungs.
Moreover, we prepared 28-day-old piglets with PDV ligation for 3.5 × 104/kg (assumed clinical dose) human iPSC-derived liver organoid transplantation. After general anesthesia induction and laparotomy, the ductus venosus was transfixed using a 4-0 needle, then an 18-G double-lumen catheter was inserted through the umbilical vein down to the left portal branch, as described above.
Detection Method of hKO
The liver, heart, lungs, and brain were removed from the 10-day-old piglets 1 week after transplantation of the hKO-positive fetal liver-derived organoids. The surface, the back surface, and cross-sections obtained every 1 cm from each organ were observed with a fluorescence stereomicroscope, and the hKO-positive areas were counted. Each tissue was embedded in an optimal cutting temperature medium, snap-frozen, and then sectioned into 7-µm thick slices that were then stained with 4′,6-diamidino-2-phenylindole to observe hKO-positive tissue. The piglets transplanted with the human iPSC-derived liver organoids were sampled 5 minutes after transplantation, and the surfaces of the liver, lungs, heart, and brain as well as cross-sections obtained every 1 cm from each organ were observed with a fluorescence stereomicroscope. Each organ was formalin-fixed and paraffin-embedded. Each tissue block was sectioned to generate 7-µm thick slices, which were then subjected to hematoxylin and eosin staining and immunostaining with an anti-Kusabira Orange antibody (antimonomeric Kusabira-Orange 2 pAb PM051 M; Medical & Biological Laboratories Co., Ltd.).
DNA Extraction and Quantitative Polymerase Chain Reaction (qPCR)
DNA from whole organs was collected to determine the translocation of organoids to organs outside the liver after transplantation of human iPSC-derived liver organoids. Dissected samples of the liver, lung, kidney, spleen, brain, and heart were treated with proteinase K (Wako Pharmaceuticals, Tokyo, Japan), and genomic DNA was purified using the phenol/chloroform method. The purified genomic DNA samples were subjected to qPCR analyses. The qPCR reaction was performed using Light Cycler 480 DNA SYBR Green I Master mix by Roche (Roche, Basel, Switzerland). Target molecules were quantified using the relative quantification method according to the manufacturer’s protocol, where the ratio of the amount of target molecules to the amount of a reference molecule within the same sample was calculated. All measurements were performed in triplicate. Pig DNA levels (exonic region of the pig GAPDH) were used to normalize the relative amplification levels of DNA from human cells (human Alu)12. Genomic DNA was amplified using the following primer sequences: human Alu forward: cgaggcgggtggatcatgaggt; human Alu reverse: tctgtcgcccaggccggact; pig GAPDH forward: agtaagagcccctggaccac; and pig GAPDH reverse: cctaagcccctccccttctt.
Serological Analyses
Whole-blood samples were centrifuged for 10 min at 4 °C and 1200×g. Supernatants were collected and stored at −80 °C until further testing using a serum multiple biochemical analyzer (Fuji Drichem; Fujifilm Inc., Tokyo, Japan) to measure the aspartate transaminase, alanine aminotransaminase, ammonia, gamma-glutamyl transpeptidase (gGTP), lactate dehydrogenase (LDH), total and direct bilirubin, and albumin levels.
Study Approval
All experimental protocols were conducted with the approval of the laboratory animal ethics committee of Meiji University and the National Center for Child Health and Development, based on the Japanese Guideline for Animal Experiments of Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor and Welfare.
Statistical Analysis
All data are expressed as the mean ± SD. The Mann-Whitney U test was used for between-group comparisons. Different variables were compared using a one-way analysis of variance. Significant data were examined using a Bonferroni-Dunn multiple comparison post hoc test. In all cases, P < 0.05 was considered significant.
Results
Fetal Liver Cell-Derived Liver Organoid Transplantation in Infantile Piglets
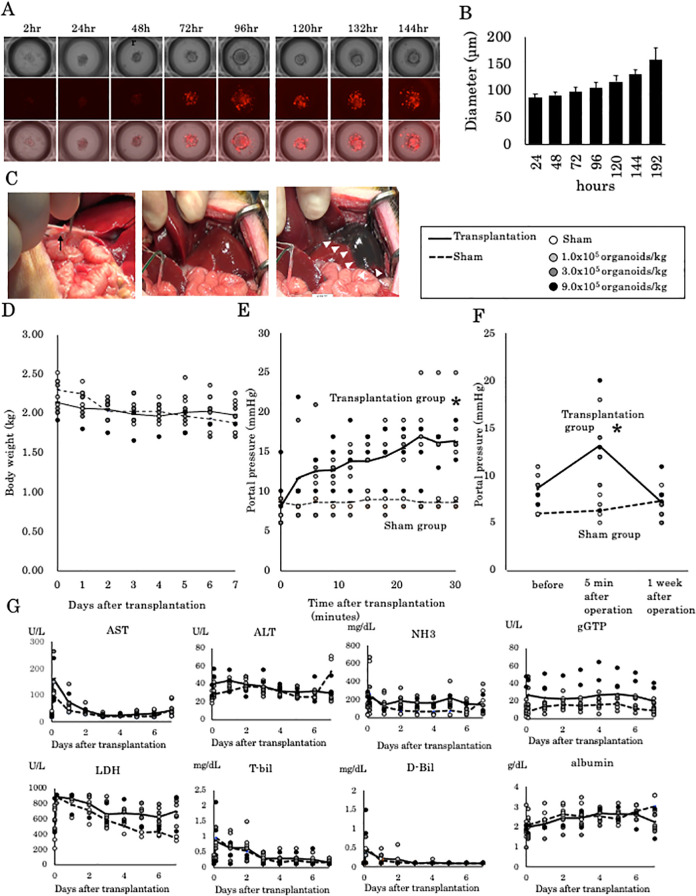
At culture initiation, a single fetal liver organoid consisted of 1000 fetal liver cells was prepared. The organoid gradually grew in size, and interestingly, hKO fluorescence was detectable as early as 72 h after culture initiation (Fig. 1A). The diameter of the organoid increased from 90 µm at 24 h to 150 µm at 192 h in a somewhat exponential manner (Fig. 1B). Since the fetal cells fused to form an organoid in 72 h and the mean diameter of the fetal liver cell-derived liver organoid becomes 100 µm, we transplanted organoids that had been cultured for 72 h. Figure 1C illustrates the insertion of an 18-G double-lumen catheter and the determination of whether the catheter outlet was placed in the correct position by injection of ICG. This experiment involved four groups of 10-day-old piglets that received transplanted cells as follows: sham group (0) and transplant groups (1.0 × 105, 3.0 × 105, and 9.0 × 105 organoids/kg, Table 1). No difference was observed in body weight between animals in the transplanted groups and animals in the sham group at 1 week after surgery (Fig. 1D). Local pressure at the outlet of the catheter placed in the left branch of the portal vein was measured during the transplantation of liver organoids (Fig. 1E). Predictably, the pressure in animals in the sham group was maintained at approximately 9 mmHg throughout the procedure, which suggests that the systemic circulation was stable under systemic anesthesia. In contrast, the pressure in animals in the transplantation groups gradually increased to 15 mmHg (range 13 to 25 mmHg). Portal pressure in the left branch of the portal vein (Fig. 1E) was significantly increased with transplantation; furthermore, no apparent correlation was observed between portal pressure and the number of transplanted cells. The main portal vein pressure was significantly increased with transplantation but normalized within 1 week after the procedure (Fig. 1F). Regarding the changes in liver function within 1 week of transplantation, gGTP and LDH levels tended to be slightly higher in the transplantation groups compared with the sham group, but no significant exacerbation was observed as a result of transplantation (Fig. 1G).
Figure 1.
Fetal liver cell-derived liver organoid transplantation in infantile piglets. (A) Liver organoid formation by hKO transgenic ED30 fetal liver cells. After 72 h of cultivation, hKO expression in the liver organoids was positive. The upper lane shows a bright-field image. The middle lane shows red fluorescence. The lower lane shows the merged images. Scale bar, 100 μm. (B) Diameter of the liver organoids. At 72 h after incubation, the diameter of the liver organoids increased to 100 µm. (C) Insertion of an 18-G double-lumen catheter into the umbilical vein. After the injection of indocyanine green, the left lobe of the liver was stained. (D) Transplantation of 1.0 × 105, 3.0 × 105, and 9.0 × 105 liver organoids/kg. One week after transplantation, no apparent weight loss was observed. (E) Portal vein pressure in the left portal branch during transplantation. *P < 0.05 versus the sham operation group. (F) Portal vein pressure in the main portal branch before, 5 min after, and 1 week after transplantation. *P < 0.05 versus the sham operation group. (G) Blood chemistry results 1 week after transplantation. hKO: humanized Kusabira Orange.
Evaluation of Extrahepatic Translocation of Fetal Liver Cell-Derived Liver Organoids
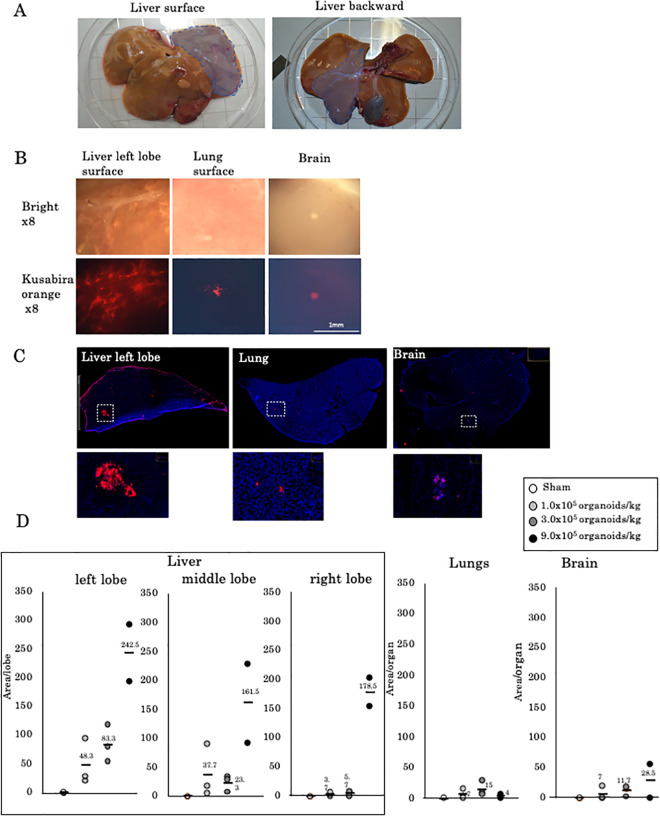
One week after surgery, the transplanted pig organs were extracted. The area to which liver organoids were attached was highlighted. No atrophy was observed at the transplanted site (Fig. 2A). Histologically, hKO-positive cells had accumulated in the left lobe of the liver, while a few positive cells were observed in the lungs and brain (Fig. 2B, C). We observed no necrotic area in the liver caused by portal vein embolism. In the group that received 9.0 × 105 organoids/kg, fetal liver organoids had accumulated mostly in the left lobe, with some organoids detected in the lungs and brain (Fig. 2D).
Figure 2.
Evaluation of extrahepatic translocation of fetal liver cell-derived liver organoids. (A) Transplantation to the left lobe of the liver was selectively performed. (B) Stereoscopic findings and fluorescence imaging of the surfaces of the left lobe, lung, and brain 1 week after the transplantation of 9.0 × 105 liver organoids/kg. (C) Microscopic findings of the cut edge. Most attached organoids were observed in the liver. However, hKO-positive tissues were observed in the lungs and brain. (D) The number of hKO-positive areas in the left lobe of the liver, middle lobe, and right lobe, lungs, and brain. hKO: humanized Kusabira Orange.
Human iPSC-Derived Organoid Transplantation in Infantile Piglets
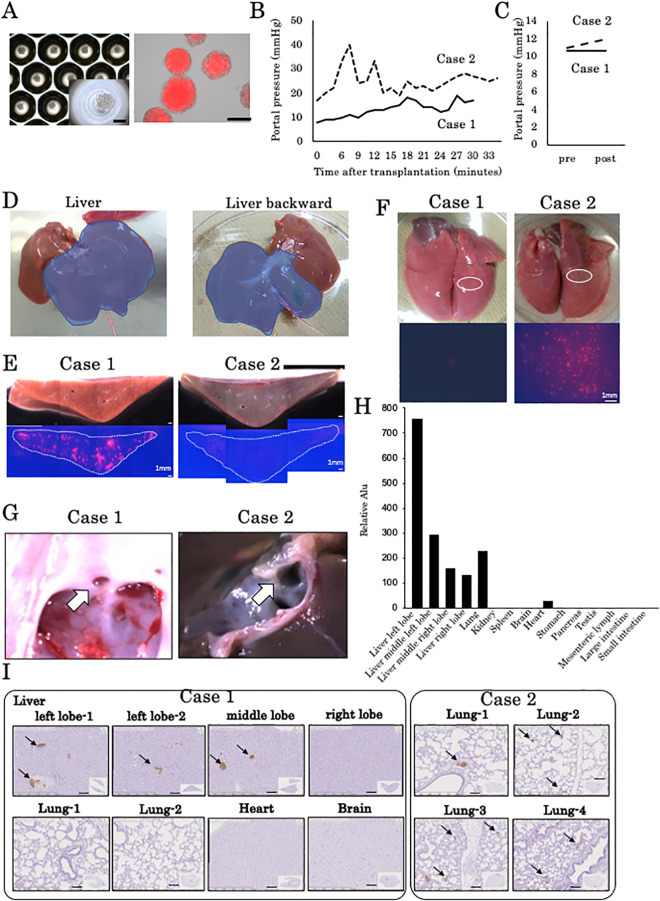
Since the tolerance of organoid transplantation was found in previous experiments with fetal liver organoids, we conducted an experiment with human iPSC liver organoids for the purpose of intraoperative portal pressure measurement and study of translocation to other organs 5 min after transplantation. The same protocol that was used for fetal pig liver-derived organoid transplantation was used for transplantation of human iPSC-derived liver organoids in a 10-day-old piglet (Table 1). Figure 3A is an image of human iPSC-derived liver organoids that express fluorescently labeled hKO. Each liver organoid contained 900 cells, which is similar to each fetal liver-derived organoid. The cells were fused within 2 days of culture, and no nonaggregated cells were observed. Therefore, we transplanted a human iPSC-derived liver organoid that had been cultured for 2 days. The mean diameter of the human iPSC-derived liver organoid is 137.2 µm (Table 1). In all, 1.0 × 105/kg human iPSC-derived liver organoids were transplanted into two 10-day-old piglets. The left portal vein pressure during transplantation was consistently 20 mmHg or less in case 1 during the transplantation procedure, but in case 2, the portal vein pressure increased transiently (Fig. 3B). A comparison of portal vein main pressures before and immediately after transplantation did not reveal an increase in case 1 but revealed a slight increase in case 2 (Fig. 3C). Engraftment of human iPSC-derived liver organoids was observed exclusively in the left lobe of the liver (Fig. 3D).
Figure 3.
Human iPSC-derived organoid transplantation in infantile piglets. (A) Human iPSC-derived liver organoids in a micropattern plate. Light microscopic and KO fluorescence images. Scale bar, 100 μm. (B) Portal vein pressure in the left portal branch during transplantation in cases 1 and 2. (C) Portal vein pressure in the main portal branch before and 5 min after transplantation. (D) Transplantation to the left lobe of the liver was selectively performed in case 1. (E) The engraftment area of the human iPSC-derived liver organoids in a cross-section of the left lobe of the liver was confirmed as an hKO-positive red area. Many hKO-positive regions were observed in case 1, but a smaller region was observed in case 2 compared with case 1. (F) Surface and magnified images of the lungs. As an hKO-positive region on the surface of the right lung was confirmed, a positive region was not detected at all in case 1, but in case 2, many hKO-positive regions were recognized on the surface. (G) Observation of the venous duct opening from the inferior vena cava. The arrow shows the opening of the ductus venosus. The venous duct was closed in case 1, but in case 2, the venous duct was patent. (H) Human Alu sequence qPCR in whole-organ samples from case 2. Human Alu sequences were most frequently detected in the left hepatic lobe but were also detected in the left middle lobe, right middle lobe, and right hepatic lobe. The detection intensity of human Alu sequences tended to decrease with increasing distance from the left hepatic lobe. Human Alu sequences were detected in the lung and heart. (I) Immunostaining of various organs with an anti-hKO antibody revealed hKO-positive clusters in the left and middle hepatic lobes in case 1 but not in the lungs, heart, or brain. In case 2, hKO-positive clusters were found in the lungs. hKO: humanized Kusabira Orange; iPSC: induced pluripotent stem cells; PDV: patent ductus venosus; qPCR: quantitative polymerase chain reaction.
The engraftment area of the human iPSC-derived liver organoids according to cross-sections of the left lobe of the liver was confirmed as an hKO-positive red area. Many KO-positive regions were observed in case 1, but fewer regions were observed in case 2 compared with case 1 (Fig. 3E). As an hKO-positive region on the surface of the right lung was confirmed, this was not detected at all in case 1, but in case 2, many hKO-positive regions were recognized on the surface (Fig. 3F). Observation of the venous duct opening from the inferior vena cava revealed that the venous duct was closed in case 1 but patent in case 2 (Fig. 3G). Human Alu sequence qPCR was performed on whole-organ samples from case 2, and the distribution of human cells was examined. Human Alu sequences were most frequently detected in the left hepatic lobe, but they were also detected in the left middle lobe, right middle lobe, and right hepatic lobe. The detection intensity of human Alu sequences tended to decrease with increasing distance from the left lobe of the liver. Human Alu sequences were detected in the lungs at a slightly higher level than in the right middle lobe. These sequences were also found in the heart. Based on these results, it was suggested that human cells translocated to other organs such as the lungs and heart, as seen in case 2 (Fig. 3H). Immunostaining of specimens from various organs with an anti-hKO antibody revealed KO-positive clusters in the left and middle lobes of the liver in case 1 but not in the lungs, heart, or brain. In case 2, hKO-positive clusters were found in the lung (Fig. 3I).
PDV Ligation Completely Prevented the Translocation of Human iPSC-Derived Liver Organoids to Other Organs after Portal Vein Transplantation
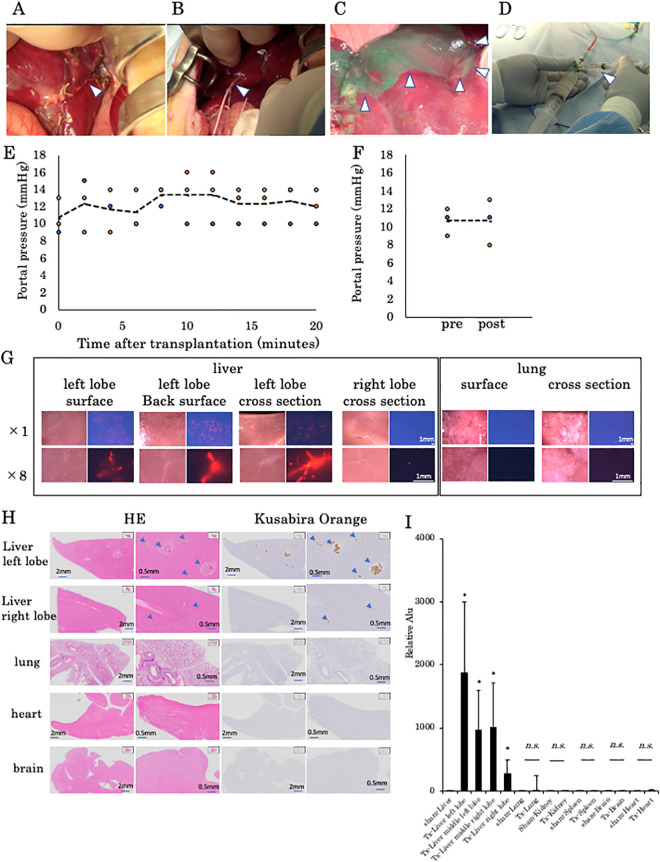
After transplantation into the portal vein, we observed translocation by fetal liver-derived organoids in a 10-day-old piglet. The PDV was supposed to be the main route of translocation in newborn piglets. To prevent translocation after liver organoid transplantation through the portal vein, the ductus venosus was ligated before transplantation in 28-day-old piglets (Fig. 4A). An 18-G double-lumen catheter was cannulated into the left portal vein through the umbilical vein (Fig. 4B), and cannulation was confirmed by injection of ICG (Fig. 4C). Transplantation of liver organoids was performed using this catheter (Fig. 4D). During transplantation of 3.5 × 104/kg human iPSC-derived liver organoids, no exacerbation in portal pressure was observed in the left portal vein branch (Fig. 4E), and the portal trunk pressure remained unchanged (Fig. 4F). In addition, tissue engraftment was centered on the left lobe of the liver (Fig. 4G). As shown, more liver organoids can be seen in the left lobe than in the right lobe. However, organoids were not found in the lungs, heart, or brain (Fig. 4G, H). Alu sequence qPCR could not detect any human cells in the lungs, kidneys, spleens, or hearts of three piglets, as in the sham group which is the same as the fetal liver-derived liver organoid experiment (Fig. 4I). By ligating the PDV, the migration of human cells to other organs was completely suppressed.
Figure 4.
Evaluation of the extrahepatic translocation of human iPSC-derived liver organoids after PDV ligation. (A) The ductus venosus was transfixed in a piglet (arrowhead). (B) Insertion of the double-lumen catheter in the umbilical vein (arrowhead). (C) Detection of the perfused area in the left lobe of the liver by injection of ICG through the catheter (arrowhead). (D) Transplantation of liver organoids from the catheter (arrowhead). (E) Portal vein pressure in the left portal branch during transplantation. No portal hypertension in the left portal branch was observed in three piglets during human iPSC-derived liver organoid transplantation. (F) Portal vein pressures in the main portal branch before and 5 min after transplantation. No portal hypertension was observed in the main trunk before and after human iPSC-derived liver organoid transplantation. (G) Fluorescence image of the surface of the left lobe of the liver, the back surface, and a cross-section, a cross-section of the right lobe of the liver, the lung surface, and a lung cross-section immediately after transplantation. Many organoid-positive tissues were confirmed in the liver but not in the lungs. (H) Hematoxylin and eosin staining and immunostaining images of the liver, lungs, and brain. hKO-positive tissues were found in the liver but not in the lungs, heart, or brain. (I) Human Alu sequence qPCR in tissues from the left lobe of the liver, middle lobe on the left side, middle lobe on the right side, and the right lobe as well as the lungs, kidneys, spleen, brain, and heart. hKO: humanized Kusabira Orange; ICG: indocyanine green; iPSC: induced pluripotent stem cells; PDV: patent ductus venosus; qPCR: quantitative polymerase chain reaction.
Discussion
This study clearly demonstrates the safety of liver organoid transplantation through the portal vein. Using human iPSC-derived liver organoids, along with PDV ligation, the translocation of human cells to sites outside the liver was clearly inhibited.
Primary hepatocyte proliferation is difficult in vitro because of the characteristics of hepatocytes12–14. Therefore, the clinical use of hepatocyte transplantation is still limited, especially with hepatocytes obtained from discarded liver grafts15–17. Spheroids, which are three-dimensional hepatocyte aggregates, may circumvent some of these issues because of their improved longevity and phenotypic durability compared with those of single-cell hepatocytes18,19. In numerous studies, spheroid-like structures have been used experimentally to enhance cell transplantation. These structures can improve transplanted cell survival and engraftment in both insulin-secreting islet cell and human cardiac progenitor cell transplantations20,21.
Nicolas et al. studied the biodistribution of single-cell hepatocytes and hepatocyte spheroids in the liver, spleen, and digestive tract and showed that the cells were progressively cleared out of the spleen and digestive tract until only the liver contained cells8. These results are consistent with those reported in other studies. When human iPSC-derived hepatocyte-like cells were transplanted in mice, the cells were found not only in the spleen but also in the liver, stomach, and large intestine22. Nevertheless, these cells were not found in the brain, heart, lung, kidney, and pancreas. The biodistribution of single-cell hepatocytes transplanted through portal vein infusion was evaluated in a clinical study of pediatric ornithine transcarbamylase deficiency23; in that study, a predominant hepatic distribution was found, with an average liver-to-spleen ratio of 9.5:1 and no significant pulmonary radiotracer activity. Furthermore, the pulmonary translocation of hepatocytes after transplantation has been described in some studies24,25. Muraca et al. showed that hepatocytes remained in the lung sinusoids for up to 48 h after infusion in all pigs; a minor basal pulmonary infarction was also identified in one of the pigs24. Moreover, Bilir et al. reported that intraportal hepatocyte transplantation in patients with acute liver failure was followed by hypoxia and the presence of pulmonary infiltrates, which were visible by chest echocardiography; however, both of these conditions improved after 24 h25. In hepatocyte transplantation in a clinical setting, hepatocytes are infused several times, and therefore, portal vein complications are a serious problem26–28. There is also a risk of pulmonary embolism due to the repeated infusion of hepatocytes, even though only a few hepatocytes pass through the liver.
Unfortunately, in case 2, which is shown in Fig. 3, we observed the translocation of human cells outside the liver. The PDV is supposed to be the primary route of translocation in newborn piglets. To support our hypothesis, Dickson reported that PDV was observed in 14-day-old pigs29. Furthermore, Meyer and Lind reported that the ductus venosus closes functionally soon after birth, but anatomically it does not close for 15 to 20 days30. In addition, several studies have suggested that closure of the PDV is affected by shorter gestation time and lower birth weight31–33. Notably, in smaller neonates, PDV has also been observed 4 weeks after birth31. Therefore, as we show in Fig. 4, in portal vein transplantation, ligation of the ductus venosus is important to prevent the transfer of transplanted cells to other organs.
The thrombogenic potential of spheroids presents an important safety issue. It has been reported that in pigs, portal pressures increase linearly with cell load and that even with single-cell hepatocyte infusion, thrombi are formed in segmental portal branches24. These concerns are magnified with the use of spheroids. In the study by Nicolas et al., portal pressures during infusion were significantly higher when spheroids were used compared with single-cell suspensions. In our experiments of limited human liver organoid transplantation, portal vein pressure in the left portal branch and main trunk did not increase.
Organoid administration through the portal vein may be associated with technical complications similar to those associated with hepatocyte transplantation34. The maximum transplantable volume of hepatocytes is 2 × 108 hepatocytes/kg body weight, but repetitive infusions can be administered35. In the fetal liver-derived organoid transplantation performed in this study, 9.0 × 105 liver organoids/kg body weight, which contains 9.0 × 108 cells/kg body weight, could be transplanted without severe liver damage. One of the reasons for this is the distribution of the transplanted cells. With a single-cell injection, diffuse sinusoidal obstruction occurs, but with organoid transplantation, only a few sinusoids were obstructed. Consequently, a multitude of cells can be transplanted.
In this study, both fetal liver organoid and hiPSC liver organoid are composed of almost the same number of cells, but the size of liver organoids is different. The pigs were observed for only 1 week after the transplantation of fetal-derived liver organoids. Since hiPSC liver organoids have different species differences, it is considered unsuitable for long-term observation due to xenoimmunity. So long-term observation after transplantation of human iPSC-derived liver organoids has not been performed. Problems such as changes in weight and blood biochemistry evaluation may occur and are only a representation of the general condition after transplantation. The therapeutic effect of liver organoid transplantation has also not been assessed. Despite the above-mentioned problems, we believe that the results of studies that suggest the safety of intraportal transplantation of liver organoids are valid.
In conclusion, liver organoid transplantation through the portal vein is a safe procedure. Ligation of the ductus venosus prevents the translocation of the liver organoids to extrahepatic sites. Therefore, human iPSC-derived liver organoids can be safely transplanted through the portal vein.
Acknowledgments
We thank N. Hijikata, N. Sasaki, H. Nozawa, N. Mizuguchi, Y. Nafune, and S. Arimatsu for kindly providing technical support and the members of our laboratory for their comments. The authors would like to thank Enago (www.enago.jp) for the English Language review.
Footnotes
Authors' Contributions: TT and SM designed the experiments; TT and SM performed the experiments; SH and SM analyzed the qPCR data; SO performed culture of human iPSCs; HM, KN, and HN generated the transgenic pigs; SE, HCH, and AF performed anesthesia, animal surgery, and perioperative care; MM, YU, and TT analyzed the data and reviewed the experiments; AM, YWZ, and HT contributed to discussions; SM and TT prepared the manuscript with the contributions by all authors.
Ethical Approval: All experimental protocols were performed with the approval of the laboratory animal ethics committee of Meiji University and National Center for Child Health and Development, based on the Japanese Guideline for Animal Experiments of Ministry of Education, Culture, Sports, Science and Technology and Ministry of Health, Labor and Welfare.
Statement of Human and Animal Rights: Animal rights were secured with the approval of the laboratory animal ethics committee of Meiji University and the National Center for Child Health and Development, based on the Japanese Guideline for Animal Experiments of Ministry of Education, Culture, Sports, Science and Technology and Ministry of Health, Labor and Welfare.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Japan Agency for Medical Research and Development through the “Center for Clinical Application Research on Specific Disease/Organ” Research Center Network for Realization of Regenerative Medicine (HT). Japan Regenerative Medicine Project(1st) “New treatment strategy in urinary cycle disorder on chronic liver failure using human iPS cell derived liver organoids” (20314422) (HT). This work has also received a Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (B) (16H05405) (SM).
ORCID iD: Soichiro Murata  https://orcid.org/0000-0001-7367-301X
https://orcid.org/0000-0001-7367-301X
References
- 1. Lancaster MA, Knoblich JA. Organoigenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194):1247125. [DOI] [PubMed] [Google Scholar]
- 2. Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499(7459):481–484. [DOI] [PubMed] [Google Scholar]
- 3. Takebe T, Zhang RR, Koike H, Kimura M, Yoshizawa E, Enomura M, Koike N, Sekine K, Taniguchi H. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc. 2014;9(2):396–409. [DOI] [PubMed] [Google Scholar]
- 4. Takebe T, Enomura M, Yoshizawa E, Kimura M, Koike H, Ueno Y, Matsuzaki T, Yamazaki T, Toyohara T, Osafune K, Nakauchi H, et al. Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell. 2015;16(5):556–565. [DOI] [PubMed] [Google Scholar]
- 5. Zhang S, Liu P, Chen L, Wang Y, Wang Z, Zhang B. The effects of spheroid formation of adipose-derived stem cells in a microgravity bioreactor on stemness properties and therapeutic potential. Biomaterials. 2015;41:15–25. [DOI] [PubMed] [Google Scholar]
- 6. Uchida S, Itaka K, Nomoto T, Endo T, Matsumoto Y, Ishii T, Kataoka K. An injectable spheroid system with genetic modification for cell transplantation therapy. Biomaterials. 2014;35(8):2499–2506. [DOI] [PubMed] [Google Scholar]
- 7. Gabriel E, Schievenbusch S, Kolossov E, Hengstler JG, Rotshteyn T, Bohlen H, Nierhoff D, Hescheler J, Drobinskaya I. Differentiation and selection of hepatocyte precursors in suspension spheroid culture of transgenic murine embryonic stem cells. PLoS One. 2012;7(9):e44912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nicolas CT, Hickey RD, Allen KL, Du Z, Guthman RM, Kaiser RA, Amiot B, Bansal A, Pandey MK, Suksanpaisan L, DeGrado TR, et al. Hepatocyte spheroids as an alternative to single cells for transplantation after ex vivo gene therapy in mice and pig models. Surgery. 2018;164(3):473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsuchida T, Murata S, Matsuki K, Mori A, Matsuo M, Mikami S, Okamoto S, Ueno Y, Tadokoro T, Zheng YW, Taniguchi H. The Regenerative effect of portal vein injection of liver organoids by retrorsine/partial hepatectomy in rats. Int J Mol Sci. 2019;21(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsunari H, Onodera M, Tada N, Mochizuki H, Karasawa S, Haruyama E, Nakayama N, Saito H, Ueno S, Kurome M, Miyawaki A, et al. Transgenic-cloned pigs systemically expressing red fluorescent protein, kusabira-orange. Cloning Stem Cells. 2008;10(3):313–323. [DOI] [PubMed] [Google Scholar]
- 11. Takebe T, Sekine K, Kimura M, Yoshizawa E, Ayano S, Koido M, Funayama S, Nakanishi N, Hisai T, Kobayashi T, Kasai T, et al. Massive and reproducible production of liver buds entirely from human pluripotent stem cells. Cell Rep. 2017;21(10):2661–2670. [DOI] [PubMed] [Google Scholar]
- 12. Zhang W, Wu M, Menesale E, Lu T, Magliola A, Bergelson S. Development and qualification of a high sensitivity, high throughput Q-PCR assay for quantitation of residual host cell DNA in purification process intermediate and drug substance samples. J Pharm Biomed Anal. 2014;100:145–149. [DOI] [PubMed] [Google Scholar]
- 13. Prigent J, Herrero A, Ambroise J, Smets F, Deblandre GA, Sokal EM. Human progenitor cell quantification after xenotransplantation in rat and mouse models by a sensitive qPCR assay. Cell Transplant. 2015;24(8);1639–1652. [DOI] [PubMed] [Google Scholar]
- 14. Lauschke VM, Vorrink SU, Moro SM, Rezayee F, Nordling Å, Hendriks DF, Bell CC, Sison-Young R, Park BK, Goldring CE, Ellis E, et al. Massive rearrangements of cellular MicroRNA signatures are key drivers of hepatocyte dedifferentiation. Hepatology. 2016;64(5):1743–1756. [DOI] [PubMed] [Google Scholar]
- 15. Hughes RD, Mitry RR, Dhawan A. Current status of hepatocyte transplantation. Transplantation. 2012;93(4):342–347. [DOI] [PubMed] [Google Scholar]
- 16. Gramignoli R, Vosough M, Kannisto K, Srinivasan RC, Strom SC. Clinical hepatocyte transplantation: practical limits and possible solutions. Eur Surg Res. 2015;54(3-4):162–177. [DOI] [PubMed] [Google Scholar]
- 17. Enosawa S, Horikawa R, Yamamoto A, Sakamoto S, Shigeta T, Nosaka S, Fujimoto J, Nakazawa A, Tanoue A, Nakamura K, Umezawa A, et al. Hepatocyte transplantation using the living donor reduced-graft in a baby with ornithine transcarbamylase deficiency: a novel source for hepatocytes. Liver Transpl. 2014;20(3):391–393. [DOI] [PubMed] [Google Scholar]
- 18. Luebke-Wheeler JL, Nedredal G, Yee L, Amiot BP, Nyberg SL. E-cadherin protects primary hepatocyte spheroids from cell death by a caspase-independent mechanism. Cell Transplant. 2009;18(12):1281–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brophy CM, Luebke-Wheeler JL, Amiot BP, Khan H, Remmel RP, Rinaldo P, Nyberg SL. Rat hepatocyte spheroids formed by rocked technique maintain differentiated hepatocyte gene expression and function. Hepatology. 2009;49(2):578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kusamori K, Nishikawa M, Mizuno N, Nishikawa T, Masuzawa A, Shimizu K, Konishi S, Takahashi Y, Takakura Y. Transplantation of insulin-secreting multicellular spheroids for the treatment of type 1 diabetes in mice. J Control Release. 2014;173:119–124. [DOI] [PubMed] [Google Scholar]
- 21. Oltolina F, Zamperone A, Colangelo D, Gregoletto L, Reano S, Pietronave S, Merlin S, Talmon M, Novelli E, Diena M, Nicoletti C, et al. Human cardica progenitor spheroids exhibit enhanced engraftment potential. PLoS One. 2015;10(9):e0137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nagamoto Y, Takayama K, Ohashi K, Okamoto R, Sakurai F, Tachibana M, Kawabata K, Mizuguchi H. Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J Hepatol. 2016;64(5):1068–1075. [DOI] [PubMed] [Google Scholar]
- 23. Bohnen NI, Charron M, Reyes J, Rubinstein W, Strom SC, Swanson D, Towbin R. Use of indium-111-labeled hepatocytes to determine the biodistribution of transplanted hepatocytes through portal vein infusion. Clin Nucl Med. 2000;25(6):447–450. [DOI] [PubMed] [Google Scholar]
- 24. Muraca M, Neri D, Parenti A, Feltracco P, Granato A, Vilei MT, Ferraresso C, Ballarin R, Zanusso GE, Giron G, Rozga J, et al. Intraportal hepatocyte transplantation in the pig: hemodynamic and histopathological study. Transplantation. 2002;73(6):890–896. [DOI] [PubMed] [Google Scholar]
- 25. Bilir BM, Guinette D, Karrer F, Kumpe DA, Krysl J, Stephens J, McGavran L, Ostrowska A, Durham J. Hepatocyte transplantation in acute liver failure. Liver Transpl. 2000;6(1):32–40. [DOI] [PubMed] [Google Scholar]
- 26. Enosawa S, Yuan W, Douzen M, Nakazawa A, Omasa T, Fukuda A, Sakamoto S, Shigeta T, Kasahara M. Consideration of a safe protocol for hepatocyte transplantation using infantile pigs. Cell Med. 2012;3(1-3):13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smets F, Najimi M, Sokal EM. Cell transplantation in the treatment of liver diseases. Pediatr Transplant. 2008;12(1):6–13. [DOI] [PubMed] [Google Scholar]
- 28. Meyburg J, Hoffmann GF. Liver cell transplantation for the treatment of inborn errors of metabolism. J Inherit Metab Dis. 2008;31(2):164–172. [DOI] [PubMed] [Google Scholar]
- 29. Dickson AD. The ductus venosus of the pig. J Anat. 1956;90(1):143–152. [PMC free article] [PubMed] [Google Scholar]
- 30. Meyer WW, Lind J. The ductus venosus and the mechanism of its closure. Arch Dis Child. 1966;41(220):597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Loberant N, Herskovits M, Barak M, Ben-Elisha M, Herschkowitz S, Sela S, Roguin N. Closure of the ductus venosus in premature infants: findings on real-time gray-scale, color-flow doppler, and duplex doppler sonography. Am J Roentgenol. 1999;172(1):227–229. [DOI] [PubMed] [Google Scholar]
- 32. Kondo M, Itoh S, Kunikata T, Kusaka T, Ozaki T, Isobe K, Onishi S. Time of closure of ductus venosus in term and preterm neonates. Arch Dis Child Fetal Neonatal Ed. 2001;85(1):F57–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murayama K, Nagasaka H, Tate K, Ohsone Y, Kanazawa M, Kobayashi K, Kohno Y, Takayanagi M. Significant correlations between the flow volume of patent ductus venosus and early neonatal liver function: possible involvement of patent ductus venosus in postnatal liver function. Arch Dis Child Fetal Neonatal Ed. 2006;91(3):F175–F179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Najimi M, Defresne F, Sokal EM. Concise review: updated advances and current challenges in cell therapy for inborn liver metabolic defects. Stem Cells Transl Med. 2016;5(8):1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iansante V, Chandrashekran A, Dhawan A. Cell-based liver therapies: past, present and future. Philos Trans R Soc Lond B Biol Sci. 2018;373(1750):20170229. [DOI] [PMC free article] [PubMed] [Google Scholar]