Abstract
Poly- and perfluoroalkyl substances (PFASs) are persistent, bioaccumulative anthropogenic compounds associated with adverse health impacts on humans and wildlife. PFAS production changed in North America and Europe around the year 2000, but impacts on wildlife appear to vary across species and location. Unlike other mammal species, cetaceans lack the enzyme for transforming an important intermediate precursor (perfluorooctane sulfonamide: FOSA), into a prevalent compound in most wildlife (perfluorooctane sulfonate: PFOS). Thus, their tissue burden differentiates these two compounds while other mammals contain PFOS from both direct exposure and precursor degradation. Here we report temporal trends in 15 PFASs measured in muscle from juvenile male North Atlantic pilot whales (Globicephala melas) harvested between 1986 and 2013. FOSA accounted for a peak of 84% of the 15 PFASs around 2000 but declined to 34% in recent years. PFOS and long-chained PFCAs (C9-C13) increased significantly over the whole period (2.8% yr−1 to 8.3% yr−1), but FOSA declined by 13% yr−1 after 2006. Results from FOSA partitioning and bioaccumulation modeling forced by changes in atmospheric inputs reasonably capture magnitudes and temporal patterns in FOSA concentrations measured in pilot whales. Rapid changes in atmospheric FOSA in polar and subpolar regions around 2000 helps to explain large declines in PFOS exposure for species that metabolize FOSA, including seafood consuming human populations. This work reinforces the importance of accounting for biological exposures to PFAS precursors.
Graphical Abstract
Introduction
Poly- and perfluoroalkyl substances (PFASs) are widely used persistent anthropogenic chemicals that are accumulating in the global oceans.1–3 Long-chain PFASs bioaccumulate in aquatic food webs,4–5 posing risks to apex predators such as whales, seals and polar bears.6–8 PFAS exposures have been associated with adverse health effects in humans and wildlife, including immunotoxicity, developmental disorders, and cancer.9–10 Global regulations and voluntary shifts in chemical manufacturing have changed the source regions and composition of PFASs and precursor compounds released to the environment.3, 11 However, impacts of changing emissions on biological PFAS concentrations and contributions of precursor compounds remain unclear.11–13
Exposure analyses focus on two major classes of PFASs, perfluoroalkyl sulfonic acids (PFSAs) and perfluoroalkly carboxylic acids (PFCAs) because of their persistence and ubiquity. Between 2000–2002, the most prevalent compound, perfluoroctane sulfonate (PFOS), and its precursors were voluntarily phased-out and eventually regulated in North America and Europe.3 Inconsistent temporal patterns in PFAS concentrations have been measured in marine mammals following this phase out.6, 14 Between 1984 and 2009, trends for perfluorooctane sulfonate (PFOS) and other PFSAs varied across geographical locations, while long-chained fluorinated carboxylates (PFCAs) in seals, porpoises and dolphins from the Arctic and Subarctic continued to increase by 7–15% per year.15–17
Temporal trends in biological concentrations can be confounded by differences in migratory patterns, dietary habits, gender and age of individuals sampled,18 as well as varying exposures to precursor compounds.12, 19–21 The suite of PFASs routinely targeted in analytical studies typically comprises a small fraction (<50%) of the environmental burden of total organic fluorine (TOF).22–24 Relative contributions of neutral volatile atmospheric precursors such as fluorotelomer alcohols (FTOH) and perfluoroalkyl sulfonamides (FASAs) to overall exposures of humans and wildlife are uncertain.25–29 For humans, the modeled fraction of total PFOS exposures contributed by precursors ranges between 8% and 60%.19, 30 Gebbink et al.20 reported that precursors contribute minimally to recent (2013–2014) bioaccumulation in a Baltic Sea food web. By contrast, a longitudinal study in fish from the Swedish coast between 1991 and 2011 suggested that exposures from precursors were greater than PFOS prior to year 2001.31
Biological exposures to precursor compounds are difficult to measure directly because in vivo biotransformation occurs in many animals, including humans.19, 21, 32 One exception is cetaceans, which are missing a key enzyme for metabolism of the commonly observed precursor, perfluorooctane sulfonamide (FOSA), into PFOS.33–35 FOSA is an intermediate degradation product of other precursors such as the FASA: N-ethyl-perfluorooctane sulfonamide (N-EtFOSA).4, 36–37 Lack of biotransformation of FOSA by cetaceans provides a unique opportunity to quantify the exposures attributable to this neutral atmospheric precursor.
Here we analyze temporal changes in 15 PFASs in juvenile male North Atlantic pilot whales (Globicephala melas) caught in the Faroe Islands between 1986–2013 (Figure S2). The Faroe Islands are located in the central North Atlantic (62°N, 7°W), and the traditional diet of the population includes pilot whale.38 PFASs are generally measured in the liver of marine mammals because contaminants often concentrate there, but measurements in muscle provide a more direct link to human exposure for marine food consuming populations. The main objectives of this work are: 1) To gain insight into the responsiveness of North Atlantic marine food-webs to the phase out in North American and European manufacturing of PFOS and its precursors around the year 2000,3 and 2) to better understand the role of the intermediate precursors in these temporal patterns. We compare measured and modeled temporal changes in FOSA to gain insight into the importance of precursor exposures for shifts in marine food web PFAS burdens.
Methods
Sample collection
Pilot whales exhibit a high degree of site-fidelity and have a relatively homogenous diet consisting mainly of squid.39–40 We selected muscle tissue for PFAS analysis from 86 pilot whales harvested in the Faroe Islands between 1994 and 2013 and archived by the Faroese Natural History Museum. In addition, we included PFAS data measured in the muscle of 38 juvenile male pilot whales that were previously analyzed by the Faroese Environment Agency to extend our temporal analysis back to 1986.41 We also collected five squid (Todarodes sagittatus) in 2010 from a research vessel off the coast of the Faroe Islands. All samples were frozen after collection and stored in high-density polyethylene bags until analysis.
Muscle samples from 49 of the 86 pilot whales collected for this study were from juvenile males with ages ranging between 5 and 15 years based on length (Supporting Information (SI) Figure S1).42 To assess variability in PFAS concentrations related to gender and size, we also analyzed samples from 9 juvenile females, 20 adult females, and 8 adult males harvested in 2013 to compare to the 16 juvenile males from this year. Adults are defined by lengths >500 cm for males and >378 cm for females.42 Detailed information on the harvest dates, size, age, and gender of whales included in this study are included in SI Tables S1 and S2.
PFAS extraction and analysis
All whale muscle and squid samples were analyzed for 15 PFASs at Aarhus University, Denmark. Duplicate squid samples were also analyzed by the Faroese Environment Agency, following methods for extraction and quantification described in Ahrens et al.43 PFASs quantified included: perfluorobutanesulfonic acid (PFBS, four carbon chain length: C-4), perfluorohexanesulfonic acid (PFHxS: C-6), perfluoroheptanesulfonic acid (PFHpS: C-7), PFOS (C-8), perfluorodecanesulfonic acid (PFDS: C-10), FOSA (C-8), perfluorohexanoic acid (PFHxA: C-6), perfluoroheptanoic acid (PFHpA: C-7), perfluorooctanoic acid (PFOA: C-8), perfluorononanoic acid (PFNA: C-9), perfluorodecanoic acid (PFDA: C-10), perfluoroundecanoic acid (PFUnA: C-11), perfluorododecanoic acid (PFDoA: C-12), perfluorotridecanoic acid (PFTrA: C-13), and perfluorotetradecanoic acid (PFTeA: C-14).
Approximately 5 g of wet tissue was homogenized and a 1 gram aliquot was weighed in a polypropylene tube and spiked with 10 ng of isotopically-labeled PFAS mixture (Wellington Laboratories; Guelph, ON, Canada) as an internal standard for quantification (SI Table S1). Tissues were extracted with 5 mL acetonitrile for 30 min in an ultrasonic bath at 30 °C. Extraction procedures were repeated, and the combined extract was reduced to 2 mL under a stream of nitrogen and 50 μL acetic acid was added. Supelclean ENVI-Carb® cartridges (100 mg, 1 mL, 100–400 mesh, Supelco, USA) were used for cleanup. The cartridges were conditioned with 2 mL acetonitrile followed by 1 mL 20% acetic acid in acetonitrile. The sample extract and 3 mL of methanol were added to the cartridge and directly collected into another vial. The extracts were reduced to dryness under a nitrogen stream and re-dissolved in 1 ml methanol/2mM ammonium acetate (50:50, v/v).
Table 1 provides a complete list of the PFASs analyzed and corresponding detection limits for this study. Analysis was performed by liquid chromatography tandem-mass spectrometry (LC-MS/MS) with electrospray ionization in negative mode.29 Chromatographic separation was performed using a C18 Kinetex column (2.1 × 150 mm, Phenomenex, Torrance, CA, USA) and an Agilent 1200 Series HPLC (Agilent, Palo Alto, CA, USA). Duplicate squid samples were analyzed on a BEH C-18 column in Water Acquity I-Class UPLC and Waters Xevo TQ-S for improved sensitivity. The ions monitored for each compound can be found in SI Table S3. Each batch of samples was analyzed with a procedural blank. Method detection limits (MDL) were calculated as three times the standard deviation of procedural blanks. Recoveries ranged from 75% to 128%, which is comparable to previous work (SI Table S4).35, 44 The relative standard deviation (RSD) of samples run in duplicate ranged from 5–24%. RSDs for PFTeA and PFTrA were higher (44%−47%) because of the tendency of longer-chained PFASs to sorb to surfaces during sample preparation and analysis.
Table 1.
Median concentrations (ng g−1 wet weight) and number of samples above detection limit (DL) in parentheses for 15 PFASs measured in juvenile male pilot whale muscle (Globicephala melas) between 1986 and 2013 from this study and from the Faroese Environment Agency (FEA).4,1
| Compound | 1986–1988 | 1994–1997 | 1998–2002 | 2006–2009 | 2010–2013 | DL (this study) | DL (FEA) |
|---|---|---|---|---|---|---|---|
| PFBS | <DL (0/5) | <DL (0/11) | <DL (0/10) | <DL (0/29) | 0.01 | 0.09 | |
| PFHxS | 0.13 (5/5) | 0.19 (11/11) | 0.11 (5/10) | 0.14 (29/29) | 0.05 | 0.03 | |
| PFHpS | <DL (1/5) | <DL (2/8) | 0.01 (15/29) | 0.05 | |||
| PFOS | 2.0 (13/13) | 2.3 (11/11) | 3.7 (15/15) | 2.8 (15/15) | 4.0 (33/33) | 0.01 | 0.01 |
| PFDS | 0.06 (5/5) | 0.06 (6/11) | <DL (4/10) | 0.06 (16/29) | 0.01 | 0.15 | |
| FOSA | 16 (5/5) | 22 (8/8) | 19 (7/7) | 7.4 (29/29) | 0.05 | ||
| PFHxA | <DL (0/5) | <DL (0/11) | 0.14 (7/10) | <DL (0/29) | 0.006 | 0.06 | |
| PFHpA | <DL(0/5) | <DL (0/11) | 0.03 (5/10) | <DL (10/29) | 0.01 | 0.06 | |
| PFOA | 0.17 (5/5) | 0.13 (11/11) | 0.10 (9/10) | 0.06 (29/29) | 0.006 | 0.18 | |
| PFNA | <DL (3/13) | 0.14 (6/11) | 0.22 (13/14) | 0.29 (13/14) | 0.51 (32/33) | 0.02 | 0.06 |
| PFDA | <DL (1/13) | 0.18 (5/11) | 0.22 (14/15) | 0.27 (13/15) | 0.73 (32/33) | 0.006 | 0.01 |
| PFUnA | 0.5 (9/11) | 0.59 (9/11) | 0.91 (13/13) | 1.6 (15/15) | 2.0 (33/33) | 0.006 | 0.02 |
| PFDoA | 0.19 (5/5) | 0.20 (11/11) | 0.46 (10/10) | 0.38 (29/29) | 0.02 | 0.02 | |
| PFTrA | 0.53 (1/6) | 1.2 (7/7) | 1.1 (12/13) | 1.4 (13/13) | 3.5 (30/31) | 0.02 | 0.01 |
| PFTeA | <DL (0/6) | 0.32 (5/7) | 0.35 (9/13) | <DL (5/13) | 0.84 (28/31) | 0.10 | 0.02 |
For pilot whale data from the Faroese Environment Agency,41 sample extraction and analysis methods are provided in Rotander et al.15 Samples were analyzed in two batches and their corresponding detection limits are listed in Table 1. The first batch contained muscle tissue from whales sampled between 1986–2010. For this batch, detection limits were higher and frequencies lower than this study for PFBS, PFHxS, PFHpS, PFDS, PFHxA, PFHpA, and PFOA. We therefore excluded these data from subsequent statistical analyses. The second batch included the years 2001/2006 and all compounds had comparable detection limits and frequencies to this work and so were included. Neither batch reported FOSA levels.
Statistical analysis
All statistical analyses were performed in R version 3.2.2. Five compounds (PFBS, PFHpS, PFDS, PFHpA and PFHxA) were infrequently detected (6%−52%) and thus removed from subsequent statistical analyses. Detection frequencies for the remaining 10 compounds were all >80%. For compounds that contained samples below the detection-limit (DL), maximum-likelihood estimation was used for inclusion in summary statistics, ANOVA, and regression analyses, as implemented by the NADA package in R.45 For plotting purposes non-detects are shown as the detection limit multiplied by 1/√2.46
We investigated the occurrence of statistically significant changes in PFAS composition over time using methods for compositional data analysis described in Aitchisen47 and implemented in the R package compositions.48 This method removes spurious correlations and other constraints inherent in compositional data by applying a log-ratio transformation prior to additional analysis. One-way ANOVA for each compound was used to investigate concentration differences resulting from gender and age as a four-level categorical variable (juvenile/adult, male/female) in whales harvested in the year 2013. We log-transformed concentrations to correct for the observed distribution of PFASs and estimated annual changes in concentrations in juvenile males by the slope of linear regression models for individual compounds.
Environmental partitioning and bioaccumulation model for FOSA
We developed a model for FOSA partitioning and bioaccumulation in whales to simulate expected temporal trends in this neutral precursor compound and quantify the importance of different uptake pathways. Changing atmospheric FOSA concentrations are driven by shifts in chemical production over time, but are poorly constrained based on emissions inventories and direct measurements.3, 12 Three cruises between 2007–2008 measured FOSA in the North Atlantic marine boundary layer.49 We estimated temporal shifts in atmospheric FOSA levels at northern latitudes by linearly scaling the average concentrations from these cruises by changes in FOSA deposition in the Devon Ice cap, Devon Island, Nunavut, Canada50 between 1994 and 2007 (SI Table S5). Temporal changes in seawater FOSA concentrations were estimated from a measured air-water partition coefficient (log Kaw = −3.7).51 We compared these values to open ocean measurements from the migratory territory of North Atlantic pilot whales indicated by satellite telemetry data (SI Figure S2).40
Pilot whale stomach contents suggest their diet consists mainly of European flying squid (Todarodes sagittatus).39 We modeled squid FOSA concentrations assuming simple equilibrium partitioning with the ocean surface mixed layer (SI Table S6). Partitioning of FOSA from seawater to squid is based on an octanol-water partition coefficient (log Kow = 5.8),52 measured lipid content (1.4%), and protein content (16%).53
We parameterized the time-dependent bioaccumulation model for neutral organic pollutants developed by Arnot and Gobas54 for FOSA in the North Atlantic pilot whale food web (SI Tables S7 and S8). This model has previously been applied to a wide-range of food-webs, including marine mammals.54–57 The model quantifies chemical uptake and elimination in biota based on dietary uptake, respiration, fecal egestion, urination, and growth dilution. We assumed metabolism of FOSA by pilot whales is negligible based on prior work.33–35 Ingestion rates for pilot whales were based on a cetacean specific allometric equation.58,59 Respiration rates were quantified from breathing frequency and tidal lung volume derived from allometric equations for marine mammals.60–61 We assumed a 100% uptake efficiency in the lungs, following previous exposure analyses for FOSA.19, 62 Growth rates and body composition were based on data from over 3,400 pilot whales from the Faroe Islands.42, 59 A complete description of the bioaccumulation model is provided in the SI (Tables S7 and S8).
Temporal changes in FOSA reported here are for juvenile males between the ages of 5 to 14 years (mean 6–9 years) (Figure S1).18 To reproduce these measurements with the bioaccumulation model, we simulated birth cohorts born between 1980 and 2025 and FOSA exposure across the lifetime of each pilot whale individual given changes in environmental concentrations. We sampled the expected FOSA concentrations in whales at ages five, 10 and 15 years from each simulation to reproduce cross-sectional body burden-age trends (CBATs).18 We evaluated the simulation by comparing modeled means and changes over time to measured FOSA concentrations and slopes of the regression model for temporal changes in observations.
Results and Discussion
Contemporary PFAS levels in pilot whale muscle
Concentrations of different PFASs in pilot whale muscle in 2013 were highest for FOSA, PFOS and the PFCAs with chain lengths between 9–14 carbons (Figure 1, Table 1). The largest fractions of total measured PFASs consist of PFOS (23%) and its neutral precursor FOSA (34%). Prior cetacean studies report concentrations of FOSA to be as high or greater than PFOS,5, 63–65 but underlying mechanisms for accumulation have not been explored.
Figure 1.
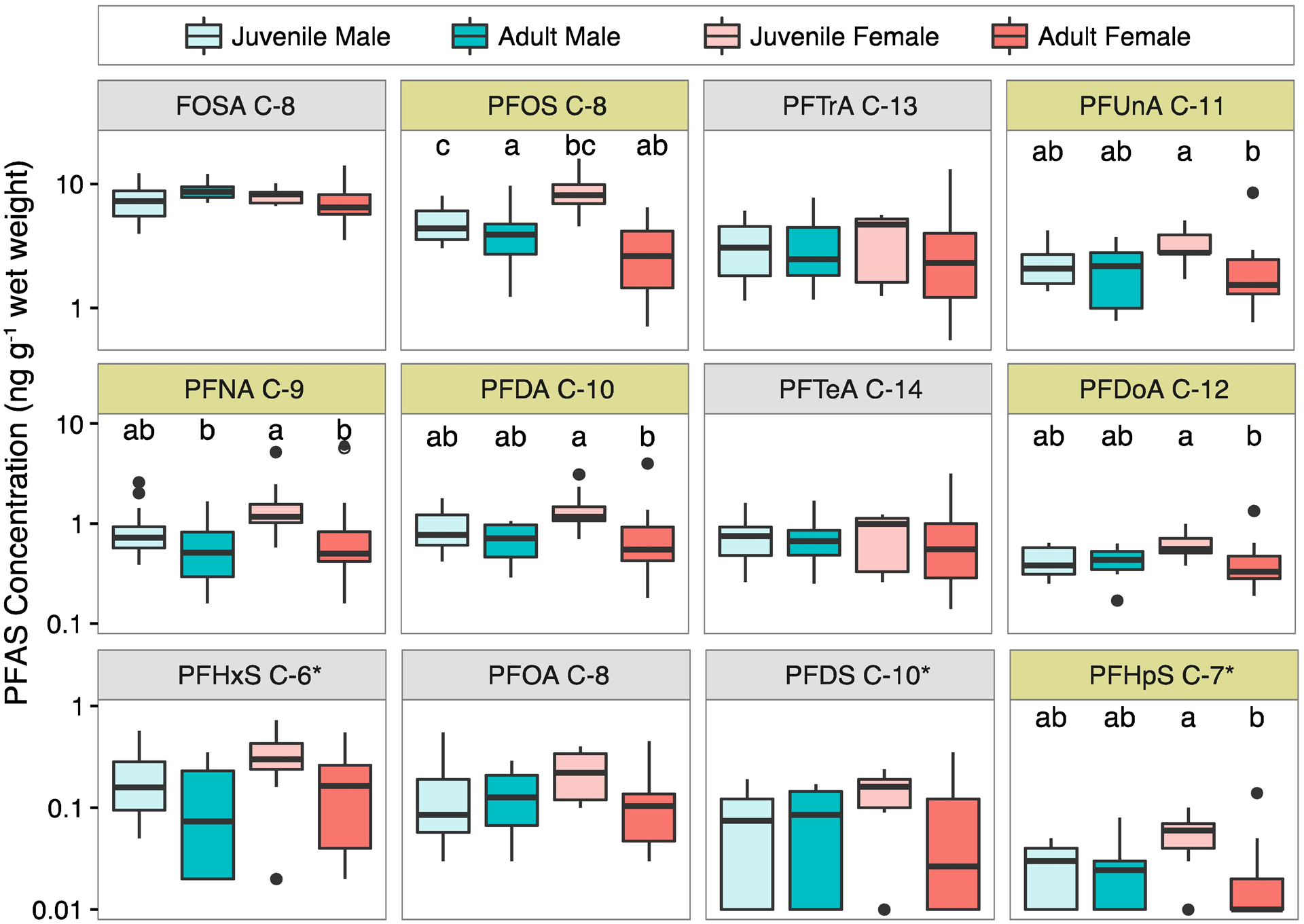
Effects of life stage and gender on measured PFAS concentrations in pilot whale muscle (Globicephala melas) sampled in 2013. Median concentrations for each group are represented by the horizontal black line in box/whisker plots. Notches represent 25th and 75th percentile concentrations, whiskers extend to 1.5 times the interquartile range and outliers are shown as circles. Compounds that differ significantly between groups based on one-way ANOVA (p < 0.05) are shaded yellow, and common letters above each box indicate groups with no significant difference in between group comparisons in post hoc analysis using Tukey’s test. Compounds from Table 1 that are not shown here were infrequently detected. For compounds denoted by ‘*’ non-detects are shown as the detection limit multiplied by 1/√2.46
The lack of statistical correlation between FOSA and other PFASs in Figure 2 highlights its contrasting origin and timescales for environmental cycling.66 Most PFASs were correlated with each other, indicating similarity in production sources and/or cycling in the ocean (Figure 2). Their lifetimes in surface seawater, where biological exposures occur, are thought to be long (decades)12, 67–68 relative to the atmospheric half-lives of precursors such as FOSA (50–80 days for FTOHs and even less for water-soluble FASAs).12, 67–68
Figure 2.
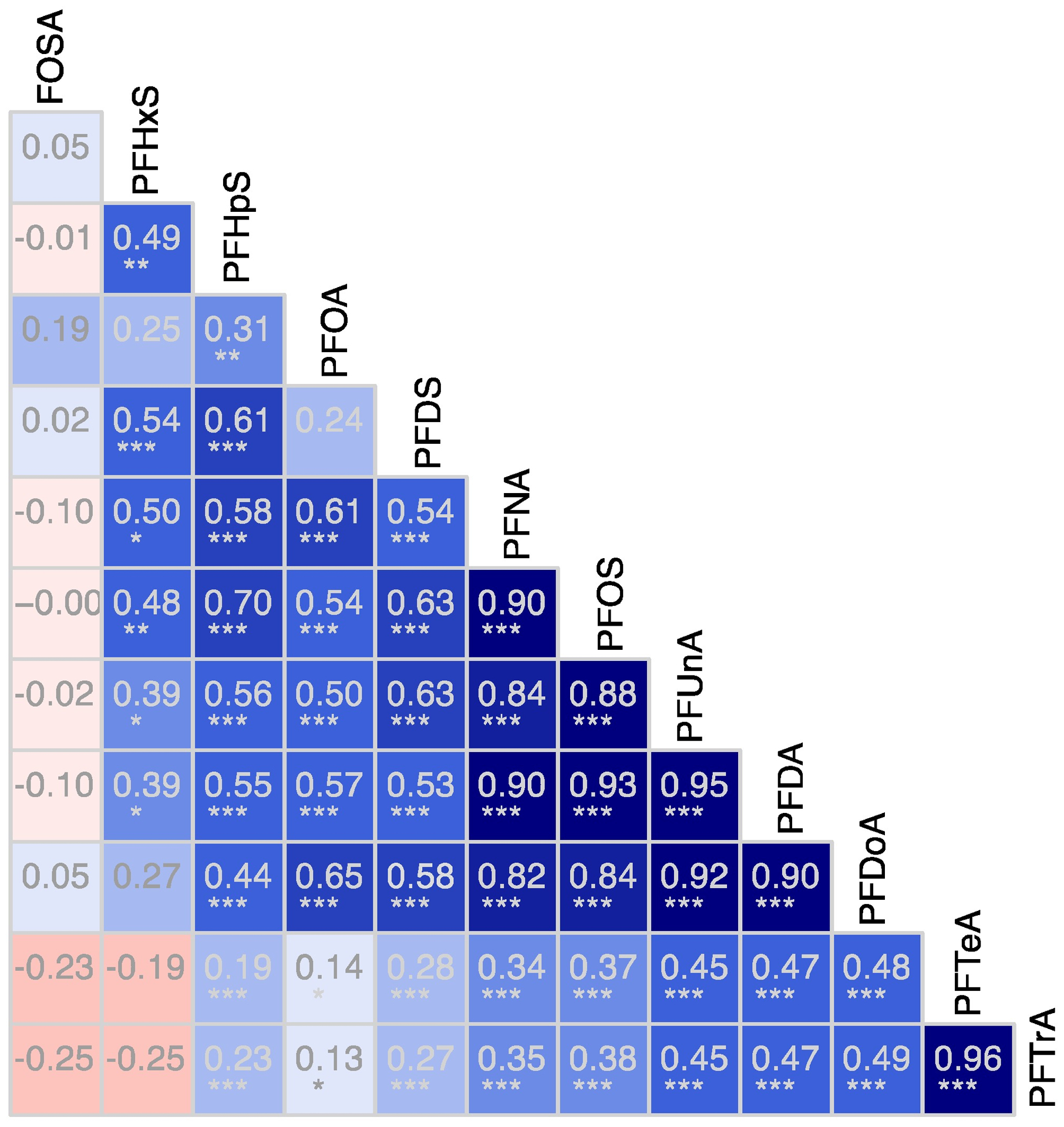
Correlation matrix for PFASs measured in pilot whale muscle tissue (Globicephala melas) in 2013. Numbers indicate Spearman correlation coefficients for a two-sided statistical test. The intensities of blue and red show the strength of positive and negative correlations, respectively. Significant correlations are denoted by asterisks (* = p<0.05; **=p<0.005, ***=p<0.0005).
For whales sampled in 2013, odd numbered long-chain PFCAs (PFTrA, PFUnA, PFNA) were comparable in magnitude to PFOS and FOSA, but other compounds (PFHxS, PFOA, PFDS and PFHpS) were all at least an order of magnitude lower (Figure 1). The enhanced propensity for PFOS and other long-chained PFASs to bioaccumulate in aquatic food-webs has been demonstrated in many other studies.69–70 Sturm and Ahrens14 suggest enrichment of the odd numbered PFCAs in many marine mammals is consistent with atmospheric fluorotelomer alcohol (FTOH) degradation as an important exposure source. Greater bioaccumulation of the longer chain (odd numbered) compounds is expected when there is equal production of odd and even PFASs during degradation of precursors such as 8:2 FTOH and 10:2 FTOH.14
We found significant differences (one-way ANOVA, p<0.05) across life stage and gender for PFOS, PFUnA, PFNA, PFDA, PFDoA, and PFHpS in pilot whales sampled in 2013 (Figure 1). For all compounds except FOSA, median concentrations were highest in nulliparous juvenile females (Figure 1). Juvenile females were significantly higher than adult females for PFNA, PFDA, PFUnA, PFDoA, and PFHpS (one-way ANOVA, p<0.05 and post-hoc Tukey test). Juvenile males were statistically elevated (p<0.05) compared to adult males and females for PFOS, but not statistically different for other compounds.
Observed differences between PFAS concentrations in juvenile and adult females are consistent with prior work showing that birth and lactation are large elimination pathways for PFASs in mammals.71–75 FOSA is the only neutral compound and is known to partition differently than the other PFASs across tissues.21 In pilot whales, female calves nurse longer than males and juvenile females are also known to consume a wider range of prey.39 For these reasons, juvenile males were selected for temporal trends analysis in this study to minimize impacts of life stage and gender related variability.
Temporal patterns in juvenile male pilot whales
Between 1994 and 2013, FOSA accounted for a large but declining fraction of the 15 PFASs (ΣPFASs) measured in juvenile male whale muscle tissue (Figure 3A). The fraction of ΣPFASs consisting of FOSA peaked in 1999 at 84% and declined after the phase out in chemical production of PFOS and its precursors around the year 2000 to a low of 34% in 2013 (Figure 3A). By contrast, long chain PFCAs (C9-C14) have continued to increase in relative importance over this same period from between 7–14% for 1994 to 2000, up to 40% of the ΣPFASs in 2013 (Figure 3A). All reported changes in composition were statistically significant based on Aitchison compositional regression. Declining concentrations of FOSA between 1994 and 2013 were offset by increases in other compounds over the same time-period, resulting in no significant change in ΣPFASs between 1994 and 2013 (Figure 3B). Peak ΣPFAS concentrations occur in 1998 (31 ng g−1 wet weight: ww) and levels in 1994 are comparable to 2013 (21 ng g−1 ww).
Figure 3.
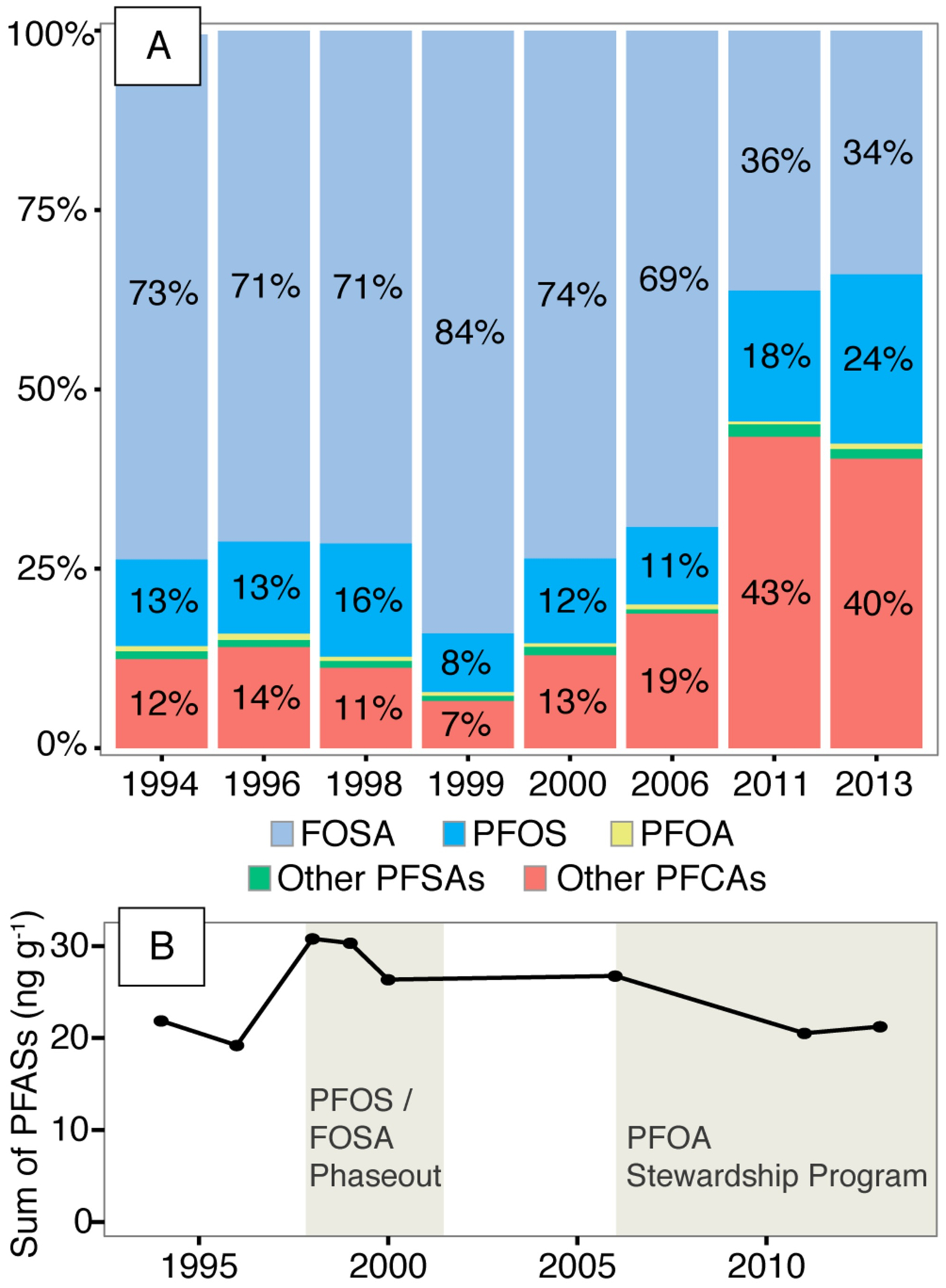
Temporal patterns in PFAS concentrations in juvenile male pilot whale muscle tissue (Globicephala melas) between 1994 and 2013. Compounds are grouped into categories reflecting one or more compound: perfluorooctane sulfonamide: FOSA, the neutral atmospheric precursor to perfluorooctane sulfonate (PFOS); perfluorooctanoic acid: PFOA; perfluorosulfonic acids: PFSAs; perfluorinated carboxylic acids: PFCAs. Panel (A) shows the changing composition of PFASs over time. Panel (B) shows the sum of the 15 detectable PFASs measured in this study.
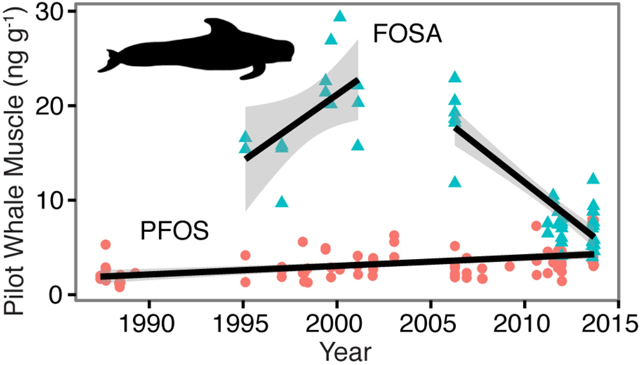
Figure 4 shows statistically significant temporal trends for six PFASs (PFOS, FOSA, PFNA, PFDA, PFUnA, PFTrA) between 1986 and 2013 inferred from log-linear regression models. All compounds show increases for the entire period except FOSA, which declined by 13% yr−1 after 2006. Increases since 1986 observed for the other five compounds range from 2.8% yr−1 (PFOS) to 8.2% yr−1 (PFDA) (Figure 4). We calculated crude trends as well as trends adjusted for pilot whale length as a proxy for age. Length was only statistically significant for PFOS, but the effect size was minimal as shown in SI Table S9.
Figure 4.
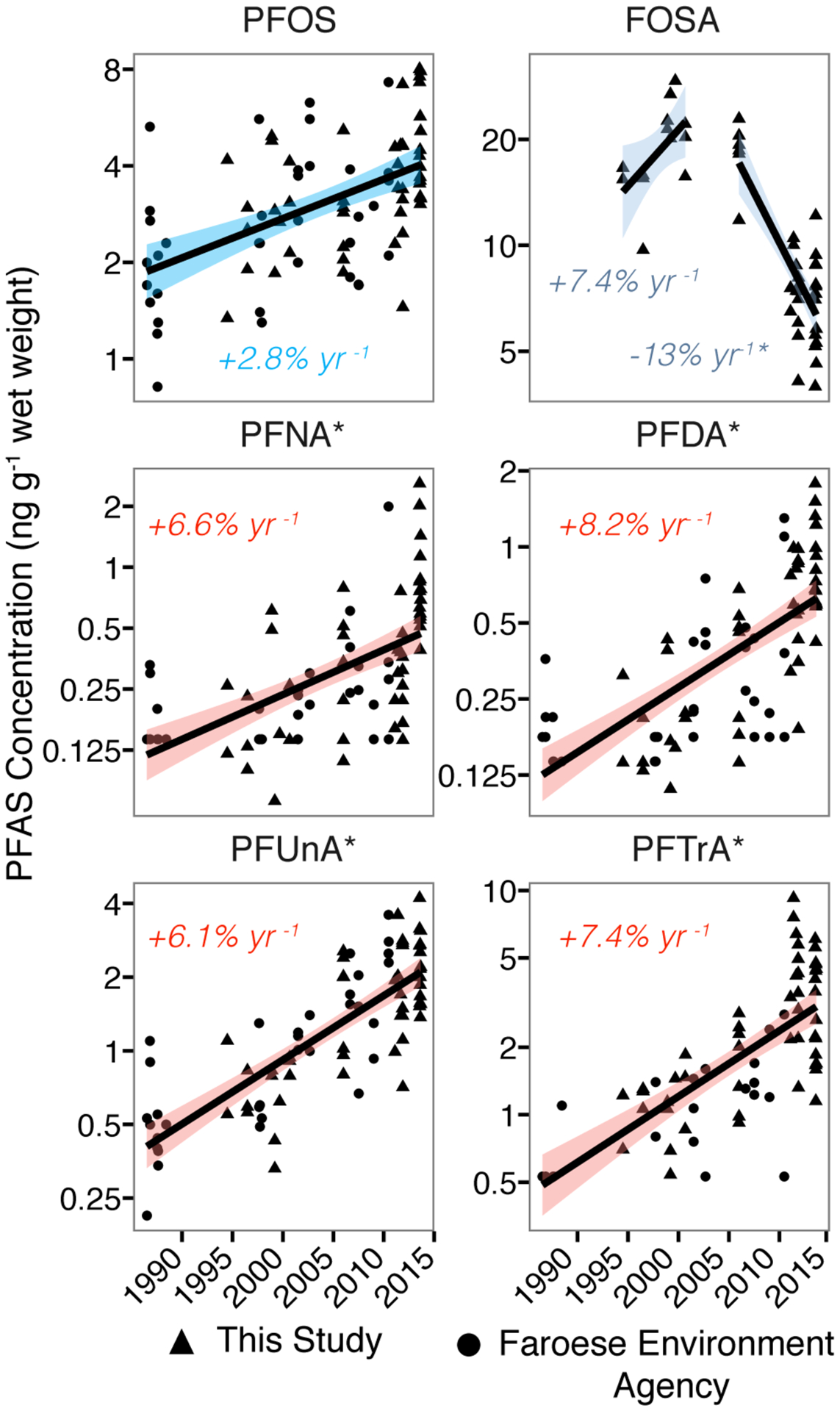
Temporal trends in concentrations of selected PFASs in juvenile male North Atlantic pilot whale muscle tissue (Globicephala melas) between 1986 and 2013. All trends (shown as percent annual changes) are significant at the p<0.05 level based on linear regression of log-transformed concentrations. Shaded areas represent 95% confidence intervals of the mean. Triangles are pilot whale analyzed for this study and circles are analyzed by the Faroese Environment Agency.41 For compounds denoted by ‘*’ non-detects are represented in plots as the detection limit multiplied by 1/√2.46, but slopes are based on maximum likelihood estimates for non-detect values.45
Increases in long-chained PFCAs in juvenile male pilot whale muscle reported here fall within ranges previously reported for other marine mammals. Swedish sea otters (6–11% yr−1) and Alaskan beluga whales (9–14% yr−1) show greater increases and Norwegian ringed seals are comparable (5–9%) to pilot whale changes observed here. However, increases in polar bears from east Greenland through 2006 (2–3% yr−1) and decreases since 2006 are lower than pilot whale trends.16–17, 66 Varying rates of change likely reflect species-specific differences in metabolism and environmental exposures, as discussed in other work.14
We find that increases in PFOS concentrations in pilot whales are smaller than those for long-chained PFCAs, consistent with shifting emissions away from PFOS. This has been confirmed by results across several wildlife species.16–17, 66 We speculate that relatively rapid decreases in PFOS reported in other studies such as for harbor seals from the German Bight (2002–2008),76 ringed seal from the Canadian Arctic (2000–2005),77 and ringed seals and polar bears from Greenland (2006–2010)16 may reflect decreases in FOSA exposure that has been biotransformed into PFOS. Previous studies have alluded to a potential role for precursors affecting biological trends,6, 12 but did not specifically identify FOSA as a major intermediate compound.
Temporal patterns of FOSA exposure in pilot whales
Figure 5a shows reconstructed atmospheric trends in FOSA from ice core measurements and ship cruise data (SI Table S5), and corresponding concentrations in seawater and squid based on simple equilibrium partitioning calculations. Results suggest FOSA levels peaked between 1997 to 2001 at ~22 pg m−3 in the atmosphere, ~110 pg L−1 in seawater, and ~1355 pg g−1 wet weight in European flying squid. By 2010, modeled levels suggest declines to ~2.2 pg m−3 in the atmosphere, ~11 pg L−1 in seawater, and ~138 pg g−1 in squid.
Figure 5.
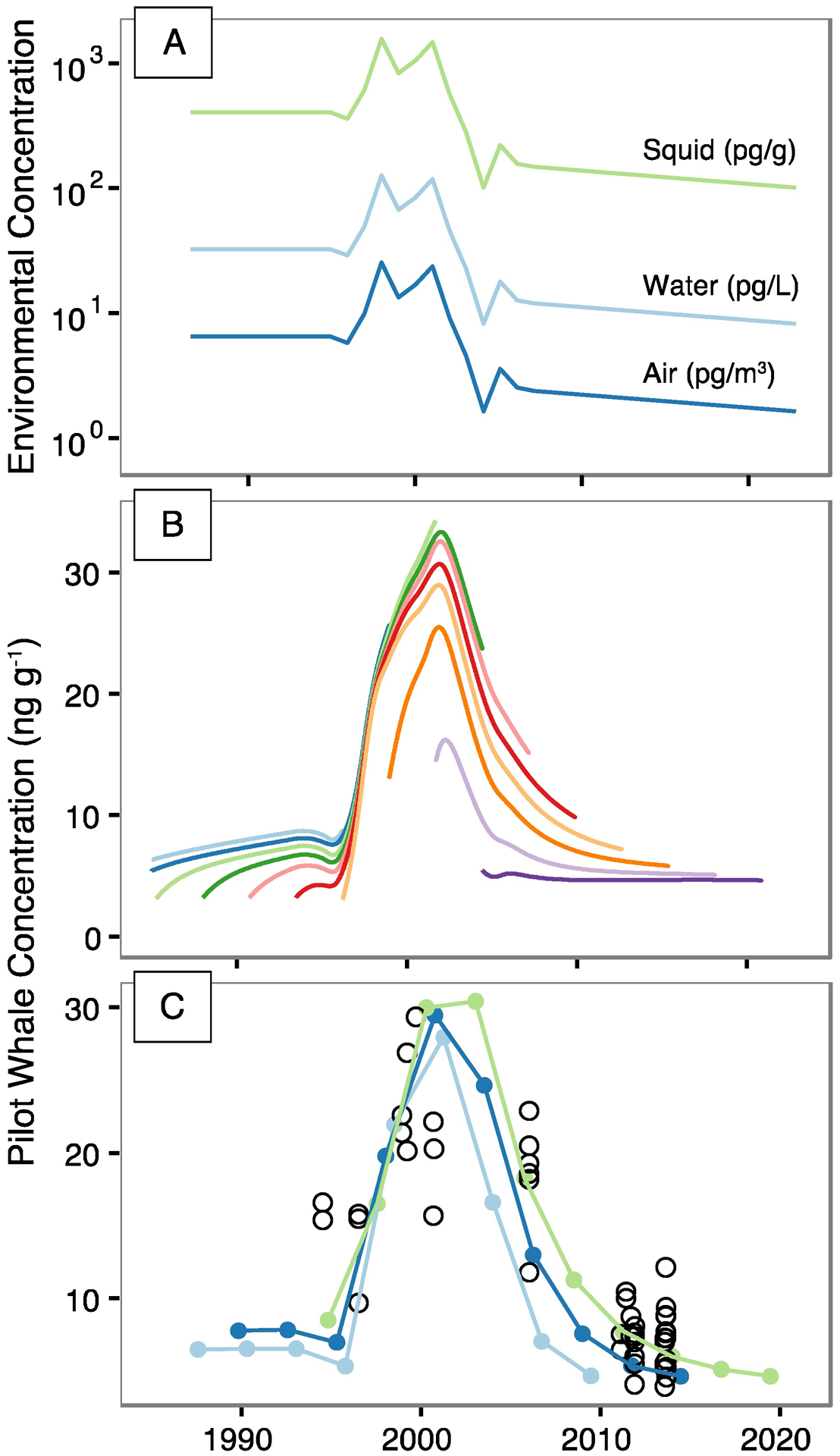
Modeled concentrations of perfluorooctane sulfonamide (FOSA) in air, water, squid (Todarodes sagitatus), and pilot whales (Globicephala melas) compared to temporal measurements collected in this study. Panel A shows reconstructed concentrations in air based on historic ice core data26, 50, and corresponding concentrations in surface seawater and squid based on equilibrium partitioning. Panel (B) shows modeled concentrations of FOSA in different pilot whale birth cohorts based on environmental levels (Panel A). Panel (C) shows average concentrations of FOSA in juvenile male pilot whales for ages ranging between 5 to 15 years to match the bounds of observations. Measured values from this study shown as black circles in Panel (C).
By comparison, a mean atmospheric FOSA concentration of 1.2 pg m−3 was measured at a remote high elevation site in Switzerland in 2010.78 A variety of studies report seawater FOSA concentrations from the North Atlantic and Arctic between 2005 and 2009 but results varied widely (1–300 pg L−1) depending on sampling methods, reported detection limits, and proximity to the coast (and thus point sources).27, 79–85 Measurements from the offshore North Atlantic Ocean in 2005 were all <17 pg L−1 and in the Norwegian Sea in 2007 were all <60 pg L−1.80–81 For squid (n=5) collected for this study in 2010, measured FOSA concentrations ranged between 177–386 pg g−1 (Table S10).
Given the simplicity of our partitioning modeling approach, approximations based on atmospheric FOSA appear to reasonably capture the magnitude of concentrations and differences across media. We slightly underestimate available observations in recent years (post-2005), but are generally within a factor of two difference, which is acceptable given spatial variability in measurements. Reported ranges from prior modeling studies on PFOS and its precursors are within a factor of five of observations.12
Reasonable agreement between observed and modeled FOSA concentrations suggests that changes in atmospheric FOSA levels and equilibration with the surface mixed layer ocean on timescales of less than one year are driving changes in biological concentrations. Such a response is more rapid than predicted for PFOS and PFOA in the ocean by prior work due to lag times introduced by penetration into subsurface waters and accumulation of legacy releases.12, 67
Modeling results for pilot whales further confirm that the relatively rapid atmospheric decline in FOSA accounts for the observed changes in pilot whales between 1986 and 2013 (Figure 5b–c). To correct for the confounding influence of age on temporal trends, we modeled FOSA in pilot whale cohorts born between 1980 and 2020 (Figure 5B). To capture the distribution of measured values, we modeled low, moderate, and high scenarios (Figure 5C) that correspond to birth cohort simulations between 5 and 15 years. Modeled mean values (3–8 ng g−1) agree well with average measured FOSA between 2011–2013 of 7.3±1.9 ng g−1. Modeled FOSA prior to 1998 (6–10 ng g−1) falls slightly below observed concentrations (mean: 14.6±2.8 ng g−1) but is generally within a factor of two of measurements.
Modeling results suggest a 9%−10% yr−1 increase in FOSA concentrations in pilot whales between 1994–2002 (Figure 5C), which is slightly greater than the observed increase of 7.4% yr−1 (Figure 2). Modeled declines in FOSA after 2006 range from approximately 6% to 10% yr−1 while observations suggest an average of 13% yr−1 (Figure 2). These differences are consistent with the underestimate in seawater and squid data based on partitioning calculations (Figure 5). Increasing whale age from five to 15 years results in up to a doubling of FOSA tissue burdens, depending on the timing of exposure. The greatest difference is during the period of declining environmental concentrations because the oldest whales had high exposures during their early life. In summary, we find that FOSA declines in pilot whale muscle can be generally reproduced by accounting for changing atmospheric concentrations, and simple equilibrium partitioning between the atmosphere, surface ocean and prey items. This implies that changing atmospheric burdens of FOSA exerted a major influence on biological exposures in the Arctic and Subarctic regions.
The average FOSA:PFOS ratios in juvenile male pilot whales peaked at 7.5 in 1998–2002 and declined to 1.6 by 2013 (Table 1). This implies that for species that biotransform FOSA to PFOS, observed decreases in PFOS may refelect a decline in exposure to precursors even if direct exposure to PFOS remained unchanged. This would help to explain inconsistent trends across species from different remote locations. Biological PFOS concentrations are expected to decline more rapidly in locations where precursors historically represented a larger exposure source (i.e., high latitude locations). An example of this can be seen in two distinct populations of beluga whales (Delphinapterus leucas) harvested off the northern and southern Alaskan coasts. FOSA:PFOS ratios in beluga whales from northern Alaska were higher and decreased more rapidly compared to those from southern Alaska.66 The authors suggest that these patterns could reflect greater direct exposures to PFOS in southern Alaska from Anchorage and potentially higher precursor contributions in the northern Alaskan Arctic.
Implications for future exposures
We find that shifts in PFASs released to the environment have led to large changes in the composition of PFAS exposures in pilot whales, but not necessarily to overall decreases in concentration. Declines in FOSA, the most prevalent PFAS around the year 2000, has been offset by increasing levels of long-chained PFCAs. Despite the phase-out of both PFOS and FOSA before 2002, PFOS concentrations have continued to increase, highlighting the relatively longer timescales of removal through ocean transport. If current trends continue, long-chained PFCAs will likely become the dominant compounds in pilot whales and total PFAS exposures may also increase. Production of long chain PFCAs (>C7) by eight major global manufacturers was phased-out in 2015 as part of the U.S. Environmental Protection Agency’s PFOA Stewardship Program.11 However, new manufactures in Asia have continued production of these compounds.11 The slow response of PFOS to its phase out prior to 2002 suggests declines in long-chained PFCAs may lag production by decades, depending on the ages, sizes and foraging depths of biota.
FOSA levels in pilot whale muscle reported here indicate that precursors are important exposure sources for marine food webs. While we know that whales cannot biotransform FOSA to PFOS, we do not know their metabolic capacity for the other precursor compounds.21 For this reason, FOSA levels in samples described here could represent an integrated signal of overall precursor concentration, a subset of precursors that degrade to FOSA, or FOSA itself. Measuring total organic fluorine (TOF) and identifying novel precursors would provide much needed insights on the contribution of fluorinated precursors to ongoing biological exposurs.23 Rapid observed declines in FOSA suggest atmospherically derived PFAS exposures in remote locations will be more responsive to changes in emissions than those originating from coastal discharges and ocean circulation.
While results from this study apply primarily to the marine environment, they may also point to a potential pathway for declining human exposures. Rapid decreases in measured concentrations of PFOS observed in humans globally86–89 since 2000 may be due in part to the large decrease in atmospheric precursors.90–91 Furthermore, increases in PFOS and other long-chained PFCAs in whales, which are consumed by the population of the Faroe Islands, implies a continued source of exposure to these contaminants from marine food consumption.
Supplementary Material
Acknowledgements
We acknowledge financial support for this study from the Smith Family Foundation, the U.S. National Science Foundation Office of Polar Programs (PLR 1203496), and the NSF-NIH Oceans and Human Health Program (OCE-1321612). CD acknowledges support from a U.S. EPA Star Program Graduate Fellowship (F13D10739). Inga Jensen (AU) is acknowledged for technical assistance in PFAS chemical analyses. Sissal V Erenbjerg and Katrin Hoydal at the Environment Agency, Faroe Islands, are acknowledged for their assistance in sampling, and Heini Viderø Johansen for performing the squid PFAS analyses. We thank Philippe Grandjean and Pál Weihe for assistance initiating this work.
Footnotes
Supporting Information
Details on samples, analytical methods, and supporting figures and tables.
References
- 1.Prevedouros K; Cousins IT; Buck RC; Korzeniowski SH, Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol 2006, 40 (1), 32–44. [DOI] [PubMed] [Google Scholar]
- 2.Yamashita N; Taniyasu S; Petrick G; Wei S; Gamo T; Lam PK; Kannan K, Perfluorinated acids as novel chemical tracers of global circulation of ocean waters. Chemosphere 2008, 70 (7), 1247–55. [DOI] [PubMed] [Google Scholar]
- 3.Paul AG; Jones KC; Sweetman AJ, A First Global Production, Emission, And Environmental Inventory For Perfluorooctane Sulfonate. Environ Sci Technol43 2009, 43 (2), 386–392. [DOI] [PubMed] [Google Scholar]
- 4.Tomy G; Budakowski W; Halldorson T; Helm PA; Stern GA; Friesen K; Pepper K; Tittlemier SA; Fisk AT, Fluorinated Organic Compounds in an Eastern Arctic Marine Food Web. Environ Sci Technol 2004, 38, 6475–6481. [DOI] [PubMed] [Google Scholar]
- 5.Tomy G; Pleskach K; Hare J; Ferguson SH; Hare J; Stern GA; Macinnis G; Marvin CH; Loseto L, Trophodynamics of Some PFCs and BFRs in a Western Canadian Arctic Marine Food Web. Environ Sci Technol 2009, 43, 4076–4081. [DOI] [PubMed] [Google Scholar]
- 6.Butt CM; Berger U; Bossi R; Tomy GT, Levels and trends of poly- and perfluorinated compounds in the arctic environment. Sci Total Environ 2010, 408 (15), 2936–65. [DOI] [PubMed] [Google Scholar]
- 7.Dietz R; Bossi R; Riget FF; Sonne C; Born EW, Increasing perfluoroalkyl contaminants in east Greenland polar bears (Ursus maritimus): a new toxic threat to the Arctic bears. Environ Sci Technol 2008, 42 (7), 2701–2702. [DOI] [PubMed] [Google Scholar]
- 8.Houde M; De Silva AO; Muir DC; Letcher RJ, Monitoring of perfluorinated compounds in aquatic biota: an updated review. Environ Sci Technol 2011, 45 (19), 7962–73. [DOI] [PubMed] [Google Scholar]
- 9.Lindstrom AB; Strynar MJ; Libelo EL, Polyfluorinated compounds: past, present, and future. Environ Sci Technol 2011, 45 (19), 7954–61. [DOI] [PubMed] [Google Scholar]
- 10.Grandjean P; Anderson EW; Budtz-Jorgensen E; Nielsen F; Molbak K; Weihe P; Heilmann C, Serum vaccine antibody concentrations in children exposed to perfluorinated comopunds. Jama 2012, 307 (4), 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z; Cousins IT; Scheringer M; Buck RC; Hungerbuhler K, Global emission inventories for C4-C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: production and emissions from quantifiable sources. Environ Int 2014, 70, 62–75. [DOI] [PubMed] [Google Scholar]
- 12.Armitage JM; Schenker U; Sceringer M; Martin JW; Macleod M; Cousins IT, Modeling the Global Fate and Transport of Perfluorooctane Sulfonate (PFOS) and Precursor Compounds in Relation to Temporal Trends in Wildlife Exposure. Environ Sci Technol 2009, 43, 9274–9280. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z; Cousins IT; Scheringer M; Buck RC; Hungerbuhler K, Global emission inventories for C4-C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, part II: the remaining pieces of the puzzle. Environ Int 2014, 69, 166–76. [DOI] [PubMed] [Google Scholar]
- 14.Sturm R; Ahrens L, Trends of polyfluoroalkyl compounds in marine biota and in humans. Environmental Chemistry 2010, 7 (6), 457. [Google Scholar]
- 15.Rotander A; Karrman A; van Bavel B; Polder A; Riget F; Auethunsson GA; Vikingsson G; Gabrielsen GW; Bloch D; Dam M, Increasing levels of long-chain perfluorocarboxylic acids (PFCAs) in Arctic and North Atlantic marine mammals, 1984–2009. Chemosphere 2012, 86 (3), 278–85. [DOI] [PubMed] [Google Scholar]
- 16.Riget F; Bossi R; Sonne C; Vorkamp K; Dietz R, Trends of perfluorochemicals in Greenland ringed seals and polar bears: indications of shifts to decreasing trends. Chemosphere 2013, 93 (8), 1607–14. [DOI] [PubMed] [Google Scholar]
- 17.Roos A; Berger U; Järnberg U; van Dijk J; Bignert A, Increasing Concentrations of Perfluoroalkyl Acids in Scandinavian Otters (Lutra lutra) between 1972 and 2011: A New Threat to the Otter Population? Environmental Science & Technology 2013, 47 (20), 11757–11765. [DOI] [PubMed] [Google Scholar]
- 18.Binnington MJ; Wania F, Clarifying relationships between persistent organic pollutant concentrations and age in wildlife biomonitoring: individuals, cross-sections, and the roles of lifespan and sex. Environ Toxicol Chem 2014, 33 (6), 1415–26. [DOI] [PubMed] [Google Scholar]
- 19.Gebbink WA; Berger U; Cousins IT, Estimating human exposure to PFOS isomers and PFCA homologues: the relative importance of direct and indirect (precursor) exposure. Environ Int 2015, 74, 160–9. [DOI] [PubMed] [Google Scholar]
- 20.Gebbink WA; Bignert A; Berger U, Perfluoroalkyl Acids (PFAAs) and Selected Precursors in the Baltic Sea Environment: Do Precursors Play a Role in Food Web Accumulation of PFAAs? Environ Sci Technol 2016, 50 (12), 6354–62. [DOI] [PubMed] [Google Scholar]
- 21.Martin JW; Asher BJ; Beesoon S; Benskin JP; Ross MS, PFOS or PreFOS? Are perfluorooctane sulfonate precursors (PreFOS) important determinants of human and environmental perfluorooctane sulfonate (PFOS) exposure? J Environ Monit 2010, 12 (11), 1979–2004. [DOI] [PubMed] [Google Scholar]
- 22.Yeung LW; De Silva AO; Loi EI; Marvin CH; Taniyasu S; Yamashita N; Mabury SA; Muir DC; Lam PK, Perfluoroalkyl substances and extractable organic fluorine in surface sediments and cores from Lake Ontario. Environ Int 2013, 59, 389–97. [DOI] [PubMed] [Google Scholar]
- 23.Loi EI; Yeung LW; Taniyasu S; Lam PK; Kannan K; Yamashita N, Trophic magnification of poly- and perfluorinated compounds in a subtropical food web. Environ Sci Technol 2011, 45 (13), 5506–13. [DOI] [PubMed] [Google Scholar]
- 24.Miyake Y; Yamashita N; Rostkowski P; So MK; Taniyasu S; Lam PK; Kannan K, Determination of trace levels of total fluorine in water using combustion ion chromatography for fluorine: a mass balance approach to determine individual perfluorinated chemicals in water. J Chromatogr A 2007, 1143 (1–2), 98–104. [DOI] [PubMed] [Google Scholar]
- 25.Stock NL; Furdui VI; Muir DC; Mabury SA, Perfluoroalkyl Contaminants in the Canadian Arctic: Evidence of Atmospheric Transport and Local Contamination. Environ Sci Technol 2007, 41, 3529–3536. [DOI] [PubMed] [Google Scholar]
- 26.Young CJ; Furdui VI; Franklin J; Koerner RM; Muir DC; Mabury SA, Perfluorinated Acids in Arctic Snow: New Evidence for Atmospheric Formation. Environ Sci Technol 2007, 41, 3455–3461. [DOI] [PubMed] [Google Scholar]
- 27.Benskin JP; Muir DC; Scott BF; Spencer C; De Silva AO; Kylin H; Martin JW; Morris A; Lohmann R; Tomy G; Rosenberg B; Taniyasu S; Yamashita N, Perfluoroalkyl acids in the Atlantic and Canadian Arctic Oceans. Environ Sci Technol 2012, 46 (11), 5815–23. [DOI] [PubMed] [Google Scholar]
- 28.Kwok KY; Yamazaki E; Yamashita N; Taniyasu S; Murphy MB; Horii Y; Petrick G; Kallerborn R; Kannan K; Murano K; Lam PK, Transport of perfluoroalkyl substances (PFAS) from an arctic glacier to downstream locations: implications for sources. Sci Total Environ 2013, 447, 46–55. [DOI] [PubMed] [Google Scholar]
- 29.Bossi R; Dam M; Riget FF, Perfluorinated alkyl substances (PFAS) in terrestrial environments in Greenland and Faroe Islands. Chemosphere 2015, 129, 164–9. [DOI] [PubMed] [Google Scholar]
- 30.Vestergren R; Berger U; Glynn A; Cousins IT, Dietary exposure to perfluoroalkyl acids for the Swedish population in 1999, 2005 and 2010. Environ Int 2012, 49, 120–7. [DOI] [PubMed] [Google Scholar]
- 31.Ullah S; Huber S; Bignert A; Berger U, Temporal trends of perfluoroalkane sulfonic acids and their sulfonamide-based precursors in herring from the Swedish west coast 1991–2011 including isomer-specific considerations. Environ Int 2014, 65, 63–72. [DOI] [PubMed] [Google Scholar]
- 32.D’Eon JC; Mabury SA, Is indirect exposure a significant contributor to the burden of perfluorinated acids observed in humans? Environ Sci Technol 2011, 45 (19), 7974–84. [DOI] [PubMed] [Google Scholar]
- 33.Letcher RJ; Chu S; McKinney MA; Tomy GT; Sonne C; Dietz R, Comparative hepatic in vitro depletion and metabolite formation of major perfluorooctane sulfonate precursors in Arctic polar bear, beluga whale, and ringed seal. Chemosphere 2014, 112, 225–31. [DOI] [PubMed] [Google Scholar]
- 34.Galatius A; Bossi R; Sonne C; Riget FF; Kinze CC; Lockyer C; Teilmann J; Dietz R, PFAS profiles in three North Sea top predators: metabolic differences among species? Environ Sci Pollut Res Int 2013, 20 (11), 8013–20. [DOI] [PubMed] [Google Scholar]
- 35.Gebbink WA; Bossi R; Riget FF; Rosing-Asvid A; Sonne C; Dietz R, Observation of emerging per- and polyfluoroalkyl substances (PFASs) in Greenland marine mammals. Chemosphere 2016, 144, 2384–91. [DOI] [PubMed] [Google Scholar]
- 36.Xu L; Krenitsky DM; Seacat AM; Butenhoff JL; Anders MW, Biotransformation of N-Ethyl-N-(2-hydroxyethyl)perfluorooctanesulfonamide by Rat Liver Microsomes, Cytosol, and Slices and by Expressed Rat and Human Cytochromes P450. Chem Res Toxicol 2004, 17, 767–775. [DOI] [PubMed] [Google Scholar]
- 37.Fu Z; Wang Y; Wang Z; Xie H; Chen J, Transformation pathways of isomeric perfluorooctanesulfonate precursors catalyzed by the active species of P450 enzymes: in silico investigation. Chem Res Toxicol 2015, 28 (3), 482–9. [DOI] [PubMed] [Google Scholar]
- 38.Gannon DP, Stomach contents of long-finned pilot whales (Globicephala melas) stranded on the U.S. mid-atlantic coast. Mar Mamm Sci 1997, 13 (3), 405–418. [Google Scholar]
- 39.Desportes G; Mouritsen R, Preliminary Results on the Diet of Long-Finned Pilot Whales off the Faroe Islands. Rep Int Whal Commn 1993, (Special Issue 14), 305–324. [Google Scholar]
- 40.Bloch D; Heide-Jorgense MP; Stefansson E; Mikkelsen B; Ofstad LH; Dietz R; Andersen LW, Short-term movements of long-finned pilot whales Globicephala melas around the Faroe Islands. Wildlife Biology 2003, 9 (1), 47–58. [Google Scholar]
- 41.Dam M; van Bavel B; Rigét F; Rotander A; Polder A; Auðunsson GA; Bloch D; Víkingsson G; Mikkelsen B; Gabrielsen GW; Sagerup K “New” POPs in marine mammals in Nordic Arctic and NE Atlantic areas during three decades; Nordic Council of Ministers: Copenhagen, Denmark, 2011. [Google Scholar]
- 42.Bloch D; Lockyer C; Zachariassen M, Age and growth parameters of the long-finned pilot whale off the Faroe Islands. Report of the International Whaling Commission 1993, (Special Issue 14), 163–207. [Google Scholar]
- 43.Ahrens L; Siebert U; Ebinghaus R, Total body burden and tissue distribution of polyfluorinated compounds in harbor seals (Phoca vitulina) from the German Bight. Mar Pollut Bull 2009, 58 (4), 520–5. [DOI] [PubMed] [Google Scholar]
- 44.Taniyasu S; Kannan K; So MK; Gulkowska A; Sinclair E; Okazawa T; Yamashita N, Analysis of fluorotelomer alcohols, fluorotelomer acids, and short- and long-chain perfluorinated acids in water and biota. J Chromatogr A 2005, 1093 (1–2), 89–97. [DOI] [PubMed] [Google Scholar]
- 45.NADA: Nondetects And Data Analysis for environmental data. R package, Version 1.5–6; 2013. [Google Scholar]
- 46.Hewett P; Ganser GH, A comparison of several methods for analyzing censored data. Ann Occup Hyg 2007, 51 (7), 611–32. [DOI] [PubMed] [Google Scholar]
- 47.Aitchison J, The statistical analysis of compositional data. Chapman and Hall: London, 1986. [Google Scholar]
- 48.Van den Boogaart KG; Tolosana-Delgado R, Analyzing compositional data with R. Springer: Berlin, 2013. [Google Scholar]
- 49.Dreyer A; Ebinghaus R, Polyfluorinated compounds in ambient air from ship- and land-based measurements in northern Germany. Atmospheric Environment 2009, 43 (8), 1527–1535. [Google Scholar]
- 50.MacInnis JJ; French K; Muir DCG; Spencer C; Criscitiello AS; DeSilva AO; Young C, Emerging investigator series: A 14-year depositional ice record of perfluoroalkyl substances in the High Arctic. Environ. Sci.: Processes Impacts 2017, 19, 22–30. [DOI] [PubMed] [Google Scholar]
- 51.Vierke L; Ahrens L; Shoeib M; Palm WU; Webster EM; Ellis DA; Ebinghaus R; Harner T, In situ air-water and particle-water partitioning of perfluorocarboxylic acids, perfluorosulfonic acids and perfluorooctyl sulfonamide at a wastewater treatment plant. Chemosphere 2013, 92 (8), 941–8. [DOI] [PubMed] [Google Scholar]
- 52.EPA U Estimation Programs Interface Suite™ for Microsoft® Windows, v 4.11, United States Environmental Protection Agency: Washington, DC, USA, 2012. [Google Scholar]
- 53.Atayeter S; Ercoskun H, Chemical composition of European squid and effects of different frozen storage temperatures on oxidative stability and fatty acid composition. J Food Sci Technol 2011, 48 (1), 83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arnot JA; Gobas F, A food web bioaccumulation model for organic chemicals in aquatic ecosystems. Environmental Toxicology and Chemistry 2004, 23 (10), 2343–2355. [DOI] [PubMed] [Google Scholar]
- 55.Kelly BC; Gobas FAPC, An Arctic Terrestrial Food-Chain Bioaccumulation Model for Persistent Organic Pollutants. Environmental Science & Technology 2003, 37 (13), 2966–2974. [DOI] [PubMed] [Google Scholar]
- 56.Gobas FAPC; Arnot JA, Food web bioaccumulation model for polychlorinated biphenyls in San Francisco Bay, California, USA. Environmental Toxicology and Chemistry 2010, 29 (6), 1385–1395. [DOI] [PubMed] [Google Scholar]
- 57.Armitage JM; Arnot JA; Wania F; Mackay D, Development and evaluation of a mechanistic bioconcentration model for ionogenic organic chemicals in fish. Environ Toxicol Chem 2013, 32 (1), 115–28. [DOI] [PubMed] [Google Scholar]
- 58.Innes S; Lavigne DM; Earle WM; Kovacs KM, Feeding rates of seals and whales. Journal of Animal Ecology 1987, 56, 115–130. [Google Scholar]
- 59.Lockyer C, Seasonal changes in body fat condition of northeast Atlantic pilot whales, and their biological significance. IWC Special Issue 14: Biology of the Northern Hemisphere Pilot Whales 1993, 205–324. [Google Scholar]
- 60.Mortola JP; Limoges MJ, Resting breathing frequency in aquatic mammals: a comparative analysis with terrestrial species. Respir Physiol Neurobiol 2006, 154 (3), 500–14. [DOI] [PubMed] [Google Scholar]
- 61.Lafortuna CL; Jahoda M; Azzellino A; Saibene F; Colombini A, Locomotor behaviours and respiratory pattern of the Mediterranean fin whale (Balaenoptera physalus). Eur J Appl Physiol 2003, 90 (3–4), 387–95. [DOI] [PubMed] [Google Scholar]
- 62.Vestergren R; Cousins IT; Trudel D; Wormuth M; Scheringer M, Estimating the contribution of precursor compounds in consumer exposure to PFOS and PFOA. Chemosphere 2008, 73 (10), 1617–24. [DOI] [PubMed] [Google Scholar]
- 63.Bossi R; Riget FF; Dietz R; Sonne C; Fauser P; Dam M; Vorkamp K, Preliminary screening of perfluorooctane sulfonate (PFOS) and other fluorochemicals in fish, birds and marine mammals from Greenland and the Faroe Islands. Environ Pollut 2005, 136 (2), 323–9. [DOI] [PubMed] [Google Scholar]
- 64.Kannan K; Corsolini S; Falandysz J; Oehme G; Focardi S; Giesy JP, Perfluorooctanesulfonate and related fluorinated hydrocarbons in marine mammals, fishes, and birds from coasts of the Baltic and the Mediterranean Seas. Environ Sci Technol 2002, 36 (15), 3210–3216. [DOI] [PubMed] [Google Scholar]
- 65.Hart K; Kannan K; Isobe T; Takahashi S; Yamada TK; Miyazaki N; Tanabe S, Time Trends and Transplacental Transfer of Perfluorinated Compounds in Melon-Headed Whales Stranded Along the Japanese Coast in 1982, 2001/2002, and 2006. Environ Sci Technol 2008, 42, 7132–7137. [DOI] [PubMed] [Google Scholar]
- 66.Reiner JL; O’Connell SG; Moors AJ; Kucklick JR; Becker PR; Keller JM, Spatial and temporal trends of perfluorinated compounds in Beluga Whales (Delphinapterus leucas) from Alaska. Environ Sci Technol 2011, 45 (19), 8129–36. [DOI] [PubMed] [Google Scholar]
- 67.Armitage JM; Macleod M; Cousins IT, Modeling the Global Fate and Transport of Perfluorooctanoic Acid (PFOA) and Perfluorooctanoate (PFO) Emitted from Direct Sources Using a Multispecies Mass Balance Model. Environ Sci Technol 2009, 43 (4), 1134–1140. [DOI] [PubMed] [Google Scholar]
- 68.Armitage JM; Macleod M; Cousins IT, Comparative Assessment of the Global Fate and Transport Pathways of Long-Chain Perfluorocarboxylic Acids (PFCAs) and Perfluorocarboxylates (PFCs) Emitted from Direct Sources. Environ Sci Technol 2009, 43, 1134–1140. [DOI] [PubMed] [Google Scholar]
- 69.Conder JM; Hoke RA; Wolf WD; Russell MH; Buck RC, Are PFCAs Bioaccumulative? A Critical Review and Comparison with Regulatory Criteria and Persistent Lipophilic Compounds. Environ Sci Technol 2008, 42 (4), 995–1003. [DOI] [PubMed] [Google Scholar]
- 70.Kelly BC; Ikonomou MG; Blair JD; Surridge B; Hoover D; Grace R; Gobas FAPC, Perfluoroalkyl Contaminants in an Arctic Marine Food Web: Trophic Magnification and Wildlife Exposure. Environ Sci Technol 2009, 43, 4037–4043. [DOI] [PubMed] [Google Scholar]
- 71.Karrman A; Ericson I; van Bavel B; Darnerud PO; Aune M; Glynn A; Lignell S; Lindstrom G, Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ Health Perspect 2007, 115 (2), 226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Loccisano AE; Longnecker MP; Campbell JL Jr.; Andersen ME; Clewell HJ 3rd, Development of PBPK models for PFOA and PFOS for human pregnancy and lactation life stages. J Toxicol Environ Health A 2013, 76 (1), 25–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Needham LL; Grandjean P; Heinzow B; Jørgensen PJ; Nielsen F; Patterson DG; Sjoödin A; Turner WE; Weihe P, Partition of environmental chemicals between maternal and fetal blood and tissues. Environ Sci Technol 2010, 45 (3), 1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mogensen UB; Grandjean P; Nielsen F; Weihe P; Budtz-Jorgensen E, Breastfeeding as an Exposure Pathway for Perfluorinated Alkylates. Environ Sci Technol 2015, 49 (17), 10466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gronnestad R; Villanger GD; Polder A; Kovacs KM; Lydersen C; Jenssen BM; Borga K, Maternal transfer of perfluoroalkyl substances in hooded seals. Environ Toxicol Chem 2017, 36 (3), 763–770. [DOI] [PubMed] [Google Scholar]
- 76.Ahrens L; Siebert U; Ebinghaus R, Temporal trends of polyfluoroalkyl compounds in harbor seals (Phoca vitulina) from the German Bight, 1999–2008. Chemosphere 2009, 76 (2), 151–8. [DOI] [PubMed] [Google Scholar]
- 77.Butt CM; Muir D; Stirling I; Kwan M; Mabury SA, Rapid Response of Arctic Ringed Seals to Changes in Perfluoroalkyl Production. Environ Sci Technol 2007, 41 (1), 42–49. [DOI] [PubMed] [Google Scholar]
- 78.Muller CE; Gerecke AC; Bogdal C; Wang Z; Scheringer M; Hungerbuhler K, Atmospheric fate of poly- and perfluorinated alkyl substances (PFASs): I. Day-night patterns of air concentrations in summer in Zurich, Switzerland. Environ Pollut 2012, 169, 196–203. [DOI] [PubMed] [Google Scholar]
- 79.Ahrens L; Felizeter S; Ebinghaus R, Spatial distribution of polyfluoroalkyl compounds in seawater of the German Bight. Chemosphere 2009, 76 (2), 179–184. [DOI] [PubMed] [Google Scholar]
- 80.Ahrens L; Barber JL; Xie Z; Ebinghaus R, Longitudinal and latitudinal distribution of perfluoroalkyl compounds in the surface water of the Atlantic Ocean. Environ Sci Technol 2009, 43 (9), 3122–3127. [DOI] [PubMed] [Google Scholar]
- 81.Ahrens L; Taniyasu S; Yeung LW; Yamashita N; Lam PK; Ebinghaus R, Distribution of polyfluoroalkyl compounds in water, suspended particulate matter and sediment from Tokyo Bay, Japan. Chemosphere 2010, 79 (3), 266–72. [DOI] [PubMed] [Google Scholar]
- 82.Busch J; Ahrens L; Xie Z; Sturm R; Ebinghaus R, Polyfluoroalkyl compounds in the East Greenland Arctic Ocean. J Environ Monit 2010, 12 (6), 1242–6. [DOI] [PubMed] [Google Scholar]
- 83.Gonzalez-Gaya B; Dachs J; Roscales JL; Caballero G; Jimenez B, Perfluoroalkylated substances in the global tropical and subtropical surface oceans. Environ Sci Technol 2014, 48 (22), 13076–84. [DOI] [PubMed] [Google Scholar]
- 84.Cai M; Zhao Z; Yin Z; Ahrens L; Huang P; Cai M; Yang H; He J; Sturm R; Ebinghaus R; Xie Z, Occurrence of perfluoroalkyl compounds in surface waters from the North Pacific to the Arctic Ocean. Environ Sci Technol 2012, 46 (2), 661–8. [DOI] [PubMed] [Google Scholar]
- 85.Theobald N; Caliebe C; Gerwinski W; Huhnerfuss H; Lepom P, Occurrence of perfluorinated organic acids in the North and Baltic seas. Part 1: distribution in sea water. Environ Sci Pollut Res Int 2011, 18 (7), 1057–69. [DOI] [PubMed] [Google Scholar]
- 86.Bjerregaard-Olesen C; Bach CC; Long M; Ghisari M; Bossi R; Bech BH; Nohr EA; Henriksen TB; Olsen J; Bonefeld-Jorgensen EC, Time trends of perfluorinated alkyl acids in serum from Danish pregnant women 2008–2013. Environ Int 2016, 91, 14–21. [DOI] [PubMed] [Google Scholar]
- 87.Toms LM; Thompson J; Rotander A; Hobson P; Calafat AM; Kato K; Ye X; Broomhall S; Harden F; Mueller JF, Decline in perfluorooctane sulfonate and perfluorooctanoate serum concentrations in an Australian population from 2002 to 2011. Environ Int 2014, 71, 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Okada E; Kashino I; Matsuura H; Sasaki S; Miyashita C; Yamamoto J; Ikeno T; Ito YM; Matsumura T; Tamakoshi A; Kishi R, Temporal trends of perfluoroalkyl acids in plasma samples of pregnant women in Hokkaido, Japan, 2003–2011. Environ Int 2013, 60, 89–96. [DOI] [PubMed] [Google Scholar]
- 89.Olsen GW; Lange CC; Ellefson ME; Mair DC; Church TR; Goldberg CL; Herron RM; Medhdizadehkashi Z; Nobiletti JB; Rios JA; Reagen WK; Zobel LR, Temporal trends of perfluoroalkyl concentrations in American Red Cross adult blood donors, 2000–2010. Environ Sci Technol 2012, 46 (11), 6330–8. [DOI] [PubMed] [Google Scholar]
- 90.Yeung LW; Robinson SJ; Koschorreck J; Mabury SA, Part II. A temporal study of PFOS and its precursors in human plasma from two German cities in 1982–2009. Environ Sci Technol 2013, 47 (8), 3875–82. [DOI] [PubMed] [Google Scholar]
- 91.Liu Y; Pereira AS; Beesoon S; Vestergren R; Berger U; Olsen GW; Glynn A; Martin JW, Temporal trends of perfluorooctanesulfonate isomer and enantiomer patterns in archived Swedish and American serum samples. Environ Int 2015, 75, 215–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


