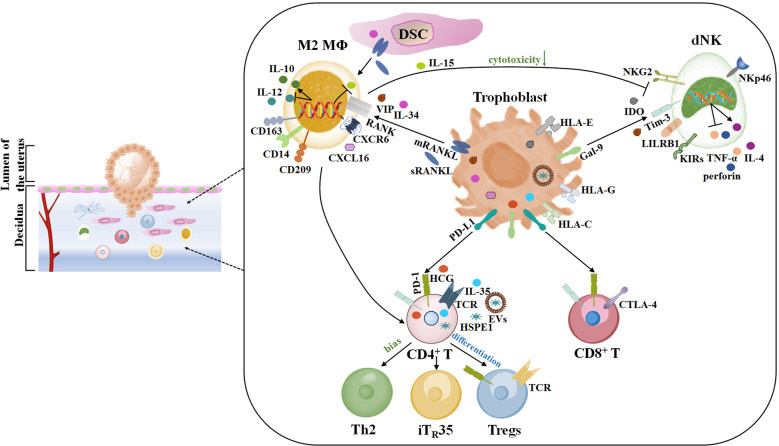
A successful pregnancy requires the maternal immune system to recognize and tolerate the allogeneic fetus while maintaining defense against infection both systemically and in placental tissues. At the maternal–fetal interface, the trophoblasts in the placenta play an essential role in the suppression of maternal immune rejection of the fetus. To ensure maternal–fetal tolerance and successful placentation, delicate crosstalk is established among fetus-derived trophoblasts, maternal immune cells, and decidual stromal cells (DSCs) during normal pregnancy. Decidual immune cells not only participate in the maintenance of immune tolerance but also regulate the function of trophoblasts to promote fetal growth. An imbalance in this crosstalk may lead to adverse pregnancy outcomes, such as recurrent spontaneous abortion, preeclampsia, preterm birth, intrauterine growth restriction, and infection. Here we outline some of the important discoveries in recent years related to the mechanisms by which trophoblast-derived molecules induce maternal–fetal immune tolerance.
Immune cells at the maternal–fetal interface
The maternal–fetal interface consists of different immune cells, such as natural killer (NK) cells, macrophages (MФs), dendritic cells (DCs), T cells, myeloid-derived suppressor cells (MDSCs), B cells, and NKT cells. Trophoblasts have a central role in the control of maternal immune homeostasis mediated by modulating uterine NK cell activity, decidual MФ polarization into an alternative phenotype, tolerogenic DC differentiation, and regulatory T cell (Treg) induction. Although some research results have been produced, the mechanisms by which trophoblasts exert these functions have not been fully elucidated.
NK cells
Most leukocytes (up to 90%) that present at the maternal–fetal interface during the first trimester of gestation are decidual NK (dNK) cells. It has become increasingly clear from both human studies and mouse studies that dNK cells differ significantly from peripheral NK (pNK) cells in both phenotype and function. In a healthy state, freshly isolated dNK cells are poorly cytotoxic and skewed toward producing high levels of cytokines and angiogenic factors. Furthermore, dNK cells have been demonstrated to facilitate extravillous trophoblast invasion into the maternal decidua during the first trimester. dNK cells can influence DC function during pregnancy by preventing the maturation of immature DCs, thus protecting trophoblasts from mature DC-initiated cytotoxic T cell responses, which may play a crucial role in modulating the balance between immune responsiveness and immune tolerance.
Macrophages
Decidual MФs (dMФs) represent approximately 15–20% of decidual leukocytes. MФs isolated from the decidua during the first trimester mostly exhibit the immunomodulatory phenotype (M2) and contribute to the creation of a tolerogenic immune environment by producing immunosuppressive cytokines to suppress responses to allogeneic fetal antigens, phagocytizing apoptotic trophoblasts to prevent the release of proinflammatory substances, and inhibiting the cytotoxic function of dNK cells. dMФs also secrete high levels of factors that facilitate placental growth and trophoblast invasion.
Dendritic cells
DCs comprise approximately 1–2% of the immune cells in the decidua and are scattered through both the decidua basalis (in which trophoblasts are infiltrated) and the decidua parietalis. DCs within the decidual microenvironment exhibit a tolerogenic profile. Human extravillous trophoblasts induce regulatory DCs that poorly induce the proliferation and activation of allogeneic T cells but promote Treg expansion.
T cells
Decidual T cells comprise 5–15% of placental leukocytes during the first trimester of gestation. Trophoblasts secrete cytokines that regulate the function and differentiation of decidual immune cells and induce epigenetic changes in DSCs, modulating the capacity of DSCs to produce the chemokines necessary for T cell recruitment. Immune tolerance induction by trophoblasts is accomplished not only by directly inhibiting effector T cells and shifting the T helper type 1 (Th1)-type immune response into a Th2-type immune response but also by promoting Treg induction and recruitment. High percentages of Tregs can be found in the decidual tissue, where they suppress fetus-specific and nonspecific lymphocyte responses.
Myeloid-derived suppressor cells
MDSCs, which are capable of inhibiting T cell responses, inducing dNK cell and Treg proliferation and differentiation, increasing the suppressive activity of dNK cells and Tregs, and polarizing CD4+ T cells toward a Th2 cytokine response, are highly increased in number in the early stage of pregnancy.
Mechanisms by which trophoblast-derived molecules induce maternal–fetal immune tolerance
During pregnancy, trophoblasts express and secrete a battery of immune inhibitory molecules targeting MФs, NK cells, DCs, T cells, or MDSCs to sustain maternal–fetal immune tolerance.
Roles of costimulatory molecules
T cell immunoglobulin mucin 3 (Tim-3)/galectin-9 (Gal-9)
Tim-3 is an important negative regulatory molecule that induces immune tolerance by interacting with its ligand Gal-9 to modulate the functions of various immune cells. Tim-3, whose expression on decidual CD4+ T cells is upregulated during pregnancy, promotes a Th2 bias at the maternal–fetal interface. Decreased Tim-3 expression on decidual CD4+ T cells may be associated with miscarriage. Trophoblasts induce a relatively high frequency of Tim-3+CTLA-4+CD8+ T cells during pregnancy; this subset has an active status and produces a relatively high amount of anti-inflammatory cytokines, and a decreased number of these cells and altered function correlate with miscarriage.1 Tim-3+ dNK cells display higher interleukin (IL)-4 production and lower tumor necrosis factor-α and perforin production than Tim-3- dNK cells. Human trophoblasts induce the transformation of pNK cells into cells with a dNK-like phenotype via the Gal-9/Tim-3 interaction. A decreased percentage of Tim-3+ dNK cells has been observed in human miscarriage and abortion-prone mouse models.2 Therefore, Tim-3/Gal-9 signaling pathways may play positive roles in the establishment and/or maintenance of maternal–fetal tolerance.
Programmed death-ligand 1 (PD-L1)/programmed death-1 (PD-1)
The immune checkpoint molecule PD-L1, a suppressor of T cell activation, is expressed in the syncytiotrophoblasts and cytotrophoblasts in the human placenta and is assumed to induce maternal immune tolerance to fetal antigens via PD-1 receptors on T cells. During human pregnancy, the levels of soluble forms of PD-L1 are reported to be elevated in the serum, and PD-L1 blockade results in spontaneous fetal resorption in pregnant mice.3 Decidual Tim-3+PD-1+CD8+ T cells recognize PD-L1 expressed on extravillous trophoblasts, which provides an inhibitory signal resulting in trophoblast antigen-specific tolerance.4
Roles of cytokines and chemokines
Interleukin-34
IL-34 has been shown to bind to the same receptor as macrophage-colony stimulating factor, which has important immunomodulatory roles at the fetal–maternal interface, for example, polarizing dMФs into an M2-like regulatory phenotype. IL-34 is produced by placental cytotrophoblasts, syncytiotrophoblasts, and DSCs. It is able to polarize blood monocytes into MФs with an immunoregulatory phenotype (CD14highCD163+CD209+) and a cytokine secretion pattern similar to that of dMФs observed in vitro, and these macrophages are involved in the establishment of the tolerant milieu found at the fetal–maternal interface.5
Interleukin-35
IL-35 is expressed in both human first-trimester primary trophoblasts and a trophoblast cell line. Trophoblasts inhibit the proliferation of human naive conventional T cells and convert these cells into IL-35-inducible regulatory T (iTR35) cells in an IL-35-dependent manner. Mice with spontaneous abortion have relatively low levels of IL-35 and iTR35 cells at the maternal–fetal interface, and exogenous IL-35 induces iTR35 cells and prevents immune-induced abortion.6
Receptor activator for nuclear factor-κB ligand (RANKL)/RANK
Compared with patients with a normal pregnancy, miscarriage patients have abnormally low levels of RANKL/RANK in the villi and decidua. RANKL, which is secreted by human embryonic trophoblasts and maternal DSCs, polarizes dMФs toward the M2 phenotype and then induces a Th2 bias that promotes maternal–fetal tolerance. Impaired expression of RANKL leads to dysfunction in dMФs in vivo and increased rates of fetal loss in mice.7
C-X-C chemokine motif ligand 16 (CXCL16)/C-X-C chemokine motif receptor 6 (CXCR6)
A highly coordinated chemokine network not only underlies recruitment of different leukocyte subsets but also orchestrates unique mechanisms of immunomodulation to maintain tolerance. Maternal MФs can interact with extravillous trophoblasts through the expression of the chemokine receptor CXCR6 and chemokine CXCL16.8 CXCL16 derived from first-trimester trophoblasts induces the polarization of human M2 MФs. M2 MΦs polarized by CXCL16 exhibit decreased IL-15 production, which facilitates the inactivation of NK cells. The cytotoxicity of NK cells is attenuated by CXCL16-polarized M2 MΦs, which thereby contribute to the homeostatic, immunotolerant milieu required for successful fetal development.9
Roles of human leukocyte antigen (HLA) molecules
Major histocompatibility complex (MHC) antigen presentation is an important mechanism to prevent immune rejection of the fetus.
Human leukocyte antigen-C
HLA-C is a key molecule that can elicit an allogeneic immune response by maternal T and NK cells and for which maternal–fetal immune tolerance needs to be established. HLA-C is also the only classical MHC molecule expressed by extravillous trophoblasts that can present a wide variety of peptides to maternal memory T cells and establish protective immunity. The expression of paternal HLA-C by extravillous trophoblasts provides a target for maternal NK and T cells, but HLA-C expression may also influence how this response is shaped. These dual functions of HLA-C require tight transcriptional regulation of HLA-C expression to balance the induction of tolerance with the induction of immunity. Extravillous trophoblasts can increase PD1hi Treg induction via HLA-C and CD3.10 dNK cells express high levels of killer immunoglobulin receptors (KIRs) specific for HLA-C allelic variants on trophoblasts,8 and binding between KIRs and HLA-C molecules protects trophoblasts from NK cell cytotoxicity.
Human leukocyte antigen-G
A growing body of evidence suggests that HLA-G modulates not only the activity of NK cells but also that of MФs, T cells, and B cells. Extravillous trophoblasts present HLA-C and the nonclassical HLA-Ib molecules HLA-E and HLA-G on the cell surface. dNK cells express high levels of leukocyte immunoglobulin-like receptor subfamily B member 1, which has a high affinity for HLA-G, and various NKG2 receptors, which recognize HLA-E on extravillous trophoblasts.8 NK cell inhibition by extravillous trophoblasts can be achieved by HLA-G directly or by HLA-E presenting an HLA-G peptide. HLA-G-induced immune tolerance can occur via the peculiar cellular process trogocytosis, which confers a transient immunosuppressive phenotype upon recipient cells, such as dNK cells and CD4+ T cells, to allow these cells to play a role in balancing immune tolerance and antiviral immunity at the maternal–fetal interface.11 Notably, cytokine activation of dNK cells results in the disappearance of internalized HLA-G in parallel with the restoration of cytotoxicity.11 Coculture of HLA-G+ extravillous trophoblasts or dMФs with blood CD4+ T cells directly increases the proportion of CD25hiFOXP3+ Tregs.10
Roles of human chorionic gonadotropin (HCG), indoleamine-2,3-dioxygenase (IDO), vasoactive intestinal peptide (VIP), and heat shock protein family E member 1 (HSPE1)
Human chorionic gonadotropin
Trophoblast-derived HCG induces CD4+FOXP3+ Treg differentiation from CD4+FOXP3− T cells and increases the suppressive activity of CD4+FOXP3− T cells through an antigen-independent pathway.12
Indoleamine-2,3-dioxygenase
Trophoblast-derived IDO can downregulate NKp46 and NKG2D expression and reduce the cytotoxicity of pNK cells; it may also contribute to maintaining dNK cell cytotoxicity at a low level, suggesting an important role for IDO in the maintenance of normal pregnancy.13
Vasoactive intestinal peptide
VIP secreted by trophoblasts has potent immunomodulatory and trophic effects on adult and embryonic tissues. It has the ability to induce a regulatory/suppressive activation profile in dMФs and dNK cells. VIP increases IL-10 synthesis and reduces IL-12 activity in dMФs.14
Hsp10/HSPE1
Extracellular vesicles exhibit either immunostimulatory properties or immunosuppressive properties depending on their origin and composition. Trophoblast-derived Hsp10/HSPE1 has an important role in Treg differentiation from CD4+ T cells in vitro, and HSPE1 is a useful marker for Treg subtype characterization.15
Conclusion
Maternal immune adaptation is required for a successful pregnancy that avoids rejection induced by embryonic antigens. We need to perform in-depth research to profile placental and uterine cells using a novel high-throughput analysis approach, which will reveal new candidates for trophoblast-mediated immunoregulatory mechanisms. Exploring the functions of trophoblasts will help provide a more complete picture of immune tolerance induction at the maternal–fetal interface, which will have a profound impact on the treatment of immune-related reproductive diseases. The mechanisms by which trophoblast-derived molecules induce immune tolerance are highlighted in Fig. 1.
Fig. 1.
Schematic diagram of trophoblast-derived molecules inducing immune tolerance at the maternal–fetal interface
Acknowledgements
This work was supported by grant number MOST 2017YFC1001400 awarded to D.-J.L.; grants from the National Natural Science Foundation of China, numbers 81971456 and 81200425 awarded to X.-Q.W.; and grants from the National Natural Science Foundation of China, numbers 81471548 and 81490744 awarded to D.-J.L.
Competing interests
The authors declare no competing interests.
References
- 1.Wang, S. C. et al. The appropriate frequency and function of decidual Tim-3(+)CTLA-4(+)CD8(+) T cells are important in maintaining normal pregnancy. Cell Death Dis. 10, 407 (2019). [DOI] [PMC free article] [PubMed]
- 2.Li YH, et al. The Galectin-9/Tim-3 pathway is involved in the regulation of NK cell function at the maternal-fetal interface in early pregnancy. Cell. Mol. Immunol. 2016;13:73–81. doi: 10.1038/cmi.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okuyama M, et al. Elevated soluble PD-L1 in pregnant women’s serum suppresses the immune reaction. Front. Immunol. 2019;10:86. doi: 10.3389/fimmu.2019.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang SC, et al. PD-1 and Tim-3 pathways are associated with regulatory CD8(+) T-cell function in decidua and maintenance of normal pregnancy. Cell Death Dis. 2015;6:e1738. doi: 10.1038/cddis.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindau R, et al. Interleukin-34 is present at the fetal-maternal interface and induces immunoregulatory macrophages of a decidual phenotype in vitro. Hum. Reprod. 2018;33:588–599. doi: 10.1093/humrep/dey037. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, et al. Human placental trophoblast cells contribute to maternal-fetal tolerance through expressing IL-35 and mediating iT(R)35 conversion. Nat. Commun. 2019;10:4601. doi: 10.1038/s41467-019-12484-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng YH, et al. RANKL-mediated harmonious dialogue between fetus and mother guarantees smooth gestation by inducing decidual M2 macrophage polarization. Cell Death Dis. 2017;8:e3105. doi: 10.1038/cddis.2017.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vento-Tormo R, et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature. 2018;563:347. doi: 10.1038/s41586-018-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang XQ, et al. Trophoblast-derived CXCL16 induces M2 macrophage polarization that in turn inactivates NK cells at the maternal-fetal interface. Cell. Mol. Immunol. 2018;15:1038–1046. doi: 10.1038/s41423-018-0019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salvany-Celades M, et al. Three types of functional regulatory T cells control T cell responses at the human maternal-fetal interface. Cell Rep. 2019;27:2537. doi: 10.1016/j.celrep.2019.04.109. [DOI] [PubMed] [Google Scholar]
- 11.Tilburgs T, et al. Human HLA-G plus extravillous trophoblasts: Immune-activating cells that interact with decidual leukocytes. Proc. Natl Acad. Sci. USA. 2015;112:7219–7224. doi: 10.1073/pnas.1507977112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poloski E, et al. JEG-3 trophoblast cells producing human chorionic gonadotropin promote conversion of human CD4(+)FOXP3(-) T cells into CD4(+) FOXP3(+) regulatory T cells and foster T cell suppressive activity. Biol. Reprod. 2016;94:106. doi: 10.1095/biolreprod.115.135541. [DOI] [PubMed] [Google Scholar]
- 13.Ban YL, et al. Effect of indoleamine 2,3-dioxygenase expressed in HTR-8/SVneo cells on decidual NK cell cytotoxicity. Am. J. Reprod. Immunol. 2016;75:519–528. doi: 10.1111/aji.12481. [DOI] [PubMed] [Google Scholar]
- 14.Paparini DE, et al. Vasoactive intestinal peptide shapes first-trimester placenta trophoblast, vascular, and immune cell cooperation. Br. J. Pharmacol. 2019;176:964–980. doi: 10.1111/bph.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovacs AF, et al. Unravelling the role of trophoblastic-derived extracellular vesicles in regulatory T cell differentiation. Int. J. Mol. Sci. 2019;20:3457. doi: 10.3390/ijms20143457. [DOI] [PMC free article] [PubMed] [Google Scholar]