Abstract
Long noncoding RNAs (lncRNAs) are crucial regulatory molecules involved in diverse biological processes and human diseases, including preeclampsia (PE). The lncRNA growth arrest associated lncRNA 1 (GASAL1) has been implicated in multiple malignant solid tumors and other diseases, while it is poorly known as the potential molecular mechanism of GASAL1 in PE. In this study, GASAL1 was significantly downregulated in the placentas’ of tissues from primipara with PE and trophoblast cell lines. Then, the upregulation of GASAL1 dramatically decreased proliferation and invasion and enhanced apoptosis in HTR-8/SVneo and JAR cells. Bioinformatics tool predicated that there is a potential interaction between GASAL1 and serine/arginine splicing factor 1 (SRSF1). RNA pull-down assays showed that GASAL1 directly binds with SRSF1 that could promote cell proliferation and invasion and suppress cell apoptosis. Further research showed that promoting effects of trophoblasts proliferation and invasion caused by co-transfecting GASAL1 and SRSF1 into HTR-8/SVneo and JAR cells were impaired by SRSF1 knockdown. Moreover, inhibition of the mammalian target of rapamycin (mTOR) activity by rapamycin influenced the effects of GASAL1 on cell proliferation, invasion, and apoptosis. Taken together, these findings suggest that lncRNA GASAL1 interacts with SRSF1 to regulate the proliferative, invasive, and apoptotic abilities of trophoblast cells via the mTOR signaling pathway.
Keywords: preeclampsia (PE), long noncoding RNA GASAL1, SRSF1, apoptosis, invasion, mTOR signaling pathway
Introduction
Preeclampsia (PE) is one of the pregnancy syndromes accounting for approximately 20% of maternal deaths and 15% to 20% of all premature deliveries1,2. It was reported that many theories were associated with the pathogenesis of PE, such as maternal vascular damage (induced by inflammatory cytokines and oxidative stress), impaired spiral artery remodeling, and anomalous inflammation-immune response3,4. Among them, increasing evidences indicate that trophoblast cell dysfunction in proliferation and invasion lead to insufficient placentation implantation and insufficient placental feeding, which has been considered as a crucial defect that causes PE and fetal growth restriction. At present, the delivery of the baby and placenta is the only known cure for PE5. Despite advances that have been made in this field, the molecular mechanisms of PE pathogenesis and the regulation of trophoblast cell functions remain largely unknown.
Long noncoding RNAs (lncRNAs) are a class of noncoding RNAs longer than 200 nucleotides in length, which are involved in many disease processes by regulating cell pluripotency, development, and cell growth6–8. Numerous evidences revealed that lncRNAs had different expression in the placentas from primipara with PE and healthy subjects, indicating that abnormal expression of lncRNA might lead to PE6,9. Some lncRNAs, such as MEG3, HIF1A-AS2, TCL6, and uc. 294, had been proved to be involved in PE by regulating the invasion and proliferation of trophoblasts10–13. For instance, low expression of lncRNA TUG1 in placentas from PE patients contributes to PE by inhibiting trophoblast migration, invasion, and proliferation14,15. LncRNA growth arrest associated lncRNA 1 (GASAL1) was frequently downregulated in many diseases and closely related to cell proliferation, apoptosis, and invasion, including gastric cancer, heart failures, and prostate carcinoma16–18. However, the underlying mechanism of lncRNA GASAL1 in modulating the functions of trophoblast cells in PE is poorly understood. Therefore, investigations of the lncRNA GASAL1 abnormally expressed in PE patients are beneficial for understanding the pathogenesis and progression of PE.
In this study, we explored the potential molecular mechanism in the relationship between GASAL1 and PE progression. We found that GASAL1 was remarkably downregulated in placentas from primipara with PE compared with those from healthy primipara, and GASAL1 promoted cell proliferation and invasion and inhibited cell apoptosis. Serine/arginine splicing factor 1 (SRSF1) as an RNA-binding protein of GASAL1 has been demonstrated to participate in multiple biological functions, including cell proliferation, invasion, and senescence19–21. Results of our research verified that GASAL1 and SRSF1 had synergistic effects on trophoblast cell proliferation, invasion, and apoptosis. Furthermore, our results indicate that GASAL1 and SRSF1 regulate proliferation, invasion, and apoptosis of trophoblast cells through activating the mammalian target of rapamycin (mTOR) pathway. In conclusion, this study may provide new insights into the key regulatory role of lncRNA GASAL1 in human PE.
Materials and Methods
Tissue Sample Collection
Thirty placental samples were obtained from primipara with PE (median age 27.5 years; range 20 to 36 years) who underwent cesarean deliveries from March 2017 to June 2018 in The Second Affiliated Hospital of Zhengzhou University (Zhengzhou, China). During the same period, 30 placental samples from healthy primipara were selected as controls. All collected samples were immediately washed with sterile phosphate-buffered saline (PBS) before being snap-frozen in liquid nitrogen and then stored at –80 °C for further RNA and protein extraction. All patients in this study signed an informed consent form prior to the study, and all experiments gained the approval of the Ethics Committee for Clinical Experiments at The Second Affiliated Hospital of Zhengzhou University.
Cell Culture and Transfection
Human trophoblastic cell lines (HTR-8/SVneo, JAR, BeWo, and JEG3) and human umbilical vein endothelial cells (HUVECs) were obtained from the Shanghai Institute of Cell Biology (Shanghai, China). HTR-8/SVneo and JAR cell lines were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (#E600028; Sangon Biotech, Shanghai, China) supplemented with 10% heat-inactivated fetal bovine serum (#E600001; Sangon Biotech), and HUVEC cells were maintained in endothelial cell growth medium (#1001; ScienCell, USA). All cells were kept at 37 °C in a humidified incubator with 5% carbon dioxide.
Plasmid vectors (plasmid cloning DNA [pcDNA]-GASAL1 and pcDNA-SRSF1) were constructed by GenScript Biotech Corp. (Nanjing, China). The short interfering RNA against GASAL1 (GASAL1 siRNA), SRSF1 (SRSF1 siRNA), and negative control siRNAs were designed, synthesized, and validated by Thermo Fisher Scientific (Waltham, MA, USA). HTR-8/SVneo and JAR cells were seeded in six-well plates at a density of 2 × 105 cells/ml. On reaching about 70% confluence, the cell transfection was performed using Lipofectamine 2000 transfection reagent (#11668019; Invitrogen, Carlsbad, CA, USA) under the suggestion of the manufacturer. Twenty-four hours after transfection with the siRNAs and plasmid vectors, the cells were harvested for further experiments.
RNA Extraction and Quantitative Polymerase Chain Reaction (qPCR)
Total RNA was isolated from placental samples and cells using Trizol reagent (#B511311; Sangon Biotech). Subsequently, a total of 1 µg RNA was subjected to reverse transcription, and single-stranded cDNA was synthesized with the PrimeScript Reagent Kit (#11752050; Thermo Fisher Scientific). Then, qPCR reactions were conducted using a Power SYBR Green PCR kit (#4367659; Thermo Fisher Scientific) and performed by CFX96 qPCR machine (Invitrogen) with the following conditions: 95°C for 3 min, followed by denaturation at 94°C for 15 s, annealing at 55°C for 25 s and extension at 72°C for 15 s for 35 cycles. The relative expression levels were calculated by the 2 −ΔΔCt method after normalization to the expression of GAPDH.
Western Blotting Analysis
HTR-8/SVneo and JAR cells transfected with plasmid vectors or siRNAs were lysed to extract total proteins in the radioimmunoprecipitation assay lysis buffer (#C500005; Sangon Biotech) supplemented with protease inhibitors. Protein concentrations in each sample were determined by Bradford assay. Then, 25 µg of protein from each sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and loaded onto polyvinylidene fluoride membranes (#F019531; Sangon Biotech) in transfer buffer. Next, the membranes were blocked with 5% nonfat milk diluted in tris-buffered saline solution containing 0.5% Tween-20 at 4 °C overnight, and then incubated with the following primary antibodies (Abcam, Cambridge, UK) at 4 °C overnight: anti-SRSF1 (ab38017; 1:1000), anti-cleaved-caspase-3 (ab2302; 1:500), anti-Bcl-2 (ab59348; 1:500), anti-pmTOR (ab84400; 1:900;), anti-p4EBP1 (1:800; ab47365), and anti-β-tubulin (ab6046; 1:500). Finally, the membranes were cultured with horseradish peroxidase-labeled secondary antibodies immunoglobulin G (IgG) for 1 h at room temperature, followed by the detection with ECL analyses kits (#D601039; Sangon Biotech). All experiments were repeated at least three times.
Cell Proliferation Assay
Cell proliferation was examined by using Cell Counting Kit-8 (CCK-8; #96992; Sigma, St. Louis, MO, USA). The transfected HTR-8/SVneo and JAR cell lines were plated into the six-well plate at a density of 2 × 104 cells per well. After incubation for 24 h, 48 h, 72 h, and 96 h, CCK-8 reagent was added in each well, followed by culturing for 2 h in the dark. Then, cell proliferation was assessed by measuring absorbance value with the optical density at 450 nm.
Cell Invasion Assay
Transwell assay was conducted to determine cell invasion by using the Transwell plates with an 8 µm pore inserts (#CLS3428; Millipore, Shijingshan, Haidian, USA). Cells (0.5 ml; 2.5 × 104 cells) were resuspended in serum-free RPMI-1640 medium and placed in the upper chamber, while RPMI-1640 medium containing 10% FBS was placed into the lower chamber. After incubation for 48 h, cells that invaded through the membrane filter were fixed with 4% paraformaldehyde (#E672002; Sangon Biotech) and stained with 0.3% crystal violet solution (#A600331; Sangon Biotech). At last, every well was photographed under a microscope (Nikon, Chiyoda-Ku, Tokyo, Japan), and the stained cells were counted in three randomly selected views of every well.
Flow Cytometry Analysis of Cell Cycle and Apoptosis
Cell cycle assay was detected by using propidium iodide (PI; #P4170; Sigma, St. Louis, MO, USA) staining. The HTR-8/SVneo and JAR cells were obtained after transfection for 48 h and made into single-cell suspension using trypsin, and then washed twice with PBS and fixed in 75% ethanol overnight at –20 °C. After that, the two cell lines were stained with the PI solution (100 ng/ml) in the dark for 20 min. MoFlo XDP flow cytometer sorting system (Thermo Fisher Scientific) was used to detect the percentage of cells in G0/G1, S, and G2/M phases in each group, and each experiment was repeated for three times. Cell apoptosis was assessed by flow cytometry after double staining of the transfected cells with fluorescein isothiocyanate (FITC)-Annexin V and PI using the FITC-Annexin V Apoptosis Detection Kit (#E606336; Sangon Biotech), and the percentages of apoptotic cells were calculated.
Prediction of the Binding Between SRSF1 and GASAL1
The online bioinformatics tool StarBase 2.0 (http://starbase.sysu.edu.cn/) was used for predicting the binding between SRSF1 and GASAL1. The SRSF1-binding motif was provided, which was aligned with the GASLA1 sequence.
RNA Binding Protein Immunoprecipitation (RIP) Assay
RIP assays were conducted using the EZ-Magna RIP kit (#17-701; Millipore) according to the manufacturer’s instructions. After grown in 15-cm plates, HTR-8/SVneo and JAR cells were collected by centrifugation and lysed in RIP lysis buffer. The lysates were incubated with 2 µg of anti-SRSF1 antibody and normal IgG at 4 °C for 2 h, and qPCR was applied for analyzing the immunoprecipitated RNA.
RNA Pull-Down Assay with Biotinylated RNA Probe
After transfected with 50 nM biotinylated RNA probe for 48 h, cells were washed with PBS after brief vortex and incubated with an RNA pull-down lysis buffer on ice for 10 min. After that, the lysates were precleared by centrifugation. The remaining lysates (about 150 μL) were incubated with M-280 streptavidin magnetic beads precoated with RNase-free BSA (#EN0531; Thermo Scientific) and yeast tRNA (#AM7119; Invitrogen) at 4 °C for 3 h. Then, the beads were washed with ice-cold lysis buffer, SDS-Tris low salt buffer (pH 8.0 containing 150 mM sodium chloride [NaCl]), and a high salt buffer (containing 500 mM NaCl). The bound complexes were purified for further analysis. Independent experiments were conducted at least three times.
RNA Pull-Down Assay with Wild Type (WT) or Mutated (MUT) Biotinylated GASAL1 Transcripts
WT GASAL1 and MUT GASAL1 were transcribed and labeled by the biotin RNA labeling mix and T7 RNA polymerase according to the manufacturer’s instructions (#18033100; Invitrogen). The pretreated biotinylated RNAs were incubated with 1 mg protein extracts at 4 °C for 1 h, gently mixed with 40 µl of washed streptavidin beads (#60210; Invitrogen), and incubated on a rotator overnight. Then the beads were washed with 1× binding washing buffer (5 mM Tris-hydrochloric acid, 1 M NaCl, 0.5 mM EDTA, and 0.005% Tween 20) for five times. The proteins were precipitated and diluted in 60 µL protein lysis buffer and measured on SDS-PAGE gels for Western blotting.
Statistical Analysis
Data are presented as the mean ± SEM which were obtained from at least three experiments. Student’s t-test and one-way analysis of variance (ANOVA) were applied for analyzing the data. P < 0.05 was considered statistically significant. All statistical analyses were performed with SPSS version 22.0.
Results
LncRNA GASAL1 was Downregulated in PE Placental Tissues
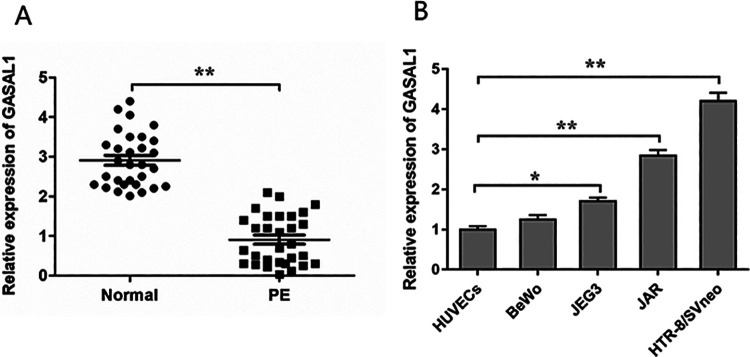
To investigate the expression of GASAL1 in PE, we analyzed the GASAL1 level in 30 placental samples from pregnant women with PE and 30 placental samples from healthy pregnant women by qPCR assay. The expression levels of GASAL1 in pregnant women with PE were significantly lower than normal counterparts (Fig. 1A). Then, we analyzed GASAL1 expression in four trophoblastic cell lines (HTR-8/SVneo, JAR, BeWo, and JEG3) after normalization to that in other cells relevant to pregnancy (HUVECs). We found that the expression of GASAL1 in HTR-8/SVneo and JAR cells was relatively higher than in the other cell lines (Fig. 1B). Thus, we chose HTR-8/SVneo and JAR cells to investigate GASAL1 functional activity. These data suggested that the downregulation of lncRNA GASAL1 might be involved in PE progress.
Figure 1.
LncRNA GASAL1 expression is downregulated in PE placental tissues. (A) Relative expression of GASAL1 was detected by qPCR assay in 30 placental samples with PE and 30 normal placental samples. (B) GASAL1 expression was assessed by qPCR assay in trophoblastic cell lines. The levels of GASAL1 in HTR-8/SVneo, BeWo, JAR, and JEG3 cells were normalized to that in HUVECs. The data were presented as the mean ± SEM, n = 3. Student’s t-test was used for comparisons between groups in this study. *P < 0.05 and **P < 0.01. GASAL1: lncRNA growth arrest associated lncRNA 1; HUVECs: human umbilical vein endothelial cells; lncRNA: long noncoding RNA; PE: preeclampsia; qPCR: quantitative polymerase chain reaction.
GASAL1 Promoted Cell Proliferation and G1-to-S Phase Progression in HTR-8/SVneo and JAR Cell Lines
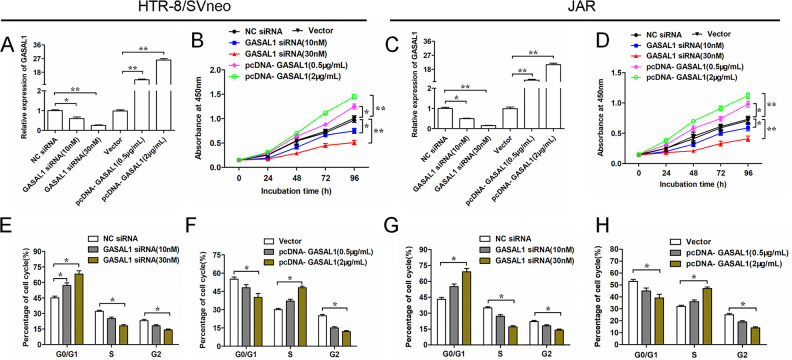
To explore how GASAL1 effects in HTR-8/SVneo and JAR cells, we silenced or overexpressed GASAL1 in cells by transfecting with GASAL1 siRNA or pcDNA GASAL1 overexpression vector (pcDNA GASAL1), respectively. As shown in Fig. 2A, C, GASAL1 expression was downregulated or upregulated by GASAL1 siRNA and pcDNA GASAL1 in a dose-dependent way in HTR/SVneo and JAR cells. Silencing GASAL1 by transfecting with GASAL1 siRNA significantly inhibited proliferation in HTR-8/SVneo and JAR cells; while GASAL1 overexpression promoted cell proliferation (Fig. 2B, D). Furthermore, to explore whether GASAL1 influenced the proliferation of HTR-8/SVneo and JAR by the regulation of the cell cycle, we detected cell cycle by flow cytometry. The results showed that silencing GASAL1 suppressed the G1-to-S phase progression (Fig. 2E, G), whereas overexpressed GASAL1 reversed this effect (Fig. 2F, H). These data revealed that lncRNA GASAL1 promotes cell proliferation and G1-to-S phase progression in HTR-8/SVneo and JAR cell lines.
Figure 2.
GASAL1 promotes cell proliferation and G1-to-S phase progression of HTR-8/SVneo and JAR cell lines. Cells were transfected with GASAL1 siRNA (10 nM or 30 nM) and pcDNA GASAL1 (0.5 μg/ml or 2 μg/ml). (A and C) The efficiencies of GASAL1 siRNA and pcDNA GASAL1 were determined by qPCR. (B and D) Cell proliferation was detected by cell counting kit-8 assay at 0 h, 24 h, 48 h, 72 h, and 96 h in HTR-8/SVneo and JAR cell lines. (E–H). Cell cycle was analyzed by flow cytometry after 24 h of cell transfection with GASAL1 siRNA or pcDNA GASAL1 in HTR-8/SVneo (E and F) and JAR (G and H) cell lines. The data were presented as the mean ± SEM, n = 3. Student’s t-test or one-way analysis of variance was used for comparisons between groups in this study. *P < 0.05 and **P < 0.01. GASAL1: lncRNA growth arrest associated lncRNA 1; lncRNA: long noncoding RNA; pcDNA: plasmid cloning DNA; qPCR: quantitative polymerase chain reaction; siRNA: short interfering RNA.
GASAL1 Inhibited Cell Apoptosis and Promoted Cell Invasion of HTR-8/SVneo and JAR Cell Lines
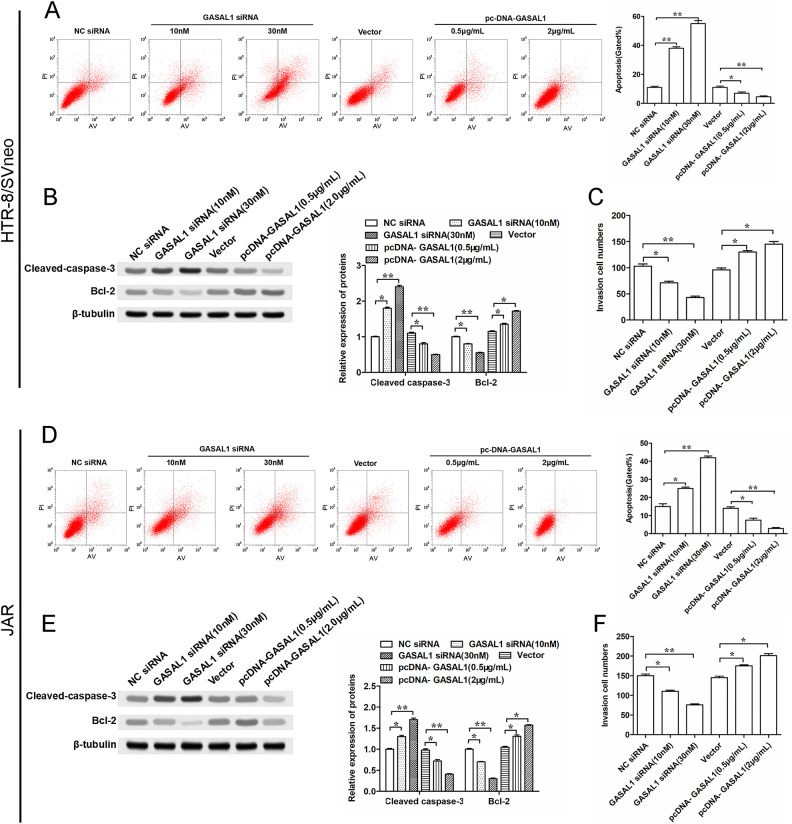
We evaluated the effects of GASAL1 on the cell apoptosis of HTR-8/SVneo and JAR cells by flow cytometry and Western blotting. The results showed that knockdown of GASAL1 increased the cell apoptosis rate (Fig. 3A, D), promoted the expression of the cleaved-caspase-3 protein, and suppressed the expression of Bcl-2 protein (Fig. 3B, E). On the other hand, overexpressed GASAL1 by transfecting pcDNA GASAL1 decreased the cell apoptosis ratio (Fig. 3A, D), inhibited cleaved-caspase-3 expression, and promoted Bcl-2 protein expression (Fig. 3B, E). As we know, the development of placental vasculature is impaired in PE patients, in which cell invasion associated with pregnancy plays an important role. In addition, silencing GASAL1 inhibited invasion of HTR-8/SVneo and JAR cells, whereas GASAL1 overexpression performed the opposite effect (Fig. 3C, F). Hence, our results suggest that lncRNA GASAL1 may influence apoptosis and invasion of HTR-8/SVneo and JAR cells.
Figure 3.
GASAL1 inhibits cell apoptosis and promotes cell invasion in HTR-8/SVneo and JAR cell lines. Cells were transfected with GASAL1 siRNA (10 nM or 30 nM) and pcDNA GASAL1 (0.5 μg/ml or 2 μg/ml). (A and D) Cell apoptosis was detected by flow cytometry after cell transfection for 24 h in HTR-8/SVneo and JAR cell lines. (B and E) Expression of apoptosis-related protein (cleaved-caspase-3 and Bcl-2) were determined by Western blotting in HTR-8/SVneo and JAR cell lines. (C and F) Transwell assay was used to analyze cell invasion in HTR-8/SVneo and JAR cell lines. The data were presented as the mean ± SEM, n = 3. Student’s t-test was used for comparisons between groups in this study. *P < 0.05 and **P < 0.01. GASAL1: lncRNA growth arrest associated lncRNA 1; lncRNA: long noncoding RNA; pcDNA: plasmid cloning DNA; siRNA: short interfering RNA.
LncRNA GASAL1 Directly Bound with SRSF1 Protein
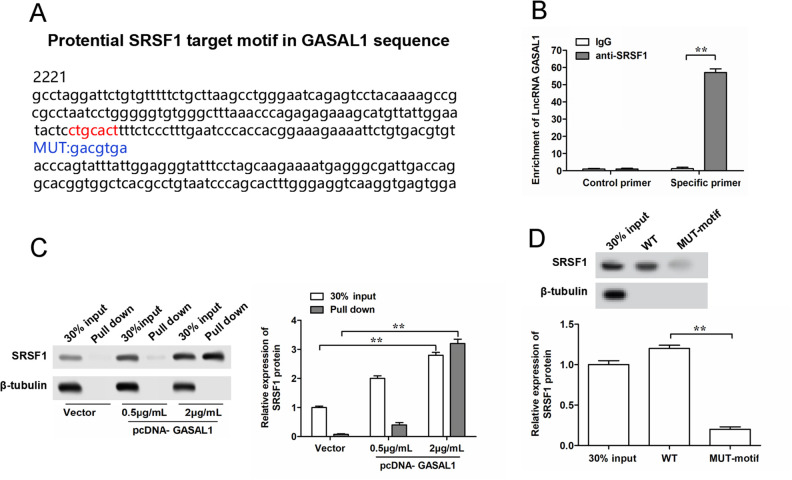
Interacting with functional RNA binding proteins (RBPs) is an important regulatory mechanism of lncRNAs. To explore the underlying molecular mechanism of GASAL1 in PE disease, we searched motifs of RBPs SRSF1 on the GASAL1 sequence by using the online bioinformatics tool StarBase 2.0 (http://starbase.sysu.edu.cn/). As shown in Fig. 4A, we found a target motif of SRSF1 protein (red fonts in Fig. 4A). Therefore, we speculated that GASAL1 might bind with SRSF1 protein to regulate the functions of HTR-8/SVneo and JAR cells. Then, we performed RNA-IP and RNA pull-down assays to verify their binding. RIP assays showed that the antibodies of SRSF1 could significantly enrich GASAL1 by using specific primers for GASAL1 compared with control primers, while not in that immunoprecipitated by IgG antibody (Fig. 4B). RNA pull-down assay revealed that the amount of the SRSF1 protein in the GASAL1–protein complexes was significantly increased with the upregulation of GASAL1 (Fig. 4C). In addition, RNA pull-down assay using WT or MUT biotinylated GASAL1 transcripts suggested that MUT-motif reduces the binding capacity of GASAL1 with SRSF1 compared with the WT motif (Fig. 4D). Together, lncRNA GASAL1 directly binds with SRSF1 protein to play an important role in PE.
Figure 4.
Prediction and validation of binding between GASAL1 and SRSF1 protein in HTR-8/SVneo cells. (A) Schema of SRSF1 binding sites in the GASAL1 sequence. Red fonts represent the binding motifs, and blue fonts represent the mutated nucleotides in the binding motifs. (B) Binding of GASAL1 and SRSF1 was validated by SRSF1-antibody-based RNA-immunoprecipitation assay. (C) Binding of GASAL1 and SRSF1 was validated by GASAL1-probe-based RNA pull-down assay. (D) Binding of GASAL1 and SRSF1 was validated by RNA pull-down assay using WT or MUT biotinylated GASAL1 transcripts. The data were presented as the mean ± SEM, n = 3. Student’s t-test was used for comparisons between groups in this study.*P < 0.05 and **P < 0.01. GASAL1: lncRNA growth arrest associated lncRNA 1; lncRNA: long noncoding RNA; MUT: mutated; SRSF1: serine/arginine splicing factor 1; WT: wild type.
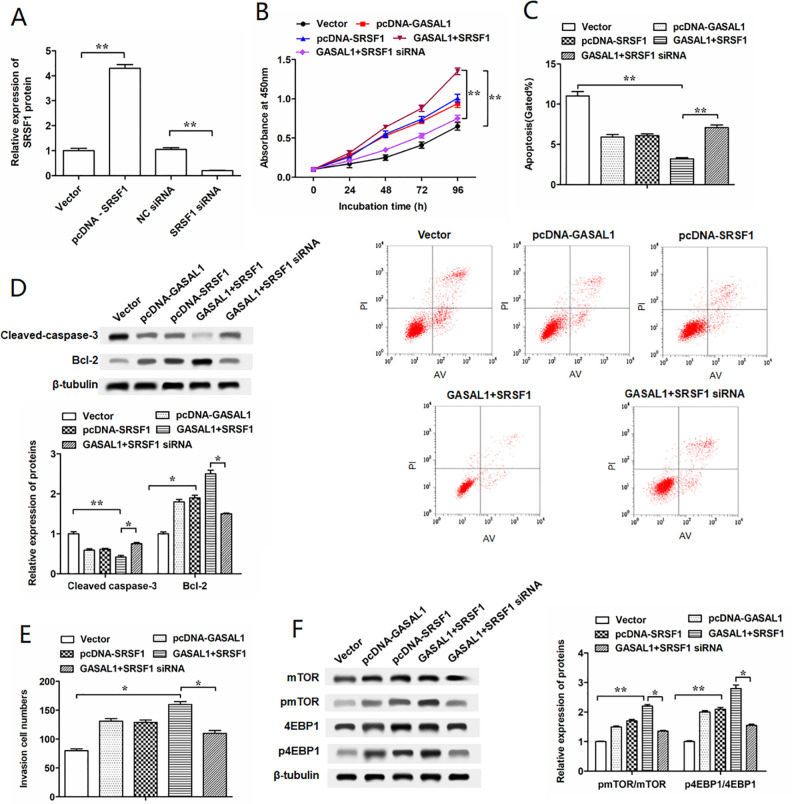
SRSF1 and GASAL1 Had a Synergistic Effect on Cell Functions in HTR-8/SVneo and JAR Cells, Whereas SRSF1 Downregulation Could Impair These Effects
To further verify the effects of GASAL1 binding with SRSF1 on regulating the proliferation, invasion, and apoptosis of HTR-8/SVneo cells, we performed antagonism experiments by transfecting with pcDNA-GASAL1 and SRSF1 siRNA into HTR-8/SVneo cells to reverse the results caused by transfecting with pcDNA-GASAL1 and pcDNA-SRSF1. Overexpression and interference efficiencies of SRSF1 were confirmed by Western blotting (Fig. 5A). Transfected with pcDNA-GASAL1 and pcDNA-SRSF1 further enhanced the proliferation and invasion of HTR-8/SVneo cells compared with transfected with pcDNA-GASAL1 or pcDNA-SRSF1 alone, but SRSF1 siRNA reversed this effect (Fig. 5B, E). Flow cytometry and Western blotting assay showed greater inhibition of cell apoptosis by transfecting with pcDNA-GASAL1 and pcDNA-SRSF1 than transfecting with pcDNA-GASAL1 or pcDNA-SRSF1 alone (Fig. 5C, D). To investigate whether GASAL1 and SRSF1 were involved in the mTOR pathway, we determined the expression of phosphorylated mTOR and 4EBP1. Our results indicated that pmTOR and p4EBP1 were upregulated by transfecting with pcDNA-GASAL1 and pcDNA-SRSF1, whereas SRSF1 siRNA had a remarkable opposite result (Fig. 5F). Besides, the effects of GASAL1 binding with SRSF1 on regulating the proliferation, invasion, and apoptosis of JAR cells were also investigated. After elevating or silencing the expression of SRSF1 in JAR cells using pcDNA-SRSF1 or SRSF1 siRNA (Supplemental Fig. 1A), we observed that the effects of GASAL1 overexpression on cell proliferation, apoptosis, and invasion were further enhanced by co-transfection with SRSF1 (Supplemental Fig. 1B-D). On the other hand, the phosphorylation levels of mTOR and 4EBP1 in JAR cells co-transfected with pcDNA-GASAL1 and pcDNA-SRSF1 were higher than that in cells transfected with pcDNA-GASAL1 or pcDNA-SRSF1 alone (Supplemental Fig. 1E). However, knockdown of SRSF1 weakened the synergistic effect of GASAL1 and SRSF1 on cell proliferation, invasion, apoptosis, and the activation of the mTOR pathway (Fig. S1B-S1E). These data indicated lncRNA GASAL1 involved in the mTOR pathway and influenced cell functions via SRSF1.
Figure 5.
GASAL1 enhances proliferation and invasion and suppresses cell apoptosis by the regulation of SRSF1 in HTR-8/SVneo cells. (A) Efficiencies of SRSF1 siRNA and pcDNA SRSF1 were confirmed by Western blotting. (B) Cell proliferation was detected by cell counting kit-8 assay at 0 h, 24 h, 48 h, 72 h, and 96 h in HTR-8/SVneo cells which were transfected with pcDNA-GASAL1, pcDNA-SRSF1, GASAL1 + SRSF1, and GASAL1 + SRSF1 siRNA. The concentrations of pcDNA-GASAL1, pcDNA-SRSF1, and SRSF1 siRNA transfected into the HTR-8SVneo cell line were 2 μg/ml, 1.5 μg/ml, and 50 nM, respectively. (C) Cell apoptosis was detected by flow cytometry in HTR-8/SVneo cell line after transfection for 24 h. (D) Cell apoptosis-related proteins (cleaved-caspase-3 and Bcl-2) were determined by Western blotting in transfected HTR-8/SVneo cell line. (E) Cell invasion was detected by the Transwell assay. (F) Relative expressions of pmTOR and p4EBP1 protein were detected with Western blotting. The data were presented as the mean ± SEM, n = 3. Student’s t-test or one-way analysis of variance was used for comparisons between groups in this study. *P < 0.05 and **P < 0.01. GASAL1: lncRNA growth arrest associated lncRNA 1; lncRNA: long noncoding RNA; pcDNA: plasmid cloning DNA; mTORl: mammalian target of rapamycin; siRNA, short interfering RNA; SRSF1: serine/arginine splicing factor 1.
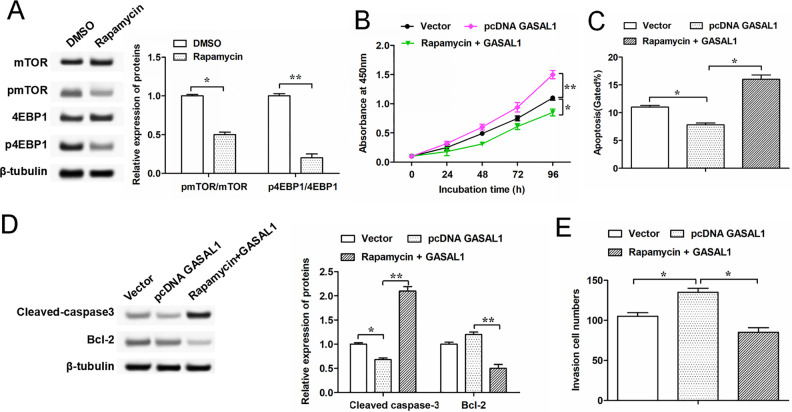
mTOR Signaling Pathway is Involved in GASAL1-Mediated Cell Proliferation, Cell Invasion, and Cell Apoptosis in HTR-8/SVneo and JAR Cells
The above results preliminarily show that GASAL1 is involved in the proliferation, invasion, and apoptosis of HTR-8/SVneo cells via the mTOR pathway. To further verify this underlying molecular mechanism, we next explored the alteration of the mTOR signaling pathway in HTR-8/SVneo cells by using the inhibitor of mTOR (rapamycin). The results indicated that the expressions of phosphorylated mTOR and 4EBP1 protein were downregulated in HTR-8/SVneo cells treated with rapamycin (Fig. 6A), which suggested that the mTOR pathway was suppressed. CCK-8 assay and Transwell assay showed that cell proliferation and invasion were promoted by transfecting with GASAL1, but rapamycin reversed this result (Fig. 6B, E). As shown in Fig. 6C, D, transfected with pcDNA-GASAL1 decreased the level of cell apoptosis, while treated with rapamycin promoted cell apoptosis (Fig. 6C, D). Moreover, JAR cells were also treated with rapamycin (Supplemental Fig. 2A). The results showed that rapamycin suppressed the effects of GASAL1 on proliferation (Supplemental Fig. 2B) and invasion (Supplemental Fig. 2C) capacity and increased apoptosis of JAR cells (Supplemental Fig. 2D), which showed that GASAL1 played the same role in the activation of mTOR pathway in JAR cells as in HTR-8/SVneo cells. These data further indicated that GASAL1 regulates proliferation, invasion, and apoptosis of HTR-8/SVneo and JAR cells through activating the mTOR signaling pathway.
Figure 6.
GASAL1 regulates HTR-8/SVneo cell proliferation, invasion, and apoptosis through activating the mTOR signaling pathway. (A) Expressions of pmTOR and p4EBP1 protein were determined in HTR-8/SVneo cells treated by dimethylsulfoxide or mTOR inhibitor (rapamycin, 10 nM for 72 h). (B) Cell proliferation was detected by cell counting kit-8 assay at 0 h, 24 h, 48 h, 72 h, and 96 h in HTR-8/SVneo cell line. After transfecting with pcDNA-GASAL1, cells were treated by rapamycin (10 nM for 72 h). (C and E) Cell apoptosis and invasion were detected by flow cytometry and Transwell assay in treated cells. (D) Expressions of cell apoptosis-related protein (cleaved-caspase-3 and Bcl-2) were determined by Western blotting in treated HTR-8/SVneo cells. The data were presented as the mean ± SEM, n = 3. Student’s t-test or one-way analysis of variance was used for comparisons between groups in this study. *P < 0.05 and **P < 0.01. GASAL1: lncRNA growth arrest associated lncRNA 1; lncRNA: long noncoding RNA; pcDNA: plasmid cloning DNA; mTOR: mammalian target of rapamycin; SRSF1: serine/arginine splicing factor 1.
Discussion
Various diseases that accompany pregnancy seriously threaten the health of pregnant women and fetuses22,23. PE is considered as a major cause of maternal and fetal morbidity and mortality, which is influenced by various factors, including parity, health status of the placenta, diet habits, and body size24. In recent years, lncRNAs had been introduced as a hotspot on the study of PE placenta. Numerous lncRNAs are abnormally expressed in placentas from PE patients and play important roles in the pathogenesis of PE6,9,25. For example, lncRNA-CCAT1 is upregulated in PE and promotes the progression of PE by inhibiting trophoblast proliferation and arresting cell cycle26. However, lncRNA-MVIH is downregulated in placentas from PE patients, and the silencing of MVIH expression inhibits cell growth, migration, invasion, and angiogenesis in trophoblast cells27. In our study, expression of lncRNA GASAL1 was significantly downregulated in placentas from PE patients suggesting that downregulation of GASAL1 in the placenta may be involved in the pathogenesis of PE.
Trophoblast cell dysfunction in proliferation, invasion, and apoptosis leads to insufficient placenta implantation and insufficient placental feeding, which induces PE occurring. LncRNA uc.187 is upregulated in PE, and silencing uc.187 enhances cell proliferation and invasion and reduces the cell apoptotic level28. The upregulation of lncRNA SPRY4-IT1 inhibits trophoblast cell migration and proliferation and enhances cell apoptosis and the epithelial–mesenchymal transition process29,30. LncRNA MALAT-1 is downregulated in PE and regulates proliferation, apoptosis, migration, and invasion of trophoblast cells31. In this study, further functional assays were conducted to prove how GASAL1 affects the biological functions of trophoblasts. Our results revealed that GASAL1 promoted trophoblast cell proliferation and invasion, whereas silencing GASAL1 reversed this effect. Furthermore, lncRNA GASAL1 is downregulated in several diseases, including gastric cancer, heart failures, and prostate carcinoma, which contributes to the changes in cell apoptosis and proliferation16–18. Our findings suggest that downregulated lncRNA GASAL1 might contribute to abnormal functions of trophoblast cells, thereby leading to placental defects and subsequent development of PE.
SR proteins are a family of RBPs that regulate both general and alternative splicing32. SRSF1 (also named ASF/SF2) is an important family member of highly conserved SR proteins and is reported to participate in various biological functions, including splicing regulation, RNA transport and translation, cell proliferation, invasion, and senescence19–21. For example, loss of the SRSF1 genes reduces embryo survival in mouse and Caenorhabditis elegans 33–35 and suppresses cell cycle and apoptosis in chicken DT-40 cells36. Recent studies showed that SRSF1 has a pro-oncogenic effect on different types of human cancers on the basis of its upregulation, which enhances proliferation and suppressed apoptosis37,38. SRSF1 facilitates the migration and proliferation of vascular smooth muscle cells through the Δ133p53/KLF5 pathway and, thus, the intimal thickening, after vascular injury39. In addition, SRSF1 has emerged as a crucial oncogene to promote cell proliferation and invasions in several solid tumors, such as hepatocellular carcinoma and gastric carcinoma40,41. In our research, we found that SRSF1 is a target of GASAL1 and plays a synergistic role with GASAL1 in PE. Moreover, our results revealed that SRSF1 is involved in regulating cell proliferation and invasion via the mTOR pathway, which is consistent with the reported studies41–43.
In conclusion, our study demonstrates that lncRNA GASAL1 is remarkably downregulated in human placentas from PE patients, which may alter the biological functions of trophoblast cells in vitro. The downregulation of GASAL1 significantly reduces the proliferation and invasion of trophoblast cells by modulating the SRSF1/mTOR axis. Taken together, this study illuminates an underlying molecular mechanism in which GASAL1 may be involved in the dysfunction of trophoblast cells, suggesting that GASAL1 may be a diagnostic and therapeutic target for PE.
Supplemental Material
Supplemental Material, Supplementary_materials for LncRNA GASAL1 Interacts with SRSF1 to Regulate Trophoblast Cell Proliferation, Invasion, and Apoptosis Via the mTOR Signaling Pathway by Jia Liu, Qing Zhang and Nan Ma in Cell Transplantation
Footnotes
Ethical Approval: This study was approved by the Ethics Committee at The Second Affiliated Hospital of Zhengzhou University (Zhengzhou, China).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the The Second Affiliated Hospital of Zhengzhou University of Ethics Committee’s or Institutional Review Board’s (approval number: 00357) approved protocols.
Statement of Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jia Liu  https://orcid.org/0000-0002-9823-925X
https://orcid.org/0000-0002-9823-925X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Kurtz WS, Glueck CJ, Hutchins RK, Sisk RA, Wang P. Retinal artery and vein thrombotic occlusion during pregnancy: markers for familial thrombophilia and adverse pregnancy outcomes. Clin Ophthalmol. 2016;10(Issue 1):935–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pedroso MA, Palmer KR, Hodges RJ, Costa FDS, Rolnik DL. Uterine artery doppler in screening for preeclampsia and fetal growth restriction. Rev Bras De Ginecol Obstet. 2018;40(05):287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pennington KA, Schlitt JM, Jackson DL, Schulz LC, Schust DJ. Preeclampsia: multiple approaches for a multifactorial disease. Dis Model Mech. 2012;5(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zárate A, Saucedo R, Valencia J, Manuel L, Hernández M. Early disturbed placental ischemia and hypoxia creates immune alteration and vascular disorder causing preeclampsia. Arch Med Res. 2014;45(7):519–524. [DOI] [PubMed] [Google Scholar]
- 5. Romero R, Chaiworapongsa T. Preeclampsia: a link between trophoblast dysregulation and an antiangiogenic state. J Clin Invest. 2013;123(7):2775–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Long W, Rui C, Song X, Dai X, Xue X, Lu Y. Distinct expression profiles of lncRNAs between early-onset preeclampsia and preterm controls. Clin Chimica Acta. 2016;463:193–199. [DOI] [PubMed] [Google Scholar]
- 7. Sun Q, Hao Q, Prasanth K V. Nuclear long noncoding rnas: key regulators of gene expression. Trends Genetics Tig. 2017;34(2):142–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo Q, Huang Y, Xu J, Wang W, Gao J, Su Y. Long non-coding RNA BANCR contributes to cervical adenocarcinoma migration by affecting epithelial-mesenchymal transition. Euro J Gynaecol Oncol. 2019;40(3):408–412. [Google Scholar]
- 9. He X, He Y, Xi B, Zheng J, Zeng X, Cai Q. LncRNAs expression in preeclampsia placenta reveals the potential role of LncRNAs contributing to preeclampsia pathogenesis. Plos One. 2013;8(11):e81437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tao H, Liu X, Liu X, Liu W, Wu D, Wang R. LncRNA MEG3 inhibits trophoblast invasion and trophoblast-mediated VSMC loss in uterine spiral artery remodeling. Mole Rep Develope. 2019;86(6):686–695. [DOI] [PubMed] [Google Scholar]
- 11. Wu D, Yang N, Xu Y, Wang S, Zhang Y, Sagnelli M, et al. lncRNA HIF1A Antisense RNA 2 Modulates Trophoblast Cell Invasion and Proliferation through Upregulating PHLDA1 Expression. Molecular therapy. Nucle acids. 2019;16(1):605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu JL, Wang YG, Gao GM, Feng L, Guo N, Zhang CX. Overexpression of lncRNA TCL6 promotes preeclampsia progression by regulating PTEN. Eur Rev Med Pharmacol Sci. 2019;23(10):4066–4072. [DOI] [PubMed] [Google Scholar]
- 13. Song X, Li C, Li J, Liu L, Meng L, Ding H. The long noncoding RNA uc.294 is upregulated in early-onset pre-eclampsia and inhibits proliferation, invasion of trophoblast cells (HTR-8/SVneo). J Cell Physiol. 2018;234(7):11001–11008. [DOI] [PubMed] [Google Scholar]
- 14. Xu Y, Ge Z, Zhang E, Zuo Q, Huang S, Yang N. The lncRNA TUG1 modulates proliferation in trophoblast cells via epigenetic suppression of RND3. Cell Death Dis. 2017;8(10):e3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Y, , Wang L, , Gao M, , Guan H. Long non-coding RNA TUG1 regulates the migration and invasion of trophoblast-like cells through sponging miR-204-5p. Clin Exp pharmacol physiol. 2018;46(4). [DOI] [PubMed] [Google Scholar]
- 16. Deng H, Ouyang W, Zhang L, Xiao X, Huang Z, Zhu W. LncRNA GASL1 is downregulated in chronic heart failure and regulates cardiomyocyte apoptosis. Cellular & Molecular Biology Letters. 2019;24(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Z, Liu H, Ju W, Xing Y, Zhang X, Yang J. LncRNA GASL1 inhibits growth and promotes expression of apoptosis-associated proteins in prostate carcinoma cells through GLUT-1. Oncolo letters. 2019;17 (6):5327–5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peng C, Li X, Yu Y, Chen J. LncRNA GASL1 inhibits tumor growth in gastric carcinoma by inactivating the Wnt/β-catenin signaling pathway. Exp Therapeutic Med. 2019;17(5):4039–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fregoso OI, Das S, Akerman M, Krainer AR. Splicing-factor oncoprotein srsf1 stabilizes p53 via rpl5 and induces cellular senescence. Mole Cell. 2013;50(1):56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Y, Xiao X, Zhang J, Choudhury R, Robertson A, Li K. A Complex network of factors with overlapping affinities repress splicing through intronic elements. Nat Struct Mole Bio. 2013;20(1):U36–U54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou X, Wang R, Li X, Yu L, Hua D, Sun C. Splicing factor SRSF1 promotes gliomagenesis via oncogenic splice-switching of MYO1B. J Clin Inves. 2019;129(2):676–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Englert-Golon M, Smolarek N, Burchardt B, Słopień R, Sajdak S. A case of cervical cancer diagnosed during pregnancy. Clin Exp Obs Gynecol. 2019;46(6):1005–1006. [Google Scholar]
- 23. Filipek A, Jurewicz E. Preeclampsia - a disease of pregnant women. Postepy biochem. 2018;64(4):232–239. [DOI] [PubMed] [Google Scholar]
- 24. Moradi MT, Rahimi Z, Vaisi-Raygani A. New insight into the role of long non-coding RNAs in the pathogenesis of preeclampsia. Hyp Pre. 2019;38(1):41–51. [DOI] [PubMed] [Google Scholar]
- 25. Song X, Luo X, Gao Q, Wang Y, Gao Q, Long W. Dysregulation of LncRNAs in placenta and pathogenesis of preeclampsia. Current Drug Targets. 2017;18(10):1165–1170. [DOI] [PubMed] [Google Scholar]
- 26. Li JL, Li R, Gao Y, Guo WC, Shi PX, Li M. LncRNA CCAT1 promotes the progression of preeclampsia by regulating CDK4. Eur Rev Med Pharmacol Sci. 2018;22(5):1216–1223. [DOI] [PubMed] [Google Scholar]
- 27. Zou Y, Li Q, Xu Y, Yu X, Zuo Q, Huang S, et al. Promotion of trophoblast invasion by lncRNA MVIH through inducing Jun-B. Journal of cellular and molecular medicine. 2018;22(2):1214–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cao C, Li J, Li J, Liu L, Cheng X, Jia R. Long Non-Coding RNA Uc.187 Is upregulated in preeclampsia and modulates proliferation, apoptosis, and Invasion of HTR-8/SVneo trophoblast cells. J Cell Bio. 2016;118(6):1462–1470. [DOI] [PubMed] [Google Scholar]
- 29. Zou Y, Jiang Z, Yu X, Sun M, Zhang Y, Zuo Q. Upregulation of Long noncoding RNA SPRY4-IT1 modulates proliferation, migration, apoptosis, and network formation in trophoblast cells HTR-8SV/neo. Plos One. 2013;8(11):e79598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zuo Q, Huang S, Zou Y, Xu Y, Jiang Z, Zou S. The Lnc RNA SPRY4-IT1 modulates trophoblast cell invasion and migration by affecting the epithelial-mesenchymal transition. Sci Rep. 2016;6(1):37183–37195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen H, Meng T, Liu X, Sun M, Tong C, Liu J. Long non-coding RNA MALAT-1 is downregulated in preeclampsia and regulates proliferation, apoptosis, migration and invasion of JEG-3 trophoblast cells. Int J Clin Exp Pathol. 2015;8(10):12718–12727. [PMC free article] [PubMed] [Google Scholar]
- 32. Karni R, Stanchina ED, Lowe SW, Sinha R, Mu D, Krainer AR. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nature Structural & Molecular Biology. 2007;14(3):185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhong XY, Wang P, Han J, Rosenfeld MG, Fu XD. SR proteins in vertical integration of gene expression from transcription to RNA processing to translation. Mol Cell. 2009;35(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Longman D, Johnstone IL, Caceres J F. Functional characterization of SR and SR-related genes in Caenorhabditis elegans. Embo j. 2000;19(7):1625–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu X, Yang D, Ding JH, Wang W, Chu PH, Nancy D, Dalton ASF/SF2-regulated CaMKIIdelta alternative splicing temporally reprograms excitation-contraction coupling in cardiac muscle. Cell. 2005;120 (1):59–72. [DOI] [PubMed] [Google Scholar]
- 36. Li X, Wang J, Manley JL. Loss of splicing factor ASF/SF2 induces G2 cell cycle arrest and apoptosis, but inhibits internucleosomal DNA fragmentation. Genes Dev. 2005;19 (22):2705–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anczukã3 WO, , Rosenberg AZ, , Akerman M, , Das S, , Zhan L, , Karni R. The splicing factor SRSF1 regulates apoptosis and proliferation to promote mammary epithelial cell transformation. Nat Struct Mole Bio. 2012;19(2):220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Das S, Anczuków O, Akerman M, Krainer AR. Oncogenic splicing factor SRSF1 is a critical transcriptional target of MYC. Cell Rep. 2012;1(2):110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xie N, Chen M, Dai R, Zhang Y, Zhao H, Song Z. SRSF1 promotes vascular smooth muscle cell proliferation through a Δ133p53/EGR1/KLF5 pathway. Nat Commun. 2017;8(1):16016–16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu Y, Zhang YM, Ma FB, Pan SR, Liu BZ. Long noncoding RNA HOXA11-AS promotes gastric cancer cell proliferation and invasion via SRSF1 and functions as a biomarker in gastric cancer. World J Gastroentero. 2019;25(22):2763–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Malakar P, Shilo A, Mogilevsky A, Stein I, Pikarsky E, Nevo Y. Long noncoding RNA MALAT1 promotes hepatocellular carcinoma development by SRSF1 Upregulation and mTOR Activation. Can Res. 2017; 77 (5):1155–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Karni R, Hippo Y, Lowe SW, Krainer AR. splicing-factor oncoprotein SF2/ASF activates mTORC1. Proce Nat Academy Sci USA. 2008;105(40):15323–15327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Michlewski G, Sanford JR, Caceres JF. The splicing factor SF2/ASF regulates translation initiation by enhancing phosphorylation of 4E-BP1. Mol Cell. 2008;30(2):179–189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Supplementary_materials for LncRNA GASAL1 Interacts with SRSF1 to Regulate Trophoblast Cell Proliferation, Invasion, and Apoptosis Via the mTOR Signaling Pathway by Jia Liu, Qing Zhang and Nan Ma in Cell Transplantation