Increasing evidence indicates that airway inflammation is linked to the generation of reactive oxygen species (ROS),1 which serve as important signaling molecules for various immune cells. Antioxidant-based interventions are therefore a potential strategy for treating airway inflammation, but the therapeutic effects require improvement by precise use in the proper situations.2 Group 2 innate lymphoid cells (ILC2s) play an important role in the early phase of airway inflammation, and their activities are dependent on metabolic activation.3 However, the molecular mechanisms underlying the metabolic activation and functions of ILC2s are not fully understood. Here, we found that the ROS levels in ILC2s increased upon cellular activation both in vivo and in vitro. An ROS scavenger, N-acetyl cysteine (NAC), compromised cytokine production and the proliferation of ILC2s, as well as ILC2-mediated eosinophilia, in response to IL-33 stimulation. Mechanistically, ROS are required for optimal IL-33-triggered activation of the metabolic program and for Akt-mechanistic target of rapamycin (mTOR) signaling in ILC2s. In mouse models, NAC alleviated IL-33-triggered ILC2 activation and inflammation in vivo in the airway and the liver. Collectively, our study indicates that IL-33-induced ROS are required for the optimal metabolic activation and functions of ILC2s and suggests that antioxidant therapy might ameliorate the ILC2-mediated immune responses and inflammation in the airway, liver, and possibly other organs.
ROS levels were increased in ILC2s upon IL-33 stimulation in a NOX2-dependent manner
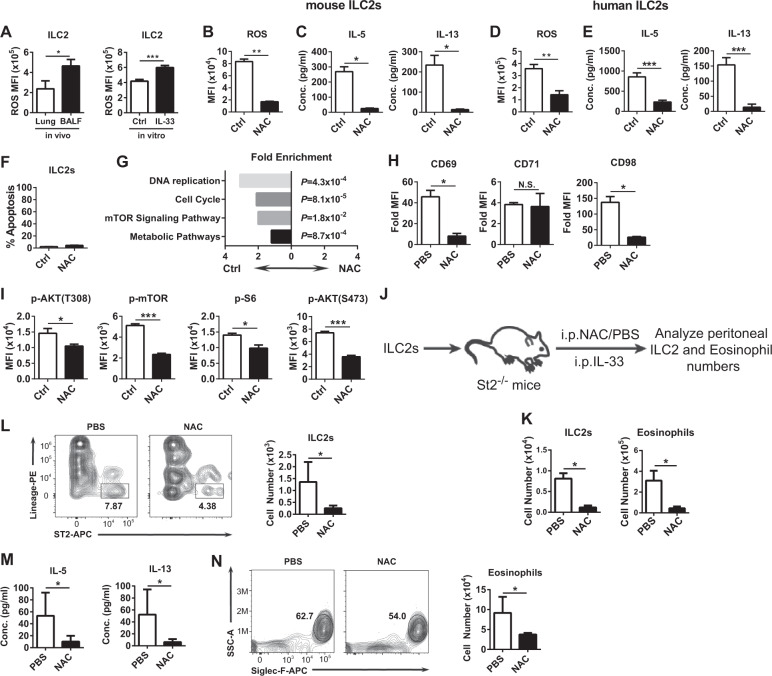
Intranasal administration of IL-33 causes activation and infiltration of ILC2s into the airway to induce inflammation. We found that activated BALF ILC2s displayed significantly higher levels of ROS than the nonactivated control cells (Fig. 1a). Consistent with this finding, we also detected increased ROS levels in ILC2s upon IL-33 stimulation in vitro (Fig. 1a). A primary source of ROS production is NADPH oxidase (NOX). Nox2/gp91phox, encoded by the Nox2/Cybb gene, is the catalytic and membrane-bound subunit of NADPH oxidase and is responsible for the production of intracellular ROS.4 Deficiency in ROS production in Nox2−/− mice or inhibition of Nox2 activity ameliorated lung inflammation in mice.5–7 NOX2 protein expression was comparable between resting and activated mouse ILC2s (data not shown). We found that the ROS levels were significantly reduced in the BALF ILC2s from the Nox2−/− mice compared with those from the wild-type mice (Fig. S2a). This result confirmed a role of NOX2 in ROS production and indicates that Nox2−/− mice could serve as a model to study the functional roles of the increased ROS levels in ILC2 functions.
Fig. 1.
IL-33-induced ROS are required for the optimal metabolic program in ILC2s. a ROS levels in ILC2s. (Left) “Lung” indicates ILC2s from the lung tissue of the untreated mice. “BALF” indicates ILC2s in the BALF from the IL-33-challenged mice (n = 6). (Right) ILC2s were stimulated with IL-33 for 2 days (“IL-33”) or left unstimulated (“ctrl”) in vitro. b, c, f–i Mouse ILC2s were pretreated with NAC or an equal volume of PBS for 2 h before stimulation with IL-33 for 2 days. b ROS levels are shown. c IL-5 and IL-13 production in the supernatant is shown. d, e Human ILC2s were pretreated with NAC or an equal volume of PBS for 2 h before stimulation with IL-33 for 2 days. d ROS levels are shown. e IL-5 and IL-13 production in the supernatant is shown. f Percentages of apoptotic cells are shown. g Fold enrichment of genes from the KEGG pathway. h Fold mean fluorescent intensity of CD69, CD71, and CD98 staining against the isotype antibody staining is shown. i Intracellular levels of phosphorylated AKT (T308), p-mTOR, p-S6, and p-AKT (S473) are shown. j, k Mouse ILC2 adoptive transfer model. Data are pooled from three independent experiments (PBS group: n = 8; NAC group: n = 6). l–n IL-33-induced airway inflammation model. l ILC2 numbers, m IL-5 and IL-13 protein levels, and n eosinophil numbers in the BALF are shown (n = 6). a–n Data are in triplicate unless otherwise indicated, are representative of at least two independent experiments and are presented as the mean ± SEM
The ILC2 numbers in the lung were slightly increased in the Nox2−/− mice compared with the wild-type mice (Fig. S2b). However, after intranasal IL-33 challenge, infiltration of ILC2s into the airway was compromised in the Nox2−/− mice (Fig. S2c). In addition, significantly reduced levels of IL-5 and IL-13 and fewer eosinophils were detected in the BALF of the Nox2−/− mice than that of the wild-type mice (Fig. S2d, e). These data demonstrated that NOX2 is required for IL-33-driven airway ILC2 responses and inflammation, suggesting that IL-33-induced ROS might play a role in ILC2 functions.
ROS are required for the IL-33-stimulated ILC2 functions in vitro
To further determine the role of ROS in ILC2 functions, we isolated mouse ILC2s and stimulated them with IL-33 in vitro. The presence of NAC significantly reduced the levels of ROS in the stimulated ILC2s (Fig. 1b) and suppressed the production of IL-5 and IL-13 in the culture supernatant (Fig. 1c). This reduction in ROS levels and IL-5/13 production by NAC was also observed in human ILC2s (Fig. 1d, e). Detailed analysis of the NAC-treated mouse ILC2s showed decreased intracellular levels of the IL-5 and IL-13 proteins (Fig. S3a), indicating that the ability to produce IL-5 and IL-13 was decreased in these cells. Additionally, the proliferation of NAC-treated mouse ILC2s was suppressed, as shown by the lower expression of Ki67 in these cells than in the control cells (Fig. S3b). In addition, apoptosis was comparable between the NAC-treated and PBS-treated mouse ILC2s (Fig. 1f). Thus, the ROS scavenger NAC suppressed the ability of ILC2s to produce cytokines, indicating that ILC2s require ROS for their effector functions.
ROS are required for IL-33-stimulated metabolic activation and AKT-mTOR signaling in ILC2s
Transcriptome analysis of IL-33-stimulated mouse ILC2s in the presence of PBS or NAC showed that genes from the KEGG pathways “DNA replication” and “cell cycle” were significantly enriched compared with those in the control ILC2s (Fig. 1g), consistent with the observation that the proliferation was decreased in the NAC-treated ILC2s (Fig. S3b). More importantly, genes of the “mTOR signaling pathway” and “metabolic pathways” were also decreased in the NAC-treated ILC2s (Fig. 1g), suggesting that the mTOR signaling pathway might be suppressed and that metabolic activation might be compromised in the IL-33-stimulated ILC2s in the presence of NAC. Consistent with this finding, electron microscopy analysis demonstrated significantly fewer numbers of mitochondria, the organelles that play central metabolic roles, in the NAC-treated ILC2s compared to the control cells (Fig. S4). Additionally, the NAC-treated ILC2s were smaller than the control cells (Fig. S4). These results coincided with lower expression of the activation marker CD69 and the nutrient receptor CD98 on the NAC-treated ILC2s than the control cells, while the CD71 expression was not changed (Fig. 1h). Moreover, the NAC-treated ILC2s displayed decreased phosphorylation of AKT at Thr308, mTOR, and S6, a downstream target of mTORC1 (Fig. 1i). Additionally, we detected reduced phosphorylation of AKT at Ser473, a target of mTORC2 (Fig. 1i). Taken together, these results demonstrated that ROS are required for the IL-33-stimulated metabolic activation and AKT-mTOR signaling in ILC2s.
ROS are required for the IL-33-driven ILC2 accumulation and ILC2-mediated eosinophilia in vivo
The requirement of ROS for ILC2 functions in vitro prompted us to investigate whether ROS are also required for ILC2 functions in vivo. We adoptively transferred ILC2s into ILC2-deficient St2−/− mice, followed by IL-33 injection for four consecutive days before analysis of the ILC2 and eosinophil numbers in the peritoneal cavity (Fig. 1j, k). IL-33 injection resulted in the accumulation of ILC2s and subsequent recruitment of eosinophils in the peritoneal cavity of this model (data not shown). We found that NAC injection significantly reduced the number of ILC2s and eosinophils harvested in the St2−/− mice (Fig. 1k). These results indicated that ROS are required for IL-33-driven ILC2 accumulation and for ILC2-dependent eosinophilia in vivo.
Antioxidant therapy alleviated the IL-33-driven ILC2 responses and inflammation in vivo
Finally, in a model of IL-33-induced airway inflammation, we found that NAC injection significantly inhibited ILC2 infiltration into the airway (Fig. 1l) and significantly reduced the IL-5 and IL-13 levels (Fig. 1m) and eosinophil numbers in the BALF (Fig. 1n), both in the B6 wild-type mice (Fig. 1l–n) and in the Rag1−/− mice (Fig. S5), suggesting that the effects were independent of the adaptive immune system. Moreover, we employed a model of IL-33-driven ILC2 infiltration into the liver,8 and we found similar effects by NAC (Fig. S6), suggesting that the role of IL-33-induced ROS in ILC2 functions might not be limited to the airway. Taken together, these results demonstrated that NAC antioxidant therapy effectively alleviated the IL-33-driven ILC2 responses and eosinophilia in the airway.
Supplementary information
Acknowledgements
This work was supported by the National Key R&D Program of China (2019YFA0906100 to X.W.), the Natural Science Foundation of Guangdong Province (2019A1515011412 to J.B.), the Shenzhen Technology Innovation Project (JCYJ20170413153158716 to X.W.), the Shenzhen Special Funds of Industry in the Future (Development and Reform Commission in Shenzhen [2015] 971 to X.W.), and the Special Funds for Major Science and Technology of Guangdong Province (2013A022100037 to X.W.).
Author contributions
C.Z. performed the experiments, analyzed the data, and wrote the manuscript. H.W. and Z.L. provided technical assistance. J.B. conceived the research, designed the experiments, performed the experiments and transcriptome analysis, analyzed the data, and wrote the manuscript. X.W. supervised the research.
Competing interests
The authors declare no competing interests.
Contributor Information
Jiacheng Bi, Email: jc.bi@siat.ac.cn.
Xiaochun Wan, Email: xc.wan@siat.ac.cn.
Supplementary information
The online version of this article (10.1038/s41423-020-0393-z) contains supplementary material.
References
- 1.Henricks PA, Nijkamp FP. Reactive oxygen species as mediators in asthma. Pulm. Pharmacol. Ther. 2001;14:409–420. doi: 10.1006/pupt.2001.0319. [DOI] [PubMed] [Google Scholar]
- 2.Dozor AJ. The role of oxidative stress in the pathogenesis and treatment of asthma. Ann. N. Y. Acad. Sci. 2010;1203:133–137. doi: 10.1111/j.1749-6632.2010.05562.x. [DOI] [PubMed] [Google Scholar]
- 3.Salmond RJ, et al. IL-33 induces innate lymphoid cell-mediated airway inflammation by activating mammalian target of rapamycin. J. Allergy Clin. Immunol. 2012;130:1159–1166. doi: 10.1016/j.jaci.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam GY, Huang J, Brumell JH. The many roles of NOX2 NADPH oxidase-derived ROS in immunity. Semin. Immunopathol. 2010;32:415–430. doi: 10.1007/s00281-010-0221-0. [DOI] [PubMed] [Google Scholar]
- 5.Vlahos R, et al. Inhibition of Nox2 oxidase activity ameliorates influenza A virus-induced lung inflammation. PLoS Pathog. 2011;7:e1001271. doi: 10.1371/journal.ppat.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang WJ, Wei H, Tien YT, Frei B. Genetic ablation of phagocytic NADPH oxidase in mice limits TNFalpha-induced inflammation in the lungs but not other tissues. Free Radic. Biol. Med. 2011;50:1517–1525. doi: 10.1016/j.freeradbiomed.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sevin CM, et al. Deficiency of gp91phox inhibits allergic airway inflammation. Am. J. Respir. Cell Mol. Biol. 2013;49:396–402. doi: 10.1165/rcmb.2012-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bi J, et al. NK cells alleviate lung inflammation by negatively regulating group 2 innate lymphoid cells. J. Immunol. 2017;198:3336–3344. doi: 10.4049/jimmunol.1601830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.