Abstract
Diabetic retinopathy (DR) is one of the common complications of diabetes mellitus. C1q/TNF-related protein 9 (CTRP9) has been demonstrated to be associated with the progression of diabetes and relative complications. However, its role in DR and underlying action of mechanism are not yet well understood. In the present study, human retinal pigment epithelial ARPE-19 cells were cultured under high concentration of glucose to simulate hyperglycemia condition in vitro. Our results showed that the expression of CTRP9 was significantly decreased in high glucose (HG)–stimulated ARPE-19 cells. CTRP9 overexpression improved HG-caused reduction in cell viability of ARPE-19 cells. CTRP9 overexpression significantly attenuated HG-induced oxidative stress, as proved by decreased levels of reactive oxygen species and malondialdehyde, and increased superoxide dismutase activity. Moreover, CTRP9 also prevented apoptosis in ARPE-19 cells in response to HG stimulation with decreased caspse-3 activity and bax expression, as well as increased bcl-2 expression. In contrast, knockdown of CTRP9 aggravated HG-induced oxidative stress and apoptosis. Furthermore, CTRP9 significantly induced the activation of AMPK/Nrf2 pathway in HG-induced ARPE-19 cells. Notably, inhibiting AMPK or Nrf2 blocked the protective effect of CTRP9 on ARPE-19 cells exposed to HG stimulation. Taken together, our findings suggested a protective effect of CTRP9 on HG-induced ARPE-19 cells and a putative mechanism involving the activation of AMPK/Nrf2 signaling pathway.
Keywords: diabetic retinopathy, C1q/TNF-related protein 9, oxidative stress, apoptosis, AMPK/Nrf2 signaling pathway
Introduction
Diabetes mellitus (DM) is a chronic metabolic condition characterized by an inability to produce or effectively use insulin1,2. Diabetic retinopathy (DR) is a common and specific microvascular complication of DM, along with diabetic neuropathy3. As the worldwide prevalence of DM continues to increase, DR remains the leading cause of moderate-to-severe vision loss and blindness. Of the people with diabetes, about a third have signs of DR, and a third of these patients might have vision-threatening retinopathy4. Apart from its effects on vision, the presence of DR is also associated with a heightened risk of life-threatening systemic vascular complications such as stroke, coronary heart disease, and heart failure4. Conventional therapy is unable to reverse the visual loss, but the outlook for future treatment modalities is promising.
In recent years, C1q/TNF-related protein (CTRP) family has attracted much interest because of its anti-inflammatory and insulin-sensitizing effects5. The CTRP family of proteins have been found to be associated with metabolic diseases including obesity, insulin resistance, and type 2 diabetes (T2DM)6. For instance, Bai et al.7 demonstrated that serum CTRP1 is significantly higher and CTRP12 is significantly lower in T2DM participants, which support CTRP1 and CTRP12 as potential novel biomarkers for the prediction and early diagnosis of T2DM. Chinese patients with obesity and T2DM exhibit lower plasma CTRP3 concentration than healthy subjects. Low levels of CTRP3 are not negatively correlated with insulin resistance8. CTRP5 levels in patients with coronary artery disease and T2DM are lower than that of in control group, and CTRP5 exerts a potential role in inflammatory process9.
Moreover, some CTRP proteins are involved in the progression of diabetic complications. Moradi et al. reported that the decreased serum level of CTRP3 in patients with diabetic nephropathy is associated with pathologic mechanism of diabetic nephropathy10. CTRP3 is implicated in the pathogenesis of DR and serves as a novel biomarker for DR11. Additionally, CTRP9, a member of CTRP family, is known to be an adipokine involved in communication among skeletal muscle, liver, and adipose tissue. It also plays a critical role in T2DM12,13. Peterson et al. reported that the circulating levels of CTRP9 were downregulated in diet-induced obese mice, and CTRP9 transgenic mice had a greatly improved metabolic profile with markedly reduced fasting insulin and glucose levels14. However, the role of CTRP9 in DR and underlying action of mechanism remain unclear. The present study was to investigate the effects of CTRP9 on oxidative stress and apoptosis in ARPE-19 cells cultured with high glucose (HG).
Materials and Methods
Cell Culture and Treatment
The human retinal pigment epithelial ARPE-19 cells purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) were cultivated with ATCC-formulated Dulbecco’s modified Eagle’s medium/F12 (ATCC) containing 10% fetal bovine serum in an incubator containing 5% CO2 at 37°C. Cells in control group were cultured in normal glucose medium (5.5 mM). Cells in HG group were cultured in HG medium (25 mM).
CTRP9 Overexpression and Cell Transfection
The CTRP9 expressing plasmid pcDNA3.0-CTRP9 was constructed by inserting the full length of CTRP9 into the pcDNA3.0 vector. The pcDNA3.0-CTRP9 or empty pcDNA3.0 vector was transfected into ARPE-19 cells using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s manuals.
Si-CTRP9 and Cell Transfection
CTRP9 small interfering RNA (si-CTRP9), si-Nrf2, and negative control siRNA (si-NC) were designed and synthesized by GenePharma (Shanghai, China). Then the siRNAs were transfected into the ARPE-19 cells using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol.
Cell Viability Assay
Cell viability of ARPE-19 cells was assessed with the cell counting kit-8 (CCK-8; Dojindo, Kumamoto, Japan). Cells (1.0 × 104 cells/well) were seeded into 96-well plates and incubated at 37°C overnight prior to the indicated treatments. After the desired transfections, cells were incubated for an additional 4 h with 10 µl of CCK-8 solution. The plates were read by a microplate reader (Bio-Rad, Hercules, CA, USA) at the wavelength of 450 nm.
Quantitative Real-time Polymerase Chain Reaction Analysis
A 10 ng total RNA isolated by TRIzol Reagent (Invitrogen) was used for the synthesis of cDNA using the RevertAid RT Reverse Transcription kit ( Thermo Fisher Scientific, Waltham, MA, USA). Then polymerase chain reaction (PCR) mixtures were prepared for the real-time (RT)-PCR reaction using the SensiFAST Real-Time PCR kit (Bioline, London, UK) under the condition of 95°C for 45 s, followed by 40 cycles of 95°C for 30 s and 56°C for 35 s. Sequences of primers used for PCR were as follows—CTRP9 forward: 5’-TGG AAG CCA GTG CCT GTC ATG T-3’ and reverse: 5’-AGC CGA CTC GAA GCT GGA GGT-3’; HO-1 forward: 5’-GAG CCT GCA GCT TCT CAG AT-3’ and reverse: 5’-CGG CCG GTC ACA TTT ATG CT-3’; NQO1 forward: 5’-CGC AGA CCT TGT GAT ATT CCA G-3’ and reverse: 5’-CGT TTC CAT CCT TCC AGG-3’; β-actin forward: 5’-GAC CTC TAT GCC AAC ACA GT-3’ and reverse: 5’-AGT ACT TGC GCT CAG GAG GA-3’. Quantitative expression levels of CTRP9, HO-1, and NQO1 were determined using the 2-ΔΔCq method.
Western Blot Analysis
ARPE-19 cells were scraped in RIPA lysis buffer (Beyotime, Shanghai, China) to prepare the cellular lysates. Nuclear extracts were prepared using Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime). After determination of protein concentration, aliquots of lysates (50 μg) were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (Takara Biotechnology, Dalian, China). The separated proteins were electrotransferred onto the pure nitrocellulose blotting membrane (Millipore, Bedford, MA, USA) and then the nonspecific bindings were blocked with 5% nonfat milk for 2 h at room temperature. After that, the membranes were incubated at 4°C overnight with the following primary antibodies: anti-CTRP9 (Abcam, Cambridge, MA, USA), anti-bax (Abcam), anti-bcl-2 (Abcam), anti-p-AMPK (Cell Signaling Technology, Boston, MA, USA), anti-AMPK (Cell Signaling Technology), anti-Nrf2 (Abcam), anti-lamin B1 (Abcam), and anti-β-actin (Cell Signaling Technology). After washing with tris-buffered saline with tween for three times, the membranes were subsequently incubated with horseradish peroxidase–coupled secondary antibodies (Cell Signaling Technology) for 1 h at room temperature. The blots were visualized using Pierce enhanced chemiluminescence western blotting reagent (Pierce Biotechnology, Rockford, IL, USA). Subsequently, the intensity of the blots was analyzed using Quantity One 4.6 software (Bio-Rad).
Detection of Reactive Oxygen Species, Malondialdehyde, and Superoxide Dismutase
The cellular lysates of ARPE-19 cells were prepared as described above and used for the detection of oxidative stress indicators. The changes in the intracellular reactive oxygen species (ROS) production, malondialdehyde (MDA) content, and superoxide dismutase (SOD) activity in the ARPE-19 cells were detected using commercial kits obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacturer’s instruction.
Cell Apoptosis Assay
Cell apoptosis of ARPE-19 cells was verified through the measurement of caspase-3 activity using a fluorometric caspase-3 assay kit (BioVision Technologies, Milpitas, CA, USA) as per manufacturer’s instructions. The samples were read using a FACSAria (Becton Dickinson, Newyork, NJ, USA) with a 400-nm excitation filter and 505-nm emission filter.
Statistical Analysis
All experiments were performed in triplicate and the results were analyzed by one-way analysis of variance followed by Tukey’s test using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). All of the values were expressed as the mean ± standard deviation. P < 0.05 was used to indicate statistical significance.
Results
CTRP9 was Lowly Expressed in HG-Induced ARPE-19 Cells
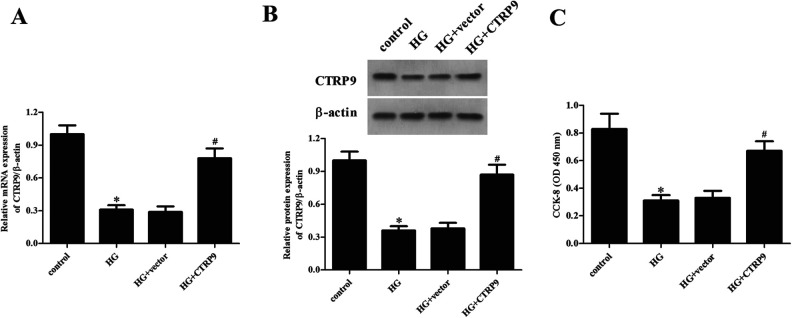
To evaluate the role of CTRP9 in DR, in vitro assays were performed using ARPE-19 cells cultured under HG condition. The quantitative real-time polymerase chain reaction (qRT-PCR) and western blot were used for the detection of the mRNA and protein levels of CTRP9. As shown in Fig. 1A, HG stimulation lead to significant decrease in the mRNA level of CTRP9 in ARPE-19 cells when compared with ARPE-19 cells in control group. Consistently, the protein level of CTRP9 was also downregulated in HG-induced ARPE-19 cells (Fig. 1B).
Figure 1.
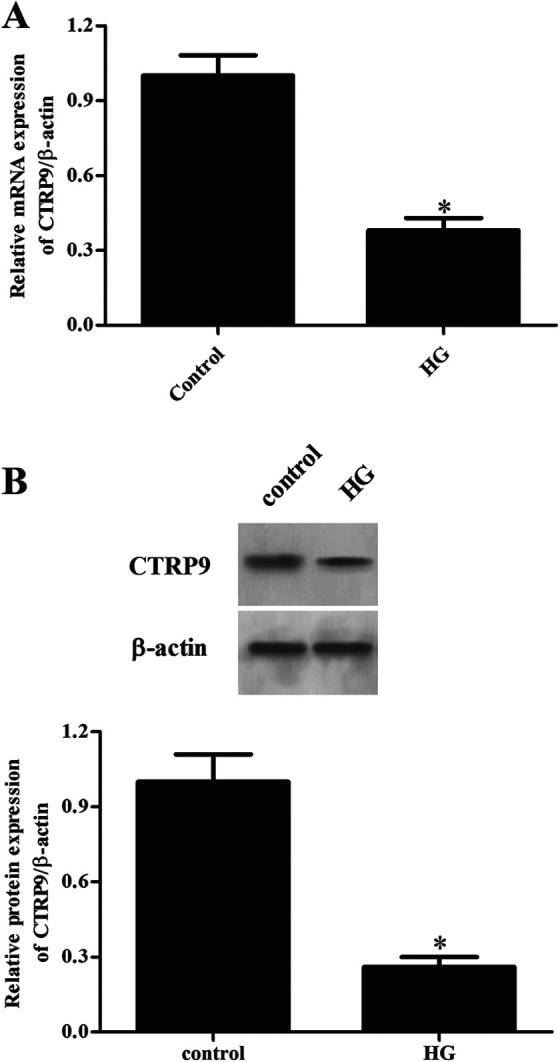
Changes in the expression levels of CTRP9 in HG-induced ARPE-19 cells. ARPE-19 cells were cultured in HG medium (25 mM). Then the qRT-PCR and western blot were used for the detection of the mRNA (A) and protein levels of CTRP9 (B). *Significantly different compared to control group. CTRP9: C1q/TNF-related protein 9; HG: high glucose; qRT-PCR: quantitative real-time polymerase chain reaction.
CTRP9 Improved Cell Viability in ARPE-19 Cells Exposed to HG
To investigate the precise role of CTRP9 in regulating HG-induced ARPE-19 cell injury, CTRP9-overexpressing ARPE-19 cells were constructed. Cells transfected with pcDNA3.0-CTRP9 showed significant increases in the mRNA and protein levels of CTRP9 in HG-stimulated ARPE-19 cells, as confirmed by western blot (Fig. 2A, B). Moreover, CCK-8 assay demonstrated a significant reduction in cell viability of ARPE-19 cells exposed to HG, as compared to the controls. However, the reduced cell viability of ARPE-19 cells was reversed by overexpression of CTRP9 (Fig. 2C).
Figure 2.
Effect of CTRP9 on cell viability in ARPE-19 cells exposed to HG. ARPE-19 cells were transfected with pcDNA3.0-CTRP9 or vector for 48 h, then they were exposed to HG stimulation. (A and B) The mRNA and protein levels of CTRP9 were determined by qRT-PCR and western blot. (C) Cell viability was detected by CCK-8 assay. *Significantly different compared to control group; #Significantly different compared to HG + vector group. CCK-8: cell counting kit-8; CTRP9: C1q/TNF-related protein 9; HG: high glucose; qRT-PCR: quantitative real-time polymerase chain reaction.
CTRP9 Suppressed HG-Induced Oxidative Stress in ARPE-19 Cells
We then examined the effect of CTRP9 on oxidative stress in HG-stimulated ARPE-19 cells. The results showed that HG stimulation significantly increased the production of ROS and MDA in ARPE-19 cells; however, overexpression of CTRP9 greatly inhibited HG-induced the production of ROS and MDA (Fig. 3A, B). Expectedly, the HG-caused decrease in the SOD activity was prevented by CTRP9 (Fig. 3C).
Figure 3.
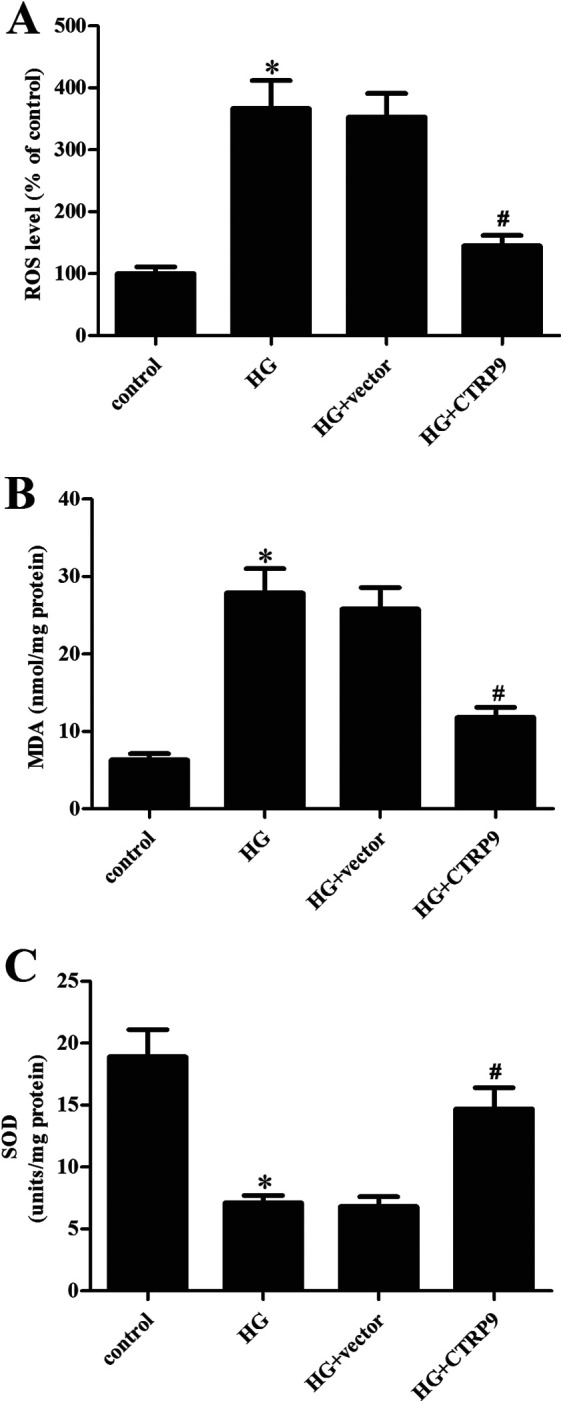
Effect of CTRP9 on oxidative stress in HG-induced ARPE-19 cells. ARPE-19 cells were transfected with pcDNA3.0-CTRP9 or vector for 48 h, then they were exposed to HG stimulation. To evaluate oxidative stress, the production of ROS (A) and MDA (B) and the SOD activity (C) were determined. *Significantly different compared to control group. #Significantly different compared to HG + vector group. CTRP9: C1q/TNF-related protein 9; HG: high glucose; MDA: malondialdehyde; ROS: reactive oxygen species; SOD: superoxide dismutase.
CTRP9 Reduced Cell Apoptosis Induced by HG in ARPE-19 Cells
Next, we evaluated the cell apoptosis using western blot to determine the changes in the expression levels of bax and bcl-2. The bax expression was significantly increased, while bcl-2 expression was decreased in HG-stimulated ARPE-19 cells. However, these changes were attenuated by CTRP9 overexpression (Fig. 4A). Cell apoptosis was also assessed by detecting the caspase-3 activity. The HG-caused increased caspase-3 activity was reduced in CTRP9-overexpressing ARPE-19 cells (Fig. 4B).
Figure 4.
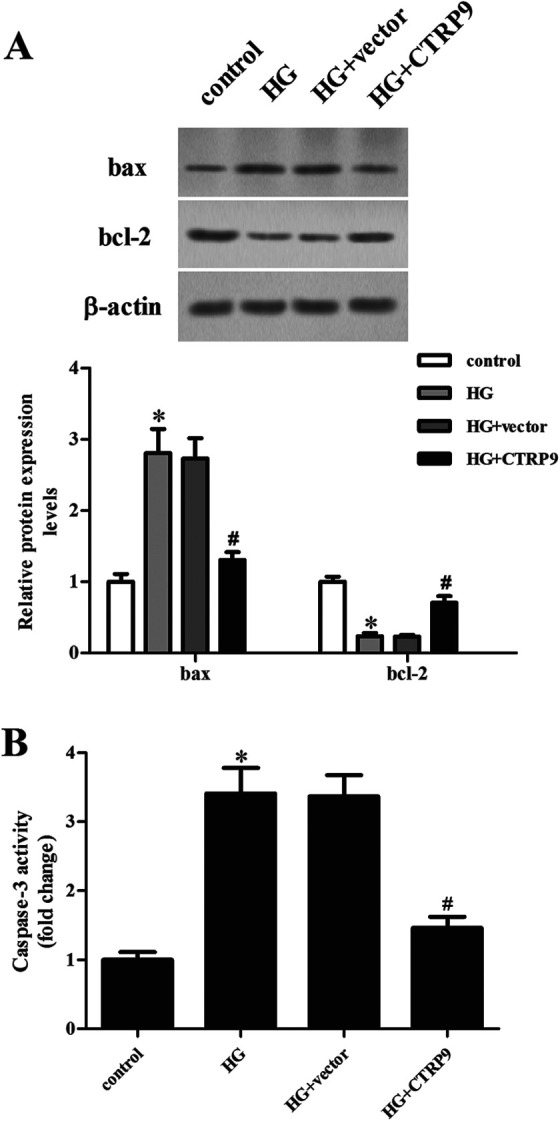
Effect of CTRP9 on cell apoptosis in HG-induced ARPE-19 cells. ARPE-19 cells were transfected with pcDNA3.0-CTRP9 or vector for 48 h, then they were exposed to HG stimulation. (A) Western blot was used for the detection of the expression levels of bax and bcl-2. (B) Caspase-3 activity was detected to indicate apoptosis. *Significantly different compared to control group; #Significantly different compared to HG + vector group. CTRP9: C1q/TNF-related protein 9; HG: high glucose.
Knockdown of CTRP9 Promoted Oxidative Stress and Apoptosis in HG-Stimulated ARPE-19 Cells
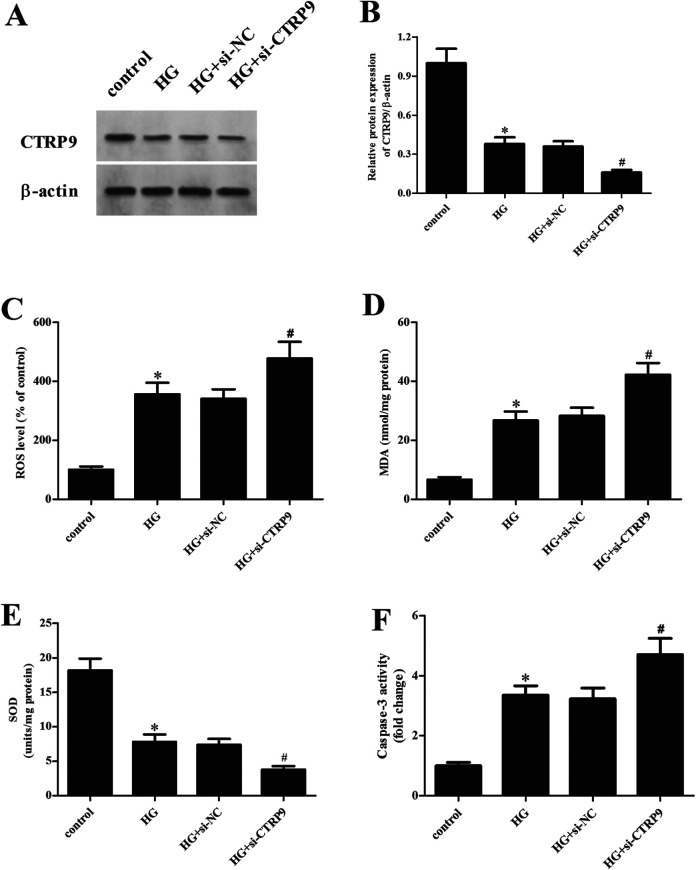
To further confirm the role of CTRP9, the CTRP9 was knocked down in ARPE-19 cells through transfection with si-CTRP9. The downregulation of CTRP9 in si-CTRP9-transfected cells was confirmed by qRT-PCR and western blot analysis (Fig. 5A, B). Furthermore, we observed that the increased ROS and MDA levels and the decreased SOD activity caused by HG stimulation were attenuated by si-CTRP9 transfection (Fig. 5C–E). In addition, the upregulated caspase-3 activity in HG-stimulated ARPE-19 cells was enhanced by knockdown of CTRP9 (Fig. 5F).
Figure 5.
Effect of CTRP9 knockdown on oxidative stress and apoptosis in HG-stimulated ARPE-19 cells. ARPE-19 cells were transfected with si-CTRP9 or si-NC for 48 h, then they were exposed to HG stimulation. (A) The protein expression of CTRP9 was measured by western blot analysis. (B) Quantification analysis of CTRP9. (C–E) The production of ROS and MDA and the SOD activity were determined to indicate oxidative stress. (F) Caspase-3 activity was determined to indicate apoptosis. *Significantly different compared to control group; #Significantly different compared to HG + si-NC group. CTRP9: C1q/TNF-related protein 9; HG: high glucose; MDA: malondialdehyde; ROS: reactive oxygen species; SOD: superoxide dismutase.
CTRP9 Enhanced the Activation of AMPK/Nrf2 Pathway in HG-Stimulated ARPE-19 Cells
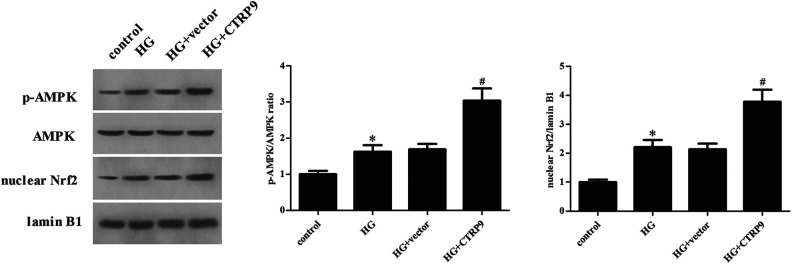
Regarding AMPK/Nrf2 signaling pathway activation, the expression levels of p-AMPK and Nrf2 were significantly increased in HG-stimulated ARPE-19 cells, whereas when ARPE-19 cells were transfected with pcDNA3.0-CTRP9, the increased expression levels of p-AMPK and Nrf2 were enhanced (Fig. 6).
Figure 6.
Effect of CTRP9 on the activation of AMPK/Nrf2 pathway in HG-stimulated ARPE-19 cells. Western blot was used for the detection of the expression levels of AMPK, p-AMPK, and Nrf2. *Significantly different compared to control group; #Significantly different compared to HG + vector group. CTRP9: C1q/TNF-related protein 9; HG: high glucose.
CTRP9 Upregulated the Expression of HO-1 and NQO1 in HG-Stimulated ARPE-19 Cells
We confirmed the changes in the mRNA levels of downstream genes of AMPK/Nrf2 including HO-1 and NQO1. Results in Fig. 7A showed that the upregulated mRNA level of HO-1 in HG-stimulated ARPE-19 cells was increased after transfection with pcRNA3.0-CTRP9. Similarly, we found that CTRP9 also enhanced the upregulation of NQO1 mRNA level in HG-stimulated ARPE-19 cells (Fig. 7B).
Figure 7.
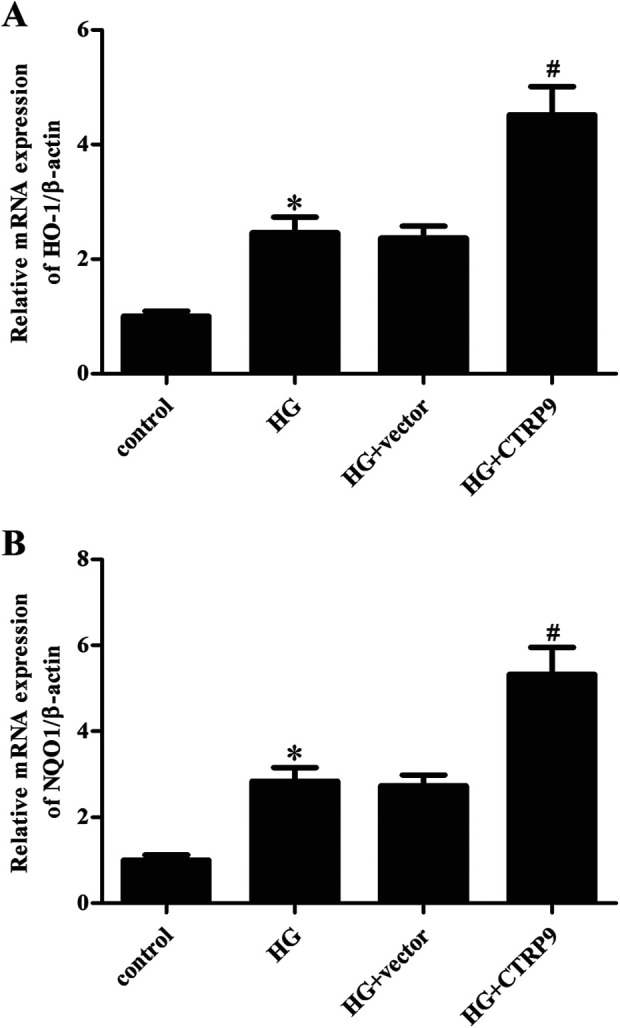
Effect of CTRP9 on the expression of HO-1 and NQO1 in HG-stimulated ARPE-19 cells. The mRNA levels of HO-1 (A) and NQO1 (B) were determined by qRT-PCR. *Significantly different compared to control group; #Significantly different compared to HG + vector group. CTRP9: C1q/TNF-related protein 9; HG: high glucose; qRT-PCR: quantitative real-time polymerase chain reaction.
AMPK Inhibition Partially Reversed CTRP9-Mediated Nrf2 Promotion and Retinal Protection
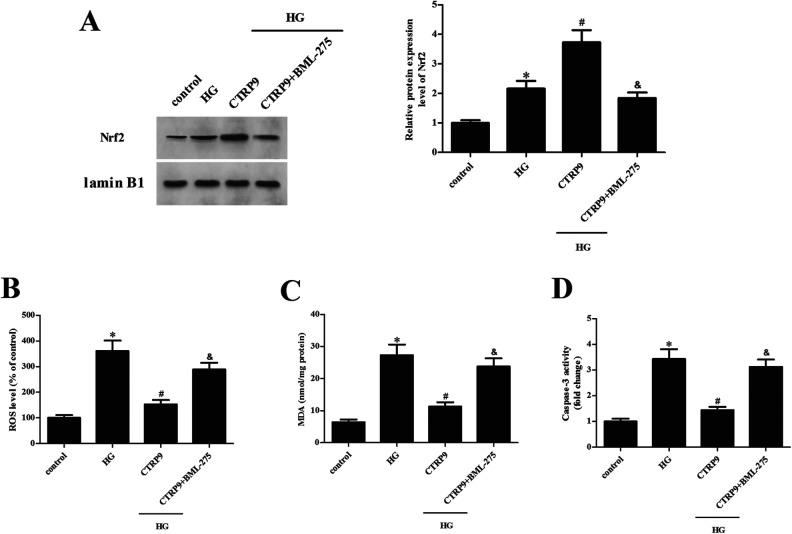
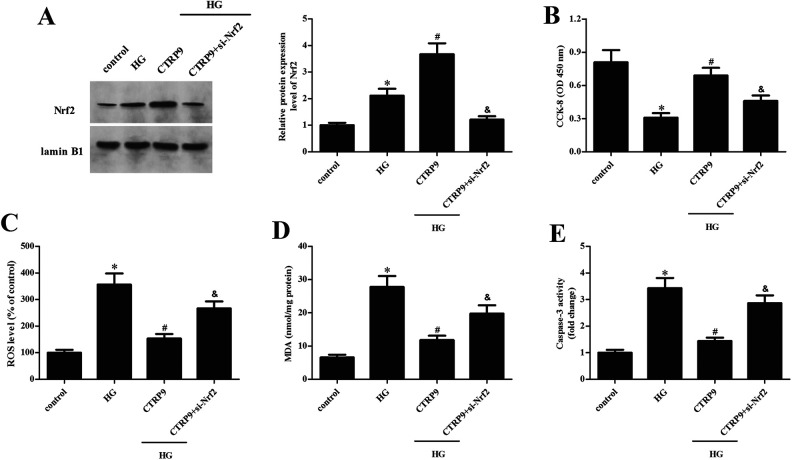
Next, ARPE-19 cells were transfected with pcRNA3.0-CTRP9 for 48 h in the presence of AMPK inhibitor BML-275, and then they were subjected to HG stimulation. The CTRP9-caused increase in the Nrf2 expression level was significantly attenuated by BML-275, which confirmed the role of AMPK in the CTRP9-mediated Nrf2 activation (Fig. 8A). CTRP9-mediated suppressive effects on HG-induced ROS production, MDA content, and caspase-3 activity were greatly abrogated by BML-275 in ARPE-19 cells (Fig. 8B–D).
Figure 8.
Effect of BML-275 on CTRP9-mediated Nrf2 promotion and retinal protection. ARPE-19 cells were transfected with pcDNA3.0-CTRP9 for 48 h in the presence of AMPK inhibitor BML-275, and then they were subjected to HG stimulation. (A) Western blot was used to assess Nrf2 expression. (B–D) Detection of ROS production, MDA content, and caspase-3 activity. *Significantly different compared to control group. #Significantly different compared to HG group. &Significantly different compared to HG + CTRP9 group. CTRP9: C1q/TNF-related protein 9; HG: high glucose; MDA: malondialdehyde; ROS: reactive oxygen species.
Knockdown of Nrf2 Reversed CTRP9-Mediated Retinal-Protective Effect
Finally, ARPE-19 cells were cotransfected with pcRNA3.0-CTRP9 and si-Nrf2 for 48 h, then subjected to HG stimulation. We found that transfection of Nrf2 siRNA significantly decreased the nuclear expression of Nrf2 in ARPE-19 cells transfected with pcRNA3.0-CTRP9 following HG stimulation (Fig. 9A). Moreover, Nrf2 silencing markedly reduced the promotion effect of CTRP9 on the viability of HG-exposed ARPE-19 cells (Fig. 9B). In addition, the suppressive effects of CTRP9 overexpression on HG-induced ROS production, MDA content, and caspase-3 activity were also markedly abolished by Nrf2 silencing (Fig. 9C–E).
Figure 9.
Effect of Nrf2 knockdown on CTRP9-mediated retinal-protective effect. ARPE-19 cells were cotransfected with pcDNA3.0-CTRP9 and si-Nrf2 for 48 h, then subjected to HG stimulation. (A) Western blot was used to assess Nrf2 expression. (B) Cell viability was detected by CCK-8 assay. (C–E) Detection of ROS production, MDA content, and caspase-3 activity. *Significantly different compared to control group. #Significantly different compared to HG group. &Significantly different compared to HG + CTRP9 group. CCK-8: cell counting kit-8; CTRP9: C1q/TNF-related protein 9; HG: high glucose; MDA: malondialdehyde; ROS: reactive oxygen species.
Discussion
DR remains the most prevalent cause of visual impairment in the working-age population. Decades of research into the pathogenesis and management of DR have revolutionized our understanding of the disease process15. A number of laboratory studies have showed that hyperglycemia is implicated in the pathogenesis of DR, which includes oxidative stress, polyol and hexosamine pathway activity, advanced glycation end-product formation, and activation of protein kinase C isoforms16. Retinal pigment epithelium (RPE) is the outer layer of the retina, which is crucial to convert light energy into electrical impulses and control our sense of vision17. Under hyperglycemia condition, intracellular excess glucose flux can damage the retina, caused by several seemingly mechanisms, i.e., mitochondrial overproduction of ROS and the ROS-driven oxidative stress and apoptosis18. Therefore, attenuating the ROS-driven oxidative stress and apoptosis of RPE cells under HG condition may prevent the development of DR.
CTRP9 has been identified as an antioxidant, which is associated with increased risk of various diseases. It serves as a promising scavenger of free radicals in myocardial ischemia/reperfusion injury under diabetes19. Globular CTRP9 protects cardiomyocytes from palmitic acid–induced oxidative stress through regulation of autophagy20. CTRP9 induces mitochondrial biogenesis and protects against endothelial oxidative damage with reduced ROS production and increased the activities of endogenous antioxidant enzymes21. CTRP9 treatment ameliorates ox-LDL-induced endothelial dysfunction via regulating antioxidant enzymes levels and eNOS expression22.
In addition, previous researches have demonstrated that CTRP9 plays a beneficial role in DM and diabetic complications. High plasma CTRP9 levels are associated with atherosclerosis in patients with T2DM, indicating a potential role of CTRP9 in atherosclerosis progression in human T2DM23. Reduction of CTRP9 production during diabetes plays a causative role in platelet hyperactivity, contributing to platelet-induced diabetic cardiovascular damage24. CTRP9 attenuates diabetic nephropathy through ameliorating glomerular and tubular glycogen accumulation, fibrosis, hyperglycemia-mediated oxidative stress, and apoptosis in diabetic db/db mice25. CTRP9 regulates growth, differentiation, and apoptosis in human keratinocytes, providing a potentially effective therapeutic strategy for diabetic wounds26. Moreover, CTRP9 can inhibit the inflammation of DR and protect blood-retinal barrier via decreasing proinflammatory cytokines in db/db mice27. Hence, we further investigated the role of CTRP9 in DR in vitro using ARPE-19 cells. We found that CTRP9 was lowly expressed in HG-induced ARPE-19 cells. CTRP9 improved cell viability and attenuated oxidative stress and apoptosis in HG-induced ARPE-19 cells, whereas knockdown of CTRP9 exhibited opposite effects with CTRP9.
AMPK is a crucial cellular regulator that can maintain the energy supply and demand balance of cells in the body28. Notably, activated AMPK promotes activation of Nrf2, which is a crucial antioxidative transcriptional factor29. The AMPK/Nrf2 pathway has been shown to play a crucial part in the process of resisting oxidative stress and inflammation30,31. Zhang et al.32 reported that CTRP9 inhibits oxLDL-induced inflammatory response in RAW 264.7 macrophages via AMPK activation. CTRP9 inhibits the cholesterol-induced vascular smooth muscle cells phenotype switch and cell dysfunction by activating the AMPK pathway33. CTRP9 protects against cardiac injury following ischemia-reperfusion via an AMPK-dependent mechanism34. Additionally, CTRP9 alleviates inflammation to ameliorate myocardial infarction in rats by activating Nrf235. Thus, we further explore the role of AMPK/Nrf2 signaling in the protective effect of CTRP9. The results showed that CTRP9 enhanced the activation of the AMPK/Nrf2 pathway in HG-stimulated ARPE-19 cells. However, inhibition of AMPK partially reversed CTRP9-mediated Nrf2 promotion and retinal protection in HG-exposed ARPE-19 cells. Additionally, knockdown of Nrf2 also reversed CTRP9-mediated retinal-protective effects. These findings suggest that CTRP9 exerted protective effect against HG-induced oxidative damage via activating the AMPK/Nrf2 signaling pathway.
In conclusion, these findings show that CTRP9 attenuates HG-induced oxidative damage and apoptosis in ARPE-19 cells. The protective effect of CTRP9 might be mediated by the activation of AMPK/Nrf2 signaling pathway. These data suggest that CTRP9 may be a promising functional target for the treatment and prevention of DR.
Footnotes
Ethical Approval: Ethical Approval is not applicable for this article.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Natural Science Basic Research Project of Shaanxi Province, China (2019JM-578).
ORCID iD: Cheng Pei  https://orcid.org/0000-0003-1190-5457
https://orcid.org/0000-0003-1190-5457
References
- 1. Schmidt AM. Highlighting diabetes mellitus: the epidemic continues. Arterioscler Thromb Vasc Biol. 2018;38(1):e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guthrie RA, Guthrie DW. Pathophysiology of diabetes mellitus. Crit Care Nurs Q. 2004;27(2):113–125. [DOI] [PubMed] [Google Scholar]
- 3. Heng LZ, Comyn O, Peto T, Tadros C, Ng E, Sivaprasad S, Hykin PG. Diabetic retinopathy: pathogenesis, clinical grading, management and future developments. Diabet Med. 2013;30(6):640–650. [DOI] [PubMed] [Google Scholar]
- 4. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. [DOI] [PubMed] [Google Scholar]
- 5. Seldin MM, Tan SY, Wong GW. Metabolic function of the CTRP family of hormones. Rev Endocr Metab Disord. 2014;15(2):111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schaffler A, Buechler C. CTRP family: linking immunity to metabolism. Trends Endocrinol Metab. 2012;23(4):194–204. [DOI] [PubMed] [Google Scholar]
- 7. Bai B, Ban B, Liu Z, Zhang MM, Tan BK, Chen J. Circulating C1q complement/TNF-related protein (CTRP) 1, CTRP9, CTRP12 and CTRP13 concentrations in Type 2 diabetes mellitus: in vivo regulation by glucose. PLoS One. 2017;12(2):e0172271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qu H, Deng M, Wang H, Wei H, Liu F, Wu J, Deng H. Plasma CTRP-3 concentrations in Chinese patients with obesity and type II diabetes negatively correlate with insulin resistance. J Clin Lipidol. 2015;9(3):289–294. [DOI] [PubMed] [Google Scholar]
- 9. Moradi N, Fadaei R, Rashidbeygi E, Bagheri Kargasheh F, Malek M, Shokoohi Nahrkhalaji A, Fallah S. Evaluation of changing the pattern of CTRP5 and inflammatory markers levels in patients with coronary artery disease and type 2 diabetes mellitus. Arch Physiol Biochem. 2020;23:1–6. [DOI] [PubMed] [Google Scholar]
- 10. Moradi N, Fadaei R, Khamseh ME, Nobakht A, Rezaei MJ, Aliakbary F, Vatannejad A, Hosseini J. Serum levels of CTRP3 in diabetic nephropathy and its relationship with insulin resistance and kidney function. PLoS One. 2019;14(4):e0215617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yan Z, Zhao J, Lu G, Zhang Y, Rui G, Cao X, Bond LW, Xin M, Wang Y, Peterson JM. CTRP3 is a novel biomarker for diabetic retinopathy and inhibits HGHL-induced VCAM-1 expression in an AMPK-dependent manner. Plos One. 2017;12(6):e0178253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahmed SF, Shabayek MI, Abdel Ghany ME, El-Hefnawy MH, El-Mesallamy HO. Role of CTRP3, CTRP9 and MCP-1 for the evaluation of T2DM associated coronary artery disease in Egyptian postmenopausal females. PLoS One. 2018;13(12):e0208038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moradi N, Fadaei R, Emamgholipour S, Kazemian E, Panahi G, Vahedi S, Saed L, Fallah S. Association of circulating CTRP9 with soluble adhesion molecules and inflammatory markers in patients with type 2 diabetes mellitus and coronary artery disease. PLoS One. 2018;13(1):e0192159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peterson JM, Wei Z, Seldin MM, Byerly MS, Aja S, Wong GW. CTRP9 transgenic mice are protected from diet-induced obesity and metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol. 2013;305(5):R522–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hammes HP. Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia. 2018;61(1):29–38. [DOI] [PubMed] [Google Scholar]
- 16. Jenkins AJ, Joglekar MV, Hardikar AA, Keech AC, O’Neal DN, Januszewski AS. Biomarkers in diabetic retinopathy. Rev Diabet Stud. 2015;12(1-2):159–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tian L, Kazmierkiewicz KL, Bowman AS, Li M, Curcio CA, Stambolian DE. Transcriptome of the human retina, retinal pigmented epithelium and choroid. Genomics. 2015; 105(5-6):253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ponnalagu M, Subramani M, Jayadev C, Shetty R, Das D. Retinal pigment epithelium-secretome: a diabetic retinopathy perspective. Cytokine. 2017;95:126–135. [DOI] [PubMed] [Google Scholar]
- 19. Zhao D, Yang J, Yang L. Insights for oxidative stress and mtor signaling in myocardial ischemia/reperfusion injury under diabetes. Oxid Med Cell Longev. 2017;2017:6437467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zuo A, Li J, Zhao X, Li T, Lei S, Chen J, Xu D, Song C, Li N, Ruan S, Lyu L, et al. Globular CTRP9 protects cardiomyocytes from palmitic acid-induced oxidative stress by enhancing autophagic flux [published online ahead of print April 10, 2020]. Chem Biol Interact. 2020:109094. [DOI] [PubMed] [Google Scholar]
- 21. Cheng L, Li B, Chen X, Su J, Wang H, Yu S, Zheng Q. CTRP9 induces mitochondrial biogenesis and protects high glucose-induced endothelial oxidative damage via AdipoR1 -SIRT1- PGC-1alpha activation. Biochem Biophys Res Commun. 2016;477:685–691. [DOI] [PubMed] [Google Scholar]
- 22. Sun H, Zhu X, Zhou Y, Cai W, Qiu L. C1q/TNF-related Protein-9 ameliorates Ox-LDL-Induced endothelial dysfunction via PGC-1alpha/AMPK-Mediated antioxidant enzyme induction. Int J Mol Sci. 2017;18(6):1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Asada M, Morioka T, Yamazaki Y, Kakutani Y, Kawarabayashi R, Motoyama K, Mori K, Fukumoto S, Shioi A, Shoji T, Emoto M, et al. Plasma C1q/TNF-Related Protein-9 levels are associated with atherosclerosis in patients with type 2 diabetes without renal dysfunction. J Diabetes Res. 2016;2016:8624313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang W, Lau WB, Wang Y, Ma X, Li R. Reduction of CTRP9, a novel anti-platelet adipokine, contributes to abnormal platelet activity in diabetic animals. Cardiovasc Diabetol. 2016;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hu H, Li W, Liu M, Xiong J, Li Y, Wei Y, Huang C, Tang Y. C1q/Tumor necrosis factor-related protein-9 attenuates diabetic nephropathy and kidney fibrosis in db/db mice. DNA Cell Biol. 2020;39(6):938–948 [DOI] [PubMed] [Google Scholar]
- 26. Jung TW, Park HS, Choi GH, Kim D, Lee T. CTRP9 regulates growth, differentiation, and apoptosis in human keratinocytes through TGFbeta1-p38-dependent pathway. Mol Cells. 2017;40(12):906–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li W, Ma N, Liu MX, Ye BJ, Li YJ, Hu HY, Tang YH. C1q/TNF-related protein-9 attenuates retinal inflammation and protects blood-retinal barrier in db/db mice. Eur J Pharmacol. 2019;853:289–298. [DOI] [PubMed] [Google Scholar]
- 28. Kim J, Yang G, Kim Y, Kim J, Ha J. AMPK activators: mechanisms of action and physiological activities. Exp Mol Med. 2016;48(4):e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Joo MS, Kim WD, Lee KY, Kim JH, Koo JH, Kim SG. AMPK facilitates nuclear accumulation of nrf2 by phosphorylating at serine 550. Mol Cell Biol. 2016;36(14):1931–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kosuru R, Kandula V, Rai U, Prakash S, Xia Z, Singh S. Pterostilbene decreases cardiac oxidative stress and inflammation via activation of AMPK/Nrf2/HO-1 pathway in fructose-fed diabetic rats. Cardiovasc Drugs Ther. 2018;32(2):147–163. [DOI] [PubMed] [Google Scholar]
- 31. Zhou F, Wang M, Ju J, Wang Y, Liu Z, Zhao X, Yan Y, Yan S, Luo X, Fang Y. Schizandrin A protects against cerebral ischemia-reperfusion injury by suppressing inflammation and oxidative stress and regulating the AMPK/Nrf2 pathway regulation. Am J Transl Res. 2019;11(1):199–209. [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang P, Huang C, Li J, Li T, Guo H, Liu T, Li N, Zhu Q, Guo Y. Globular CTRP9 inhibits oxLDL-induced inflammatory response in RAW 264.7 macrophages via AMPK activation. Mol Cell Biochem. 2016;417(1-2):67–74. [DOI] [PubMed] [Google Scholar]
- 33. Liu Q, Zhang H, Lin J, Zhang R, Chen S, Liu W, Sun M, Du W, Hou J, Yu B. C1q/TNF-related protein 9 inhibits the cholesterol-induced Vascular smooth muscle cell phenotype switch and cell dysfunction by activating AMP-dependent kinase. J Cell Mol Med. 2017;21(11):28232836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kambara T, Ohashi K, Shibata R, Ogura Y, Maruyama S, Enomoto T, Uemura Y, Shimizu Y, Yuasa D, Matsuo K, Miyabe M, et al. CTRP9 protein protects against myocardial injury following ischemia-reperfusion through AMP-activated protein kinase (AMPK)-dependent mechanism. J Biol Chem. 2012;287(23):18965–18973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu T, Xu B, Liu Z. CTRP9 alleviates inflammation to ameliorate myocardial infarction in rats by activating Nrf2 [published online ahead of print December 3, 2019]. Minerva Endocrinol. 2019. [DOI] [PubMed] [Google Scholar]