Abstract
Background:
Ankle foot orthoses (AFOs) are commonly prescribed to individuals with Charcot-Marie-Tooth disease (CMT). The aim of this study was to evaluate patient reported satisfaction with orthotic devices and services in individuals with CMT to provide preliminary data for advancing AFO development and improving clinical care.
Methods:
The Orthotics and Prosthetics Users Survey was distributed via e-mail through the Inherited Neuropathy Consortium (INC) Contact Registry and includes 11 device-specific questions and 10 service-related questions. Participants were also asked open-ended questions about their experiences with AFOs.
Results:
Three hundred and fourteen individuals completed the survey. Over one-third of participants provided negative responses, including dislike of AFO appearance, discomfort, abrasions or irritations, and pain. Ratings of orthotic services were generally positive.
Conclusions:
Lower scores related to discomfort, abrasions and pain identified areas for AFO improvement. Continued research in these areas will be beneficial to informing and advancing AFO development and improving clinical care.
Keywords: AFO, ankle foot orthoses, braces, Charcot Marie Tooth disease, satisfaction, survey
1 |. INTRODUCTION
Charcot-Marie-Tooth disease (CMT) is the most common inherited peripheral neuropathy.1 Affected patients usually have progressive distal weakness, muscle atrophy and sensory loss, first in the feet and lower legs, followed by the hands.2–5 The early loss of lower limb function results in impaired gait and balance deficits.6 Ankle foot orthoses (AFOs) are commonly prescribed to individuals with CMT to restore mobility by compensating for progressive distal limb weakness, fatiguability and decreased sensation. Despite the potential functional benefits associated with AFO use, many factors can negatively influence satisfaction including discomfort, device appearance, limited footwear options and the quality of clinical services provided when receiving an AFO.7–10 Unfortunately, data related to the level of satisfaction individuals with CMT have with their AFOs and related clinical services are limited, and generally are from small cohorts of individuals at a single site, limiting the generalizability of the information.6–9 The primary purpose of this study was to gather input from a large cohort of individuals with CMT who use an AFO to identify opportunities to improve AFO related clinical care. Specifically, we sought to gain insight into the (a) relative satisfaction with daily use AFOs and (b) patient experience with the clinical fitting process.
2 |. METHODS
The study was approved by the Institutional Review Board of the University of Iowa. In a method similar to that used in previous studies,11 we used the Web-based Inherited Neuropathies Consortium (INC) Contact Registry to distribute the study survey. The INC Contact Registry is part of the INC Rare Disease Clinical Research Consortium (RDCRC), which in turn is part of the Rare Disease Clinical Research Network (RDCRN), supported by the National Institutes of Health (NIH). Individuals in the Contact Registry have a similar distribution of CMT type to those participating in the natural history studies within CMT clinics. In the contact registry, 53.45% have CMT1A, 3.77% have CMT1B, 9.69% have CMT2A, 1.97% have CMT4, 4.93% have CMTX, and 26.19% have CMT without a subtype or genetic classification. Nearly 3500 individuals participate in the registry; however, the proportion who use AFOs is unknown.
All registry participants between the ages of 18 to 90 were invited to participate, and consent was required prior to accessing the survey. Data regarding age, gender, height, weight, ethnicity, and CMT type were collected. The Orthotics and Prosthetics Users Survey (OPUS)12 with satisfaction with devices (CSD) and satisfaction with services (CSS) subscales were distributed in English via e-mail through the Contact Registry. The OPUS is a reliable and valid patient reported outcome measure designed to evaluate patient satisfaction with both devices and the clinical services provided, and has been validated in multiple languages.13–18 The OPUS is well suited for the systematic evaluation of AFO satisfaction and perception in individuals with CMT. The OPUS includes 11 device-specific questions and 10 service-related questions, with patients rating their level of agreement to statements using a five-level Likert scale. Stronger agreement indicates greater satisfaction. Participants were also given to option to elaborate on what they would change about their orthosis, and what activities their orthoses help with and limit.
Descriptive statistics were first performed to generate the mean and standard deviation for gender, height, weight, age, genetic diagnosis, and location of participant.
Percentages were determined for each question for each response category.
3 |. RESULTS
A total of 314 individuals completed both subscales of the OPUS. Approximately 56% of completers were female, and the average (SD) age was 56.8 (15) y. Additional demographic and clinical characteristics of the participants are shown in Table 1. The CMT subtype distribution of participants in the survey was similar to the registry with CMT1A indicated most often, and the majority of participants were from North America.
TABLE 1.
Demographic and clinical characteristics of survey respondents (n = 314)
| Characteristic | Mean (SD) or % |
|---|---|
| Age (y) | 56.8 (15) |
| Female (%) | 57 |
| Height (cm) | 168 (12) |
| Weight (kg) | 79.8 (23.7) |
| Geographic location | |
| North America | 82.8 |
| Europe | 14 |
| Asia | 1.6 |
| Australia | 1.0 |
| South America | 0.6 |
| Type of CMT | |
| CMT1A | 42.04 |
| CMT1B | 3.45 |
| CMT2A | 10.6 |
| CMT4 | 1.59 |
| CMTX | 7.96 |
| Unknown | 34.36 |
Ratings of devices showed that greater than 50% of individuals responded in the affirmative (Strongly Agree or Agree) to most questions. However, over -hird of individuals who used AFOs provided negative responses to multiple questions regarding their AFOs (Figure 1). This included 42% who indicated they dislike the appearance of their AFO (average age 57 ± 16.2 [range 30–87]), 32% who experienced discomfort, 35% who experienced abrasions or irritations, and 36% who experienced pain with AFO use.
FIGURE 1.
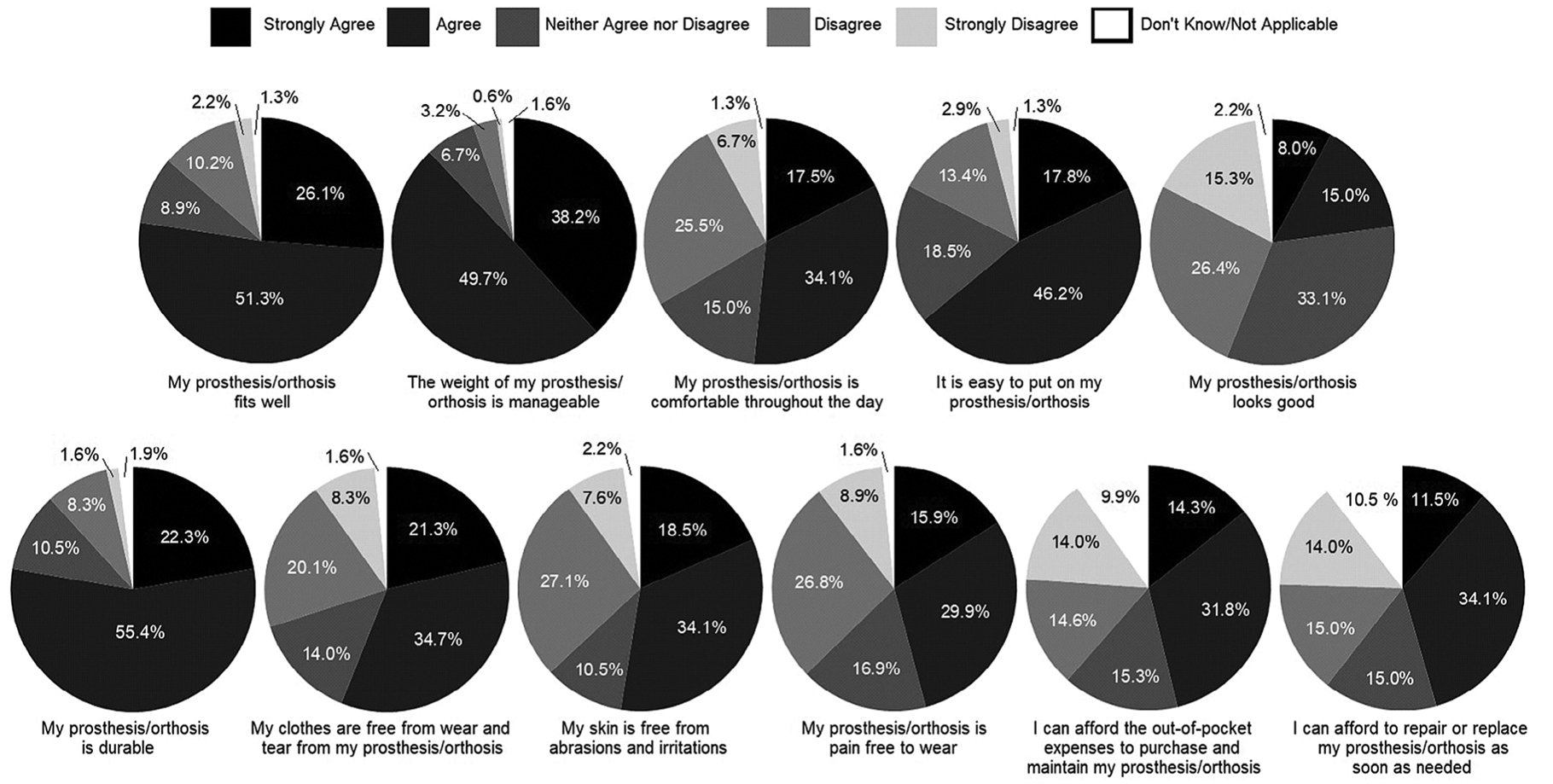
Satisfaction with device. The majority of survey participants responded positively to questions concerning their AFOs. Topics such as device fit, comfort throughout the day, skin abrasions and irritations, pain, and cost to purchase and repair devices had higher rates of negative responses, indicating room for improvement
Participants generally gave positive ratings for the orthotic services they received, with approximately 80% or more of participants indicating they received timely access to care, waited a reasonable time to be seen and were shown courtesy and respect by the staff. Seventy-nine percent of participants also indicated that their orthotist gave them the opportunity to express their concerns and were responsive to their concerns and questions. Participants indicated there was room for improvement in communication. Only 63% indicated they were a partner in decision-making with clinic staff regarding their device. Fifty-five percent indicated they were informed of different AFO options, and 51% indicated they were informed of potential problems they might encounter with their AFOs (Figure 2).
FIGURE 2.
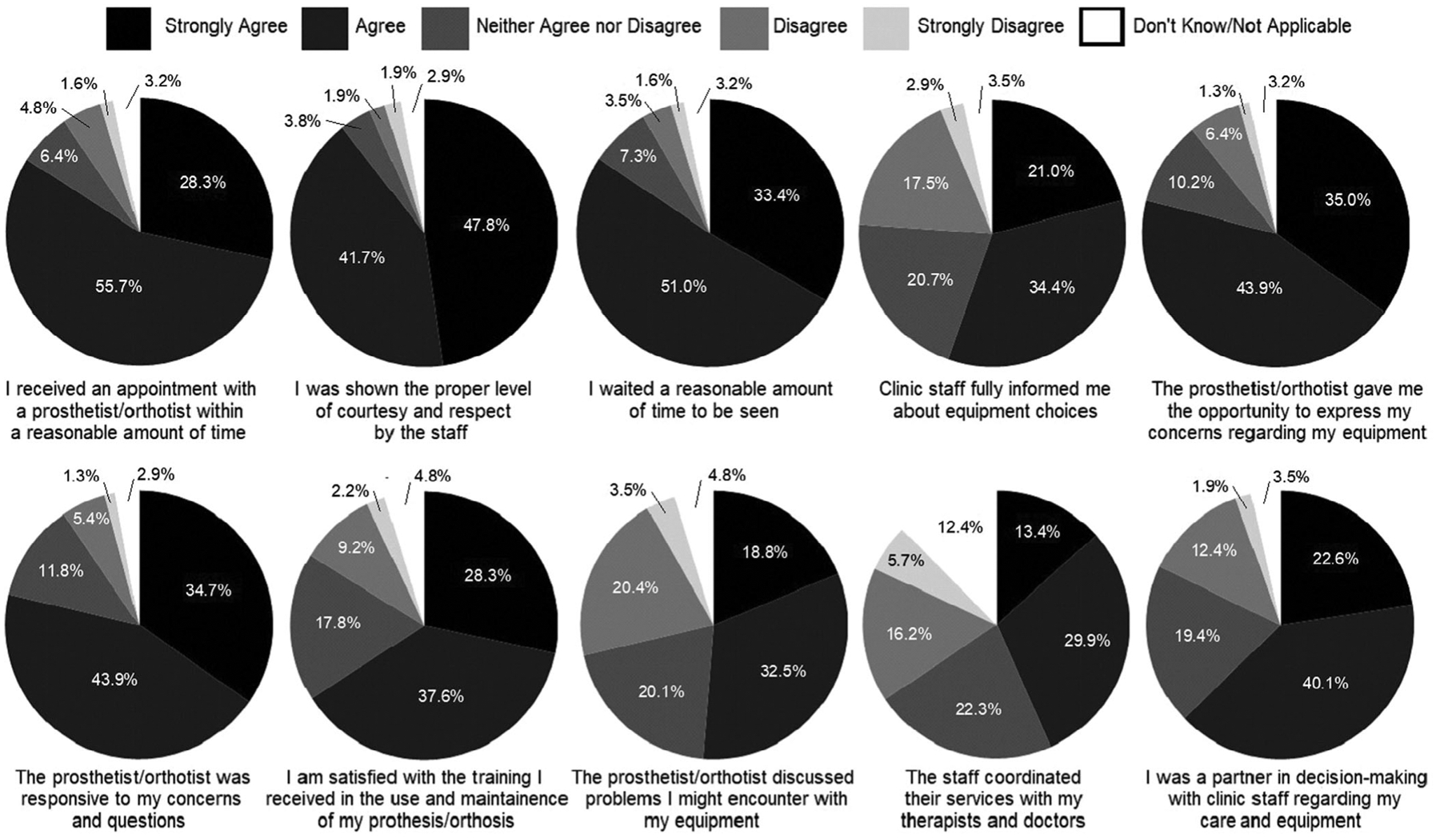
Satisfaction with service. The majority of survey respondents indicated satisfaction with the services they were provided, however, areas for improvement included information of available devices, the ability to express concerns, Clinicians/Prosthetist/Orthotist response to concerns, training with the device, discussion of potential problems, and coordination of care
Participants were able to provide free text statements regarding their AFOs, which generally aligned with questionnaires responses. This included negative comments about appearance, comfort, and function such as: “Their appearance and the inability to wear shoes that do not have lacing capabilities”; “I would like something that is a bit less bulky and looks a little less obvious”; “They hardly fit under any of my clothes and I’m not comfortable wearing them with skirts.” Moreover, many patients commented on wearing orthoses in daily life and embarrassment in social situations. Comments included: “My AFO limits the choices I make in shoes and clothing”; “Looks (so ugly-frightened a grandchild!)”; “It’s sad that humans are so intelligent, and yet we are stuck wearing awful looking braces”; “Feeling normal, wearing shorts and dresses, It’s impossible to wear without being identified and stared at”; “I’m disabled, don’t want to look like a disabled person in the limited, ugly, bulky shoe.” Participants also directly commented on difficulties in climbing and going downstairs or slopes or performing specific activities such as driving the car. However, many indicated the AFOs improved their balance and ability to walk.
4 |. DISCUSSION
Although many participants appreciated their AFOs and the teams that provide them, there are multiple areas where both the AFO devices and the manner in which they are provided could be improved to increase overall patient satisfaction.
Most study-participants indicated they were satisfied with the overall fit of their AFOs. However, the overall positive impression was contrasted by many individuals identifying deficits associated with the comfort of their device. One-third of participants indicated their device was not comfortable throughout the day and reported pain and skin irritation. Although issues associated with comfort are expected due to foot deformity associated with CMT, and difficulties associated with accommodating that altered geometry during AFO fittings7,8,19, it is also clearly an area for improvement. Possible targets to address comfort, pain, and irritation include reducing areas of high pressure on the foot, trim lines and padding to unload sensitive areas, and reducing friction or rubbing at the device skin interface.
There was an overall positive response to questions concerning AFO weight and durability, but not with responses to topics such as appearance and damage to clothes. Both dissatisfaction with appearance and damage to clothing may result from device design and geometry. Plastic AFOs are commonly provided to individuals with CMT, and even though, excess polypropylene is removed, the resulting orthotics are often bulky. The bulkiness of the AFO can prove unappealing and result in a hard surface or edges that result in wear or damage to clothing. Custom-made composite-fiber braces are gaining popularity; however, they are also more difficult to fit and fabricate, and materials such as carbon fiber and Kevlar reinforced polymers require pressure or high heat to cure, and errors during fabrication can result in delamination.20
Advances in AFO fitting and fabrication methods are needed to improve satisfaction with comfort, appearance, and design of ankle foot orthoses.
In recent years, technology has emerged permitting the use of three-dimensional (3D) foot scanning, computer aided design (CAD), and computer aided manufacturing (CAM) in the fabrication of foot molds and custom foot orthosis components.21 3D printing, one of the most recent forms of CAM, has proven efficacy in the fabrication of AFO with reports of excellent dimensional accuracy, good manufacturing precision, and performance that is at least equivalent to hand-crafted AFOs.22 Further research is needed to evaluate the success of 3D scanning and 3D printing of AFOs in CMT populations.23
Another aspect of AFO design that could benefit from further study is the effect of design on putting on and taking off the device. While the majority of participant responded positively when asked about the ease of putting on 16% were dissatisfied. It is important to consider involvement of the upper limbs in patients with CMT, as the dissatisfaction with putting on may be due, in part, to the progressive hand weakness experienced by some individuals with CMT. This finding suggests a potential need for adaptive closing systems in individuals with upper extremity involvement.
The majority of participants responded positively concerning the clinical services they experienced while obtaining their orthosis. Participants were generally happy with their access to care and providers, but dissatisfied with factors such as cost of the devices, the information provided about the available options, and care coordination. Clinical interaction could be improved by increasing patient participation in the clinical decisions making process. Providing individuals with CMT access to educational materials and different types of AFOs could improve their engagement in the process and facilitate an informed and engaged decision. These approaches could allow clinicians to better explain the differences and benefits of available AFOs. These data indicate improved and more transparent coordination between healthcare providers and individuals with CMT could improve overall care and satisfaction.
The reliance on a registry for distribution, and a survey to gather data for this study, resulted in limitations to this study. First, the total number of individuals in the registry who use AFOs is unknown, resulting in an inability to calculate a meaningful response rate or evaluate potential bias in respondent demographics. The mean age of study respondents indicates the results may be more indicative of older individuals with CMT. The use of a questionnaire also prevented the objective assessment of physical performance, device type, sensory impairment and other indicators of disease severity or progression. Although it does not allow for direct evaluation of the association between these factors and orthosis use and satisfaction, the results provide meaningful insight into the experiences and opinions of several hundred individuals with CMT and provide foundational information to guide further study.
In summary, responses indicate overall satisfaction but identify clear areas for improvement. Responses to services questions highlighted the importance of putting the patient at the center of the orthosis prescription path dedicating time to listening and addressing questions or concerns.
The results of this study demonstrate the need for future prospective studies to enhance clinical outcomes for individuals with CMT. Specifically, studies addressing the interaction between patient characteristics, medical and orthotic management, satisfaction, and function are needed. There are many factors to consider including activity level, foot deformity, disease progression, as well as device fit, material, and mechanical characteristics, indicating room for improvement in these areas.
Population specific outcome measures such as the CMT Pediatric Scale (CMTPedS) and CMT-Functional Outcome Measure (CMT-FOM) could play an important role, and be used to evaluate patients with and without their AFOs.24,25
Future studies could also examine the relationship between objective validated measures of physical performance and satisfaction and preference for AFOs. Further, comparison across populations will help to identify factors that are unique to individuals with CMT and those relevant to individuals with other neuromuscular disorders. To our best knowledge only two studies addressed the issue of AFOs satisfaction in individuals with other neuromuscular diseases, but neither study used validated scales for satisfaction, and enrolled fewer than 30 participants.26,27 Therefore, a robust understanding of the key factors will help ensure that the AFO prescription, fitting, and fabrication process results in care that maximizes outcomes.
Further, focused efforts are needed to improve AFO device comfort and appearance, while also reducing irritations or abrasions, pain with use, and damage to clothes. Continued research in these areas would be beneficial to informing and advancing AFO development and improving clinical care.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the Inherited Neuropathy Consortium, Prof Joshua Burns for his input regarding study approach and the support and Nicholas Yencer for his help building the survey documents for distribution. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002537. MES receives support from the NCATS and the NIH (U54NS065712), NINDS (R21TR003034; U01 NS1094301, R01NS105755), the MDA and the Charcot Marie Tooth Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding information
Muscular Dystrophy Association; National Center for Advancing Translational Sciences, Grant/Award Number: UL1TR002537; National Institute of Neurological Disorders and Stroke, Grant/Award Number: U54NS065712
Abbreviations:
- AFOs
ankle foot orthoses
- CAM
computer aided manufacturing
- CMT
Charcot-Marie-Tooth disease
- OPUS
Orthotics and Prosthetics Users Survey
Footnotes
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Reilly MM, Murphy SM, Laura M. Charcot-Marie-Tooth disease. J Peripher Nerv Syst. 2011;16:1–14. [DOI] [PubMed] [Google Scholar]
- 2.Pareyson D, Marchesi C. Diagnosis, natural history, and management of Charcot-Marie-Tooth disease. Lancet Neurol. 2009;8(7):654–667. [DOI] [PubMed] [Google Scholar]
- 3.Reilly MM, Shy ME. Diagnosis and new treatments in genetic neuropathies. J Neurol Neurosurg Psychiatry. 2009;80:1304–1314. [DOI] [PubMed] [Google Scholar]
- 4.Harding AE, Thomas PK. The clinical features of hereditary motor and sensory neuropathy types I and II. Brain. 1980;103:259–280. [DOI] [PubMed] [Google Scholar]
- 5.Shy ME, Lupski JR, Chance PF, Klein CJ, Dyck PJ. Hereditary motor and sensory neuropathies: an overview of clinical, genetic, electro-physiologic, and pathologic features In: Dyck PJ, Thomas PK, eds. Peripheral Neuropathy. 4th ed. Philadelphia, PA: Saunders; 2005: 1623–1658. [Google Scholar]
- 6.Estilow T, Glanzman AM, Burns J, et al. Balance impairment in pediatric charcot-marie-tooth disease. Muscle Nerve. 2019;60(3):242–249. 10.1002/mus.26500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor J, McCaughan D, McDaid C, et al. Orthotic management of instability of the knee related to neuromuscular and central nervous system disorders: systematic review, qualitative study, survey and costing analysis. Health Technol Assess. 2016;20(55):1–262. 10.3310/hta20550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips MF, Radford K, Willis A. Ankle foot orthoses for people with Charcot Marie Tooth disease: views of users and orthotists on important aspects of use. Disabil Rehabil Assist Technol. 2011;6:491–499. 10.3109/17483107.2010.549899 [DOI] [PubMed] [Google Scholar]
- 9.Vinci P, Gargiulo P. Poor compliance with ankle-foot-orthoses in Charcot-Marie-Tooth disease. Eur J Phys Rehabil Med. 2008;44:27–31. [PubMed] [Google Scholar]
- 10.Ramdharry G, Pollard A, Marsden J, Reilly M. Comparing gait performance of people with Charcot-Marie-Tooth disease who do and do not wear ankle foot orthoses. Physiother Res Int. 2012;17:191–199. 10.1002/pri.531 [DOI] [PubMed] [Google Scholar]
- 11.Johnson EN, Sowden J, Dilek N, et al. Prospective study of muscle cramps in Charcot Marie Tooth disease. Muscle Nerve. 2015;51(4): 485–488. 10.1002/mus.24333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinemann AW, Bode RK, O’Reilly C. Development and measurement properties of the Orthotics and Prosthetics Users’ Survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int. 2003;27(3):191–206. [DOI] [PubMed] [Google Scholar]
- 13.Jarl GM, Heinemann AW, Hermansson LMN. Validity evidence for a modified version of the Orthotics and Prosthetics Users’ Survey. Disabil Rehabil Assist Technol. 2012;7:469–478. [DOI] [PubMed] [Google Scholar]
- 14.Jarl G, Heinemann AW, Lindner HY, Norling Hermansson LM. Cross-cultural validity and differential item functioning of the orthotics and prosthetics users’ survey with Swedish and United States users of lower-limb prosthesis. Arch Phys Med Rehabil. 2015;96:1615–1626. [DOI] [PubMed] [Google Scholar]
- 15.Jarl GM, Hermansson LMN. Translation and linguistic validation of the Swedish version of Orthotics and Prosthetics Users’ Survey. Prosthet Orthot Int. 2009;33:329–338. [DOI] [PubMed] [Google Scholar]
- 16.Jarl G, Holmefur M, Hermansson LM. Test-retest reliability of the Swedish version of the Orthotics and Prosthetics Users’ Survey. Prosthet Orthot Int. 2014;38:21–26. [DOI] [PubMed] [Google Scholar]
- 17.Bravini E, Franchignoni F, Ferriero G, et al. Validation of the Italian version of the client satisfaction with device module of the Orthotics and Prosthetics Users’ Survey. Disabil Health J. 2014;7:442–447. [DOI] [PubMed] [Google Scholar]
- 18.Bakhsh H, Franchignoni F, Bravini E, Ferriero G, Giordano A, Foti C. Validation of the Arabic version of the client satisfaction with device module of the Orthotics and Prosthetics Users’ Survey. Ann Saudi Med. 2014;34:320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burns J, Ryan MM, Ouvrier RA. Evolution of foot and ankle manifestations in children with CMT1A. Muscle Nerve. 2009;39(2):158–166. [DOI] [PubMed] [Google Scholar]
- 20.Janet S, Dufek N, Neumann SE, Hawkins MC, O’Toole B. Functional and dynamic response characteristics of a custom composite ankle foot orthosis for Charcot–Marie–Tooth patients. Gait Posture. 2014;39:308–313. [DOI] [PubMed] [Google Scholar]
- 21.Salles AS, Gyi DE. An evaluation of personalised insoles developed using additive manufacturing. J Sports Sci. 2013;31(4):442–450. [DOI] [PubMed] [Google Scholar]
- 22.Schrank ES, Hitch L, Wallace K, Moore R, Stanhope SJ. Assessment of a virtual functional prototyping process for the rapid manufacture of passive-dynamic ankle-foot orthoses. J Biomech Eng. 2013;135(10): 101011–101017. [DOI] [PubMed] [Google Scholar]
- 23.Wojciechowski E, Chang AY, Balassone D, et al. Feasibility of designing, manufacturing and delivering 3D printed ankle-foot orthoses: a systematic review. J Foot Ankle Res. 2019;12:11 10.1186/s13047-019-0321-6. eCollection 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burns J, Ouvrier R, Estilow T, et al. Validation of the Charcot-Marie-Tooth disease pediatric scale as an outcome measure of disability. Ann Neurol. 2012;71(5):642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eichinger K, Burns J, Cornett K, et al. The Charcot-Marie-Tooth functional outcome measure (CMT-FOM). Neurology. 2018;91(15):e1381–e1384. 10.1212/WNL.0000000000006323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garralda ME, Muntoni F, Cunniff A, Caneja AD. Knee-ankle-foot orthosis in children with duchenne muscular dystrophy: user views and adjustment. Eur J Paediatr Neurol. 2006;10(4):186–191. [DOI] [PubMed] [Google Scholar]
- 27.Mnatsakanian A, Kissel JT, Terry P, King WM. One clinic’s experience with carbon fiber orthoses in neuromuscular disease. Muscle Nerve. 2017;55(2):202–205. 10.1002/mus.25233 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


