Idiopathic pulmonary fibrosis (IPF) is the prototype of a large and heterogeneous group of chronic progressive pulmonary disorders. No treatment is able to halt or reverse pulmonary fibrosis, although pirfenidone (Pirf) and nintedanib (Nint) have been shown to slow lung function decline and disease progression but with significant side effects. We evaluated the safety and efficiency of GED-0507, an original selective peroxisome proliferator-activated receptor (PPAR)-γ modulator, in a murine model of bleomycin (BLM)-induced pulmonary fibrosis compared to those of Pirf and Nint. Preventive and curative administration of GED-0507 improved the loss of body weight and extension of lung fibrotic lesions. When given as a preventive treatment, GED-0507 also abrogated the mortality rate and histological fibrosis score, as well as the lung levels of hydroxyproline.
IPF is an aggressive disorder that is characterized by inexorable scarring of the lung parenchyma and loss of pulmonary function. With more than 100,000 individuals affected, 30,000–40,000 new cases are diagnosed each year in the United States alone, and with an average life expectancy of 3–5 years after diagnosis, IPF represents a substantial economic burden on global health care.1 Therapeutic approaches to IPF have evolved considerably over the last two decades, and in 2015, the ATS/ERS/JRS/ALAT guidelines made a conditional (i.e., weak) recommendation for the use of two drugs, pirfenidone (Pirf) and nintedanib (Nint).2 These two drugs are similarly efficacious in slowing the functional decline and disease progression in IPF; however, their safety and tolerability profile requires careful patient education and attention to potential side effects.3 In this study, we explored the safety and efficacy of the original PPARγ modulator GED-0507-34-Levo (GED-0507) in halting or even reversing pulmonary fibrosis in mice.
Drugs that target PPARs have been the subject of intense debate for several years, as their use has been associated with the development of numerous and significant side effects. However, due to their ability to bind to different chemical entities and their key role in organ homeostasis and control of inflammatory and fibrotic processes,4 PPARγ remains an important target for the development of more efficacious therapeutic approaches for a number of conditions.
The pathobiological mechanisms of pulmonary fibrosis remain poorly understood, despite favored concepts involving recurrent subclinical injuries to a genetically predisposed alveolar epithelium, followed by the failure of alveolar re-epithelialization and repair.5 Hence, the injured alveoli release a plethora of cytokines and growth factors, which induce the recruitment, proliferation, and differentiation of alveolar epithelial cells and lung fibroblasts into myofibroblasts, which are αSMA-positive cells that are able to produce large amounts of collagen. PPARγ is highly expressed and functional in alveolar epithelial cells and lung fibroblasts and is one of the main antagonists of the profibrotic TGFβ pathway.
We propose a novel therapeutic approach to lung fibrosis based on the selective PPARγ modulator (SPPARM) GED-0507 to circumvent the potential adverse effects, which are often related to the chemical structure of PPARγ agonists. GED-0507 is an aminophenyl-methoxy-propionic acid with powerful anti-inflammatory and antifibrotic properties, reduced adverse effects and limited interference in lipid and glucose metabolism based on 5-aminosalicylic acid computational chemistry-based studies.6,7 GED-0507 did not show any side effects even at very high and repeated concentrations in toxicological studies that were performed in animals (i.e., rats, rabbits, and dogs) and was well tolerated in previous phase 1 and 2 clinical studies in patients with inflammatory bowel disease.8 Mechanisms that account for the antifibrotic activities of GED-0507 include counteracting the profibrogenic effect of the TGFβ and Smad 3 pathway components, inhibiting the Snail1 and Zeb1 E-box binding repressors and extracellular matrix (ECM)-related genes, inhibiting collagen and fibronectin synthesis, reducing expression of profibrotic molecules such as IL-13 and connective tissue growth factor (CTGF) and modulating the epithelial/endothelial-to-mesenchymal transition.4,9
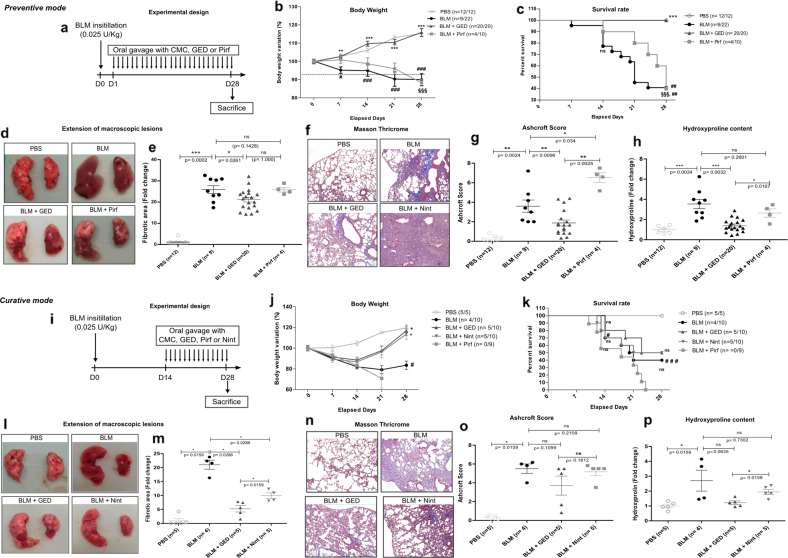
In the present study, GED-0507 was administered as preventive and curative treatments in a mouse model of bleomycin (BLM)-induced pulmonary fibrosis. Among the numerous animal models of pulmonary fibrosis, the murine intratracheal BLM model remains the best characterized animal model for preclinical testing. BLM was administered as a single intratracheal instillation at a dose of 0.8 mg/kg (0.025 U/kg). Optimal concentrations of GED-0507 (100 mg/kg/day), Pirf (400 mg/kg/day), and Nint (60 mg/kg/day) were administered daily by oral gavage both as preventive and curative treatments (Fig. 1a, i). Preventive administration of GED-0507 abolished weight loss, and the 60% mortality observed 3 weeks after BLM administration, whereas Pirf did not improve the survival rate or weight loss (Fig. 1b, c). Consistent with the body weight loss and high mortality, BLM-treated mice showed an increased area of macroscopic pulmonary lesions. These lesions were quantified using web-based ImageJ software. Briefly, after converting color images into grayscale images, macroscopic lesions were characterized as dark, dense, and translucent zones, quantified for each sample and compared by a threshold method to the total area of both lungs (Fig. 1d, l). When administered as a preventive treatment, GED-0507 significantly reduced the extension of fibrotic lesions by 18% compared to that of BLM-treated mice (Fig. 1e). Histological assessment and quantification of lung fibrosis were performed by Masson’s trichrome staining to determine the Ashcroft score (Fig. 1f, n)10 and by using a colorimetric hydroxyproline kit (Sigma-Aldrich) to determine collagen accumulation. When given as a preventive treatment, GED-0507 decreased the Ashcroft score and lung hydroxyproline content by 47% and 57%, respectively, in mice with BLM-induced lung fibrosis. As a curative treatment, GED-0507 reduced the loss of body weight and extension of the fibrotic lesions, similar to what was observed in BLM-treated mice that were treated with Nint (Fig. 1j, l). In BLM-treated mice, the mortality rate (Fig. 1k), Ashcroft score (Fig. 1o), and hydroxyproline expression (Fig. 1p) were not significantly ameliorated by GED-0507 or Nint. In this context, curative administration of Pirf induced 30% body weight loss at day 21 and 100% mortality at day 24 (Fig. 1j, k).
Fig. 1. Study design and main findings following preventive and curative administration of GED-0507.
Study design and features of a preventive and b curative treatments. a, i Outlines of the experimental design. A total of 108 mice were randomized to a PBS (n = 12), BLM (n = 22), BLM + GED (n = 20), and BLM + Pirf (n = 10) for preventive treatment and i PBS (n = 5), BLM (n = 10), BLM + GED (n = 10), BLM + Nint (n = 10), and BLM + Pirf (n = 9) for curative treatment. BLM (0.025 U/kg) was administered by a single intratracheal instillation. Compounds were given by daily oral gavage (100 mg/kg for GED, 400 mg/kg for Pirf and 60 mg/kg for Nint) at days 1 and 14 for preventive and curative treatments, respectively. b, j Curves representing the percent of initial (at day 0) body weight for each week. c, k Kaplan–Meier curves depicting percent survival versus time post-BLM. d, l Representative photographs of macroscopic lung lesions. e, m Extension of pulmonary fibrotic lesions/total lung area were quantified on digital images by the threshold method and represent the fold change compared to that of PBS mice. f, n Representative microphotographs of Masson’s trichrome-stained lung sections scanned at ×20 magnification on an LSM 510 (Zeiss). Digital images were processed with a Zeiss LSM Browser. g, o Ashcroft score established based on Masson’s trichrome-stained lung sections for each sample. h, p Graph depicting hydroxyproline levels that were measured in 10 mg of frozen lungs. Statistical analysis was performed using Kruskal–Wallis nonparametric ANOVA. Post hoc comparisons between pairs of groups were performed by the Wilcoxon rank sum test. The data are expressed as the mean ± SEM. *p < 0.05 vs BLM; **p < 0.01 vs BLM; ***p < 0.001 vs BLM; #p < 0.05 vs PBS; ##p < 0.01 vs PBS; ###p < 0.001 vs PBS. §§§p < 0.001 vs BLM + GED; ns not significant
In conclusion, in vivo studies in BLM-induced pulmonary fibrosis in mice demonstrated that GED-0507 displays beneficial effects on lung fibrosis and may represent a promising therapeutic option for pulmonary fibrosis in humans. GED-0507 is ready to be tested in a phase Ib clinical trial in patients with IPF.
Acknowledgements
We thank Nogra Pharma Ltd., Dublin, Ireland, for partly funding this study. Nogra Pharma Ltd. has filed a patent for the use of GED-0507 in inflammatory/fibrotic diseases.
Competing interests
P.D. reports personal fees from PPM Services, during the conduct of the study; personal fees from Ferring, Switzerland; personal fees from Intralytix, USA; personal fees from Lesaffre, France; personal fees from KitoZyme, Belgium; personal fees from Abbott, France; personal fees from Janssen, France; personal fees from MSD, France; personal fees from Norgine, France; personal fees from Pfizer, France; personal fees from Takeda, France, outside the submitted work. P.S. reports personal fees from PPM Services during the conduct of the study; grants, personal fees and non-financial support from Roche; grants and non-financial support from PPM Services; grants, personal fees and non-financial support from Boehringer-Ingelheim; personal fees from RedX Pharma; personal fees from Galapagos; personal fees from Chiesi, outside the submitted work. S.S., C.D., C.R., and P.C. have nothing to disclose.
References
- 1.Raghu G, et al. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18–64 years old. Eur. Respir. J. 2016;48:179–186. doi: 10.1183/13993003.01653-2015. [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, et al. Raghu, G. et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guidelines: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2015;192:e3–e19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 3.Skandamis A, et al. Systematic review and network meta-analysis of approved medicines for the treatment of idiopathic pulmonary fibrosis. J. Drug Assess. 2019;8:55–61. doi: 10.1080/21556660.2019.1597726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speca S. et al. Novel PPARγ modulator GED-0507-34 levo ameliorates inflammation-driven intestinal fibrosis. Inflamm. Bowel Dis.22, 279–292 (2016). [DOI] [PMC free article] [PubMed]
- 5.Spagnolo P, Cottin V. Genetics of idiopathic pulmonary fibrosis: from mechanistic pathways to personalised medicine. J. Med. Genet. 2017;54:93–99. doi: 10.1136/jmedgenet-2016-103973. [DOI] [PubMed] [Google Scholar]
- 6.Pirat C, et al. Targeting peroxisome proliferator-activated receptors (PPARs): development of modulators. J. Med. Chem. 2012;55:4027–4061. doi: 10.1021/jm101360s. [DOI] [PubMed] [Google Scholar]
- 7.Fumery M, et al. Peroxisome proliferator-activated receptor gamma (PPARγ) regulates lactase expression and activity in the gut. EMBO Mol. Med. 2017;9:1471–1481. doi: 10.15252/emmm.201707795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rousseaux, C. et al. Preclinical evaluation of intestinal anti-inflammatory/analgesic properties and phase I clinical trial of a new PPAR Agonist Ged-0507-34-Levo. Gastroenterology140, S-515 (2011).
- 9.Di Gregorio J, et al. Role of glycogen synthase kinase-3β and PPAR-γ on epithelial-to-mesenchymal transition in DSS-induced colorectal fibrosis. PLoS ONE. 2017;12:e0171093. doi: 10.1371/journal.pone.0171093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashcroft T, et al. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 1988;41:467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]