Abstract
Despite a decline in overall incidence rates for cancer in the past decade, due in part to impressive advancements in both diagnosis and treatment, breast cancer (BC) remains the leading cause of cancer-related deaths in women. BC alone accounts for ∼30% of all new cancer diagnoses in women worldwide. Triple-negative BC (TNBC), defined as having no expression of the estrogen or progesterone receptors and no amplification of the HER2 receptor, is a subtype of BC that does not benefit from the use of estrogen receptor-targeting or HER2-targeting therapies. Differences in socioeconomic factors and cell intrinsic and extrinsic characteristics have been demonstrated in Black and White TNBC patient tumors. The emergence of patient-derived xenograft (PDX) models as a surrogate, translational, and functional representation of the patient with TNBC has led to the advances in drug discovery and testing of novel targeted approaches and combination therapies. However, current established TNBC PDX models fail to represent the diverse patient population and, most importantly, the specific ethnic patient populations that have higher rates of incidence and mortality. The primary aim of this review is to emphasize the importance of using clinically relevant translatable tumor models that reflect TNBC human tumor biology and heterogeneity in high-risk patient populations. The focus is to highlight the complexity of BC as it specifically relates to the management of TNBC in Black women. We discuss the importance of utilizing PDX models to study the extracellular matrix (ECM), and the distinct differences in ECM composition and biophysical properties in Black and White women. Finally, we demonstrate the crucial importance of PDX models toward novel drug discovery in this patient population.
Keywords: African ancestry, health disparities, extracellular matrix, metastasis, triple-negative breast cancer, patient-derived xenograft
Introduction
Breast cancer (BC) remains a global issue despite impressive advances in therapeutic strategies, accounting for 30% of all new cancer diagnoses in women.1 Overall, BC remains the most frequently diagnosed cancer worldwide and is the leading cause of cancer-related death.2 BC is broadly categorized into three subtypes based on receptors that are expressed or amplified.
Estrogen receptor (ER) and progesterone receptor (PR) positive and HER2/Neu receptor amplified subtypes can be treated with endocrine-targeted or HER2/Neu-targeted therapies, respectively. Triple negative BC (TNBC), a subtype that lacks ER and PR expression and HER2/Neu amplification, is clinically aggressive with high rates of metastasis, chemoresistance, recurrence, and develops in women at a younger age.3 TNBC mortality is more pronounced in patients of African and Hispanic ancestries, suggesting specific contributing factors within these patients cohorts contributes to overall survival and prognoses.4–8 Socioeconomic factors (low income and poor access to health care) in these patient cohorts significantly affects TNBC incidence and mortality outcomes in Black and Hispanic populations, more so than in non-Black and non-Hispanic populations, referenced as White populations throughout this review.
Preclinical and clinical studies have discovered inherent risk factors and oncogenic pathways upregulated in Black TNBC,8,9 providing evidence that cell intrinsic factors contribute to differences in TNBC presentation and drug response. Specific regional variations of TNBC mortality is also evident: mortality is highest in less developed countries such as Fiji, the Bahamas, Nigeria (and other Middle African countries), Macedonia, and Pakistan, whereas more developed countries such as North America, Mexico, and Eastern Asia have significantly lower rates.10 BC mortality rates in sub-Saharan Africa, especially Nigeria, are ranked the highest globally.11,12
Alarmingly, BC incidence rates have been rising in transitioning countries that had historically low rates; projections for 2035 indicate that less-developed regions will have an increase of new cancer cases by up to 144%, compared with 54% in more developed areas.13 In addition to TNBC mortality, overall metastatic BC and TNBC incidence rates also differ. More non-Hispanic Black patients were diagnosed with metastatic cancer compared with other ethnicities (8% and 5%–6%, respectively).14 With respect to TNBC incidence, multiple studies have identified higher frequency of TNBC in Black women compared with cohorts with White patients.15,16 Better representation of Black patients in TNBC drug discovery research is crucial to understanding the biology of this disease in the population it most affects.
Promising new targeted therapies and drug regimens in BC have emerged in recent years due to impressive advancements in target discovery and translational approaches to assess oncology drug effects. Preclinical model systems that accurately recapitulate human tumor biology to test oncology drug therapies facilitates direct translation of the findings into the clinical setting.17,18 Patient-derived xenograft (PDX) models are currently the most accurate models to mimic the microanatomy of human solid tumors in vivo (using implanted tumors) and in vitro (using patient-derived organoids [PDO] and tumor explants).
The use of PDX models in preclinical studies has vastly improved basic and clinical study outcomes in a variety of solid cancer types, including BC.19,20 Because most TNBC-related research and knowledge has been acquired from patients who self-identify as White, this research does not reflect the patient population that is most affected by TNBC: patients of African and Hispanic ancestry. In this study, we discuss the current use of PDX models in TNBC drug discovery research, highlight efforts from various institutions to develop PDX models that represent TNBC patients with diverse ethnicities, and the application of ethnically diverse PDX models to identify cell intrinsic and extrinsic signaling pathways unique to ethnic cohorts.
Use of PDX Models to Understand the Complexity of BC, Particularly for Ethnic Disparities
Before the emergence of PDX models, the standard research model for solid tumors was immortalized, established cell lines and orthotopic xenografts.21 Although these models provided invaluable knowledge regarding cancer biology and drug effects on cellular systems, they were limited in the inability to re-create patient-specific features of tumors. More specifically, these models cannot accurately reflect the tumor architecture, defined as the three-dimensional structure and alignment of tumor matrix, and surrounding stroma and cannot reproduce the cellular heterogeneity that is present in the original patient tumor.22–24 Passaging of immortalized cell lines that have been growing in cell culture for years results in the introduction of irreversible alterations in genetic information and behavioral characteristics that were not present in the original tumor.25
Using physiologically accurate models in cancer research is crucial to investigating previously unrecognized targets and mechanisms of neoplastic diseases. A translational model system that was introduced in cancer research a few years ago and is now extensively utilized in cancer research are PDX models. These models facilitate direct translation of laboratory discoveries and findings to clinical practice; the reverse is also true, in that these models facilitate translation of clinical observation into therapeutic discovery in the laboratory setting.20,26 PDX models are imperative in cancer research efforts as they allow for testing of drugs or drug combinations before testing in actual patients.27 PDX models are especially important when studying malignancies that exhibit a complex tumor heterogeneity, including TNBC tumors,28 both in cellular composition and cell extrinsic properties due to cancer-protective properties of the tumor microenvironment.28 TNBC tumors exhibit robust heterogeneity29–31 and each established PDX model provides an opportunity to better understand TNBC biologic properties.
The introduction of PDO, and organoids established from PDX tumors serially transplanted in murine models (PDX-O) in drug development focused research studies provides critical translational links between in vitro culture systems and in vivo observations.32 Organoid cultures preserve much of the complex and unique microenvironment as well as the cellular composition of individual patient tumors.32–34 These models are crucial because although tumor explants derived from patients are the most translational model, they cannot be indefinitely maintained in cell culture conditions for long-term experiments. Conversely, organoids preserve the microanatomy of the tumors and can be maintained in culture under “low-attachment” conditions. This is especially important when evaluating drug interactions with the various components that comprise a tumor, such as fibroblasts, extracellular protein composition and structure, tumor cells, and immune components.
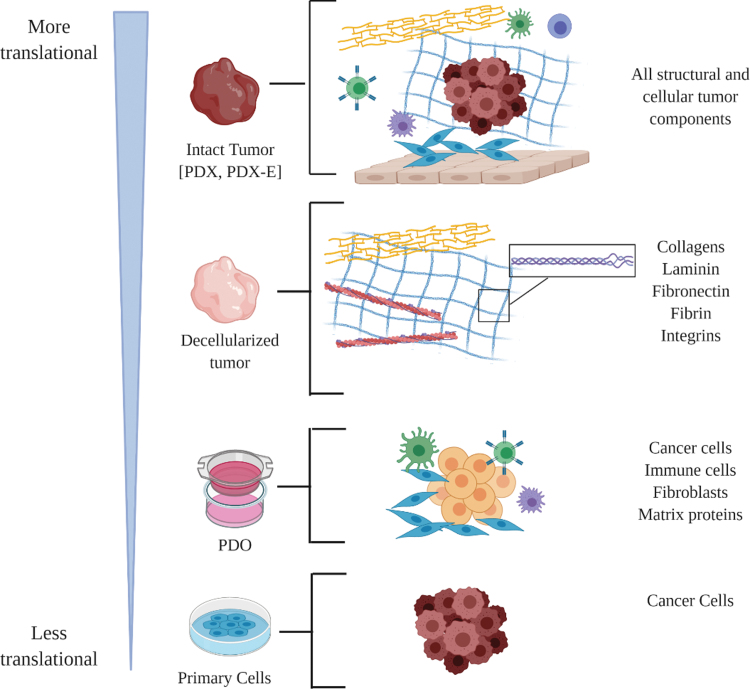
Intact tumor pieces can be treated in a dish, to mimic treating patient tumors in the clinical setting. However, this approach can only be used when sufficient amounts of PDX tumors are available. PDX-derived organoids can be generated from smaller pieces of tissue and expanded. PDX-Os and PDOs have become valuable translational tools to assess drug responses on a larger scale. Incorporating PDX-O and PDO models in cancer research has become an integral part of this era of discovering personalized therapies for individual patients.34 A schematic demonstrating how PDX models can be utilized to assess various aspects of TNBC biology is outlined in Figure 1.
FIG. 1.
Diverse applications of TNBC PDX models to study various aspects of TNBC biology. Based on the derivation of the PDX model used, different aspects of solid tumors can be analyzed. Examples of model derivations include primary cells isolated from the tumors, PDO that preserve various cell types from the primary tumor, the decellularized tumor to study drug effects on tumor structural components, and the intact tumor either ex vivo or in vivo (PDX-E). Schematic created with BioRender website. PDO, patient-derived organoids; PDX, patient-derived xenograft; PDX-E, PDX-explants; TNBC, triple-negative breast cancer.
Utilizing TNBC PDX Models to Identify Genes and Signaling Pathways in Ethnic Variants
Unique cell intrinsic molecular and gene signatures have been described in TNBC patients of African ancestry compared with other ethnicities.35–37 These findings support the hypothesis that ethnicity and TNBC-specific biomarkers exist for early cancer detection and therapeutic sensitivities.38,39 For example, using single-cell gene approaches, Azizi et al. observed significant differences in gene signatures of BC PDX samples derived from Black and White patients.40 These included EMT-associated genes (Vimentin, EpCAM, HER2, CDH1, CDH2, TGFβ1, cytokeratins, GATA3, MKI67), CSC markers (ALDH1a1, ALDH1a3, CD44, CD24, CD133), and other genes (YAP1, TM4SF1, TSPAN6, AMOTL2, STAP2, ANXA3). Another approach to identify specific gene or signaling pathways that can be targeted is taking advantage of environmental factors that have higher incidence in Black patients, including obesity.36,41 Obesity activates tissue inflammatory responses, activating cancer cell survival, proliferation, and metastasis,41 which introduces a plethora of potential targets in TNBC research.42–45 Because activation of this pathway has higher incidence in Black patients, agents targeting the obesity/inflammation axis can be a potential strategy to treat certain patients in this cohort. The introduction of PDX models into preclinical drug testing facilitated a translational tool to evaluate single and combination drug therapies in the laboratory setting to test these candidate targets, mimicking clinical trials.18
However, the implications of testing candidate targetable genes and signaling pathways in TNBC tumors representing patients of African ancestry in the clinical setting are limited by the number of PDX models available that represent Black and Hispanic patients.17,18,46 Of all BC subtypes, TNBC tumors are well represented in PDX models, as demonstrated by Dobrolecki et al. who found that 56% of all breast PDX models (506 patients total) represented TNBC patients.20 However, there remains a disparity in the overall representation and understanding of non-European patients among these models. There is an apparent under-representation of African and Black patients with TNBC, highlighting the need to focus upon this higher risk population.
Current therapeutic discovery-focused TNBC research does not adequately address the knowledge gap regarding ethnic disparity in TNBC incidence/mortality rates and biology. To date, most TNBC-related research and knowledge has been acquired from White patients, even though patients with African and Hispanic ancestries represent the majority of TNBC cases. Several institutions in the United States with established biobanks show similar findings with only a fraction of TNBC PDX models that represent Black patients.46 The PDXFinder and PDMR public databases were accessed to evaluate racial/ethnic patterns in available PDX models that represent TNBC patients. Out of the 16 total BC models in the PDMR database, 15 had self-reported race data and of those cases 3 represented Black patients and 12 represented White patients. Out of the four specified TNBC PDX models in the PDMR BC database, all were from self-reported White non-Hispanic patients who were European by inferred ancestry. The PDMR site also has 10 2D/3D BC cultures available, and 3 out of 10 models were from self-reported Black or African American patients.47 Out of the 104 invasive BC models in the PDXFinder site, 49 had race/ethnicity data available and of those models, 9 were Black patients and 20 were White. Because TNBC is often categorized as the basal-like BC subtype, we accessed basal-like BC samples in the PDXFinder database. Out of the 11 uploaded cases, 5 patients were Black and 6 were White. It is important to note that not all TNBC PDX models from institutions are uploaded to the public databases, and many of the uploaded model information does not specify race/ethnicity of some patients. Furthermore, uploaded PDX models were not characterized by TNBC subtypes, but rather “invasive” or “basal-like.” However, these findings demonstrate an overall disparity in the number of invasive or basal-like BC models representing Black patients. To acquire a more comprehensive understanding of TNBC biology and to evaluate the efficacy of novel therapeutic strategies, we emphasize the importance and necessity of incorporating cohorts of Black patients and specifically patients with African ancestry in TNBC research. Part of addressing this need is to continue to develop a comprehensive and high-content network of tissue specimens from Black TNBC patients. Importantly, these models must be shared among intrainstitutional laboratories, as well as among different institutions, in a collaborative effort. Incorporation of academic institution's research strengths is necessary to address the common goal of characterizing unique gene signatures and the tumor biology of TNBC. We have an important opportunity to pool resources and utilize samples from cancer centers across the United States to explore novel therapeutic options and discover novel targets that are more representative of the population from which the majority of TNBC-afflicted cases are found.
Notably, several groups throughout the United States that are attempting to address this knowledge gap. In 2017, the University of Michigan initiated efforts to establish PDX tumors representative of TNBC patients with African ancestry.38,46 Recently, they have expanded their registry to include the Sisters Network in Houston, TX, to recruit more BC patients, with a long-term goal to study germline BC risk in women with diverse racial and ethnic backgrounds in the United States.48 At the University of Illinois, Chicago (UIC), there exist similar efforts.49 In the Southern United States, MD Anderson Cancer Centers (MDACC), a total of 46/49 of their BC models collected represented patients with TNBC with 19 of these samples procured from Black patients. These programs, in addition to other groups not mentioned, have demonstrated impressive advancements in addressing the limited number of available TNBC PDX models.
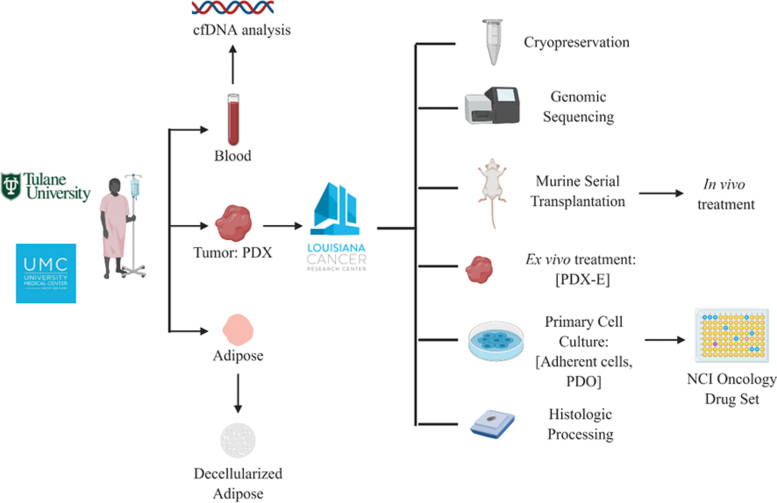
However, to draw more accurate conclusions of ethnic variation and to distinguish ethnic variation from interindividual heterogeneity, hundreds of more representative models are required. Importantly, there exists a paucity of TNBC PDXs that represent Black patients in locations that have high populations of this patient cohort. For example, New Orleans has one of the highest incidence rates of TNBC in the United States, claiming the highest rates in 2016.50 By further developing TNBC PDX models from patients in these under-represented communities, we can further address how to optimize treatment regimens for patients representing a variety of ethnicities in TNBC biology (Fig. 2).
FIG. 2.
Schematic demonstrating how TNBC PDX tumors that represent Black patients are established and developed from the hospital to laboratory settings. Furthermore, examples of how to potentially use various iterations of PDX tumors to assess ethnic variations of TNBC biology. The NCI oncology drug set contains clinically approved systemic and targeted drugs to test. Schematic created with BioRender website. NCI, National Cancer Institute.
Application of TNBC PDX Models to Address Drug Resistance
Overall, BC (all subtypes) derived from Black patients exhibit a higher degree of intratumoral genetic heterogeneity and more basal-like gene expressions.51 Acquisition of drug resistance is one of the defining features of TNBC.52 Apoptosis resistance is another contributing factor to BC mortality; important differences in downstream apoptosis regulating genes has been found to be differentially upregulated in tumors representing Black patients compared with respective White patient cohorts.9 Although TNBC tumors have a high initial response to chemotherapy, patients with residual disease after completing neoadjuvant chemotherapy have a worse prognosis compared with non-TNBC patients.52 Owing to the higher incidence of TNBC in Black populations, and the fact that TNBC tumors have high rates of drug resistance, it is warranted to further investigate the association between African ancestry and acquisition of drug resistance in this tumor type, using translational PDX models.
One potential application of PDX models in TNBC research is to compare treatment-naive tumors with matched post-treatment tumors. These paired tumor tissues are quite valuable, providing insight into the complex changes that occur at the tumor level. Using human tumors from the same patient pre- and post-BC treatment facilitates interrogation of not only cell intrinsic but also cell extrinsic pathways that have been altered in response to drug exposure. Because the extracellular matrix (ECM) of tumors changes in response to drug exposure,53–57 investigating how these changes drive drug resistance is important to interrogate, and using human tumors that represent individual tumor's ECM is crucial to these discoveries. There is also value in studying TNBC tumors from patients who have undergone a full course of neoadjuvant chemotherapy, only to have a minimal response to treatment with minimal tumor regression or shrinkage of the primary tumor. These experiments examine possible mechanisms of drug resistance in these patients with TNBC, further highlighted by our ability to compare, in some instances, the specific alterations in gene expression after neoadjuvant chemotherapy.
Using PDX Models to Understand the Role of the ECM in TNBC Biology of Tumors That Represent Black Patients
TNBC cells depend on the tumor microenvironment, defined as the ECM and other cellular components (immune modulatory cells and fibroblasts) surrounding the cancer cells within a tumor, for survival and tumor progression.48,49,58 Comprehensive dissection and examination of the intact tumor in the laboratory setting is crucial to examine the interplay of complex cell–cell and cell–stromal interactions.59 PDX models are one of the most accurate systems, to date, for assessing the tumor's unique matrix composition and cellular interactions within the ECM.60,61 Although cancer-on-a-chip models can be used to study distinct cell–stroma–microenvironment interactions, they do not truly reflect the intact original patient tumor.62 Furthermore, treatment of PDX models provides more accurate data when compared with cell-line-derived xenografts, cell line-based experiments, and other ex vivo models.63 Targeting the ECM is emerging as a novel therapeutic approach in invasive cancers and is especially important in cancer subtypes that do not have commonly targetable receptors, such as with TNBC.64–66 Schedin and Borges discovered important links between breast tissue development and the tumor microenvironment.67–69 They found that breast tissue involution after lactation resulted in tissue inflammation and wound healing pathways that deposited high-risk crosslinked fibrillar collagen, which has been associated with poor survival in European American women with invasive BC.69,70 These studies demonstrate the importance of the ECM in breast development and provides support for additional research into these interactions to improve the current understanding of the ECM's role in regulating BC development.
Differences in the tumor microenvironment composition, specifically pertaining to the ECM, exist in Black compared with White women.71,72 For example, mouse mammary glands humanized fibroblasts derived from premenopausal White patients compared with mammary glands humanized with fibroblasts harvested from Black patients resulted in differential expression of ECM-regulated gene pathways.72 Aside from ECM-regulated pathways, other microenvironment components tested (tumorigenicity, metastatic behavior, and protease activity) differed in the two tested groups.73 Another study found biologic processes related to chemotaxis, angiogenesis, endoplasmic reticulum function, and cell cycle control were differentially expressed based on race and ethnicity in BCs.73 Microvessel density and macrophage infiltration were higher in self-identified AA tumors,73 further supporting that ethnic variation contributes to distinct changes in the tumor microenvironment.
Another important feature of tumors that can be studied using PDX models is the three-dimensional tissue architecture, as it is unique to individual patients. The tissue architecture, specifically the alignment/orientation biophysical and structural properties of tumors as well as the composition of the ECM fibers within the tumor, has a powerful and influential role in facilitating tumor growth rates and their propensity to metastasize.74–76 The mechanics of the heterogeneous matrix directs tumor progression and cell interactions.77 In cancer subtypes with heterogeneous cellular components and clinical presentations, such as TNBC, studying the ECM in-depth is critical for the discovery of novel therapeutic strategies. Distinct differences in ECM alignment and organization have been demonstrated in skin of Black and White patients, suggesting unique properties of the ECM structure in relation to patients of different ethnic backgrounds. Disruption of the elastic fiber arrangement and reduced collagen organization were detrimental to the biochemical properties in aged skin of Black persons.78 In addition, biomechanical behaviors have also demonstrated the ability to predict tumor cell invasiveness and metastatic potential. From this, it may be inferred that collagen and elastic fiber organization are important in cancer development, with composition of these tumor microenvironment aspects differing between Black and White populations. Using PDX models, specifically whole and intact PDX tumors from primary tissue specimens, preserves the unique ECM fiber alignment and mechanosensing biomechanical properties within human breast tumors.
Overall, ethnic variations may exist in these cell extrinsic tumor characteristics, but the current models utilized to study these tumor properties utilize synthetic or artificial three-dimensional matrix platforms, which do not mimic native breast tissue.79 Furthermore, employing the novel technique of tissue decellularization, or removing the cellular background of tumors while preserving the ECM, facilitates examination of tumor architecture and ECM integrity in patient tumors representing various patient ethnicities.80 Applying PDX models in these settings is integral, as it represents the only model in which the true representation of individual patients' ECM architecture and composition can be preserved in the laboratory setting.
Distinguishing Interindividual from Ethnic Variability in PDX-Based Oncologic Research
The utilization of PDX models in oncologic drug discovery research introduced possible confounding factors into studies evaluating the role of ethnicity in tumor biology. Because PDX models accurately represent features of human tumors in the laboratory setting, these models also maintain the features of tumors that are unique to individuals. This presents the possibility that data demonstrating unique expression of gene/molecular/protein expression found in patients representing diverse ethnicities are due to interindividual variation, and not ethnic variation. Distinguishing between interindividual and ethnic variation is important in oncology research, especially in testing drug response and pharmacokinetic studies.81–85 Examining biomarker expression in larger cohorts of patients, or using meta-analyses, can help reduce the risk of this confounding effect.81,86 As one study concluded, thousands of samples are required to accurately contribute gene lists for predicting outcome in cancer.87 Inadequate estimation of interindividual variation leads to inclusion of nonstatistically significant genes.88
Research that identifies “gene signatures” in ethnic cohorts and molecular tumor subtypes examine large numbers of representative tumors to reduce the risk of the findings being contributed to interindividual variation. Population studies not only uncover interindividual heterogeneity but also reveal gene signatures that are longitudinally stable within individuals.89 Attempts through computational analyses and algorithms have been made to address the role of interindividual variation in population studies.84,86–88 We propose that these analyses must be applied in studies examining ethnic variation in TNBC. Given the limitation of TNBC PDX models that represent ethnic patients, more established models are required to draw accurate conclusions with respect to ethnic variation in TNBC.
Conclusions
Overall, TNBC has higher rates of mortality and poorer prognoses in Black patients, specifically patients of African ancestries. The purpose of this review is to discuss the diverse applications of PDX models in TNBC biology research to identify ethnic variations in this disease. There have been impressive efforts to address the limited number of established TNBC PDX models that represent Black patients to more comprehensively understand TNBC biology by studying patients who are most afflicted by the disease. However, additional models are required to identify biomarkers, gene and molecular signatures, and biophysical properties of TNBC tumors that are unique to patients who represent specific ethnicities. Having a large database of samples is crucial to assess which candidate gene and signaling pathways are due to ethnic variation, and not due to interindividual variation. These efforts will lead to multiple areas of drug discovery and novel therapeutic approaches, providing valuable insight and understanding of the genomic and phenotypic differences for this difficult-to-treat population.
Acknowledgments
Our collaborative project would not have been possible without the support and efforts put forth by the Louisiana Cancer Center Biospecimen Core in procuring the PDX specimens. Thank you to Dr. Yvonne Edvard, PhD, from the National Cancer Institute Patient-Derived Models Repository for providing information regarding current representation of racial disparities in TNBC PDX models. Furthermore, we thank Krewe de Pink, an organization of breast cancer survivors, their families, and community members based in New Orleans who are devoted to supporting local breast cancer research. We also thank the patients who donate their breast cancer tissue.
Abbreviations Used:
- BC
breast cancer
- ECM
extracellular matrix
- ER
estrogen receptor
- MDACC
MD Anderson Cancer Centers
- PDO
patient-derived organoids
- PDX
patient-derived xenograft
- TME
tumor microenvironment
- TNBC
triple-negative breast cancer
- UIC
University of Illinois, Chicago
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This project received funding from the National Institutes of Health 1R01CA174785-01A1 (B.M.C.-B.) and the National Institute of General Medical Sciences of the National Institute of Health, which funds the Louisiana Clinical & Translational Science Center 1-U54-GM104940 (B.A.B.).
Cite this article as: Matossian MD, Giardina AA, Wright MK, Elliott S, Loch MM, Nguyen K, Zea AH, Lau FH, Moroz K, Riker AI, Jones SD, Martin EC, Bunnell BA, Miele L, Collins-Burow BM, Burow ME (2020) Patient-derived xenografts as an innovative surrogate tumor model for the investigation of health disparities in triple negative breast cancer, Women's Health Report 1:1, 383–392, DOI: 10.1089/whr.2020.0037.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2019. CA Cancer J Clin 2019;69:7–34 [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin 2018;68:394–424 [DOI] [PubMed] [Google Scholar]
- 3. Anders C, Carey LA. Understanding and treating triple-negative breast cancer. Oncology 2008;22:1233–1243 [PMC free article] [PubMed] [Google Scholar]
- 4. Brewster AM, Chavez-MacGregor M, Brown P. Epidemiology, biology, and treatment of triple-negative breast cancer in women of African ancestry. Lancet Oncol 2014;15:e625-e634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pacheco JM, Gao F, Bumb C, Ellis MJ, Ma CX. Racial differences in outcomes in triple-negative breast cancer. Breast Cancer Res Treat 2013;138:281–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yedjou CG, Tchounwou PB, Payton M, Miele L, Fonesca DD, Lowe L, et al. . Assessing the racial and ethnic disparities in breast cancer mortality in the United States. Int J Environ Res Public Health 2017;14: 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women. JAMA Surg 2017;152:485–493 [DOI] [PubMed] [Google Scholar]
- 8. Newman LA, Stark A, Chitale D, et al. . Association between benign breast disease in African American and White American women and subsequent triple-negative breast cancer. JAMA Oncol 2017;3:1102–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Siddharth S, Sharma D. Racial disparity and triple-negative breast cancer in African-American women: A multifaceted affair between obesity, biology, and socioeconomic determinants. Cancers (Basel) 2017;10:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghoncheh M, Pournamda Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev 2016;9:43–46 [DOI] [PubMed] [Google Scholar]
- 11. Parkin DM, Bray F, Ferlay J, Jemal A. Cancer in Africa 2012. Cancer Epidemiol Biomarkers Prev 2014;23:953–966 [DOI] [PubMed] [Google Scholar]
- 12. Azubuike SO, Muirhead C, Hayes L, McNally R. Rising global burden of breast cancer: The case of sub-Saharan Africa (with emphasis on Nigeria) and implications for regional development: A review. World J Surg Oncol 2018;16:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pilleron S, Sarfati D, Janssen-Heijnen M, et al. . Global cancer incidence in older adults, 2012 and 2035: A population-based study. Int J Cancer 2019;144:49–58 [DOI] [PubMed] [Google Scholar]
- 14. DeSantis CE, Ma J, Jemal A. Trends in stage at diagnosis for young breast cancer patients in the United States. Breast Cancer Res Treat 2019;173:743–747 [DOI] [PubMed] [Google Scholar]
- 15. Plasilova MI, Hayse B, Killelea BK, Horowitz NR, Chagpar AB Lannin DR. Features of triple-negative breast cancer. Medicine 2016;95:e4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sturtz LA, Melley J, Mamula K, Shriver CD, Ellsworth RE. Outcome disparities in African American women with triple negative breast cancer: A comparison of epidemiological and molecular factors between African American and Caucasian women with triple negative breast cancer. BMC Cancer 2014;14:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ter Brugge P, Kristel P, van der Burg E, et al. . Mechanisms of therapy resistance in patient-derived xenograft models of BRCA1-deficient breast cancer. J Natl Cancer Inst 2016;108. [DOI] [PubMed] [Google Scholar]
- 18. Koga Y, Ochiai A. Systematic review of patient-derived xenograft models for preclinical studies of anti-cancer drugs in solid tumors. Cells 2019;8:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murayama T, Gotoh N. Patient-derived xenograft models of breast cancer and their application. Cells 2019;8:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dobrolecki LE, Airhart SD, Alferez DG, et al. . Patient-derived xenograft (PDX) models in basic and translational breast cancer research. Cancer Metastasis Rev 2016;35:547–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boehm JS, Hahn WC. Immortalized cells as experimental models to study cancer. Cytotechnology 2004;45:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burdall SE, Hanby AM, Lansdown MRJ, Speirs V. Breast cancer cell lines: Friend or foe? Breast Cancer Res 2003;5:89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cifani P, Kirik U, Waldemarson S, James P. Molecular portrait of breast-cancer-derived cell lines reveals poor similarity with tumors. J Proteome Res 2015;14:2819–2827 [DOI] [PubMed] [Google Scholar]
- 24. Manning HC, Buck JR, Cook RS. Mouse models of breast cancer: Platforms for discovering precision imaging diagnostics and future cancer medicine. J Nuclear Med 2016;57(S1):60S–68S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gillet JP, Clacagno AM, Varma S, et al. . Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance. Proc Natl Acad Sci U S A 2011;108:18709–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hidalgo M, Amant F, Biankin AV, et al. . Patient-derived xenograft models: An emerging platform for translational cancer research. Cancer Discov 2016;4:998–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deshmukh SK, Azim S, Ahmad A, et al. . Biological basis of cancer health disparities: Resources and challenges for research. Am J Cancer Res 2017;7:1–12 [PMC free article] [PubMed] [Google Scholar]
- 28. Sulaiman A, Wang L. Bridging the divide: Preclinical research discrepancies between triple-negative breast cancer cell lines and patient tumors. Oncotarget 2017;8:113269–113281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elkhalifa D, Alali F, Al Moustafa AE, Khalil A. Targeting triple negative breast cancer heterogeneity with chalcones: A molecular insight. J Drug Target 2019;27:0–838 [DOI] [PubMed] [Google Scholar]
- 30. Bareche Y, Venet D, Ignatiadis M, et al. . Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann Oncol 2018;29:895–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mills MN, Yang GQ, Oliver DE, et al. . Histologic heterogeneity of triple negative breast cancer: A National Cancer Centre Database analysis. Eur J Cancer 2018;98:48–58 [DOI] [PubMed] [Google Scholar]
- 32. Sasmita AO, Wong YP. Organoids as reliable breast cancer study models: An update. Int J Oncol Res 2018. [Epub ahead of print]; DOI: 10.23937/IJOR-2017/1710008 [DOI]
- 33. Chonghaile TN. Patient-derived organoids: Are PDOs the new PDX? Sci Transl Med 2018;10:eaau7377 [Google Scholar]
- 34. Yang H, Sun L, Liu M, Mao Y. Patient-derived organoids: A promising model for personalized cancer treatment. Gastroenterol Rep 2018;6:243–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. You W, Symonds I, Ruhli FJ, Henneberg M. Decreasing birth rate determining worldwide incidence and regional variation of female breast cancer. Adv Breast Cancer Res 2018;7:1–14 [Google Scholar]
- 36. Dietze EC, Sistrunk C, Miranda-Carboni G, O'Regan R, Seewaldt VL. Triple-negative breast cancer in African-American women: Disparities versus biology. Nat Rev Cancer 2015;15:248–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lindner R, Sullivan C, Offor O, et al. . Molecular phenotypes in triple negative breast cancer from African-American patients suggest targets for therapy. PLoS One 2013;8:e71915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jiagge E, Bensenhaver J, Celina K, et al. . Creating models to identify new therapeutic options for aggressive African breast cancers. J Global Oncol 2018. [Epub ahead of print] DOI: 10.1200/jgo.18.83500 [DOI]
- 39. Field LA, Love B, Deyarmin B, Hooke JA, Shriver CD, Ellsworth RE. Identification of differentially expressed genes in breast tumors from African American women compared to Caucasian women. Cancer 2012;118:1334–1344 [DOI] [PubMed] [Google Scholar]
- 40. Azizi E, Jiagge EM, Fouladdel S, et al. (April 18–22, 2015; Philadelphia, PA) Single cell multiplex gene expression analysis to unravel heterogeneity of PDX samples established from tumors of breast cancer patients with different ethnicity. In: Proceedings of the 106th Annual Meeting of the American Association for Cancer Research. Vol. 75 April 18–22, 2015. Philadelphia, PA: Cancer Research [Google Scholar]
- 41. Abraham PA, Kazman JB, Zeno SA, Deuster PA. Obesity and African Americans: Physiologic and behavioral pathways. ISEN Obes 2013;2013:314295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Teslow EA, Mitrea C, Bao B, et al. . Obesity-induced MBD2_v2 expression promotes tumor-initiating triple-negative breast cancer stem cells. Mol Oncol 2019;13:894–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sabol RA, Bowles AC, Cote A, et al. . Leptin produced by obesity-altered adipose stem cells promotes metastasis but not tumorigenesis of triple-negative breast cancer in orthotopic xenograft and patient-derived xenograft models. Breast Cancer Res 2019;21:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dietze EC, Chavez TA, Seewaldt VL. Obesity and triple-negative breast cancer: Disparities, controversies, and biology. Am J Pathol 2018;188:280–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun H, Zou J, Chen L, Zu X, Wen G, Zhong J. Triple-negative breast cancer and its association with obesity. Mol Clin Oncol 2017;7:935–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Luther TK, Jiagge E, Lewis MT, et al. . Creating a comprehensive patient-derived xenograft (PDX) bank to represent racial disparities in triple-negative breast cancer (TNBC). 2015. In: Proceedings of the Seventh AACR Conference on The Science of Health Disparities in Racial/Ethnic Minorities and the Medically Underserved, Nov 9–12, 2014. Philadelphia, PA: AACR [Google Scholar]
- 47. The NCI Patient-Derived Models Repository (PDMR), NCI-Frederick, Frederick National Laboratory for Cancer Research, Frederick, MD. Available at: https://pdmr.cancer.gov Accessed June23, 2020
- 48. Jiagge E, Oppong JK, Bensenhaver J, et al. . Breast Cancer and African Ancestry: Lessons learned at the 10-year anniversary of the Ghana-Michigan Research Partnership and International Breast Registry. J Global Oncol 2019;2:302–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhao H, Pham T, Emmadi R, et al. . Abstract B19: Breast cancer patient-derived xenografts: The University of Illinois at Chicago Cancer Center experience. Clin Cancer Res 2016;22 [Epub ahead of print]; DOI: 10.1158/1557-3265.PDX16-B19 [DOI] [Google Scholar]
- 50. Loch MM, Li X, Hsieh M-C, Chen VW, Wu X-C. New Orleans has the highest incidence rates of triple negative breast cancer. In: Proceedings of the Thirty-Eighth Annual CTRC-AACR San Antonio Breast Cancer Symposium [Abstract] 2015. Cancer Res 2016;76(4 Suppl):Abstract nr P1-09-04. [Google Scholar]
- 51. Keenan T, Moy B, Mroz EA, et al. . Comparison of the genomic landscape between primary breast cancer in African American versus white women and the association of racial differences with tumor recurrence. J Clin Oncol 2015;33:3621–3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Carey LA, Dees EC, Sawyer L, et al. . The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007;13:2329–2334 [DOI] [PubMed] [Google Scholar]
- 53. Lovitt CJ, Shelper TB, Avery VM. Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins. BMC Cancer 2018;18: [Epub ahead of print]; DOI: 10.1186/s12885-017-3953-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brown Y, Hua S, Tanwar PS. Extracellular matrix-mediated regulation of cancer stem cells and chemoresistance. Int J Biochem Cell Biol 2019;109:90–104 [DOI] [PubMed] [Google Scholar]
- 55. Morin PJ. Drug resistance and the microenvironment: Nature and nurture. Drug Resist Updat 2003;6:169–172 [DOI] [PubMed] [Google Scholar]
- 56. Oskarsson T. Extracellular matrix components in breast cancer progression and metastasis. Breast 2013;22:S66–S72 [DOI] [PubMed] [Google Scholar]
- 57. Elliott T, Sethi T. Integrins and extracellular matrix: A novel mechanism of multidrug resistance. Expert Rev Anticancer Ther 2002;4:449–459 [DOI] [PubMed] [Google Scholar]
- 58. Yuan Y, Jian Y-C, Sun C-K, Chen Q-M. Role of the tumor microenvironment in tumor progression and the clinical applications (Review). Oncol Rep 2016;35:2499–2515 [DOI] [PubMed] [Google Scholar]
- 59. Eftekhari R, Esmaeili R, Mirzaei R, et al. . Study of the tumor microenvironment during breast cancer progression. Cancer Cell Int 2017;17:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lindner D. Animal models and the tumor microenvironment: Studies of tumor-host symbiosis. Semin Oncol 2014;41:146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yamaguchi R, Perkins G. Animal models for studying tumor microenvironment (TME) abd resistance to lymphocytic infiltration. Cancer Biol Ther 2018;9:745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sleeboom JJF, Amirabadi HE, Nair P, Sahlgren CM, den Toonder JMJ. Metastasis in context: Modeling the tumor microenvironment with cancer-on-a-chip approaches. Dis Models Mech 2018;11: dmm033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shafi AA, Schiewer MJ, de Leeuw R, et al. . Patient-derived models reveal impact of the tumor microenvironment on therapeutic response. Eur Urol Oncol 2018;1:325–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lampi MC, Reinhart-King CA. Targeting extracellular matrix stiffness to attenuate disease: From molecular mechanisms to clinical trials. Science 2018;10:eaao0475. [DOI] [PubMed] [Google Scholar]
- 65. Venning FA, Wullkopf L, Erler JT. Targeting ECM disrupts cancer progression. Front Oncol 2015;5:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kaushik S, Pickup MW, Weaver VM. From transformation to metastasis: Deconstructing the extracellular matrix in breast cancer. Cancer Metastasis Rev 2016;35:655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Harisi R, Jeney A. Extracellular matrix as target for antitumor therapy. Onco Targets Ther 2015;8:1387–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schedin P, Borges V. Breaking down barriers: The importance of the stromal microenvironment in acquiring invasiveness in young women's breast cancer. Breast Cancer Res 2009;11:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lyons TR, O'Brien J, Borges VF, et al. . Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med 2011;17:1109–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Conklin MW, Eickhoff JC, Riching KM, et al. . Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol 2011;178:1221–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Deshmukh SK, Srivastava SK, Tyagi N, et al. . Emerging evidence for the role of differential tumor microenvironment in breast cancer racial disparity: A closer look at the surroundings. Carcinogenesis 2017;38:757–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fleming JM, Miller TC, Quinones M, et al. . The normal breast microenvironment of premenopausal women differentially influences the behavior of breast cancer cells in vitro and in vivo. BMC Med 2010;8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Martin DN, Boersma BJ, Yi M, et al. . Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS One 2009;4:e4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Han W, Chen S, Yuan W, et al. . Oriented collagen fibers direct tumor cell intravasation. Proc Natl Acad Sci U S A 2016;113:11208–11213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Carey SP, Martin KE, Reinhart-King CA. Three-dimensional collagen matrix induces a mechanosensitive invasive epithelial phenotype. Sci Rep 2017;7:42088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ford AJ, Rajagopalan P. Extracellular matrix remodeling in 3D: Implications in tissue homeostasis and disease progression. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2018;10:e1503. [DOI] [PubMed] [Google Scholar]
- 77. Malandrino A, Mak M, Kamm RD, Moeendarbary E. Complex mechanics of the heterogeneous extracellular matrix in cancer. Extreme Mech Lett 2018;21:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Langton AK, Alessi S, Hann M, et al. . Aging in skin of color: disruption to elastic fiber organization is detrimental to skin's biomechanical function. J Investig Dermatol 2019;139:779–788 [DOI] [PubMed] [Google Scholar]
- 79. Belgodere JA, King CT, Bursavich JB, Burow ME, Martin EC, Jung JP. Engineering breast cancer microenvironments and 3D bioprinting. Front Bioeng Biotechnol 2018;6:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Matossian MD, Burks HE, Elliott S, et al. . Panobinostat suppresses the mesenchymal phenotype in a novel claudin-low triple negative patient-derived breast cancer model. Oncoscience 2018;5:99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Liu X, Fiocco M, Swen JJ, Guchelaar H-J. Assessment of ethnic differences in sunitinib outcome between Caucasian and Asian patients with metastatic renal cell carcinoma: A meta-analysis. Acta Oncol 2017;56:582–589 [DOI] [PubMed] [Google Scholar]
- 82. Phan VH, Moore MM, McLachlan AJ, Piquette-Miller M, Xu H, Clarke SJ. Ethnic differences in drug metabolism and toxicity from chemotherapy. Expert Opinion Drug Metab Toxicol 2009;5:243–257 [DOI] [PubMed] [Google Scholar]
- 83. Petain A, Zhong D, Chen X, et al. . Effect of ethnicity on vinorelbine pharmacokinetics: A population pharmacokinetics analysis. Cancer Chemother Pharmacol 2019;84:373–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ning J, Rietjens IMCM, Strikwold M. Integrating physiologically based kinetic (PBK) and Monte Carlo modelling to predict inter-individual and inter-ethnic variation in bioactivation and liver toxicity of lasiocarpine. Arch Toxicol 2019;93:2943–2960 [DOI] [PubMed] [Google Scholar]
- 85. Touma JA, McLachlan AJ, Gross AS. The role of ethnicity in personalized dosing of small molecule tyrosine kinase inhibitors used in oncology. Transl Cancer Res 2017;6:10 [Google Scholar]
- 86. Chen J, Wang Y, Shen B, Zhang D. Molecular signatures of cancer at gene level or pathway level? Case studies of colorectal cancer and prostate cancer microarray data. Comput Math Models Med 2013; [Epub ahead of primt]; DOI: 10.1155/2013/909525 [DOI] [PMC free article] [PubMed]
- 87. Ein-Dor L, Zuk O, Domany E. Thousands of samples are needed to generate a robust gene list for predicting outcome in cancer. PNAS 2006;103:5923–5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cheng W-C, Shu W-Y, Li C-Y, et al. . Intra- and inter-individual variance of gene expression in clinical studies. PLoS One 2012. [Epub ahead of primt]; DOI: 10.1371/journal.pone.0038650 [DOI] [PMC free article] [PubMed]
- 89. Scheid AD, Van Keulen VP, Felts SJ, et al. . Gene expression signatures characterized by longitudinal stability and interindividual variability delineate baseline phenotypic groups with distinct responses to immune stimulation. J Immunol 2018;200:1917–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]