Cao et al. report on cryo-EM structures of human cyclic GMP-AMP synthase (cGAS) sensor bound to the nucleosome core particle, thereby defining the molecular mechanism of how cGAS avoids activation by chromatinized self-DNA. Notably, disruption of cGAS dimer formation and prevention of dsDNA recognition by the sensor at the nucleosome level impede cGAS activation.
Cyclic GMP-AMP synthase (cGAS) was first identified as a dsDNA sensor in the cytoplasm,1 providing a surveillance mechanism for monitoring pathogenic DNA invasion, in which the cGAS-STING pathway triggers production of type I interferons and pro-inflammatory cytokines associated with the innate immune response and stimulates inflammatory signaling, autophagy and apoptosis. Given that cGAS exhibits a dsDNA length-dependent mode of activation, several groups have undertaken structure determination of a series of mouse and human cGAS–dsDNA complexes with increasing complexity,2–6 which in turn have revealed three distinct DNA binding surfaces on cGAS (labeled sites A, B and C) that contribute to dsDNA sensing by cGAS.2–6 cGAS, which adopts a dimeric topology,6 undergoes a conformational change upon binding of naked dsDNA, and forms complexes with dsDNA through targeting separate dsDNAs by cGAS sites A and B, thereby triggering formation of the cGAS activation loop and a catalytically competent pocket for cyclic dinucleotide second messenger 2′,3′-cGAMP synthesis.2,7 Furthermore, human cGAS has an additional site C interface to further promote dsDNA-induced enzymatic activation and liquid phase condensation5 (Fig. 1a).
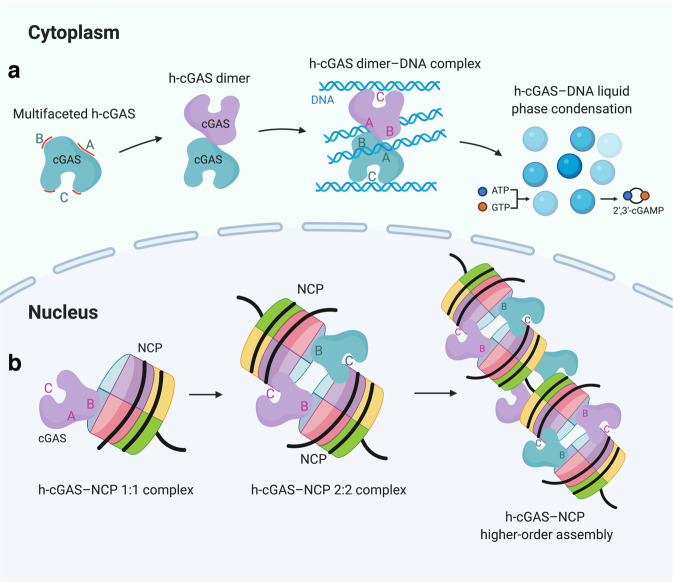
Fig. 1. Proposed models for inactivation of human cGAS by the nucleosome.
a In the cytoplasm, human cGAS (h-cGAS) proteins form dimers to sense cytosolic DNA with three DNA-binding sites (labeled A, B, and C), resulting in the formation of h-cGAS dimer–DNA clusters and h-cGAS–DNA liquid phase condensates, to activate the production of 2′,3′-cGAMP. b In the nucleus, the monomeric h-cGAS can bind NCPs with 1:1 and 2:2 stoichiometries, mediated by DNA-binding sites B and C. Furthermore, a proposed higher-order assembly of the h-cGAS–NCP complex could be indicative of a tighter tethering of h-cGAS by the nucleosomes due to a cooperative binding mechanism.
A critical feature of the cGAS regulatory mechanism requires avoidance of cGAS activation by self-DNA under normal conditions since aberrant activation of cGAS causes autoimmune diseases. It has been established in the nucleus that reconstituted nucleosomes could directly bind cGAS more tightly than corresponding naked dsDNA but cannot stimulate second messenger cGAMP synthesis.8
In the paper published in Cell Research, Cao et al. provide a structural explanation of nucleosome-mediated suppression of cGAS activity based on cryo-EM structures of human cGAS–nucleosome complexes.9 They reconstituted the cGAS–nucleosome complex using the catalytic domain dimer of human cGAS and nucleosome core particles (NCPs) and determined the cryo-EM structures of cGAS–NCP complexes with 1:1 and 2:2 stoichiometries. The structures of both complexes showed that bound cGAS interacts with the nucleosome as a monomer, implying that nucleosome can competitively disrupt the dimeric state of cGAS. Given that cGAS dimer showed stronger dsDNA-triggered enzymatic activity than its monomer counterpart, tethering of cGAS monomer/disruption of cGAS dimer by the NCP appears to be the first layer of the inhibitory mechanism.
Analysis by Cao et al. of their structure of the 1:1 cGAS–NCP complex mainly revealed that basic residues on cGAS site B projecting from loop elements are tightly bound to the nucleosome acidic patch component of the H2A–H2B heterodimer (Fig. 1b), with Arg236 and Arg255 of cGAS playing key anchoring roles in complex formation. Notably, the acidic patch on H2A–H2B heterodimer has been shown to be a universal recognition site for a range of chromatin-binding proteins.10 The absence of bound dsDNA at site A prevents activation loop formation within the cGAS catalytic pocket. Thus, prevention of dsDNA recognition by cGAS site A/B at the NCP level, serves as a second layer of the inhibitory mechanism.
Interestingly, Cao et al.’s structure of the 2:2 cGAS–nucleosome complex showed that two nucleosomes could be symmetrically bridged by a pair of cGAS monomers, with each of the cGAS monomers binding to the adjacent nucleosomal DNA through cGAS site C (Fig. 1b). Furthermore, they observed a ladder-shaped higher-order assembly of the cGAS–nucleosome complex at low resolution, mediated by protein–protein contacts through cGAS site B and protein–DNA contacts through site C (Fig. 1b). cGAS–nucleosome oligomerization appears to contribute a third layer of the inhibitory mechanism.
In parallel with this study, five other groups also reported on the cryo-EM structures of human and mouse cGAS–nucleosome complexes.11–15 We note that the 2:2 and higher-order assemblies mediated by cGAS site C are observed in all three human cGAS–NCP structures9,12,15 but not in their mouse counterparts.11,13,14 Consistent with this distinction, site C has been shown to be an evolutionarily strengthened DNA-binding interface when proceeding from mouse to humans.5 Such a distinction could also be reflective of the enhanced control of aberrant activation by human cGAS. Given that site C provides an additional valence interaction for promoting DNA condensation and phase separation in the cytoplasm,5 the topology of the higher-order human cGAS–nucleosome complex implies that inactive nuclear cGAS may play a scaffolding role in compressing chromatin and in triggering nuclear liquid phase condensation.
The studies discussed here9,11–15 provide compelling evidence of cGAS sequestration and inhibition by the nucleosome, with such nuclear tethering trapping cGAS in an inactive conformation.
Contributor Information
Wei Xie, Email: xiew@mskcc.org.
Dinshaw J. Patel, Email: pateld@mskcc.org
References
- 1.Sun L, et al. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao P, et al. Cell. 2013;153:1094–1107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreeva L, et al. Nature. 2017;549:394–398. doi: 10.1038/nature23890. [DOI] [PubMed] [Google Scholar]
- 4.Zhou W, et al. Cell. 2018;174:300–311. doi: 10.1016/j.cell.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie W, et al. Proc. Natl. Acad. Sci. USA. 2019;116:11946–11955. doi: 10.1073/pnas.1905013116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, et al. Immunity. 2013;39:1019–1031. doi: 10.1016/j.immuni.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Civril F, et al. Nature. 2013;498:332–337. doi: 10.1038/nature12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zierhut C, et al. Cell. 2019;178:302–315. doi: 10.1016/j.cell.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Can, D. et al. Cell Res.10.1038/s41422-020-00422-4 (2020).
- 10.Zhou K, et al. Nat. Struct. Mol. Biol. 2019;26:3–13. doi: 10.1038/s41594-018-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao, B. et al. Nature 10.1038/s41586-020-2749-z (2020).
- 12.Pathare, G. R. et al. Nature10.1038/s41586-020-2750-6 (2020).
- 13.Michalski, S. et al. Nature10.1038/s41586-020-2748-0 (2020).
- 14.Boyer, J. A. et al. Science eabd0609, 10.1126/science.abd0609 (2020).
- 15.Kujirai, T. et al. Science eabd0237, 10.1126/science.abd0237 (2020).