The tumor microenvironment plays a key role in malignant tumor progression. As a place where tumor cells survive, the tumor microenvironment contains not only tumor cells themselves but also biochemical and biophysical elements. Tumor cells can change the microenvironment, which in turn affects the survival and development of tumor cells. Tumor malignancy is a highly regulated pathological process following a series of complex cellular-biological events called invasion–metastasis cascades. In this correspondence, we intend to provide a comprehensive background on the tumor environment to inspire further innovations in relevant research areas in the future.
Invasion–metastasis cascades produce clinically detectable metastatic lesions.1 Throughout these processes, biochemical and biophysical factors are involved. First, invasion requires the dynamics of intercellular adhesions and the extracellular matrix (ECM). Cancer cells undergo epithelial-to-mesenchymal transition (EMT) at a certain stage accompanied by a decrease in E-cadherin and an increase in N-cadherin.2 Meanwhile, the ability of cells to degrade the ECM is enhanced, which is dependent on the chemical microenvironment, such as the activation of matrix metalloproteinases (MMPs) and urokinase plasminogen activator (uPa),3 and these changes increase the ability of cancer cells to invade and metastasize. Once in the blood vessels, circulating tumor cells (CTCs) face several natural barriers that hinder the metastatic process. The leading barriers are the huge shear stress generated by the blood flow and its collision with blood cells. Studies have shown that CTCs with EMT are more resistant to shear stress.4 Last, in the blood, CTCs face the body’s immune system. CTCs can upregulate CD47 to prevent attack from macrophages and dendritic cells to achieve immune escape.5 Therefore, biochemical and biophysical factors both contribute to cancer cell malignancy.
The biochemical microenvironment mainly comprises chemokines and inflammatory factors. Chemokines are a family of soluble proteins that regulate cell migration by forming concentration gradients. Chemokines can act on tumor cells, recruit inflammatory cells, and use secretory factors to support tumor development. For example, monocyte chemotactic protein 1 (MCP-1) is closely associated with tumor angiogenesis and malignancy.6,7 It belongs to the Cys–Cys (CC) chemotactic family, and is highly conserved in humans, rats, mice, and rabbits. It can recruit monocytes, memory T cells, dendritic cells, bone marrow cells, and natural killer (NK) cells. CCR2 is a specific receptor for MCP-1, and it is a class of GTP protein-coupled seven-transmembrane protein receptors (GPCRs) that mediate the functions of chemokines. The MCP-1/CCR2 system plays a major role in tumorigenesis. MCP-1 can induce tumor cell CCR2-positive expression and promote angiogenesis; it can also infiltrate tumor tissues through tumor-associated macrophages (TAMs) and secrete cytokines, such as TGF-β, TNF-α, VEGF, and MMP, participating in cell invasion.8 Apart from MCP-1, stromal-derived factor 1 (SDF-1) is another chemokine expressed in resting cells, and is highly expressed in target tissues or organs during breast cancer cell metastasis. The SDF-1/CXCR4 signal axis has significant chemotactic targeting in a variety of tumor cell lines, especially in breast cancer.9 The transcriptional coactivator YAP and TAZ are mostly related to the growth of tissues and organs in the traditional concept. In addition, they also play a key role in the progression of cancer. As a transcriptional coactivator, YAP can regulate cell proliferation, migration, invasion, metastasis, EMT, chemoresistance, and the self-renewal of cancer stem cells.
Inflammation is another important component of tumor progression, and many cancers originating from sites of chronic inflammation will form an inflammatory microenvironment. Inflammatory cells include neutrophilic granulocytes, dendritic cells, macrophages, eosinophils, mastocytes, and lymphocytes, which produce cytokines or reactive oxygen species (ROS), serine, cysteine protease, MMP, interleukin, and interferon. A tumor environment rich in inflammatory cells and growth factors prolongs cell proliferation. Tumor cell malignant transformations produce a variety of cytokines and chemokines that can recruit inflammatory cells, which further participate in the malignant process. TAMs are an important component of the tumor tissue inflammatory response, and are mainly derived from monocytes obtained by the recruitment of monocyte chemotactic protein (MCP). On the one hand, they can directly kill tumor cells by activating IL-2, interferon, and IL-12. On the other hand, they can produce a variety of cytokines, growth factors, and proteases that promote angiogenesis and lymphangiogenesis, which is conducive to tumor development. In addition, TAMs can also induce the expression of vascular cell adhesion molecule 1 (VCAM-1) in mesothelial cells, thus promoting the peritoneal metastasis of tumor cells. It is not only macrophages that have tumor-promoting effects in inflammatory cells. Other cells, such as neutrophils, mast cells, eosinophils, and activated T lymphocytes, also release extracellular proteases, proangiogenic factors, and chemokines to accelerate the malignant tumor process. Decreasing the levels of inflammatory cells, cytokines, and chemokines is significant for inhibiting tumor progression from a biochemical perspective.
The biophysical tumor microenvironment can also remold cell behaviors,10 and it is closely associated with the chemical microenvironment. The biophysical tumor microenvironment is mainly composed of the shear stress that cells are exposed to, the stiffness of the matrix during progression, the topological structure, surface chemistry, etc. On the one hand, the growth and development of tumors are accompanied by changes in mechanical factors in the tumor microenvironment, such as the stress caused by the infinite proliferation of tumors, the increase in matrix stiffness, the increase in interstitial fluid pressure and the enhanced interstitial fluid flow. On the other hand, the change in mechanical factors will also remold the tumor microenvironment. For example, our previous study showed that the functions of two ROCK isoforms are different; ROCK1 mainly phosphorylates the myosin light chain, and ROCK2 mainly inhibits the depolymerization of the actin cytoskeleton network. Throughout the process, the maturity of focal adhesion and the dynamics of myosin directly participate in the characteristics of tumor cell motility.11 Moreover, residual stress will squeeze blood vessels and lymphatics, leading to the loss of function of blood vessels and lymphatics, thereby affecting the metabolic microenvironment of tumors and causing interstitial hypertension. In addition, mechanical factors also affect tumor treatment and metastasis. The interstitial matrix of tumors can be squeezed by rapidly proliferating tumor cells into a tortuous matrix that obstructs drug transport. Moreover, in hypoxic or acidic microenvironments, the cell mechanical microenvironment will change, and tumor cell phenotypes result in more malignant potential.12,13 High levels of lactic acid can inhibit the proliferation of cytotoxic T cells and the secretion of cytokines, interfere with the normal metabolism and function of cells, and inhibit the activity of dendritic cells and the migration of monocytes, helping tumor cells escape the immune system from identification and attack.14 Cells that undergo EMT also have enhanced abilities of movement and migration, processes that are accompanied by dynamic changes in the actin cytoskeleton and focal adhesions, inducing downstream intracellular signaling pathways. This evidence shows the synergetic effect of biochemical and biophysical properties in the tumor microenvironment (Fig. 1).
Fig. 1.
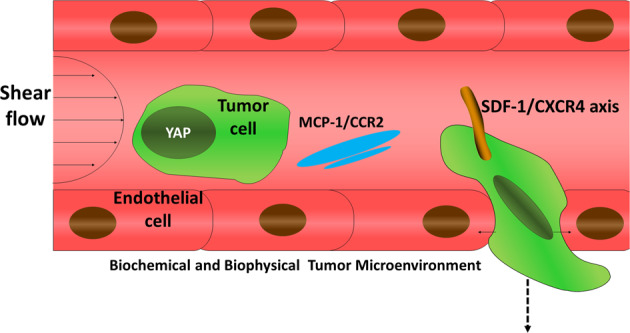
Schematic of biochemical and biophysical tumor microenvironments
As one of the important research contents of tumor biology, the tumor microenvironment has received increasing attention. The extracellular matrix, chemokines, and inflammatory factors in the microenvironment can affect the dynamics of adhesions and EMT processes, thus contributing to the invasion and metastasis ability of tumor cells. Some cells adhere to the vascular endothelium and migrate out of the blood vessels to undergo mesenchymal–epithelial-transformation (MET) to regain proliferation. Throughout the process, mechanical factors are involved in every step of tumor metastasis. This suggests that the mechanical microenvironment is inextricably linked to tumor cells, the extracellular matrix, chemokines, and inflammatory factors in the tumor microenvironment. An in-depth understanding of various elements in the tumor microenvironment as well as the EMT process is extremely valuable for the early diagnosis and prognosis of cancers, which would further reveal the physicochemical properties of the tumor microenvironment, especially immune-related tumor invasion and metastasis.
Acknowledgements
This work was supported, in part or in whole, by the National Natural Science Foundation of China (11772088, 31700811, 11802056, 31800780, and 81671821), the Research Program of Sichuan Science and Technology (2017JY0019, 2017JY0217, 2019YJ0183, and 2019YJ0184), the China Postdoctoral Science Foundation (2017JY0217, 2018M640904, and 2019T120831), and the Fundamental Research Funds for the Central Universities (ZYGX2016Z001).
Competing interests
The authors declare no competing interests.
References
- 1.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 2.Yilmaz M, Christofori G. Mechanisms of motility in metastasizing cells. Mol. Cancer Res. 2010;8:629–642. doi: 10.1158/1541-7786.MCR-10-0139. [DOI] [PubMed] [Google Scholar]
- 3.Dano K, et al. Plasminogen activation and cancer. Thromb. Haemost. 2005;93:676–681. doi: 10.1160/TH05-01-0054. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell MJ, King MR. Computational and experimental models of cancer cell response to fluid shear stress. Front Oncol. 2013;3:44. doi: 10.3389/fonc.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinert G, et al. Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res. 2014;74:1694–1704. doi: 10.1158/0008-5472.CAN-13-1885. [DOI] [PubMed] [Google Scholar]
- 6.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li S, et al. MCP-1-induced ERK/GSK-3beta/Snail signaling facilitates the epithelial-mesenchymal transition and promotes the migration of MCF-7 human breast carcinoma cells. Cell Mol. Immunol. 2017;14:621–630. doi: 10.1038/cmi.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastas-. Rev. 2006;25:315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y, Cao HB, Li WJ, Zhao L. The CXCL12 (SDF-1)/CXCR4 chemokine axis: oncogenic properties, molecular targeting, and synthetic and natural product CXCR4 inhibitors for cancer therapy. Chin. J. Nat. Med. 2018;16:801–810. doi: 10.1016/S1875-5364(18)30122-5. [DOI] [PubMed] [Google Scholar]
- 10.Qin X, et al. Low shear stress induces ERK nuclear localization and YAP activation to control the proliferation of breast cancer cells. Biochem Biophys. Res. Commun. 2019;510:219–223. doi: 10.1016/j.bbrc.2019.01.065. [DOI] [PubMed] [Google Scholar]
- 11.Peng Y, et al. ROCK isoforms differentially modulate cancer cell motility by mechanosensing the substrate stiffness. Acta Biomater. 2019;88:86–101. doi: 10.1016/j.actbio.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Gruber G, et al. Hypoxia-inducible factor 1 alpha in high-risk breast cancer: an independent prognostic parameter? Breast Cancer Res. 2004;6:R191–R198. doi: 10.1186/bcr775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, et al. Copper depletion inhibits CoCl2-induced aggressive phenotype of MCF-7 cells via downregulation of HIF-1 and inhibition of Snail/Twist-mediated epithelial-mesenchymal transition. Sci. Rep. 2015;5:12410. doi: 10.1038/srep12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goetze K, Walenta S, Ksiazkiewicz M, Kunz-Schughart LA, Mueller-Klieser W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J. Oncol. 2011;39:453–463. doi: 10.3892/ijo.2011.1055. [DOI] [PubMed] [Google Scholar]


