Abstract
Radiation therapy is crucial in the therapeutic arsenal to cure cancers; however, non-neoplastic tissues around an abdominopelvic tumor can be damaged by ionizing radiation. In particular, the radio-induced death of highly proliferative stem/progenitor cells of the colonic mucosa could induce severe ulcers. The importance of sequelae for patients with gastrointestinal complications after radiotherapy and the absence of satisfactory management has opened the field to the testing of innovative treatments. The aim of this study was to use adult epithelial cells from the colon, to reduce colonic injuries in an animal model reproducing radiation damage observed in patients. We demonstrated that transplanted in vitro-amplified epithelial cells from colonic organoids (ECO) of C57/Bl6 mice expressing green fluorescent protein implant, proliferate, and differentiate in irradiated mucosa and reduce ulcer size. To improve the therapeutic benefit of ECO-based treatment with clinical translatability, we performed co-injection of ECO with mesenchymal stromal cells (MSCs), cells involved in niche function and widely used in clinical trials. We observed in vivo an improvement of the therapeutic benefit and in vitro analysis highlighted that co-culture of MSCs with ECO increases the number, proliferation, and size of colonic organoids. We also demonstrated, using gene expression analysis and siRNA inhibition, the involvement of bone morphogenetic protein antagonists in MSC-induced organoid formation. This study provides evidence of the potential of ECO to limit late radiation effects on the colon and opens perspectives on combined strategies to improve their amplification abilities and therapeutic effects.
Keywords: colonic epithelial cells, co-transplantation, mesenchymal stromal cells, BMP antagonists, radiotherapy side effects
Introduction
Radiation therapy is used in at least 50% of cancer patients and plays a critical role in 25% of cancer cures. Many cancer survivors undergo radiation therapy for tumors located in the pelvis or abdomen, rendering the bowel at risk for injury, due to the presence of some parts of it in the irradiation field. In spite of improvements in radiotherapy techniques that aim to target precisely prostate, bladder, or uterine tumors, the colorectum may be located in the irradiation field as well. Early radiation therapy side effects affect the quality of life at the time of treatment in about 90% of patients. Delayed bowel radiation toxicity is a highly important issue for long-term cancer survivors. It is a progressive condition with few therapeutic options and substantial long-term morbidity and mortality1. Following radiation exposure, inflammation, death of highly dividing progenitor cells, microvascular apoptosis, and local ischemia impair epithelial self-renewal, thereby inducing mucosal atrophy. The resultant colonic barrier dysfunction leads to nutrient and fluid loss as well as penetration of luminal pathogens in the tissue exacerbating the mucosal inflammation and homeostatic imbalance in the epithelium. Disturbances of the repair process can lead to loss of tissue (ulceration) or pathological healing (fibrosis, fistula). Thus, rapid resealing of the epithelial surface barrier following injury is essential for the preservation of normal homeostasis. In patients with inflammatory bowel disease, conventional strategies aim to readjust the immune system. However, mucosal healing has been associated with a more favorable prognosis, lower relapse, long-term improvement, and diminished risk of surgery2.
During homeostasis, the intestinal epithelium undergoes rapid and continuous stem cell-driven renewal that is finely regulated to maintain the stem cell pool and lineage differentiation. This process takes part in an organized microanatomic structure called a crypt. The self-renewing colonic stem cells (CSCs) are located at the base of the crypt and also divide to produce progenitor cells. The progenitor cells undergo several additional cell divisions, migrate along the crypt axis, and then terminally differentiate into a variety of lineages that contribute to the structure and function of the epithelium. When these cells reach the top of the crypt, they undergo anoikis and exfoliate into the lumen. The regulation of CSCs occurs within a surrounding microenvironment known as stem cell niche, which provides a variety of molecular, cellular, biochemical, and physical components essential for CSC regulation. This microenvironment consists of mesenchymal stroma containing extracellular matrix (ECM), enteric neurons, endothelial cells, smooth muscle cells, immune cells, and myofibroblasts. Communication between epithelial cells and their supportive stroma is established by a two-way circuit. The paracrine secretion of molecules by the niche component acts according to a gradient to their target cells3. Stem cell-driven epithelial homeostasis is regulated by key signaling pathways: WNT, NOTCH, bone morphogenetic protein (BMP), Hedgehog, epidermal growth factor (EGF), and BMPs4. WNT ligands act predominantly at the base of the crypt to maintain stem cell function and progenitor cell proliferation. NOTCH signaling is also effective at the base of the crypt, and it regulates cell fate through cell-to-cell contact in the stem cell and proliferative zones. BMP ligands repress WNT signaling and promote cell differentiation and apoptosis, acting predominantly in the upper part of the crypt. There is reduced BMP activity at the base of the crypt, partly through diffusion gradients of the ligands but also secondary to the restricted expression of ligand-sequestering BMP-antagonists. Noggin is the major BMP antagonist but other members like Gremlin-1 and -2 and Twsg-1 have been described. Hedgehog signaling mediated by the main ligand, Indian Hedgehog, promotes BMP ligand expression in the upper part of the crypt3,5.
Recent advances have enabled the culture and expansion of epithelial stem cells of the small intestine and colon6,7. A long-term in vitro culture system was established using Matrigel matrix and used to grow three-dimensional intestinal epithelial organoids from single LGR5 cell or adult crypts from biopsies. A defined cocktail of factors was defined and minimal and essential stem cell factors were used, namely R-Spondin, EGF, Noggin, and WNT3a, to maintain the colonic crypt culture. With the advent of such technologies, there has emerged a growing interest in the use of these cultured adult stem cells in replacement therapy for intestinal injuries. Another approach in therapy is to manipulate the stem cell microenvironment or niche to facilitate and improve tissue repair by endogenous stem cells. In this study, we tested the co-injection of in vitro-amplified epithelial cells from colonic organoids (ECO) and mesenchymal stromal cells (MSCs) to propose a new strategy to treat colonic radiation-induced damage.
Materials and Methods
MSC Culture and Characterization
C57BL/6 mouse BM MSCs from Cyagen Biosciences Inc. (Chicago, IL, USA) were cultured in growth medium (MEMα [Gibco RRL, Grand Island, NY, USA], SVF 10% Pan Biotech, PS 1%, Glut 1%, rh βFGF à 2 ng/ml). The culture medium was replaced twice a week. Cells at passages 4 to 6 were used for subsequent experiments. The phenotype of amplified BM-MSCs was verified by flow cytometry. The percentage of Sca-1 (Ly6A/e BD Pharmingen #558162, BD-Bioscience, Becton-Dickinson, Franklin Lakes, NJ, USA) and CD29 (Ha2/5; #562153)-positive cells was analyzed and the absence of hematopoietic cells was verified with CD45 (30-F11; #553081) and CD34 (RAM34; #560230) markers. Isotype identical antibodies served as controls. On average, BM-MSCs expressed Sca-1 at 97.2% (±1.91) and CD29 at 99.9% (±0.31), CD34 at 7.9% (±3.56) and CD45 at 0.1% (±0.01).
CSC Culture and MSC-CSC Co-culture
The colon of green fluorescent protein (GFP)-transgenic C57BL/6 Tg(UBC-GFP)30Scha/J (from Jackson Laboratories, Bar Harbor, ME, USA) was removed and washed with cold phosphate-buffered saline (PBS) until the supernatant was clear. Next, the colon was cut into fragments, and crypts were prepared and cultured as previously described by Sato and Clevers7. The culture medium supplemented with Wnt3a (100 ng/ml, Biotechne, MN, USA), R-spondin 1 (1 μg/ml), Noggin (50 ng/ml), and EGF (50 ng/ml), B27 (100 µl), Glutamax (Gibco), Hepes (50 µl), N2 supplement (Invitrogen, Carlsbad, CA, USA), N-acethylcystein (Sigma-Aldrich), and Primocin (Invitrogen) was changed every 48 h. For co-culture experiments, 10,000 MSCs were incorporated in Matrigel and mixed with crypt pellets (200 crypts). When mentioned, half of the concentration of added growth factors (GFs) was applied. For quantification, colonic crypts were counted at day 0 and colonoids were counted from day 1 to 7 under the microscope. The number of colonoids was normalized to the number of crypts seeded at day 0. The surface area of colonoids was quantified using a Nikon microscope (Nikon instruments, Melville, NY, USA). Images from a minimum of four fields of view per slide at an objective magnification of ×10 were examined. Images were captured using a coupled camera MOTICAM 580 5.0 MP. Analysis was performed using Image J Analysis software. Each organoid contour was sharply demarcated and the mean was calculated.
Gene Expression Analysis
Cell total RNA was prepared with the RNeasy mini kit (Qiagen, Courtaboeuf, France) and cDNA was obtained with the High Capacity Reverse Transcriptase cDNA kit (Thermo Fisher Scientific, Waltham, MA, USA). Real-time quantitative polymerase chain reaction (RT-qPCR) was performed using Taqman gene expression assays (Applied Biosystems). The samples were loaded in duplicate, and glyceraldehyde-3-phosphate dehydrogenase (gapdh) was used as an internal control. Results of gene expression were calculated using the ▵Ct method normalizing to gapdh.
Inhibition of Genes in MSCs
Grem-1 and Twsg-1 (BMP antagonists) were silenced in MSCs using small interference RNA (si-RNA). ON-TARGET plus Mouse SMART pool siRNAs designed for knockdown efficiency and other transfection reagents were from GE Healthcare, SA (Chicago, IL, USA). si-RNA against Grem-1 (L-043505-01-0010) or Twsg-1 (L-044605-01-0010) or both and ON-TARGET plus the nontargeting pool were used. The transfection was performed according to the manufacturer’s instructions. Briefly, mouse MSCs were seeded at 3,000 cells/cm2. The cells were transfected at 80% confluence with a solution containing 3 µl/ml Dharmafect-1 (T-2001-03) and 0.1 µM si-RNA in complete MSC growth medium for 3 d. Gene expression levels were verified after RNA extraction and RT-qPCR, gene silencing was more than 95% for each target gene 3 d after transfection, and inhibition fell to 25% after 8 d in co-culture medium (data not shown). Then, 3 d after transfection, MSCs were trypsinized and co-cultured for 4 d with freshly prepared CSCs.
Ethics Statement, Animals and Experimental Protocol
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals as published in the French regulations for animal experiments (Ministry of Agriculture Order No. B92-032-01, 2006) with European Directives (86/609/CEE) and French Decree 2013-118. The animal facilities of the institute have the agreement number C92-032-01. The experimental protocol was reviewed by the Ethics Committee of the Institute, which is registered by the “Comité national de réflexion éthique sur l’expérimentation animale” under the number 81. This study was approved by the Administration Committee of Experimental Animals, Paris, France. The experimental protocol was registered under the permit number APAFlS#6504-20160823 1626287 v2. Male C57BL/6 mice from Janvier (Janvier SA, Le Genest St Isle, France) were maintained in pathogen-free conditions and housed in a temperature-controlled room (21°C ± 1°C). The mice were used at 8 to 10 wk of age. They were allowed free access to water and fed standard pellets. Mice were anesthetized by isoflurane inhalation and a single 27 Gray (Gy) dose was delivered by Elekta Synergy Platform (Elekta SAS France, Boulogne, France) through a 1 × 1 cm window centered on the colorectal region (energy max. 4 MV with an average energy of about 1.5 MV; 30 kA; the dose rate was 2.3 Gy per min).
Three weeks after irradiation, mice were injected locally with CSCs, MSCs, or CSCs+MSCs in the submucosa of the colon with a 30 Gauge needle, after laparotomy. Two injection points (2 × 100 µl) were done in the irradiated zone. For CSC preparation, after 6 d in culture, Matrigel was removed from the three-dimensional-embedded colonoids with Matrigel Recovery Solution (BD Bioscience), incubating for 1 h at 4°C. The resulting solution was passed through a 27 G needle to dissociate colonoids. CSCs were obtained after the dissociation of colonoids from one well with or without 1 × 106 BM-MSCs in 10% Matrigel. Animals were then sacrificed 3, 7, and 14 d later and distal colons were collected.
Immunofluorescence
After 4 d of culture in Matrigel, the medium from each chamber slide was removed. The organoid samples embedded in Matrigel were fixed in 4% paraformaldehyde/PBS for 20 min at room temperature, followed by permeabilization in 0.5% TritonX-100/PBS for 10 min at room temperature. After washing, samples were incubated with anti-Ki67 antibody (ab15580, Abcam, Cambridge, MA, USA) at a 1:100 dilution for 1.5 h at room temperature. After washing, the samples were incubated with donkey anti-rabbit Alexa 594 (A21207, Invitrogen) at a 1:500 dilution and goat anti-GFP fluorescein isothiocyanate (FITC) (ab6662, Abcam) at a 1:400 dilution for 1 h at room temperature and protected from light. Slides were incubated for 10 min with a DAPI solution to counterstain nuclei and then coverslipped with ProLong Diamond Antifade Mountant (Thermo Fisher Scientific) and allowed to dry overnight at room temperature. Fluorescence images were captured using a laser confocal microscope (LSM 780 NLO, Carl Zeiss Microimaging, Germany) using Z-correction.
Immunohistochemistry
Sections were dewaxed and hydrated and following antibodies were applied for 1 h at 37°C: GFP (goat anti-GFP FITC; ab6662, Abcam) diluted to 1/200, KI67 (rabbit anti-KI67; ab15580, Abcam Cambridge, UK) diluted to 1/100 or rabbit anti-SOX 9 (ab185230) diluted at 5/1,000. For secondary antibodies, donkey anti-rabbit Alexa 594 (A21207, Thermo Fisher Scientific) diluted to 1/200 was applied for 30 min at 37°C. Slides were then mounted with Vectashield hard set anti-fade medium with DAPI (H1500, VectorLabs, Burlingame, CA, USA). For MUCIN-2 immunohistochemistry, mouse anti-MUC2 1/50 (Sc-515032, SantaCruz Biotechnology, CA, USA) was applied, then anti-mouse horseradish peroxidase was incubated at room temperature. Staining was developed with Histogreen substrate (E109; Abcys, les Ullis, France) and sections were counterstained with Fast nuclear red (S1963; DakoCytomation, Agilent Santa Clara, CA, USA), dehydrated, and mounted. Isotype control antibodies are used as negative controls.
Histological and Lesion Quantification
Animals were euthanized under anesthesia, the colon was collected, cut longitudinally, fixed immediately in 4% formaldehyde, and embedded in paraffin. Paraffin-embedded colons were cut on a rotary microtome (Leica Microsystems AG, Wetzlar, Germany) into serial sections of 5 µm, spaced 150 µm, and stained with hematoxylin-eosin-saffron. The severity of colorectal damage induced by irradiation was assessed by measuring the length of the ulcerated and atypical areas using Histolab software (Microvision Instruments, Lisses, France).
Statistical Analysis
All data are presented as mean ± standard error of the mean (SEM). Statistical analyses were performed using Graph Pad Prism 5.0 (GraphPad, San Diego, CA, USA). Statistical significance was determined using one-way analysis of variance (ANOVA) followed by Tukey’s post-test for multiple groups, or two-way ANOVA followed by Bonferroni’s post-test for multiple groups with different variables, or t-test when changes were compared between two groups. Statistical significance was set at P <0.05.
Results
ECO Implant, Proliferate, and Persist Over Time in Irradiated Colonic Mucosa
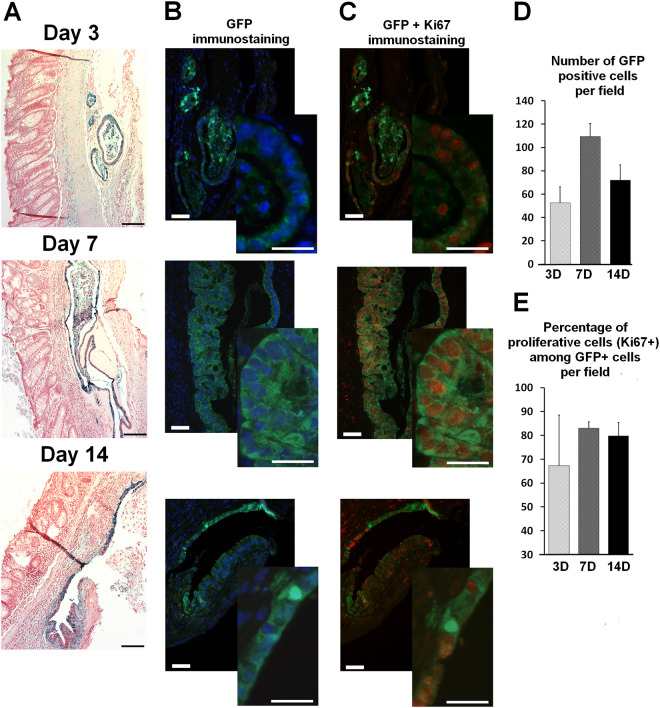
We analyzed the ability of ECO to implant and survive in irradiated colonic mucosa. To reproduce colonic damage developed in patients suffering from severe chronic side effect of pelvic radiotherapy, immunocompetent mice were irradiated at 27 Gy through a colorectal window. After 3 wk, the mice developed colonic mucosal ulcer and inflammation. At this time, colonic organoids from crypts of GFP mice, cultured for 6 d in Matrigel, were dissociated and injected locally in the irradiated zone of the colon (Fig. 1). Animals were sacrificed 3, 7, and 14 d after injection. GFP-ECO were readily detectable in the colon (Fig. 2A) as early as 3 d after injection. Various small patches of GFP-ECO were observed. Seven and 14 d after injection, the size of the patches increased and we observed a peak of implanted cells at 7 d (Fig. 2C). Proliferative GFP-ECO were investigated using Ki-67 and GFP double staining. We observed marked heterogeneity in the number of Ki-67+GFP+ 3 d after injection (Fig. 2B–D), which could depend on the microenvironment. At 7 d, the percentage of proliferative ECO among GFP-positive cells was around 85% and was not modified at 14 d. Thus, in vitro amplified ECO implants in irradiated colonic mucosa, at 3 d, form small patches, some of which show high proliferative potential that could lead to a large zone of implantation as visualized at day 7. At 14 d, the number of GFP-ECO was lower, but their proliferative potential was not modified.
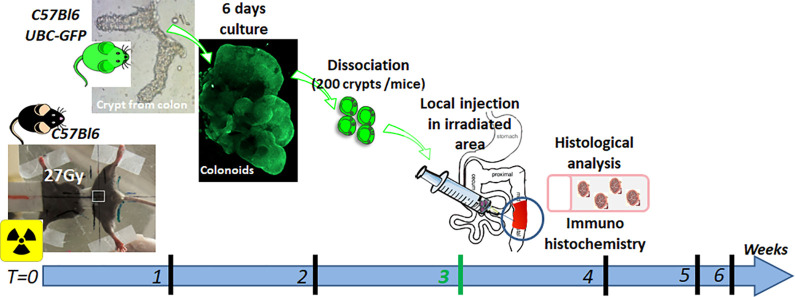
Figure 1.
Experimental procedure of ECO transplantation in colorectal-irradiated mice. C57BL/6 mice were locally irradiated at 27 Gy through a colorectal window with medical-type linear accelerator. Crypts were isolated from C57BL/6 Tg UBC-GFP and cultured as described below6. Two hundred crypts were seeded per well and cultured for 6 days. The day of injection, thus 3 weeks after irradiation, organoids were dissociated and ECO were embedded in 200 µL of 10% Matrigel. One well (200 crypts) was injected per mice. The cell suspension was locally injected in the colonic mucosa of mice, after laparotomy (2 injection points were made in the irradiated area). Colonic tissue was collected 3, 7 and 14 days after injection according to the experiments. For co-transplantation experiments (Results in Fig. 4), MSC (1 million cells) or ECO+MSC groups, mice were injected using the same experimental procedure. MSC were also embedded in 10% Matrigel (n = 6 to 8 animals per group; results expressed as mean ± SEM).
Figure 2.
GFP-ECO from cultured colonoids implant and proliferate in colonic mucosa with chronic lesions at 3, 7 and 14 days after injection. (A) Large view pictures of engrafted ECO-GFP in colonic mucosa (GFP immunostaining in blue). Scale bars represent 100 µm. (B) GFP immunostaining merged with DAPI (low and enhanced-power views). (C) Co-immunostaining of GFP (green) and Ki-67 (red) (low and enhanced-power views). Scale bars for low power views represent 25 µm and enhance power views represent 50 µm. (D) Quantification of the number of injected GFP-ECO per field. (E) Quantification of the number of proliferative cells among the GFP-positive-ECO per field. All results are expressed as mean ± SEM.
In vivo Implanted ECO Show Progenitor Cells and Differentiated Characteristics
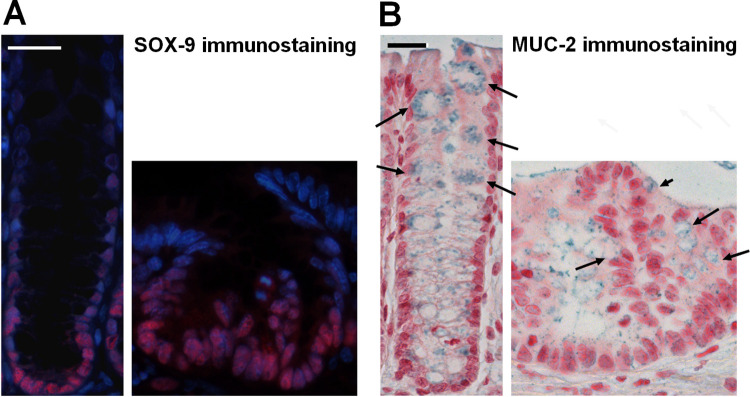
Seven days after injection, some transplanted GFP-ECO cells formed folded structures resembling a crypt, of lesser height, composed of proliferative cells at the base of the crypt and polarized with differentiated cells at the apex. Thus, in these structures, we verified by immunohistochemistry the presence of SOX-9 and a marker of mucin: MUC2. We observed that SOX9-positive cells were restricted to the bottom of the crypt, as in normal crypt epithelium (Fig. 3A). MUC2 differentiated cells could also be retrieved in the engrafted structures (Fig. 3B).
Figure 3.
In vivo stem cell and differentiation potential of ECO 7 days after injection. To demonstrate the normal behavior of injected cells in the irradiated colon (A) stem/progenitor cells were immunostained by Sox9 in normal crypt (left panel) and in engrafted structure (right panel). Scale bar represents 25 µm. (B) We also observed differentiated cells as immunostained by MUC2 in normal crypt (left panel) and in engrafted structure (right panel). Arrows indicate the mucin granules (labelled in blue) of the colonocytes. Scale bar represents 25 µm.
In Vivo ECO Co-injection with MSCs Reduces Colonic Damage Following Irradiation
We tested the therapeutic potential of ECO to reduce colonic ulcers induced after colonic irradiation. In vivo, MSCs function as a stromal niche for hematopoietic stem cells, so in the present study we tested whether this function could be applied to endogenous CSCs and participate in the therapeutic benefit of co-injection of MSCs with ECO. Using the same experimental procedure as in Fig. 1, 1 million MSCs were injected alone or mixed with ECO. Seven days after treatment, the mice were euthanized and colons were taken for histologic analyses. The size of the ulcer was measured. In irradiated animals without stem cell treatment, the ulcer size reached 2 mm (Fig. 4). We observed a statistical reduction in ulcer size after MSCs or ECO treatment (*P < 0.05) compared with sham irradiated controls. When MSCs and ECO were co-injected, the ulcer was further reduced in size (**P < 0.01).
Figure 4.
Co-treatment of ECO with MSC reduces radiation-induced colonic ulcers. Representative histological pictures of the colon in the different groups. Scale bars represent 200 µm. Quantification of the therapeutic benefit for the epithelial compartment of the colon in the different treated groups. Ulcer size was measured on histologic slides. Ulcers were pointed out by dark bars. Number of animals per group varied from n = 6 to n = 8. Results are expressed as mean ± SEM (*p < 0.05 versus IRR with Matrigel, **p < 0.01 versus IRR with Matrigel). IRR = Irradiated.
In vitro Colonic Organoid Formation Is Improved by MSC Co-culture
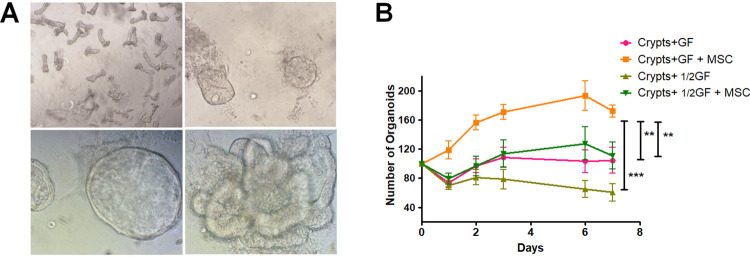
Isolated crypts were prepared from UBC-GFP-mouse colon and seeded on Matrigel, in culture medium with additive stem cell epithelial GFs as described by Sato7. Day 1 after seeding, epithelial cells located at the top of the crypt died and the crypt bottoms closed to form small spheres (Fig. 5A, upper panels). With time, the spheres started to bud and we observed colonoids with crypt-like structures (budded morphology) along with spheroid structures (cystic morphology) (Fig. 5A lower panels). To analyze the ability of MSCs to support organoid formation, co-culture experiments were done. Two culture conditions with varying GF concentrations were tested (complete or half GF concentration). When half concentration of GFs was used, the number of organoids from colonic crypts decreased statistically (Fig. 5B). To analyze the ability of MSCs to support organoid formation, co-culture experiments in each of these GF conditions were done. The results demonstrated that co-culture with MSCs, with GFs or 1/2GFs in the culture medium, increases the number of organoids compared with crypts cultured alone (Fig. 5B). As expected without GF, very few organoids were observed at day 2, an outcome not affected by the co-culture of colonic crypts with MSCs (data not shown).
Figure 5.
Culture of crypts from mouse colon with MSC improves organoid formation. (A) Pictures of crypts from colon on the day of seeding. The number of crypts was counted in each culture well (upper left panel) ×4 magnification. The day after, buds visualized in 3D were counted (upper right panel). Colonoids (lower left panel) and spheroids (lower right panel) were formed over time. ×10 magnification (B) Graph representing the number of organoids with or without co-culture with MSC in different culture conditions (normal concentration of growth factors and half concentration). Normalization according to the number of seeded crypts at day 0 was applied. Experiments were performed in triplicate with 3 different preparations of MSC. Results are expressed as mean ± SEM.
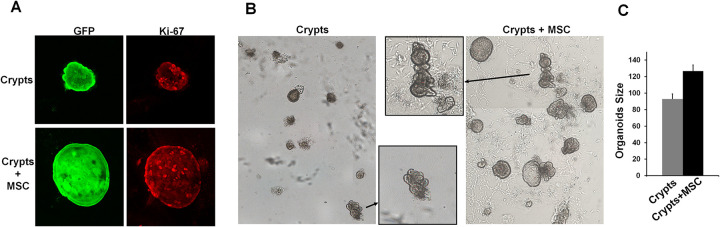
Colonic Epithelial Cell Proliferation Is Increased in the Presence of MSCs
To study the impact of MSC co-culture on colonic epithelial cell proliferation in self-organizing organoids, we performed Ki67 immunostaining. We observed that colonic organoids contain a higher proportion of Ki67-positive cells when they are in co-culture with MSCs (Fig. 6A). We quantified the organoid surface area with and without MSC co-culture in Matrigel after formaldehyde fixation (Fig. 6B). We demonstrated that organoid size is statistically bigger in the presence of MSCs (Fig. 6C). These results demonstrate that MSCs increase the proliferative potential of colonic epithelial cell leading to increased organoid size.
Figure 6.
MSC co-culture improves organoid formation. (A) Pictures of organoids from GFP mice (left panels) cultivated with or without MSC in half growth factor concentration. Proliferation of cells was visualized using Ki-67 immunostaining (right panels). (B) Pictures of organoids fixed in formaldehyde at 5 days of culture at lower and greater magnifications (in the middle small squares) to allow size measurement by computational counting. (C) Graph representing the size of organoids (µm2) when crypts were cultured in the presence of MSC or not. These results were obtained in 2 separate experiments performed in duplicate. Results are expressed as mean ± SEM
BMP Antagonists Are Involved in Organoid Formation Supported by MSCs
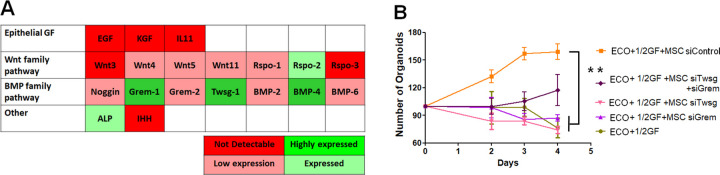
To determine the factors secreted by MSCs that support the organoid formation and increase colonic epithelial cell proliferation, we analyzed the MSC gene expression of molecules involved in epithelial cell proliferation, niche maintenance, and intestinal stem cell (ISC) fate (Fig. 7A). We observed that MSCs do not express some factors essential for ISC in vitro proliferation described by Sato as Egf and Wnt3. We also determined that the expression of genes as Rspo-1 and Noggin is low. However, this experiment highlighted that MSCs express Grem-1 and Twsg-1 genes at a high level (Fig. 7A). To demonstrate the involvement of these two BMP-antagonist genes in MSC-induced organoid formation, we inhibited Grem-1, Twsg-1, and both of them in MSCs by Si-RNA before co-culture experiments. First of all, we verified the inhibition and observed more than 95% of inhibition for the three conditions that decreased to 25% of inhibition after 8 d in the co-culture medium. We observed as early as 2 d of co-culture a statistically significant decrease in the number of organoids with MSC_SiGrem-1, MSC_SiTwsg-1, and MSC_SiGrem-1+ SiTwsg-1 compared with native MSCs (Fig. 7B).
Figure 7.
Reversion of MSC-induced increase in organoid formation by inhibition of BMP antagonists in MSC. (A) Graphic table representing the expression levels of epithelial growth factors on MSC highlighting expression of Grem-1 and Twsg-1 quantified by real-time PCR. The abundance of each transcripts is assessed according to the Ct number and normalized to Ct of Gapdh internal control. (B) Quantification of the number of organoids formed according to gene inhibition using si-RNA in MSC. Normalization according to the number of seeded crypts at day 0. Results are expressed as mean ± SEM
Discussion
Radiotherapy is powerful in treating malignant tumors. However, damage to healthy surrounding tissues remains unavoidable despite the latest ballistic innovations. For abdominopelvic radiotherapy, the intestine and the colorectum are defined as organs at risk. Toxicities to the bowel induced after irradiation evolve with time, are complex, and could result in severe complications such as acute hemorrhage and obstruction8. This pathology involves various cell compartments, in particular the epithelial mucosa whose damage leads to defective absorption and to barrier dysfunction, which exacerbates inflammatory processes already initiated during the early phase after irradiation. Thus, resealing of the epithelial barrier seems a good option for restoring mucosal function, limiting inflammation, and favoring tissue regeneration. A historical breakthrough was provided by the studies of Sato et al., who succeeded in establishing a long-term culture method to expand epithelial cells from adult tissue7. From there, new perspectives of cell therapy emerged9 with the demonstration of cultured-ISC implantation in mice with acute damage induced by DSS10.
In the present study, we demonstrate, for the first time, implantation of ECO after irradiation and chronic inflammation. We observed the integration of ECO into the recipient colonic tissue, where they formed a normal crypt structure with the presence of SOX9-positive cells at the bottom of the invagination and also differentiated MUC2 cells. An alternative strategy of injection has been used in a mouse model of ulcer induced by DSS, demonstrating ECO engraftment after intraluminal injections7. Transplantation by enema is widely used in animal models; however, a large volume is needed and it is complicated to upgrade to human colon volume/surface area, and thus endoscopic transplantation seems the simplest and the most accessible method. Moreover, endoscopic procedures are widely used nowadays by gastroenterologists. This procedure will rather control the number of injected cells and will allow standardization of the protocol of stem cell injection for transfer in clinical practice.
Numerous data described the high proliferative capacity of ISCs and their sensitivity to radiation. However, the intestine exhibits rapid and effective renewal abilities and could be related with the colon. During homeostasis, the turnover of the intestinal epithelium is driven by LGR5 + CBC progenitor cells located at the bottom of the crypt, which rapidly divide and differentiate along the crypt11. When this highly proliferative cell compartment is depleted by radiation or other toxic agents, a second population expressing Bmi-1, Lrig1, and Tert has been proposed as dedicated reserve stem cell population able to reform the Lgr5+ pool for the regeneration of the crypt. Thus, epithelial regeneration capacity after damage is dependent on the resting stem cell pool, the plasticity of the cells to dedifferentiate, and the ability of the cells to proliferate12. All these processes are dependent on and finely regulated by signals from the surrounding niche microenvironment, which provides highly orchestrated signaling pathways. Recent studies have demonstrated that essential signals from the ISC niche are provided by specialized cells in the underlying stroma, including intestinal subepithelial mesenchymal cells, enteric neurons, endothelial cells, and immune cells, as well as epithelial elements as Paneth cells, which are specific to the intestine. Irradiation, used at therapeutic doses to cure cancer, leads to a huge modification of the healthy surrounding tissue. In consequence, the therapeutic index of radiotherapy is limited by normal tissue injury of organs at risk. In the literature, numerous modifications of the cell microenvironment have been described that could perturb the epithelial regenerative process after irradiation. We previously demonstrated, in our preclinical model, an increase of immune cell infiltrates in acute and delayed lesions after irradiation of the colon13–15. Moreover, it has been reported that macrophages play a key role in intestinal crypt regeneration following irradiation damage16. The microvasculature underlying the crypt base could also affect colonic epithelial progenitors, and reduction of endothelial apoptosis by beta FGF protects mice from gastrointestinal radiation syndrome17 and intestinal radiation exposure of PAI-1-/- mice showed less endothelial cell apoptosis, which is associated with the reduction of small intestinal damage18. Added to endothelial cell death, following irradiation, vascular endothelium acquires a proinflammatory phenotype with increased secretion of soluble mediators such as cytokines, chemokines, and GF and adhesion molecules. The submucosal plexus of enteric neurons communicates with intestinal progenitors19. It has been shown that irradiation modifies nerve fibers,20 which could have important deleterious effects for patients21. Interest is growing in understanding the contribution of the underlying stroma in the maintenance and regeneration potential of CSCs. Several studies over recent years have addressed the individual roles of stromal cells that produce factors required for ISC niche maintenance. Indeed, it has been demonstrated that epithelial WNT are dispensable and stromal production can fully support normal murine intestinal homeostasis22. Recent studies have described overlapping subsets of stromal cells expressing markers including CD34+GP38, Gli1, FoxL1, and Pdgfrα that play a key role regulating ISC maintenance and differentiation by production of diverse ligands, including those regulating the WNT/beta-catenin pathway (for review4). However, what is less known is the modification of secretion of these ligands by the stromal compartment during delayed radiation-induced lesion formation. The stromal compartment is resistant to radiation-induced cell death, but a phenotype switch has been described, as a modification of the secretion of mediators23. Defining stromal cell subsets and modifications after irradiation, with regard to the expression of molecules involved in stem cell maintenance and progenitor proliferation, will likely yield new insights into therapeutic options to favor the regenerative process to cure the side effects of radiotherapy. It is admitted that all these modifications of the cell microenvironment could perturb the epithelial regenerative process after irradiation. However, it would be interesting to define the modifications in terms of secreted molecules involved in the epithelial regenerative process, such as GFs, WNT/Hedgehog/NOTCH/BMP molecules, in each cell compartment after irradiation. The advent of single-cell technology associated with gene expression could yield information valuable in defining new therapeutic targets.
In bone marrow, MSC has been described as the hematopoietic stem cell niche, so in this study we hypothesized that MSCs might also be able to reproduce components of the CSC niche, providing an optimal microenvironment for epithelial regeneration (plasticity and proliferation) after irradiation. MSCs by improving angiogenesis could also improve the microenvironment24,25. The results of this study demonstrate that MSC injection reduces established colonic ulceration, which is further improved after the co-injection of ECO. However, as previously demonstrated by others and us, we did not detect MSCs7. Cell therapy using MSCs is widely used in clinical practice, with more than 700 clinical trials ongoing, including in inflammatory bowel diseases (Alofisel®, darvadstrocel clinical trial). The outstanding feature of MSCs as regards their clinical use, besides their easy availability and culture amplification, are their low immunogenicity, and immunomodulatory and regenerative functions. After irradiation, we and others have demonstrated their anti-inflammatory13,14, antifibrotic,26 and proregenerative effects27–29. Moreover, study from Chang et al. demonstrated in vitro that the co-culture of MSCs with irradiated crypts leads to organoids with more proliferative cells and few apoptotic cells30. Thus, improvement of all these parameters due to MSC injection, associated with ECO injection, could explain our results. However, in our preclinical model, we did not detect more ECO implantation in the colonic mucosa or more ECO proliferation when ECO were co-injected with MSCs (data not shown). As implantation is largely dependent on the injection procedure, it seems difficult to draw a conclusion regarding this parameter. Concerning in vivo proliferation, we found a high proportion (∼80%) of proliferating ECO that did not increase after MSC co-injection. The high level of ECO proliferation could be due to the modification of the microenvironment after irradiation. Indeed, various studies have demonstrated that, following injury, epithelial cells at the wound margin dynamically increase their proliferation rate to cover the denuded area31,32. Two weeks after a high dose of colorectal irradiation, we previously demonstrated increased epithelial proliferation at the margins of the ulcer with an increase in crypt size and crypt branching. We also demonstrated increased Wnt4 /5 and R-Spondin gene expression, but no increase in GFs involved in epithelial proliferation or angiogenesis in the irradiated mucosa29. However, it has been demonstrated that WNT signaling is necessary but not sufficient for ISC activation and self-renewal. A second signal to antagonize BMP is required33. In the mouse intestine, the BMP antagonist, NOGGIN, may contribute to this second signal. It has been shown that BMP signaling suppresses WNT signaling to ensure a balanced control of stem cell self-renewal. In vitro, intestinal/colonic organoids cannot be maintained in culture without NOGGIN, which emphasizes the importance of BMP antagonists7. In the present study, in vitro co-culture experiments demonstrated that MSCs favor organoid formation and colonic epithelial cell proliferation. Analysis of MSC gene expression highlighted poor Wnt expression but an expression of the BMP antagonists Grem-1 and Twsg-1. Si-RNA experiments demonstrated the dependence of MSC-BMP antagonist expression in the increased formation of organoids. Interestingly, in vivo gene expression analysis on human colon demonstrated that BMP antagonists are expressed by cryptal myofibroblasts34. They demonstrated expression of Grem-1 and -2 in basal colon crypts, whereas BMP-1,-2, -5, and -7 were highly expressed in colon tops. In vitro the authors also showed that GREM-1 promotes nuclear translocation of beta-catenin and activates Axin-2, a specific Wnt target gene that could participate in maintaining WNT activity at the bottom of the crypt. More recently, pericryptal CD34+Gp38+ mesenchymal cells closely associated with LGR5+ epithelial cells in the mouse intestine have been shown to be major producers of Wnt2b, Grem-1, and R-spondin. This subset promotes the expansion of intestinal organoids, an effect mainly dependent on Grem-1. After intestinal damage and inflammation, this stromal population upregulates epithelial GFs, as well as chemokines and cytokines with key roles in intestinal immunity and tissue repair after damage35. Altogether these data demonstrate that BMP antagonists are important factors for the niche microenvironment during intestinal homeostasis and repair. MSCs possess similar properties and thus are good candidates for co-injection during cell therapy. Indeed, co-injection of ECO and MSCs could offer an innovative and plausible alternative. Applications of adult stem cell organoids have been proposed to provide a unique opportunity for treatment strategies (for reviews9,36). First of all, co-culture of ECO with MSCs during the amplification phase could improve cost-effectiveness. Indeed, we demonstrated that the use of half concentration of recombinant proteins with MSCs gave similar organoid numbers as culture media with normal GF concentration. Then, colonic injection of MSCs in addition to ECO could have twoeffects: i) spur epithelial healing and ii) limit inflammation. These will be a great advantage for therapy after irradiation, but also for the treatment of intestinal inflammatory diseases in the case of autologous biopsies36,37. However, today, organoid technology for regenerative and translational medicine is severely limited by the use of the undefined tumor-derived ECM: Matrigel. Fortunately, given the high potential of such treatments, various labs are working on bioengineered and synthetic matrices that recapitulate the ISC niche38–40. Thus, culture protocols to increase ECO amplification efficiency and diversity are continuously being explored to allow for successful applications appropriate for use in humans41. Combination of ECO transplantation with MSCs helps improve innovative organoid therapy with a view to clinical application.
In conclusion, we demonstrated that the transplantation of adult ECO improves in vivo the therapeutic outcome of radiation-induced lesions. With the aim to go toward clinical application, we propose a new concept to improve the therapeutic benefit by co-injection with MSCs. This combined approach could open up new perspectives for improving the therapeutic potential of MSC therapy, not only for radiation-induced lesions but also for other bowel disorders.
Acknowledgments
We thank Yoann Ristic for management of the irradiation facility and the GSEA team for their assistance in animal care.
Authors’ Contributions: Lara Moussa contributed to collection and assembly of data, data analysis and interpretation, and provided final approval of the manuscript; Alexia Lapiere contributed to collection and assembly of data, and data analysis and interpretation, and provided final approval of the manuscript; Claire Squiban and Christelle Demarquay contributed to collection and assembly of data and provided final approval of the manuscript; Fabien Milliat provided financial and administrative supports and final approval of the manuscript; and Noelle Mathieu contributed to conception and design, data analysis and interpretation, and manuscript writing.
Ethical Approval: This study was approved by the National Administration Committee of Experimental Animals, Paris, France (approval number APAFlS#6504-20160823 1626287 v2).
Statement of Human and Animal Rights: All of the experimental procedures involving animals were conducted in accordance with the Institutional Animal Care guidelines of the Institute of Radioprotection and Nuclear Safety Ethics Committees, France, and approved by the Administration Committee of Experimental Animals, Paris, France.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Agence Nationale pour la Recherche (ANR-13-RPIB-0008) and the Institute of Radioprotection and Nuclear Safety. LM was supported by the ANR 13-RPIB-0008 “ANTHOS”.
ORCID iD: Noëlle Mathieu  https://orcid.org/0000-0002-5753-7154
https://orcid.org/0000-0002-5753-7154
References
- 1. Andreyev HJ, Wotherspoon A, Denham JW, Hauer JM. Defining pelvic-radiation disease for the survivorship era. Lancet Oncol. 2010;11(4):310–312. [DOI] [PubMed] [Google Scholar]
- 2. Shah SC, Colombel JF, Sands BE, Narula N. Mucosal healing is associated with improved long-term outcomes of patients with ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14(9):1245–1255. [DOI] [PubMed] [Google Scholar]
- 3. Biswas S, Davis H, Irshad S, Sandberg T, Worthley D, Leedham S. Microenvironmental control of stem cell fate in intestinal homeostasis and disease. J Pathol. 2015;237(2):135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greicius G, Virshup DM. Stromal control of intestinal development and the stem cell niche. Differentiation. 2019;108(July-August):8–16. [DOI] [PubMed] [Google Scholar]
- 5. Wang S, Chen YG. BMP signaling in homeostasis, transformation and inflammatory response of intestinal epithelium. Sci China Life Sci. 2018;61(7):800–807. [DOI] [PubMed] [Google Scholar]
- 6. Ootani A, Li X, Sangiorgi E, Ho QT, Ueno H, Toda S, Sugihara H, Fujimoto K, Weissman IL, Capecchi MR, Kuo CJ. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15(6):701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–265. [DOI] [PubMed] [Google Scholar]
- 8. Moussa L, Usunier B, Demarquay C, Benderitter M, Tamarat R, Semont A, Mathieu N. Bowel Radiation Injury: complexity of the pathophysiology and promises of cell and tissue engineering. Cell Transplant. 2016;25(10):1723–1746. [DOI] [PubMed] [Google Scholar]
- 9. Bartfeld S, Clevers H. Stem cell-derived organoids and their application for medical research and patient treatment. J Mol Med (Berl). 2017;95(7):729–738. [DOI] [PubMed] [Google Scholar]
- 10. Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K, Clevers H, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat Med. 2012;18(4):618–623. [DOI] [PubMed] [Google Scholar]
- 11. Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478(7368):255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bankaitis ED, Ha A, Kuo CJ, Magness ST. Reserve stem cells in intestinal homeostasis and injury. Gastroenterology. 2018;155(5):1348–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bessout R, Demarquay C, Moussa L, Rene A, Doix B, Benderitter M, Semont A, Mathieu N. TH17 predominant T cell responses in radiation-induced bowel disease are modulated by treatment with adipose-derived mesenchymal stromal cells. J Pathol. 2015;237(4):435–446. [DOI] [PubMed] [Google Scholar]
- 14. Bessout R, Semont A, Demarquay C, Charcosset A, Benderitter M, Mathieu N. Mesenchymal stem cell therapy induces glucocorticoid synthesis in colonic mucosa and suppresses radiation-activated T cells: new insights into MSC immunomodulation. Mucosal Immunol. 2014;7(3):656–669. [DOI] [PubMed] [Google Scholar]
- 15. Blirando K, Hneino M, Martelly I, Benderitter M, Milliat F, Francois A. Mast cells and ionizing radiation induce a synergistic expression of inflammatory genes in endothelial cells by a mechanism involving p38alpha MAP kinase and (p65) NF-kappaB activation. Radiat Res. 2012;178(6):556–567. [DOI] [PubMed] [Google Scholar]
- 16. Saha S, Aranda E, Hayakawa Y, Bhanja P, Atay S, Brodin NP, Li J, Asfaha S, Liu L, Tailor Y, Zhang J, et al. Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury. Nat Commun. 2016;7(October 13):13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293(5528):293–297. [DOI] [PubMed] [Google Scholar]
- 18. Abderrahmani R, Francois A, Buard V, Tarlet G, Blirando K, Hneino M, Vaurijoux A, Benderitter M, Sabourin JC, Milliat F. PAI-1-dependent endothelial cell death determines severity of radiation-induced intestinal injury. PLoS One. 2012;7(4):e35740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bjerknes M, Cheng H. Modulation of specific intestinal epithelial progenitors by enteric neurons. Proc Natl Acad Sci USA. 2001;98(22):12497–12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Durand C, Pezet S, Eutamene H, Demarquay C, Mathieu N, Moussa L, Daudin R, Holler V, Sabourin JC, Milliat F, Theodorou V, et al. Persistent visceral allodynia in rats exposed to colorectal irradiation is reversed by mesenchymal stromal cell treatment. Pain. 2015;156(8):1465–1476. [DOI] [PubMed] [Google Scholar]
- 21. Otterson MF. Effects of radiation upon gastrointestinal motility. World J Gastroenterol. 2007;13(19):2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kabiri Z, Greicius G, Madan B, Biechele S, Zhong Z, Zaribafzadeh H, Edison, Aliyev J, Wu Y, Bunte R, Williams BO, et al. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development. 2014;141(11):2206–2215. [DOI] [PubMed] [Google Scholar]
- 23. Haydont V, Vozenin BMC. Maintenance of radiation-induced intestinal fibrosis: cellular and molecular features. World J Gastroenterol. 2007;13(19):2675–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van de PD, Demarquay C, Van Daele E, Moussa L, Vanhove C, Benderitter M, Ceelen W, Pattyn P, Mathieu N. Adipose-derived mesenchymal stromal cells improve the healing of colonic anastomoses following high dose of irradiation through anti-inflammatory and angiogenic processes. Cell Transplant. 2017;26(12):1919–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manieri NA, Mack MR, Himmelrich MD, Worthley DL, Hanson EM, Eckmann L, Wang TC, Stappenbeck TS. Mucosally transplanted mesenchymal stem cells stimulate intestinal healing by promoting angiogenesis. J Clin Invest. 2015;125(9):3606–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Linard C, Busson E, Holler V, Strup PT C, Lacave Lapalun JV, Lhomme B, Prat M, Devauchelle P, Sabourin JC, Simon JM, Bonneau M, et al. Repeated autologous bone marrow-derived mesenchymal stem cell injections improve radiation-induced proctitis in pigs. Stem Cells Transl Med. 2013;2(11):916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang P, Qu Y, Liu Y, Cui S, Zhu D, Wang H, Jin X. Multi-therapeutic effects of human adipose-derived mesenchymal stem cells on radiation-induced intestinal injury. Cell Death Dis. 2013;4:e685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang PY, Qu YQ, Wang J, Dong LH. The potential of mesenchymal stem cells in the management of radiation enteropathy. Cell Death Dis 2015;6:e1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Semont A, Demarquay C, Bessout R, Durand C, Benderitter M, Mathieu N. Mesenchymal stem cell therapy stimulates endogenous host progenitor cells to improve colonic epithelial regeneration. PLoS One. 2013;8(7):e70170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chang PY, Jin X, Jiang YY, Wang LX, Liu YJ, Wang J. Mesenchymal stem cells can delay radiation-induced crypt death: impact on intestinal CD44(+) fragments. Cell Tissue Res. 2016;364(2):331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iizuka M, Konno S. Wound healing of intestinal epithelial cells. World J Gastroenterol 2011;17(17):2161–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seno H, Miyoshi H, Brown SL, Geske MJ, Colonna M, Stappenbeck TS. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci U S A. 2009;106(1):256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36(10):1117–1121. [DOI] [PubMed] [Google Scholar]
- 34. Kosinski C, Li VS, Chan AS, Zhang J, Ho C, Tsui WY, Chan TL, Mifflin RC, Powell DW, Yuen ST, Leung SY, et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci U S A. 2007;104(39):15418–15423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stzepourginski I, Nigro G, Jacob JM, Dulauroy S, Sansonetti PJ, Eberl G, Peduto L. CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc Natl Acad Sci U S A. 2017;114(4):E506–E513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holmberg FE, Seidelin JB, Yin X, Mead BE, Tong Z, Li Y, Karp JM, Nielsen OH. Culturing human intestinal stem cells for regenerative applications in the treatment of inflammatory bowel disease. EMBO Mol Med. 2017;9(5):558–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Okamoto R, Watanabe M. Investigating cell therapy for inflammatory bowel disease. Expert Opin Biol Ther. 2016;16(8):1015–1023. [DOI] [PubMed] [Google Scholar]
- 38. Cruz Acuna R, Quiros M, Farkas AE, Dedhia PH, Huang S, Siuda D, Garcia-Hernandez V, Miller AJ, Spence JR, Nusrat A, García AJ, et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat Cell Biol. 2017;19(11):1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordonez-Moran P, Clevers H, Lutolf MP. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539(7630):560–564. [DOI] [PubMed] [Google Scholar]
- 40. Wang Y, Kim R, Hinman SS, Zwarycz B, Magness ST, Allbritton NL. Bioengineered systems and designer matrices that recapitulate the intestinal stem cell niche. Cell Mol Gastroenterol Hepatol. 2018;5(3):440–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fujii M, Matano M, Toshimitsu K, Takano A, Mikami Y, Nishikori S, Sugimoto S, Sato T. Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition. Cell Stem Cell. 2018;23(6):787–793. [DOI] [PubMed] [Google Scholar]