Abstract
Nitrification is a fundamental process in terrestrial nitrogen cycling. However, detailed information on how climate change affects the structure of nitrifier communities is lacking, specifically from experiments in which multiple climate change factors are manipulated simultaneously. Consequently, our ability to predict how soil nitrogen (N) cycling will change in a future climate is limited. We conducted a field experiment in a managed grassland and simultaneously tested the effects of elevated atmospheric CO2, temperature, and drought on the abundance of active ammonia-oxidizing bacteria (AOB) and archaea (AOA), comammox (CMX) Nitrospira, and nitrite-oxidizing bacteria (NOB), and on gross mineralization and nitrification rates. We found that N transformation processes, as well as gene and transcript abundances, and nitrifier community composition were remarkably resistant to individual and interactive effects of elevated CO2 and temperature. During drought however, process rates were increased or at least maintained. At the same time, the abundance of active AOB increased probably due to higher NH4+ availability. Both, AOA and comammox Nitrospira decreased in response to drought and the active community composition of AOA and NOB was also significantly affected. In summary, our findings suggest that warming and elevated CO2 have only minor effects on nitrifier communities and soil biogeochemical variables in managed grasslands, whereas drought favors AOB and increases nitrification rates. This highlights the overriding importance of drought as a global change driver impacting on soil microbial community structure and its consequences for N cycling.
Subject terms: Climate-change ecology, Biogeochemistry, Microbial ecology
Introduction
Nitrogen (N) cycling is a fundamental process in terrestrial ecosystems, critical for the functioning of all living organisms. Soil ecosystems are currently undergoing substantial changes, as a consequence of anthropogenic activities [1, 2]. Microorganisms catalyze most soil N transformation processes, and thus play a central role in mediating N exchange between the atmosphere, plants, and soils [3]. Thus, understanding the nature and the intensity of the responses of soil microorganisms to climate change is of utmost importance to predict the future terrestrial N cycle, including its repercussion on plant productivity and climate-carbon feedbacks [3, 4]. Yet, our understanding of the relative role of specific microbial taxa in mediating N transformations in terrestrial environments in response to climate change is still incomplete. In spite of current climate projections [5] the interactive effects of elevated temperature, elevated atmospheric CO2, and changes in precipitation patterns have rarely been assessed together with regards to microbially mediated soil N transformations [6, 7].
Nitrification represents a key process in the terrestrial N cycle. It has long been considered to be a two-step process initiated with the oxidation of ammonia to NO2− by ammonia-oxidizing bacteria (AOB) and ammonia-oxidizing archaea (AOA), followed by NO2− oxidation to NO3− by nitrite-oxidizing bacteria (NOB) [8]. Based on metagenomic surveys, marker gene assays and functional gene studies targeting the amoA gene, (gene that codes for the alpha subunit of ammonia monooxygenase) several studies demonstrated the influence of different environmental factors on the abundance and composition of nitrifying communities, most notably substrate concentration, soil water content and soil pH [9–11]. Furthermore, all known ammonia-oxidizers contribute to nitric (NO) and nitrous (N2O) oxide emissions, but the yield by which they do differs strongly between groups, with AOB showing the highest N2O yield per mol of oxidized ammonia when compared with other groups [12]. Several studies have reported a numeric dominance of AOB over AOA in agricultural and grassland soils to which fertilizers are applied on a regular basis, leading to the idea that AOB are responsible for the ammonia-oxidation step in these environments [13, 14]. In contrast, AOA were thought to be the main ammonia-oxidizing taxa in N poor environments, as well as in acidic soils [15]. Notably, all known AOB have a relatively low substrate affinity, whereas AOA encompass members with a broad range of substrate affinities [12]. NOB span six genera, out of which Nitrospira is the most phylogenetically diverse and widespread across different habitat types including terrestrial ecosystems [16]. Recently, it was discovered that some members of this genus (Comammox Nitrospira; CMX) can catalyze both steps of nitrification [17, 18]. Although our knowledge on the distribution and diversity of comammox Nitrospira is still comparatively limited, several studies have reported their presence and activity in terrestrial environments [19, 20]. Furthermore, it has been demonstrated that the only cultured comammox species N. inopinata, has a high substrate affinity [12]. Thus, a joint assessment of the relative contribution of the aforementioned microbial groups to nitrification is needed, as well as of their specific responses to climate change.
Current global change in temperate ecosystems is characterized, amongst other changes, by rising air and soil temperature, elevated CO2 concentrations, and changes in water availability, such as increased frequencies of drought events. Elevated atmospheric CO2 concentrations affect soil microorganisms by promoting plant growth and photosynthetic activity [21]. This can result in increased plant belowground C allocation and rhizodeposition [22, 23], which stimulates heterotrophic microbial activity and mobilizes N. However, higher plant growth can indirectly affect soil microorganisms by gradually depleting the soil N pool available for microbial uptake [24, 25], potentially causing microorganisms to become N limited. Previous studies revealed a decrease in the relative abundance of AOA and AOB in response to elevated CO2 [26, 27]. In contrast to elevated CO2, elevated temperature directly accelerates microbial processes and turnover rates [28], alters water and nutrient availability, extends the plant growing season [29] and may favor the growth of slow growing bacteria [30]. Concomitantly, studies have reported significant changes in amoA gene abundance and expression of AOA and AOB in response to temperature [27, 31]. The growth, activity, and community structure of AOA, but not of AOB have been shown to change in response to elevated temperature in soil mesocosms [31]. On the other hand, a comprehensive study across 23 different soil types has highlighted temperature as the most important factor affecting AOB community structure [32]. Finally, extreme events such as droughts can cause soil microorganisms to decrease their activity levels, to accumulate osmoprotectants/compatible solutes, and often to switch to dormancy [33]. A study in mountain grasslands showed that drought had distinct effects on the relative abundance of ammonia-oxidizing microorganisms, with reduced AOA amoA abundance with drought and no observed changes in AOB amoA abundance [11].
Multifactorial studies that particularly target nitrifier communities are scarce, and most studies are rather focused on the response of broad ecosystem N processes to global change [34–36].
Therefore, the goal of this study was to understand how elevated atmospheric CO2 (eCO2), elevated temperature (eT) and drought (D), alone or in combination, affect the relative abundance and diversity of nitrifier communities and their activity (i.e., gross nitrification rates) in a managed submontane grassland. We expected to observe: (i) a shift in active nitrifier community structure towards high affinity nitrifiers (AOA and CMX) as a consequence of lower NH4+ availability at eCO2; (ii) an overall increase in nitrification rates and amoA expression levels of AOA, AOB, and potentially CMX in response to eT; and (iii) a reduction of the abundance and activity of all nitrifying groups by drought. Our study also intended to explore whether the combined effect of eCO2 and eT—with or without drought—would have additive or nonadditive effects on the diversity and abundance of nitrifiers.
Methods
Site description and experimental design
The experimental site is located at the agricultural research institute HBLFA Raumberg‐Gumpenstein in Styria, Austria (47°29′38″N, 14°06′03″E) and is part of a long-term climate change experiment (ClimGrass). With a mean annual temperature of 8.2 °C and a mean annual precipitation of 1056 mm, this area is representative of a large part of alpine grasslands. The grassland under study was established in 2007 and is dominated by the grasses Arrhenatherum elatius and Festuca pratensis together with legume species such as Lotus corniculatus and Trifolium pratense. It also includes a few subdominant non‐leguminous forbs such as Taraxacum officinale and Plantago lanceolata. The soil type is a Cambisol (WRB classification) with loamy sand texture and a pH of 5.
The experimental design was originally based on a response surface regression approach that aimed to study the nature and intensity of the grassland’s responses to manipulations of elevated atmospheric temperature and CO2, and drought [37]. The experimental site comprised a total of 54 plots showing individual and combined effects of three levels of temperature (+1.5 °C, + 3 °C), atmospheric CO2 concentrations (ambient, +150 ppm, +300 ppm) and drought (Fig. S1). A detailed explanation regarding the reasons behind the choice of treatments and corresponding number of replicates can be found in [37]. Each treatment had a different number of replicates, which reflected a balance between statistical power and budget constraints [37].
For this specific experiment, we selected only a set of soil samples from 26 plots (4 × 4 m each) during peak growing season in July 2017 (Table 1; Fig. S1). Treatments included soil plots on which temperature (eT; +3 °C) and CO2 (eCO2; +300 ppm CO2) were manipulated individually or in combination, as well as plots on which drought (D) was simulated through the use of automatic rainout shelters installed 8 weeks before sampling (Table 1; Fig. S1). Manipulations in atmospheric CO2 concentrations were done through a miniFACE—free air CO2 enrichment system, whereas atmospheric temperature was manipulated through the use of infrared heaters. Since 2014, infrared heaters were continuously on except when snow cover exceeded a depth of 10 cm. The soil temperatures measured by soil probes in selected plots between June and July 2017 showed an average of +1.86 °C for plots affected by elevated temperature, compared with the controls (data not shown). CO2 fumigation was only applied during the growing season (April to November). Site management included mowing (three times a year) and mineral fertilization (90 kg N ha−1 a−1 as NH4NO3, 65 kg P ha−1 a−1 as Ca(H2PO4)2 and 170 kg K ha−1 a−1 as K2O), which corresponds approximately to the amount of NPK removed by biomass harvest, in order to replicate local farming practices.
Table 1.
Soil plots sampled in this study.
| Treatments used for statistical analyses | |||
|---|---|---|---|
| Treatment | Description | Individual and combined effects of temperature and CO2 | Individual and combined effects of future climate and drought |
| [Amb] | Ambient conditions (n = 8) | × | × |
| [eT] | +3 °C above ambient (n = 3) | × | |
| [eCO2] | +300 ppm CO2 above ambient (n = 3) | × | |
| [eT] × [eCO2] | +3 °C and +300 ppm CO2 above ambient (n = 4) | × | × |
| [Amb] × [D] | Ambient conditions + rainout shelter (n = 4) | × | |
| [eTeCO2] × [D] | +3 °C and +300 ppm CO2 above ambient + rainout shelter (n = 4) | × | |
Biological replicates are shown in parenthesis. Specific soil plots were selected in order to test for either the individual and combined effects of elevated temperature and CO2 or the individual and combined effects of future climate and drought. This separation was done in order to have a full factorial design for each set. The spatial arrangement of all the plots is shown in Fig. S1.
Sample collection and soil physicochemical analyses
Immediately after removal of aboveground biomass several (4–11) soil cores were taken per plot to a depth of 10 cm using a stainless-steel soil corer with 2 cm inner diameter. Stones and roots were picked, washed, dried and weighed, and soils were sieved through a 2 mm sieve directly after collection. Fresh soil subsamples for molecular analyses were immediately frozen in liquid N. The remaining fresh soil was transported to the laboratory and incubated shortly (2–3 days) at the respective in situ temperatures measured at the time of collection (17 °C for ambient temperature plots and 20 °C for elevated temperature plots) until analysis.
Gravimetric soil water content was determined by oven drying (105 °C for 24 h) 5 g of fresh, sieved soil. Dissolved soil organic C (DOC), nitrogen (DON) and total dissolved N (TDN) were measured in 1 M KCl extracts (using a soil to solution ratio of 1:7.5 w–v) after filtering through ash-free cellulose filters (Whatman, GE Healthcare Life Sciences) on a TOC/TN analyzer (TOC-VCPH/TNM-1, Shimadzu, Austria). In parallel, soils were chloroform fumigated for 48 h in order to estimate microbial biomass nitrogen (MBN) [38]. After fumigation, soils were also extracted with 1 M KCl, and MBN was calculated as the difference in TDN between fumigated and non-fumigated soils. Ammonium (NH4+) and nitrate (NO3−) concentrations were determined photometrically in the 1 M KCl extracts of non-fumigated soils [39]. Soil pH was determined in a 1:5 mix of dry soil and 0.01 M CaCl2 solution with a pH meter (Sentron Europe BV).
Isotope pool dilutions
Gross rates of N mineralization, and nitrification were determined and calculated from fresh, sieved soil samples using 15N pool dilution assays [40].
Two to three grams of duplicate soil samples were incubated with 15NH4Cl and K15NO3 (98 at%) tracer solutions for 4 and 24 h at the corresponding in situ field temperatures. We aimed to increase the 15N enrichment of the target pool to ~20 at%15N and added 100 µl of tracer solution per gram of fresh soil, after a previous photometrical determination of background NH4+ and NO3−pool sizes. The addition of liquid the tracer increased the soil water in ambient and drought plots by 1.79 and 4.56-fold respectively. Incubations were stopped after 4 and 24 h by the addition of 1 M KCl (1:7.5 w–v), extraction by horizontal shaking on an orbital shaker for 30 min (150 rpm) and filtration through ash-free cellulose filters. Mineralization extracts were prepared using a microdiffusion method [41] followed by the measurement of 15/14N isotope ratios and concentrations of NH4+ in the acid traps by elemental analyzer-isotope ratio mass spectrometry (EA-IRMS; EA1110 coupled via ConFlo III interface to a DeltaPLUS IRMS, Thermo Fisher, Bremen, DE [42]. Concentrations and 15/14N isotope ratios of NO3− in 1 M KCl extracts were determined by converting NO3− to NO2− with vanadium (III) chloride (VCl3) and further reduction of NO2− to N2O by sodium azide (NaN3) [42]. Concentrations and 14/15N isotope ratios of the resulting N2O were determined by purge-and-trap isotope ratio mass spectrometry (PT-IRMS), using a Gasbench II headspace analyzer (Thermo Fisher, Bremen, DE) with a cryo-focusing unit, coupled to a Finnigan Delta V Advantage IRMS (Thermo Fisher, Bremen, DE).
TNA extraction, RNA purification, and cDNA synthesis
Total nucleic acids (TNA) were extracted from 0.4 g frozen soil by bead-beating in the presence of a cetyl trimethylammonium bromide buffer and phenol, according to a previously published extraction protocol [43] and eluted in 250 µl of Low-Tris-EDTA buffer. Following extraction, samples were purified using the OneStepTM PCR Inhibitor Removal Kit (Zymo, Irvine, CA, USA) and TNA was quantified using the Quant-iTTM PicoGreen® Kit (Thermo Fisher Scientific), according to the manufacturers’ instructions. DNA was digested in 1–3 µg of TNA extract with Turbo DNase (Thermo Fisher Scientific), and RNA was purified using the GeneJET RNA Cleanup and Concentration Micro Kit (Thermo Fisher Scientific) and eluted in 25 µl of RNase Storage Solution (Thermo Fisher Scientific). The RNA yield was quantified using the Quant-iTTM RiboGreen® assay (Thermo Fisher Scientific). Successful DNA digestion was confirmed by negative results on a gel electrophoresis after a DNA-targeted SSU rRNA gene PCR assay on the purified RNA extracts using the primer pair 515F-mod/806R-mod [44, 45]. Afterwards, cDNA was synthesized from 400 ng of RNA extract using random hexamer primers and SuperScriptTM IV reverse transcriptase (Thermo Fisher Scientific) following the manufacturer´s instructions and eluted in 54 µl of nuclease-free water.
amoA/nxrB gene and transcript amplification and sequencing
The diversity of the ammonia-oxidizing microbial community, as well as the NOB community was assessed via amplification and sequencing of the ammonia monooxygenase enzyme subunit A (amoA) gene, and the nitrite oxidoreductase subunit beta (nxrB) gene respectively, with functional group specific primers modified to include a linker sequence (‘head’) for barcoding PCRs at the 5′ end, as described previously [46]. Detailed amplification conditions can be found in the Supplementary Material and Methods file. Library preparation and sequencing services were provided by Microsynth (Balgach, Switzerland). The library was prepared by adapter ligation and PCR using the TruSeq Nano DNA Library Prep Kit according to the TruSeq nano protocol (Illumina, FC-121–4003), but excluding the fragmentation step. Sequencing was performed on a MiSeq platform, v3, 2 × 300 bp (Illumina). Paired MiSeq reads were processed according to [46]. AOB, and AOA amoA gene OTUs were clustered at 95% and 96% sequence identity, respectively [47, 48]. A sequence identity cutoff of 95% was used for NOB nxrB OTUs and for CMX clade B amoA OTUs. Taxonomic classification of AOA, AOB-related (Nitrosomonas), and NOB OTUs was performed using the evolutionary placement algorithm implemented in RAxML to place OTUs into a reference tree using a set of classified full-length nucleotide sequences [49, 50]. Uncultured Nitrosospira (AOB) amoA OTUs were classified by local nucleotide BLAST (version 2.8.1+) searches against a Nitrosospira reference database [51] since phylogenetic trees among reference Nitrosospira showed no stable tree topology for mapping reads. Represented sequences for all CMX clade B amoA were aligned against a reference pmoA and amoA database [52] using the SINA aligner [53] and a maximum likelihood phylogenetic tree was calculated based on this alignment using W-IQ-Tree [54] with 1000 bootstrap iterations and ModelFinder to determine the best fitting base substitution model [55]. The resulting tree was visualized with iTOL [56].
All sequence alignments were performed with Mafft [57] and manually inspected. OTUs with no similarity to the functional group of interest were considered unspecific co-amplification and excluded from further analysis (Table S1).
AmoA gene and transcript quantification by qPCR
Gene and transcript copy numbers of amoA genes of betaproteobacterial AOB, AOA, and CMX clade B were determined by qPCR. Gene and transcript copy numbers of Nitrospira nxrB were not assessed in this study due to the highly variable gene copy number per genome, which would hamper precise quantifications. In addition, the primer pair used for amplicon sequencing co-amplifies several nonspecific amplicons, which would also make quantifications inaccurate [58].
Assays with different sample dilutions (5× to 10,000×), resulting in template amounts ranging from 0.004 to 24 ng, were performed to minimize possible PCR inhibition caused by excess template or co-extracted inhibitory substances. For all samples, the highest dilutions that yielded consistent results were used to calculate gene and transcript copy numbers. All assays were performed in triplicate for each dilution. Detailed amplification conditions can be found in the Supplementary Material and Methods file. For each assay, standard series were generated by tenfold serial dilutions (108–101 gene copies μl−1) from purified M13-PCR products obtained from environmental samples (CMX clade B amoA) or pure cultures of Nitrosomonas europaea and Nitrososcosmicus hydrocola, as described previously [52, 59]. The average amplification efficiency for the comammox clade B amoA, archaeal amoA, and betaproteobacterial amoA assays was 90.6%, 93.6% and 89,2% respectively.
Statistical analyses
All statistical analyses were performed in R (version 3.5.1). Statistical analyses were performed with two separate linear models using the Anova() function of the package “car” following a full factorial design that allowed to test for interactions between all factors under analysis. This approach was chosen because the drought treatment was imposed on a reduced set of treatments (Table 1; Fig. S1) that did not include soil plots where eT and eCO2 were manipulated individually. Therefore, the first model (eT vs. eCO2 dataset) included [eCO2], [eT] and their interactions. The second model (drought dataset) included drought, [eT × eCO2] and their interactions. Both models were used to assess if individual global change effects were modified when variables were combined (interactive effects) in order to reveal potential additive, synergistic or antagonistic effects (Tables 2 and 3, Fig. 1, Table S2). Model assumptions of normality and homoscedasticity were checked on the model residuals [60] and variables were transformed when needed to meet model assumptions. Nonparametric tests were conducted in case assumptions were not met after data transformation. Multiple comparisons were performed on the interactive effects using the Least Square Means within the R package “lsmeans” (Table S3). In order to account for the potential effect of different treatment replicates, we used a type II ANOVA, except whenever an interaction effect was observed and a type III ANOVA was used instead [61]. Sequencing data were analyzed with the “phyloseq” package [62]. The Shannon diversity index was calculated after removing OTUs not observed more than three times in at least 10% of the samples. Specific sequencing barcodes per sample, and the number of reads that passed the filtering criteria can be found in the Supplementary Datafile 1. We used a permutational analysis of variance (PERMANOVA) with 9999 permutations on a dissimilarity matrix (Morisita–Horn on top of a Hellinger transformed OTU table) to assess the effect of the variables under study on functional gene diversity using the function adonis() in the “vegan” package with a two-factorial design [63] (Table S4). Similarly to the ANOVA analyses, we developed two models, the first model (eT vs. eCO2 dataset) included [eCO2], [eT], and their interactions, the second model (drought dataset) included drought, [eT × eCO2] and their interactions. This test is sensitive to dispersions among groups, which might be more pronounced in situations where there is a different number of replicates per treatment and thus confound significant PERMANOVA results. Therefore, we confirmed a priori that groups did not differ significantly in their dispersion by performing an analysis of multivariate homogeneity (PERMDIST) using the function betadisper() of the package ‘vegan’, with the bias.adjust=T argument, to account for differences in sample size [64, 65]. All raw sequencing data were deposited into the NCBI SRA under BioProject ID PRJNA612521. All the remaining data are available as Supplementary Material.
Table 2.
Average values for soil parameters, organic and inorganic pools and process rates (means ± SE).
| Mean values per treatment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [Ambient] | [eT] | [eCO2] | [eT] × [eCO2] [eT × eCO2] | [Ambient] × [D] | [eT × eCO2] × [D] | |||||||
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | |||||||
| (n = 8) | (n = 3) | (n = 3) | (n = 4) | (n = 4) | (n = 4) | |||||||
| Soil parameters | ||||||||||||
| SWC | 0.34 | 0.01 | 0.32 | 0.01 | 0.34 | 0.00 | 0.33 | 0.01 | 0.08 | 0.01 | 0.06 | 0.01 |
| pH | 4.95 | 0.03 | 5.02 | 0.05 | 4.99 | 0.03 | 4.97 | 0.03 | 4.96 | 0.04 | 5.01 | 0.02 |
| Soil N | 0.31 | 0.01 | 0.27 | 0.02 | 0.26 | 0.02 | 0.29 | 0.02 | 0.32 | 0.01 | 0.33 | 0.03 |
| MBN | 79.31 | 5.89 | 93.39 | 14.16 | 63.65 | 9.89 | 93.34 | 6.59 | 99.44 | 7.09 | 120.26 | 6.03 |
| Extractable organic and inorganic pools | ||||||||||||
| NO3− | 2.97 | 0.64 | 5.18 | 2.30 | 4.19 | 1.47 | 5.95 | 2.46 | 5.98 | 1.72 | 5.26 | 1.09 |
| NH4+ | 1.11 | 0.20 | 0.87 | 0.12 | 0.95 | 0.13 | 0.89 | 0.11 | 12.23 | 4.54 | 13.13 | 2.99 |
| DIN | 4.09 | 0.76 | 6.04 | 2.25 | 5.13 | 1.45 | 6.84 | 2.51 | 18.21 | 6.15 | 18.39 | 3.56 |
| DON | 14.54 | 2.21 | 8.51 | 1.90 | 12.37 | 0.67 | 12.65 | 1.83 | 19.34 | 1.18 | 23.78 | 3.45 |
| TDN | 18.63 | 2.78 | 8.51 | 1.91 | 12.37 | 0.68 | 19.49 | 1.07 | 37.55 | 7.08 | 42.16 | 4.48 |
| DOC | 87.28 | 8.65 | 93.79 | 3.26 | 79.99 | 0.88 | 94.07 | 7.93 | 165.68 | 45.37 | 215.09 | 59.17 |
| Gross process rates | ||||||||||||
| NH4+ mineralizationa | 2.72 | 0.38 | 1.75 | 0.76 | 2.62 | 0.35 | 2.97 | 0.49 | 4.03 | 0.30 | 6.97 | 0.81 |
| NH4+ immobilizationab | 2.08 | 0.49 | 1.14 | 0.51 | 1.82 | 0.23 | 3.44 | 1.05 | 5.18 | 1.73 | 10.59 | 3.89 |
| Nitrification | 1.77 | 0.42 | 2.24 | 0.80 | 2.73 | 0.20 | 2.38 | 0.73 | 3.89 | 0.17 | 4.56 | 0.62 |
The number of biological replicates is shown in parenthesis. Soil water content (SWC) is expressed in % of fresh soil; MBN microbial biomass N and TDN total dissolved nitrogen are expressed in µg N g−1 DW; DIN dissolved inorganic nitrogen and DON dissolved organic nitrogen are expressed in µg N g−1 DW. Ammonium (NH4+) and nitrate (NO3−) are expressed in µg N g−1 DW. DOC dissolved organic carbon is expressed in µg C g−1 DW. All process rates are expressed in µg N g−1 DW d−1.
an = 3 for treatment [Ambient] × [D].
bn = 3 for treatment [eT × eCO2].
Table 3.
Individual and combined effects of eT, eCO2, and drought in soil parameters, organic and inorganic pools and process rates.
| Treatment effects | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eT vs. eCO2 dataset | Drought dataset | |||||||||||
| [eT] | [eCO2] | [eT] × [eCO2] | [eTeCO2] | [D] | [eT × eCO2] × [D] | |||||||
| F | p | F | p | F | p | F | p | F | p | F | p | |
| Soil parameters | ||||||||||||
| SWC | 3.140 | 0.09 | 0.72 | 0.40 | 1.180 | 0.29 | 1.740 | 0.21 | 1164.59 | 2.24 × 10−16 | 0.621 | 0.42 |
| pH | 0.557 | 0.47 | 0.01 | 0.93 | 1.280 | 0.27 | 0.890 | 0.36 | 0.28 | 0.60 | 0.320 | 0.57 |
| Soil N | 0.128 | 0.73 | 1.39 | 0.26 | 3.140 | 0.09 | 0.262 | 0.62 | 1.08 | 0.31 | 0.422 | 0.52 |
| MBN | 5.620 | 0.03 | 0.99 | 0.33 | 0.769 | 0.40 | 6.110 | 0.02 | 11.30 | 3.00 × 10−3 | 0.240 | 0.63 |
| Extractable organic and inorganic pools | ||||||||||||
| NO3− | 1.570 | 0.23 | 0.40 | 0.53 | 0.010 | 0.89 | 0.990 | 0.33 | 1.03 | 0.32 | 1.720 | 0.20 |
| NH4+ | 0.510 | 0.48 | 0.05 | 0.82 | 0.080 | 0.77 | 0.030 | 0.86 | 93.35 | 4.23 × 10−8 | 0.670 | 0.42 |
| DIN | 1.230 | 0.28 | 0.31 | 0.58 | 0.005 | 0.94 | 0.700 | 0.41 | 15.24 | 1.00 × 10−3 | 0.100 | 0.75 |
| DON | 3.120 | 0.09 | 0.54 | 0.47 | 2.260 | 0.15 | 0.007 | 0.93 | 10.96 | 4.00 × 10−3 | 1.070 | 0.31 |
| TDN | 0.140 | 0.71 | 1.40 | 0.25 | 1.250 | 0.28 | 0.770 | 0.39 | 26.35 | 1.00 × 10−4 | 0.037 | 0.84 |
| DOC | 5.330 | 0.02 | 0.01 | 0.89 | NA | NA | 1.680 | 0.21 | 24.44 | 1.00 × 10−4 | 0.000 | 0.99 |
| Gross process rates | ||||||||||||
| NH4+ mineralization | 0.560 | 0.46 | 0.84 | 0.37 | 1.550 | 0.23 | 8.180 | 0.01 | 22.81 | 2.00 × 10−4 | 5.830 | 0.03 |
| NH4+ immobilization | 0.020 | 0.88 | 1.19 | 0.29 | 3.110 | 0.10 | 2.210 | 0.16 | 5.60 | 0.03 | 0.068 | 0.79 |
| Nitrification | 0.030 | 0.85 | 0.95 | 0.34 | 0.430 | 0.51 | 1.320 | 0.26 | 15.26 | 1.40 × 10−3 | 0.002 | 0.96 |
Statistical effects were assessed by a 2-way ANOVA type II or a 2-way ANOVA type III whenever a significant interaction term was found. Least square means (lsmeans) multiple comparison tests were ran on interactive effects. Significant effects from the ANOVA are shown in bold. NA depict variables for which nonparametric tests were used and therefore an interactive effect could not be calculated.
SWC soil water content, MBN microbial biomass nitrogen, DIN dissolved inorganic nitrogen, DON dissolved organic nitrogen, TDN total dissolved nitrogen, DOC dissolved organic nitrogen.
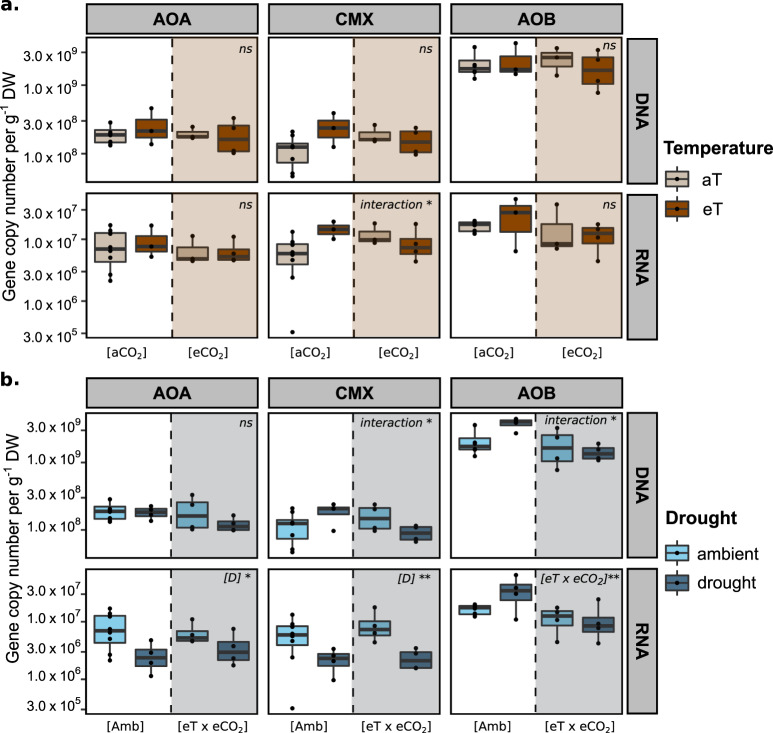
Fig. 1. qPCR quantifications of amoA gene and transcript copy numbers per gram of dry soil from the ammonia-oxidizing microbial groups.
a Quantifications from plots affected by elevated temperature (eT) and atmospheric CO2 concentrations (eCO2). The dashed lines represent plots affected by elevated atmospheric CO2 conditions (eCO2). The colors represent plots affected by either ambient temperature (aT) or elevated temperature (eT). b Quantifications from plots affected by future climate (eT x eCO2) and drought (D). The dashed lines separate plots affected by drought (D). The colors represent the drought treatment. The caption on the upper right corner of each subplot represents the ANOVA results. *p value <0.05; **p value <0.01. Whenever the ANOVA results showed a significant interaction term, lsmeans multiple comparison tests were ran. For details on the ANOVA and post-hoc tests, see Tables S2 and S3.
Results
Soil biogeochemical variables and processes are sensitive to drought but not to other global change factors
Manipulation of CO2 concentration and temperature had little or no effect on soil N and C pools, and N processes in the studied grassland (Table 3). No significant statistical effect was found for [eCO2], neither alone nor in combination with [eT], for soil N, SWC, pH and gross N process rates. In contrast, only microbial MBN and DOC increased significantly in response to [eT] alone (Table 3).
Drought had a significant effect on most of the measured variables and processes. SWC decreased from 33% in the ambient plots to around 6% in the drought plots (Tables 2 and 3, F = 1164.6, p = 2.25 × 10−16). In addition, drought caused a loss of 24.6 % (in ambient conditions) and 50% (in future climate scenarios) of aboveground plant biomass, which corresponded to a reduction in plant N uptake of 15.8% and 46.6% respectively (data not shown). Furthermore, MBN was significantly higher in both the drought plots (F = 11.31, p = 0.003) and in [eT × eCO2] plots (F = 6.11, p = 0.025), with no significant interaction term (Table 3).
There was a significant increase of DIN (F = 15.24, p = 0.001), DON (F = 10.96, p = 0.004), TDN (F = 26.35, p = 0.0001), and DOC (F = 24.44, p = 0.0001) in the drought plots relative to ambient plots, but no significant effect of [eT × eCO2] conditions. The response of inorganic N forms to drought was mainly driven by increases in NH4+ contents under drought (Table 3; F = 93.35, p = 4.23 × 10−8), since NO3− levels remained unchanged. Moreover, N mineralization rates increased significantly in response to individual and combined effects of drought and [eT × eCO2] (Table 3). Concomitantly, nitrification rates were significantly higher in the drought plots (Tables 2 and 3). These results should however be interpreted with caution due to the potential short-term rewetting effect introduced by the application of a liquid tracer during the isotope pool dilution experiments [66].
AOB are the most abundant ammonia-oxidizer group and did not decrease with drought
Betaproteobacterial AOB amoA gene copy numbers ranged between 1.43 × 109 and 3.79 × 109 copies g−1 DW, indicating that they were the most abundant of all ammonia-oxidizing microorganisms in these montane-grassland plots (Fig. 1). Archaeal amoA gene copy numbers ranged between 1.21 × 108 and 2.70 × 108 copies g−1 DW, and CMX clade B amoA gene copy numbers ranged from 9.10 × 107 to 2.50 × 108 copies g−1 DW. We observed no significant effect of individual and combined [eT] and [eCO2] effects on amoA gene abundance (Fig. 1a). We found an interactive effect of [eTeCO2] and [D] in both CMX and AOB (Fig. 1b), although post-hoc analyses revealed significant differences between individual treatments for AOB only (Table S3).
Betaproteobacterial AOB amoA transcript copy numbers ranged between 1.14 × 107 and 3.62 × 107 copies g−1 DW, whereas AOA and CMX clade B had similar amoA transcript copies g−1 DW (0.27–0.99 × 107 and 0.23–1.46 × 107, respectively) (Fig. 1). We observed no significant individual [eT] and [eCO2] effects on amoA transcription (Fig. 1a; Table S2). There was an interactive effect in CMX amoA transcription (Fig. 1a), although not supported by the post-hoc analysis (Table S3). On the other hand, both AOA and CMX clade B amoA transcript copy numbers decreased significantly in response to drought (Fig. 1b). Notably, AOB amoA transcription was not affected by drought, but a significant decrease in AOB amoA transcript copy numbers was observed in plots affected by [eT × eCO2].
AOA and NOB community structure is significantly affected by drought
The richness of nitrifying communities in all treatments was relatively low. We recovered 12 CMX amoA OTUs, 23 AOA amoA OTUs, and 15 AOB amoA OTUs which were robustly assigned to the corresponding phylogenetic clades after excluding unspecific OTUs (Figs. S2, S3). Unspecific OTUs comprised 0.01%, 0.05%, and 0.3% of all reads for AOA, CMX, and AOB, respectively, (Table S1) and are therefore unlikely to interfere with the qPCR quantifications. In addition to excluding nonspecific AOA OTUs, the coverage of the qPCR primers was checked in silico due to the use of different AOA primer pairs for archaeal amoA quantification and sequencing. This resulted on the exclusion of three more AOA OTUs, which comprised 32% of all reads (Table S1). Notably, CMX clade A amoA amplification was not detectable in all samples. Furthermore, 40 nxrB OTUs also displayed a robust taxonomic classification within the reference tree after excluding nonspecific OTUs (Table S1, Fig. S4).
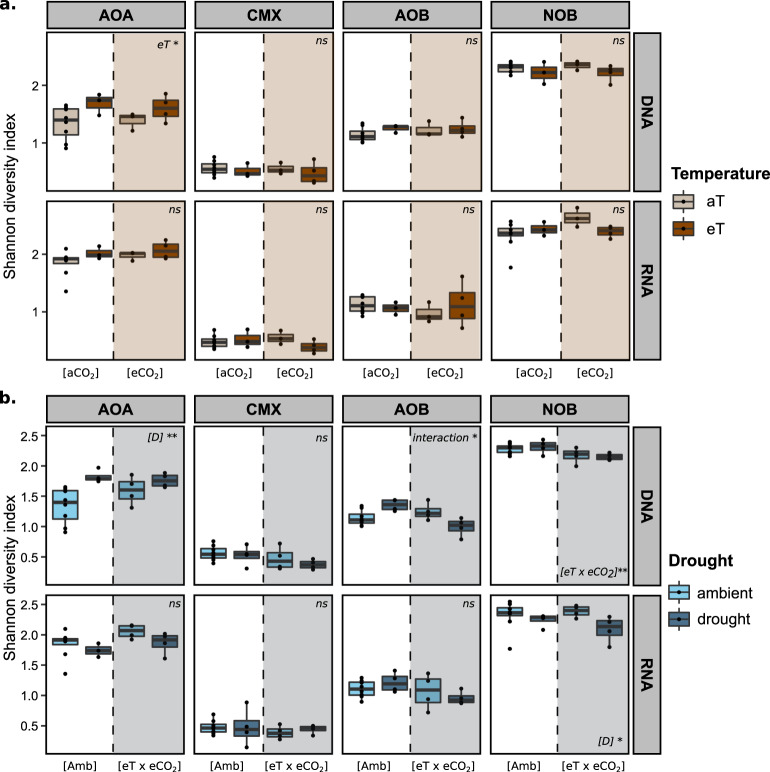
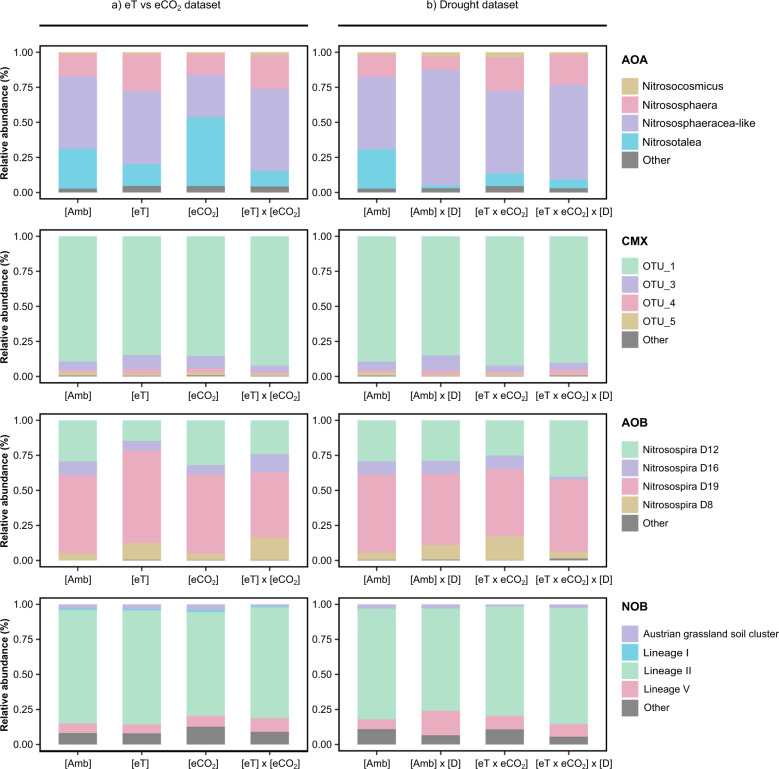
The most abundant AOA amoA gene and transcript OTU was affiliated with genus Nitrosotalea and comprised 12–65% of the reads per treatment. The remaining OTUs were affiliated with members of the family Nitrososphaeraceae, which comprises the genera Nitrososcosmicus and Nitrososphaera. Within the Nitrososphaeraceae, we observed a sister group to the genus Nitrososphaera, comprising ten OTUs and 13–37% of the reads per treatment (Fig. S2a). Statistical analyses showed that the AOA amoA gene community structure was significantly affected by [eT] alone (PERMANOVA, F = 8.50; p = 0.02; Table S4; Fig. S5a), and plots on which temperature was manipulated individually showed a higher Shannon index (ANOVA, F = 5.18; p = 0.03; Fig. 2a). In addition, variance partitioning revealed a significant difference in AOA amoA gene and transcript community structure in response to drought (PERMANOVA, F = 36.48, p ≤ 0.001; and F = 3.91, p = 0.04, respectively) (Fig. 3b; Table S4; Fig. S5b). Changes in community structure resulted in a significantly higher Shannon diversity index of AOA amoA gene (ANOVA, F = 10.908, p = 0.004) in the drought plots (Fig. 2b). The changes in AOA community composition at both transcript and gene levels reflected a clear decrease in the relative abundance of Nitrosotalea-affiliated OTUs in response to individual manipulations of drought (Fig. 3a; Fig. S5b) and [eT] (Fig. 3a; Fig. S5a).
Fig. 2. Shannon diversity index based on amoA/nxrB gene and transcript sequences from all microbial groups.
a Quantifications from plots affected by elevated temperature (eT) and atmospheric CO2 concentrations (eCO2). The dashed lines represent plots affected by elevated atmospheric CO2 conditions (eCO2). The colors represent plots affected by either ambient temperature (aT) or elevated temperature (eT). b Quantifications from plots affected by future climate (eT x eCO2) and drought (D). The dashed lines separate plots affected by drought (D). The colors represent the drought treatment. The caption on the upper right corner of each subplot represents the ANOVA results. *p value <0.05; **p value <0.01. Whenever the ANOVA results showed a significant interaction term, lsmeans multiple comparison tests were ran. For details on the ANOVA and post-hoc tests, see Tables S2 and S3.
Fig. 3. Relative abundance (%) of all nitrifying groups obtained by amoA and nxrB transcript sequences.
a Data for plots affected by single and interactive effects of elevated temperature (eT) and atmospheric CO2 (eCO2) concentrations. b Plots affected by single and interactive effects of future climate conditions (eT x eCO2) and drought (D). The color code represents different genera/OTU/clades/lineages. “Amb” stands for “ambient conditions”. Multiple horizontal lines within the same color represent individual OTUs within a group. Taxa that comprised <0.1% of all reads per treatment are grouped as “Other”.
Regarding CMX clade B amoA gene and transcript diversity, a clear dominance of one single OTU, which comprised an average of 87.3% and 87.5% of gene and transcript reads per treatment, respectively, (Fig. 3; Fig. S5), was observed. There were no significant effects of any tested variable on CMX clade B community composition and diversity indexes.
The betaproteobacterial AOB amoA diversity was dominated by OTUs affiliated with the genus Nitrosospira, and the abundance of Nitrosomonas-related amoA OTUs was very low (average of 0.24% and 0.73% of amoA gene and transcript reads per treatment, respectively). At the transcript level, the most abundant OTU was most similar to an uncultured Nitrosospira clade D19 OTU (100% identity), which comprised an average of 53% of the reads per treatment (Fig. 3), dropping to 24.4% at the gene level (Fig. S4). On the other hand, the most abundant OTU at the gene level was most similar to an uncultured Nitrosospira clade D12 OTU (100% identity), with an average of 56.7% of the reads per treatment (Fig. S4). The most abundant transcript OTU was 87.6% identical to the most abundant gene OTU. Variance partitioning showed a significant interaction effect of [eT × eCO2] × [D] on the diversity and relative abundance of AOB amoA transcripts (PERMANOVA, F = 5.34, p = 0.02; Table S4). Notably, this treatment showed the lowest Shannon diversity index across all treatments (Shannon = 0.95 ± 0.05; Fig. 2b). At the AOB amoA gene level, a significant increase in the Shannon index was observed in ambient plots versus [eT × eCO2] plots, only under drought conditions (Fig. 2b; Tables S2 and S4).
Nitrospira nxrB OTUs were affiliated with lineage 1, 2, 5, and 6, with a clear dominance of lineage 2, which comprised an average of 88.9% and 79.9% of gene and transcripts, respectively (Fig. 3; Fig. S5). The closest culturable representative of the most abundant OTU was Nitrospira japonica (99.92% identity), and most of the OTUs clustered together with environmental sequences from Austrian and Namibian soil clusters (Fig. S4). Amplicon sequencing showed that NOB were the most diverse group of nitrifiers, showing the highest Shannon index values of all microbial groups. (Fig. 2). Variance partitioning showed that [eT] alone caused significant differences in nxrB gene community structure (PERMANOVA, F = 4.06, p = 0.02; Table S4; Fig. S5a), although there were no significant differences in alpha diversity. Drought caused significant differences in both nxrB gene and transcript community composition (PERMANOVA, F = 5.34, p = 0.02, PERMANOVA, F = 6.53, p < 0.01; Table S4). These changes were coupled with significantly lower levels of alpha diversity under drought at the transcript level (ANOVA, F = 5.75, p = 0.03; Fig. 2b), although the same trend was not observed at the gene level. Contrastingly, nxrB gene community structure was significantly affected by [eT × eCO2] (PERMANOVA, F = 5.52, p = 0.02; Table S4), which was reflected in significantly lower Shannon index values (ANOVA, F = 9.12, p = 0.008; Fig. 2b).
Discussion
Ammonia-oxidation, usually regarded as the rate-limiting step of nitrification [67], is mediated by specific groups of bacteria and archaea. Therefore, climate change induced alterations in nitrifier community structure could affect soil nitrification to a higher degree than other N cycling processes, such as protein decomposition [11, 68] which are carried out by broader sets of microorganisms. In addition, since the discovery of complete nitrifiers, there is a need for the reassessment of the relative role of each microbial group in ammonia oxidation under climate change. Here we report an in-depth assessment on the effect of multiple climate change drivers on soil nitrification, including (i) the quantification of functional genes and transcripts by quantitative PCR, (ii) a census of the nitrifying microorganisms by sequencing at the functional gene and transcript levels, and (iii) an estimation of gross ammonification and nitrification rates.
In contrast to our first hypothesis we found that the nitrification process was relatively resistant to increases in atmospheric CO2 with no significant changes in gross nitrification and in nitrifier functional gene abundance and expression. Effects of increased atmospheric CO2 on soil microbial processes are primarily determined by changes in plant belowground carbon inputs and in plant nutrient uptake [69], where rhizodeposition can promote heterotrophic microbial activity, but greater plant N uptake may cause substrate (NH4+) limitation for nitrifiers. At our site, no increase in above and belowground net primary productivity was found in response to elevated CO2 or temperature (Canarini et al., in preparation). Likewise, eCO2 did not affect gross N mineralization and NH4+ levels, highlighting that substrate availability for nitrifiers did not change in response to eCO2. In addition, other studies also reported very few effects of elevated atmospheric CO2 concentration on belowground N processes in heathlands and temperate grasslands [70–72] showing that soil parameters such as pH and inorganic N concentrations likely play a more significant role than increased atmospheric CO2 on overall nitrification.
Elevated temperature alone also did not significantly affect most of the soil processes and variables studied. More importantly, in contrast to our second hypothesis, elevated temperature did not affect the abundance, composition, and activity of soil nitrifier communities, indicating a high tolerance of the nitrification process to temperature in this ecosystem. Also, our results are in line with meta-analyses that observed that the effect of temperature on N cycling processes is less pronounced in grasslands, in comparison with other ecosystem types [36, 73]. In addition, other studies in grassland systems also reported no significant effects of elevated temperature on nitrification [72, 74]. Our results indicate that increased temperature might have altered abiotic and biotic soil parameters that may be masking individual temperature effects. Specifically, the soil water content at our field site was found to decrease throughout summer (Simon et al., under review) which can constrain substrate availability and/or accessibility to nitrifiers and mask potential stimulatory effect of warming on the nitrification process. Also, plant production of biological nitrification inhibitors (BNIs) could explain these results. However, BNIs were not assessed in this study, and their importance has mostly been reported in N-limited ecosystems [75, 76].
We further hypothesized that drought would reduce the abundance and activity of all nitrifying groups but found that responses to drought were group specific. The amoA expression levels of AOA and CMX clade B significantly decreased, whereas AOB either maintained (eT × eCO2 plots) or increased (under ambient temperature and CO2) their amoA transcription levels under drought. Drought can directly affect microbial growth by reducing the soil water potential and forcing microorganisms to invest into osmoregulation rather than growth and replication [33]. It can also have indirect effects on microbial communities by reducing plant belowground C allocation [77, 78], and by changing the availability and mobility of nutrients through a reduced soil pore connectivity and reduced plant uptake [79]. Studies have shown that plants can alter their root exudate abundance and composition during drought periods [80] as an attempt to better cope with the osmotic stress and/or to recruit particular fungi to the vicinity of the roots [81, 82]. Within root exudates, plants can also actively secrete BNIs, but given that fertilized grasslands are often not N limited, and our results point to higher NH4+ levels under drought, there is little evidence to support a relevant role for BNIs in these systems [76, 83]. Also, at the same study site, a decrease in plant biomass has been observed under drought, as well as a reduction in total plant N uptake. As a consequence of the plant response to drought, organic and inorganic dissolved N forms accumulated in the soil (Table 2) as reported in other studies [33]. The same pattern was observed with regards to microbial biomass N. Previous studies reported an increase in microbial biomass with drought, and have indicated that major pools of C- and N-based microbial metabolites are dynamic in response to drought [84, 85]. The accumulation of organic and inorganic N forms might have created optimal conditions for the proliferation of AOM with low ammonia (NH3) affinities and high Vmax, such as AOB, as opposed to AOA and CMX [12]. AOA have been reported to be more sensitive to perturbations and variations in NH4+ concentrations when compared with AOB [11, 86]. Also, the nitrification activity of both AOA and CMX saturates faster due to lower maximum rates of NH3 oxidation, compared with AOB [12]. However, no pure culture representative exists for CMX clade B yet, and the kinetic parameters of Nitrospira inopinata—the only CMX Nitrospira clade A isolate so far—might not be representative of all CMX members [12]. AOB, and particularly members of the genus Nitrosospira, are ubiquitous in grasslands and tolerate high concentrations of NH4+ [87]. In addition, genes involved in the protection of bacterial cells from hypoosmotic stress have been found in N. multiformis, showing that these organisms are well adapted to changes in soil water potential. However, osmoprotectants have also been detected in genomes of cultivated AOA [88–91], indicating that these organisms can also adapt to osmotic stress. Nevertheless, a dominance of AOB in managed grasslands has been reported due to frequent fertilization events [13, 14], and AOB are known to outcompete AOA in substrate-rich environments [9].
Along with an overall decrease in archaeal amoA gene and transcript copy number in response to drought, we found a reduction in the relative abundance of AOA OTUs affiliated to Nitrosotalea. This could be explained by the accumulation of organic compounds under drought, some of which were shown to inhibit Nitrosotalea activity and growth in pure cultures [92] compared with other AOA such as N. viennensis and N. gargensis [88, 93]. Furthermore, inhibition by organic compounds has been shown to be stronger in AOA than in AOB, although it varies between AOA strains [94, 95].
Even though we lack quantitative data on nxrB gene and transcript abundance due to still unresolved methodological challenges, we observed that the existing Nitrospira-like NOB community structure differed significantly in the drought plots compared with the non-drought plots, which was reflected on a decrease in the Shannon diversity index at the transcript level. Nitrospira were shown to have a high affinity for nitrite, and often dominate NOB communities in N-limited soils [96]. Given that NO2− often does not accumulate in soils, Nitrospira are considered to be the main nitrite-oxidizing genus in these ecosystems [97, 98]. Regardless of the decrease in diversity under drought, Nitrospira sublineage II remained the most abundant sublineage. Pure culture studies have reported a high metabolic flexibility of members of this sublineage, and it is possible that some microorganisms affiliated with sublineage II are better able to cope with the higher osmotic stress and levels of inorganic N imposed by drought [99, 100]. In addition, another study has reported higher rates of autotrophic growth of a Nitrospira sublineage II phylotype upon the addition of NH4+ [101].
Emission of the greenhouse gas N2O has been shown to increase up to eightfold following the end of a drought period in grassland ecosystems, and a single short rain event can increase the annual net N2O flux between 2 and 50% [102]. Therefore, our findings may also have broader implications since a drought-induced alteration in nitrifier community structure in favor of AOB over AOA and CMX might lead to higher N2O emissions upon rewetting. In soil, N2O is produced by denitrifying and ammonia-oxidizing microorganisms [103]. Within the latter group, AOB produce relatively high yields of N2O via hydroxylamine oxidation under oxic conditions [104], while AOA and CMX produce much lower yields of N2O during aerobic ammonium oxidation [105, 106]. Changes in redox potential could affect nitrification processes, specifically at the interface between aerobic and (partially) anaerobic soil portions. However, the soils in this study did not reach anaerobic conditions (maximum 34% soil water content), therefore making it unlikely that redox changes would have played a significant role.
Finally, we found few to no significant interactive effects between the different climate change drivers. Global reviews have shown that the interaction of different climate change factors can lead to nonadditive effects [6], although a more recent meta-analysis showed that antagonistic and synergistic effects are quite rare [7]. A recent study in a grassland system assessed the effect of elevated temperature and elevated CO2 and found that while combinatory effects were antagonistic, they were mostly nonsignificant [72]. Regarding nitrifier community composition, only active AOB were significantly affected by the interactive effect of future climate conditions and drought [eTeCO2 × [D]). Under these conditions, the AOB diversity and abundance were lowest, as assessed by amoA amplicon sequencing and gene and transcript copy number quantification, which agrees with our last hypothesis.
In conclusion, in our study we demonstrated that nitrifying communities in grassland soils were remarkably unresponsive to elevated CO2 and elevated temperature, alone or in combination, resulting in unaltered nitrification rates. We also showed that drought significantly changed the structure of the existing nitrifying communities, possibly through a strong reduction of plant biomass and N uptake. We hypothesize that the specific conditions created by drought (such as the accumulation of NH4+ and organic N, and, reduced pore connectivity) resulted in favorable niches for AOB, that showed higher or unaltered levels of amoA transcription and drove the observed peak in nitrification rates at the expense of ammonium-oxidizing archaea and comammox Nitrospira. Nevertheless, caution should be taken when interpreting nitrification rates due to the potential short-term rewetting effect introduced by the application of the liquid tracer (see Methods section). Future climate conditions further interacted with drought and caused an antagonistic effect on the diversity and abundance of this group, without reducing gross nitrification rates. Given that a shift in nitrifier community structure towards AOB could potentially result in higher N2O emission rates at the end of the drought period, special emphasis should be put into understanding the sensitivity of different nitrifiers to individual and combined global change variables in terrestrial ecosystems.
Supplementary information
Acknowledgements
We would like to thank the team of the Austrian Research and Education Centre Raumberg-Gumpenstein (HBLFA) for their support during the sampling campaign and for the provision of the experimental site, which was supported by the DaFNE project ClimGrassEco (101067). In addition, we thank T. Böckle, M. Ječmenica, and V. Martin for assisting with sample processing, as well as M. Watzka for help with IRMS measurements. This project was financially supported by the Austrian Science Fund (FWF P 28572) and by the Austrian Science Fund (FWF) DK+ program ‘Microbial Nitrogen Cycling’ (W1257-B20).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alberto Canarini, Email: alberto.canarini@hotmail.it.
Andreas Richter, Email: andreas.richter@univie.ac.at.
Supplementary information
The online version of this article (10.1038/s41396-020-00735-7) contains supplementary material, which is available to authorized users.
References
- 1.Foley JA, DeFries R, Asner GP, Barford C, Bonan G, Carpenter SR, et al. Global consequences of land use. Science. 2005;309:570–4. doi: 10.1126/science.1111772. [DOI] [PubMed] [Google Scholar]
- 2.Rockström J, Steffen W, Noone K, Persson Å, Chapin FS, Lambin EF, et al. A safe operating space for humanity. Nature. 2009;461:472–5. doi: 10.1038/461472a. [DOI] [PubMed] [Google Scholar]
- 3.Graham EB, Knelman JE, Schindlbacher A, Siciliano S, Breulmann M, Yannarell A, et al. Microbes as engines of ecosystem function: when does community structure enhance predictions of ecosystem processes? Front Microbiol. 2016;7:1–10. doi: 10.3389/fmicb.2016.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavicchioli R, Ripple WJ, Timmis KN, Azam F, Bakken LR, Baylis M, et al. Scientists’ warning to humanity: microorganisms and climate change. Nat Rev Microbiol. 2019;17:569–86. doi: 10.1038/s41579-019-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoegh-Guldberg O, Jacob D, Taylor M, Bindi M, Brown S, Camilloni I, et al. Impacts of 1.5 °C global warming on natural and human systems. In: Masson-Delmotte V, Zhai P, Pörtner HO, Roberts D, Skea J, Shukla PR, et al., editors. Geneva, Switzerland: World Meteorological Organization Technical Document; 2018.
- 6.Dieleman WIJ, Vicca S, Tingey D, De Angelis P, Hagedorn F, Morgan JA, et al. Simple additive effects are rare: a quantitative review of plant biomass and soil process responses to combined manipulations of CO 2 and temperature. Glob Chang Biol. 2012;18:2681–93. doi: 10.1111/j.1365-2486.2012.02745.x. [DOI] [PubMed] [Google Scholar]
- 7.Song J, Wan S, Piao S, Knapp AK, Classen AT, Vicca S, et al. A meta-analysis of 1,119 manipulative experiments on terrestrial carbon-cycling responses to global change. Nat Ecol Evol. 2019;3:1309–20. doi: 10.1038/s41559-019-0958-3. [DOI] [PubMed] [Google Scholar]
- 8.Kuypers MMM, Marchant HK, Kartal B. The microbial nitrogen-cycling network. Nat Rev Microbiol. 2018;16:263–76. doi: 10.1038/nrmicro.2018.9. [DOI] [PubMed] [Google Scholar]
- 9.Prosser JI, Nicol GW. Archaeal and bacterial ammonia-oxidisers in soil: the quest for niche specialisation and differentiation. Trends Microbiol. 2012;20:523–31. [DOI] [PubMed]
- 10.Martens-Habbena W, Berube PM, Urakawa H, De La Torre JR, Stahl DA. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature. 2009;461:976–9. doi: 10.1038/nature08465. [DOI] [PubMed] [Google Scholar]
- 11.Fuchslueger L, Kastl EM, Bauer F, Kienzl S, Hasibeder R, Ladreiter-Knauss T, et al. Effects of drought on nitrogen turnover and abundances of ammonia-oxidizers in mountain grassland. Biogeosciences. 2014;11:6003–15. [Google Scholar]
- 12.Kits KD, Pjevac P, Daebeler A, Han P, Albertsen M, Romano S, et al. Kinetic analysis of a complete nitrifier reveals an oligotrophic lifestyle. Nature. 2017;549:269–72. doi: 10.1038/nature23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di HJ, Cameron KC, Shen JP, Winefield CS, Ocallaghan M, Bowatte S, et al. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat Geosci. 2009;2:621–4. [Google Scholar]
- 14.Jia Z, Conrad R. Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ Microbiol. 2009;11:1658–71. doi: 10.1111/j.1462-2920.2009.01891.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhalnina K, Dörr de Quadros P, Camargo FAO, Triplett EW. Drivers of archaeal ammonia-oxidizing communities in soil. Front Microbiol. 2012;3:1–9. doi: 10.3389/fmicb.2012.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruber-Dorninger C, Pester M, Kitzinger K, Savio DF, Loy A, Rattei T, et al. Functionally relevant diversity of closely related Nitrospira in activated sludge. ISME J. 2015;9:643–55. doi: 10.1038/ismej.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daims H, Lebedeva EV, Pjevac P, Han P, Herbold C, Albertsen M, et al. Complete nitrification by Nitrospira bacteria. Nature. 2015;528:504–9. doi: 10.1038/nature16461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Kessel MAHJ, Kartal B, MSM Jetten, Albertsen M, Op den Camp HJM, Lücker S, et al. Complete nitrification by a single microorganism. Nature. 2015;528:555–9. doi: 10.1038/nature16459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poghosyan L, Koch H, Lavy A, Frank J, van Kessel MAHJ, Jetten MSM, et al. Metagenomic recovery of two distinct comammox Nitrospira from the terrestrial subsurface. Environ Microbiol. 2019;00:1–11. doi: 10.1111/1462-2920.14691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Cao Y, Zhu-Barker X, Nicol GW, Wright AL, Jia Z, et al. Comammox Nitrospira clade B contributes to nitrification in soil. Soil Biol Biochem. 2019;135:392–5. [Google Scholar]
- 21.Dusenge ME, Duarte AG, Way DA. Plant carbon metabolism and climate change: elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. N. Phytol. 2019;221:32–49. doi: 10.1111/nph.15283. [DOI] [PubMed] [Google Scholar]
- 22.de Graaff MA, van Groenigen KJ, Six J, Hungate B, van Kessel C. Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Glob Chang Biol. 2006;12:2077–91. [Google Scholar]
- 23.Kuzyakov Y, Horwath WR, Dorodnikov M, Blagodatskaya E. Review and synthesis of the effects of elevated atmospheric CO2 on soil processes: No changes in pools, but increased fluxes and accelerated cycles. Soil Biol Biochem. 2019;128:66–78. [Google Scholar]
- 24.Luo Y, Su B, Currie WS, Dukes J. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience. 2004;54:731–9.
- 25.Liang J, Qi X, Souza L, Luo Y. Processes regulating progressive nitrogen limitation under elevated carbon dioxide: a meta-analysis. Biogeosciences. 2016;13:2689–99. [Google Scholar]
- 26.He Z, Xu M, Deng Y, Kang S, Kellogg L, Wu L, et al. Metagenomic analysis reveals a marked divergence in the structure of belowground microbial communities at elevated CO2. Ecol Lett. 2010;13:564–75. doi: 10.1111/j.1461-0248.2010.01453.x. [DOI] [PubMed] [Google Scholar]
- 27.Horz HP, Barbrook A, Field CB, Bohannan BJM. Ammonia-oxidizing bacteria respond to multifactorial global change. Proc Natl Acad Sci USA. 2004;101:15136–41. doi: 10.1073/pnas.0406616101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradford MA, Davies CA, Frey SD, Maddox TR, Melillo JM, Mohan JE, et al. Thermal adaptation of soil microbial respiration to elevated temperature. Ecol Lett. 2008;11:1316–27. doi: 10.1111/j.1461-0248.2008.01251.x. [DOI] [PubMed] [Google Scholar]
- 29.Liu Q, Piao S, Janssens IA, Fu Y, Peng S, Lian X, et al. Extension of the growing season increases vegetation exposure to frost. Nat Commun. 2018;9:426. doi: 10.1038/s41467-017-02690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lax S, Abreu CI, Gore J. Higher temperatures generically favour slower-growing bacterial species in multispecies communities. Nat Ecol Evol. 2020;4:560–657. doi: 10.1038/s41559-020-1126-5. [DOI] [PubMed] [Google Scholar]
- 31.Tourna M, Freitag TE, Nicol GW, Prosser JI. Growth, activity and temperature responses of ammonia-oxidizing archaea and bacteria in soil microcosms. Environ Microbiol. 2008;10:1357–64. doi: 10.1111/j.1462-2920.2007.01563.x. [DOI] [PubMed] [Google Scholar]
- 32.Fierer N, Carney KM, Horner-Devine MC, Megonigal JP. The biogeography of ammonia-oxidizing bacterial communities in soil. Micro Ecol. 2009;58:435–45. doi: 10.1007/s00248-009-9517-9. [DOI] [PubMed] [Google Scholar]
- 33.Schimel JP. Life in dry soils: effects of drought on soil microbial communities and processes. Annu Rev Ecol Evol Syst. 2018;49:409–32. [Google Scholar]
- 34.Kuzyakov Y, Horwath WR, Dorodnikov M, Blagodatskaya E. Review and synthesis of the effects of elevated atmospheric CO2 on soil processes: No changes in pools, but increased fluxes and accelerated cycles. Soil Biol Biochem. 2019;128:66–78.
- 35.Yue K, Peng Y, Fornara DA, Van Meerbeek K, Vesterdal L, Yang W, et al. Responses of nitrogen concentrations and pools to multiple environmental change drivers: a meta-analysis across terrestrial ecosystems. Glob Ecol Biogeogr. 2019;28:690–724. [Google Scholar]
- 36.Bai E, Li S, Xu W, Li W, Dai W, Jiang P. A meta-analysis of experimental warming effects on terrestrial nitrogen pools and dynamics. N. Phytol. 2013;199:431–40. doi: 10.1111/nph.12252. [DOI] [PubMed] [Google Scholar]
- 37.Piepho HP, Herndl M, Pötsch EM, Bahn M. Designing an experiment with quantitative treatment factors to study the effects of climate change. J Agron Crop Sci. 2017;203:584–92. [Google Scholar]
- 38.Vance ED, Brookes PC, Jenkinson DS. An extraction method for measuring soil microbial biomass C. Soil Biol Biochem. 1987;19:703–7. [Google Scholar]
- 39.Hood-Nowotny R, Umana NH-N, Inselbacher E, Oswald- Lachouani P, Wanek W. Alternative methods for measuring inorganic, organic, and total dissolved nitrogen in soil. Soil Sci Soc Am J. 2010;74:1018–27. [Google Scholar]
- 40.Wanek W, Mooshammer M, Blöchl A, Hanreich A, Richter A. Determination of gross rates of amino acid production and immobilization in decomposing leaf litter by a novel 15N isotope pool dilution technique. Soil Biol Biochem. 2010;42:1293–302. [Google Scholar]
- 41.Sørensen P, Jensen ES. Sequential diffusion of ammonium and nitrate from soil extracts to a polytetrafluoroethylene trap for 15N determination. Anal Chim Acta. 1991;252:201–3. [Google Scholar]
- 42.Lachouani P, Frank AH, Wanek W. A suite of sensitive chemical methods to determine the δ 15N of ammonium, nitrate and total dissolved N in soil extracts. Rapid Commun Mass Spectrom. 2010;24:3615–23. doi: 10.1002/rcm.4798. [DOI] [PubMed] [Google Scholar]
- 43.Angel R, Claus P, Conrad R. Methanogenic archaea are globally ubiquitous in aerated soils and become active under wet anoxic conditions. ISME J. 2012;6:847–62. doi: 10.1038/ismej.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Apprill A, McNally S, Parsons R, Weber L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat Micro Ecol. 2015;75:129–37. [Google Scholar]
- 45.Parada AE, Needham DM, Fuhrman JA. Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ Microbiol. 2016;18:1403–14. doi: 10.1111/1462-2920.13023. [DOI] [PubMed] [Google Scholar]
- 46.Herbold CW, Pelikan C, Kuzyk O, Hausmann B, Angel R, Berry D, et al. A flexible and economical barcoding approach for highly multiplexed amplicon sequencing of diverse target genes. Front Microbiol. 2015;6:1–8. doi: 10.3389/fmicb.2015.00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Purkhold U, Wagner M, Timmermann G, Pommerening-Röser A, Koops HP. 16S rRNA and amoA-based phylogeny of 12 novel betaproteobacterial ammonia-oxidizing isolates: extension of the dataset and proposal of a new lineage within the nitrosomonads. Int J Syst Evol Microbiol. 2003;53:1485–94. doi: 10.1099/ijs.0.02638-0. [DOI] [PubMed] [Google Scholar]
- 48.Alves RJE, Minh BQ, Urich T, Von Haeseler A, Schleper C. Unifying the global phylogeny and environmental distribution of ammonia-oxidising archaea based on amoA genes. Nat Commun. 2018;9:1–17. doi: 10.1038/s41467-018-03861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berger SA, Krompass D, Stamatakis A. Performance, accuracy, and web server for evolutionary placement of short sequence reads under maximum likelihood. Syst Biol. 2011;60:291–302. doi: 10.1093/sysbio/syr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–3. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aigle A, Prosser JI, Gubry-Rangin C. The application of high-throughput sequencing technology to analysis of amoA phylogeny and environmental niche specialisation of terrestrial bacterial ammonia-oxidisers. Environ Microbiome. 2019;14:3. doi: 10.1186/s40793-019-0342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pjevac P, Schauberger C, Poghosyan L, Herbold CW, van Kessel MAHJ, Daebeler A, et al. AmoA-targeted polymerase chain reaction primers for the specific detection and quantification of comammox Nitrospira in the environment. Front Microbiol. 2017;8:1–11. doi: 10.3389/fmicb.2017.01508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pruesse E, Peplies J, Glöckner FO. SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics. 2012;28:1823–9. doi: 10.1093/bioinformatics/bts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trifinopoulos J, Nguyen L-T, von Haeseler A, Minh BQ. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016;44:W232–5. doi: 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 2017;14:587–9. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Letunic I, Bork P. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 2019;47:W256–9. doi: 10.1093/nar/gkz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–80. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pester M, Maixner F, Berry D, Rattei T, Koch H, Lücker S, et al. NxrB encoding the beta subunit of nitrite oxidoreductase as functional and phylogenetic marker for nitrite-oxidizing Nitrospira. Environ Microbiol. 2014;16:3055–71. doi: 10.1111/1462-2920.12300. [DOI] [PubMed] [Google Scholar]
- 59.Sauder LA, Albertsen M, Engel K, Schwarz J, Nielsen PH, Wagner M, et al. Correction: cultivation and characterization of Candidatus nitrosocosmicus exaquare, an ammonia-oxidizing archaeon from a municipal wastewater treatment system. ISME J. 2020. [DOI] [PMC free article] [PubMed]
- 60.Kozak M, Piepho HP. What’s normal anyway? Residual plots are more telling than significance tests when checking ANOVA assumptions. J Agron Crop Sci. 2018;204:86–98. [Google Scholar]
- 61.Langsrud Ø. ANOVA for unbalanced data: use type II instead of Type III sums of squares. Stat Comput. 2003;13:163–7. [Google Scholar]
- 62.McMurdie PJ, Holmes S. phyloseq: an R Package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. vegan: Community Ecology Package. R package version 2.5-6. https://CRAN.R-project.org/package=vegan.
- 64.Stier AC, Geange SW, Hanson KM, Bolker BM. Predator density and timing of arrival affect reef fish community assembly. Ecology. 2013;94:1057–68. doi: 10.1890/11-1983.1. [DOI] [PubMed] [Google Scholar]
- 65.Anderson MJ. Permutational multivariate analysis of variance (PERMANOVA). Wiley StatsRef: Statistics Reference Online. Chichester, UK: John Wiley & Sons, Ltd; 2017. p 1–15.
- 66.Fierer N, Schimel JP. A proposed mechanism for the pulse in carbon dioxide production commonly observed following the rapid rewetting of a dry soil. Soil Sci Soc Am J. 2010;67:798. [Google Scholar]
- 67.Lehtovirta-Morley LE. Ammonia oxidation: ecology, physiology, biochemistry and why they must all come together. FEMS Microbiol Lett. 2018;365:1–9. doi: 10.1093/femsle/fny058. [DOI] [PubMed] [Google Scholar]
- 68.Schimel JP, Schaeffer SM. Microbial control over carbon cycling in soil. Front Microbiol. 2012;3:1–11. doi: 10.3389/fmicb.2012.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fierer N. Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol. 2017;15:579–90. doi: 10.1038/nrmicro.2017.87. [DOI] [PubMed] [Google Scholar]
- 70.Larsen KS, Andresen LC, Beier C, Jonasson S, Albert KR, Ambus P, et al. Reduced N cycling in response to elevated CO2, warming, and drought in a Danish heathland: synthesizing results of the CLIMAITE project after two years of treatments. Glob Chang Biol. 2011;17:1884–99. [Google Scholar]
- 71.Brenzinger K, Kujala K, Horn MA, Moser G, Guillet C, Kammann C, et al. Soil conditions rather than long-term exposure to elevated CO2 affect soil microbial communities associated with N-cycling. Front Microbiol. 2017;8:1–14. doi: 10.3389/fmicb.2017.01976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rütting T, Hovenden MJ. Soil nitrogen cycle unresponsive to decadal long climate change in a Tasmanian grassland. Biogeochemistry. 2020;147:99–107. [Google Scholar]
- 73.Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, et al. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia. 2001;126:543–62. doi: 10.1007/s004420000544. [DOI] [PubMed] [Google Scholar]
- 74.Fuchslueger L, Wild B, Mooshammer M, Takriti M, Kienzl S, Knoltsch A, et al. Microbial carbon and nitrogen cycling responses to drought and temperature in differently managed mountain grasslands. Soil Biol Biochem. 2019;135:144–53. [Google Scholar]
- 75.Coskun D, Britto DT, Shi W, Kronzucker HJ. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat Plants. 2017;3:17074. doi: 10.1038/nplants.2017.74. [DOI] [PubMed] [Google Scholar]
- 76.Subbarao GV, Yoshihashi T, Worthington M, Nakahara K, Ando Y, Sahrawat KL, et al. Suppression of soil nitrification by plants. Plant Sci. 2015;233:155–64. doi: 10.1016/j.plantsci.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 77.Canarini A, Dijkstra FA. Dry-rewetting cycles regulate wheat carbon rhizodeposition, stabilization and nitrogen cycling. Soil Biol Biochem. 2015;81:195–203. [Google Scholar]
- 78.Karlowsky S, Augusti A, Ingrisch J, Akanda MKU, Bahn M, Gleixner G. Drought-induced accumulation of root exudates supports post-drought recovery of microbes in mountain grassland. Front Plant Sci. 2018;871:1–16. doi: 10.3389/fpls.2018.01593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Manzoni S, Schimel JP, Barbara S. Results from a responses of soil microbial communities to water stress: results from a meta-analysis. Ecology. 2017;93:930–8. doi: 10.1890/11-0026.1. [DOI] [PubMed] [Google Scholar]
- 80.Canarini A, Merchant A, Dijkstra FA. Drought effects on Helianthus annuus and Glycine max metabolites: from phloem to root exudates. Rhizosphere. 2016;2:85–97. [Google Scholar]
- 81.Hashem A, Kumar A, Al-Dbass AM, Alqarawi AA, Al-Arjani A-BF, Singh G, et al. Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi J Biol Sci. 2019;26:614–24. doi: 10.1016/j.sjbs.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams A, de Vries FT. Plant root exudation under drought: implications for ecosystem functioning. N. Phytol. 2020;225:1899–1905. doi: 10.1111/nph.16223. [DOI] [PubMed] [Google Scholar]
- 83.Subbarao GV, Rondon M, Ito O, Ishikawa T, Rao IM, Nakahara K, et al. Biological nitrification inhibition (BNI)—Is it a widespread phenomenon? Plant Soil. 2007;294:5–18. [Google Scholar]
- 84.Homyak PM, Allison SD, Huxman TE, Goulden ML, Treseder KK. Effects of drought manipulation on soil nitrogen cycling: a meta-analysis. J Geophys Res Biogeosci. 2017;122:3260–72. [Google Scholar]
- 85.Fuchslueger L, Bahn M, Fritz K, Hasibeder R, Richter A. Experimental drought reduces the transfer of recently fixed plant carbon to soil microbes and alters the bacterial community composition in a mountain meadow. N. Phytol. 2014;201:916–27. doi: 10.1111/nph.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thion C, Prosser JI. Differential response of nonadapted ammonia-oxidising archaea and bacteria to drying-rewetting stress. FEMS Microbiol Ecol. 2014;90:380–9. doi: 10.1111/1574-6941.12395. [DOI] [PubMed] [Google Scholar]
- 87.Norton JM, Klotz MG, Stein LY, Arp DJ, Bottomley PJ, Chain PSG, et al. Complete genome sequence of Nitrosospira multiformis, an ammonia-oxidizing bacterium from the soil environment. Appl Environ Microbiol. 2008;74:3559–72. doi: 10.1128/AEM.02722-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spang A, Poehlein A, Offre P, Zumbrägel S, Haider S, Rychlik N, et al. The genome of the ammonia-oxidizing CandidatusNitrososphaera gargensis: Insights into metabolic versatility and environmental adaptations. Environ Microbiol. 2012;14:3122–45. doi: 10.1111/j.1462-2920.2012.02893.x. [DOI] [PubMed] [Google Scholar]
- 89.Kerou M, Offre P, Valledor L, Abby SS, Melcher M, Nagler M, et al. Proteomics and comparative genomics of Nitrososphaera viennensis reveal the core genome and adaptations of archaeal ammonia oxidizers. Proc Natl Acad Sci USA. 2016;113:E7937–46. doi: 10.1073/pnas.1601212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nicol GW, Hink L, Gubry-Rangin C, Prosser JI, Lehtovirta-Morley LE. Genome Sequence of “ Candidatus Nitrosocosmicus franklandus” C13, a terrestrial ammonia-oxidizing archaeon. Microbiol Resour Announc. 2019;8:1–3. doi: 10.1128/MRA.00435-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sauder LA, Albertsen M, Engel K, Schwarz J, Nielsen PH, Wagner M, et al. Cultivation and characterization of Candidatus Nitrosocosmicus exaquare, an ammonia-oxidizing archaeon from a municipal wastewater treatment system. ISME J. 2017;11:1142–57. doi: 10.1038/ismej.2016.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lehtovirta-Morley LE, Ge C, Ross J, Yao H, Nicol GW, Prosser JI. Characterisation of terrestrial acidophilic archaeal ammonia oxidisers and their inhibition and stimulation by organic compounds. FEMS Microbiol Ecol. 2014;89:542–52. doi: 10.1111/1574-6941.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stieglmeier M, Klingl A, Alves RJE, Rittmann SKMR, Melcher M, Leisch N, et al. Nitrososphaera viennensis gen. nov., sp. nov., an aerobic and mesophilic, ammonia-oxidizing archaeon from soil and a member of the archaeal phylum Thaumarchaeota. Int J Syst Evol Microbiol. 2014;64:2738–52. doi: 10.1099/ijs.0.063172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jung MY, Kim JG, Sinninghe Damsté JS, Rijpstra WIC, Madsen EL, Kim SJ, et al. A hydrophobic ammonia-oxidizing archaeon of the Nitrosocosmicus clade isolated from coal tar-contaminated sediment. Environ Microbiol Rep. 2016;8:983–92. doi: 10.1111/1758-2229.12477. [DOI] [PubMed] [Google Scholar]
- 95.Gwak JH, Jung MY, Hong H, Kim JG, Quan ZX, Reinfelder JR, et al. Archaeal nitrification is constrained by copper complexation with organic matter in municipal wastewater treatment plants. ISME J. 2020;14:335–46. doi: 10.1038/s41396-019-0538-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nowka B, Daims H, Spieck E. Comparison of oxidation kinetics of nitrite-oxidizing bacteria: nitrite availability as a key factor in niche differentiation. Appl Environ Microbiol. 2015;81:745–53. doi: 10.1128/AEM.02734-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Prosser JI. The ecology of nitrifying bacteria. In: Bothe H, Ferguson SJ, editors. Newton WEBT-B of the NC. Biology of the Nitrogen Cycle. Amsterdam: Elsevier; 2007. p 223–43.
- 98.Norton JM, Stark JM. Regulation and measurement of nitrification in terrestrial systems. In: Klotz MGBT-M in E. Research on nitrification and related processes, Part A. 2011. Academic Press, United States, p 343–68. [DOI] [PubMed]
- 99.Koch H, Galushko A, Albertsen M, Schintlmeister A, Gruber-Dorninger C, Lücker S, et al. Growth of nitrite-oxidizing bacteria by aerobic hydrogen oxidation. Science. 2014;345:1052 LP–1054. doi: 10.1126/science.1256985. [DOI] [PubMed] [Google Scholar]
- 100.Koch H, Lücker S, Albertsen M, Kitzinger K, Herbold C, Spieck E, et al. Expanded metabolic versatility of ubiquitous nitrite-oxidizing bacteria from the genus Nitrospira. Proc Natl Acad Sci USA. 2015;112:11371–6. doi: 10.1073/pnas.1506533112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Daebeler A, Bodelier PLE, Yan Z, Hefting MM, Jia Z, Laanbroek HJ. Interactions between Thaumarchaea, Nitrospira and methanotrophs modulate autotrophic nitrification in volcanic grassland soil. ISME J. 2014;8:2397–410. doi: 10.1038/ismej.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim DG, Vargas R, Bond-Lamberty B, Turetsky MR. Effects of soil rewetting and thawing on soil gas fluxes: a review of current literature and suggestions for future research. Biogeosciences. 2012;9:2459–83. [Google Scholar]
- 103.Wrage N, Velthof GL, Van Beusichem ML, Oenema O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol Biochem. 2001;33:1723–32. [Google Scholar]
- 104.Stein LY. Surveying N2O-producing pathways in bacteria. In: Klotz MGBT-M in E. Research on nitrification and related processes, Part A. 2011. Academic Press, United States, pp 131–52. [DOI] [PubMed]
- 105.Kozlowski JA, Stieglmeier M, Schleper C, Klotz MG, Stein LY. Pathways and key intermediates required for obligate aerobic ammonia-dependent chemolithotrophy in bacteria and Thaumarchaeota. ISME J. 2016;10:1836–45. doi: 10.1038/ismej.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kits KD, Jung MY, Vierheilig J, Pjevac P, Sedlacek CJ, Liu S, et al. Low yield and abiotic origin of N2O formed by the complete nitrifier Nitrospira inopinata. Nat Commun. 2019;10:1–12. doi: 10.1038/s41467-019-09790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.