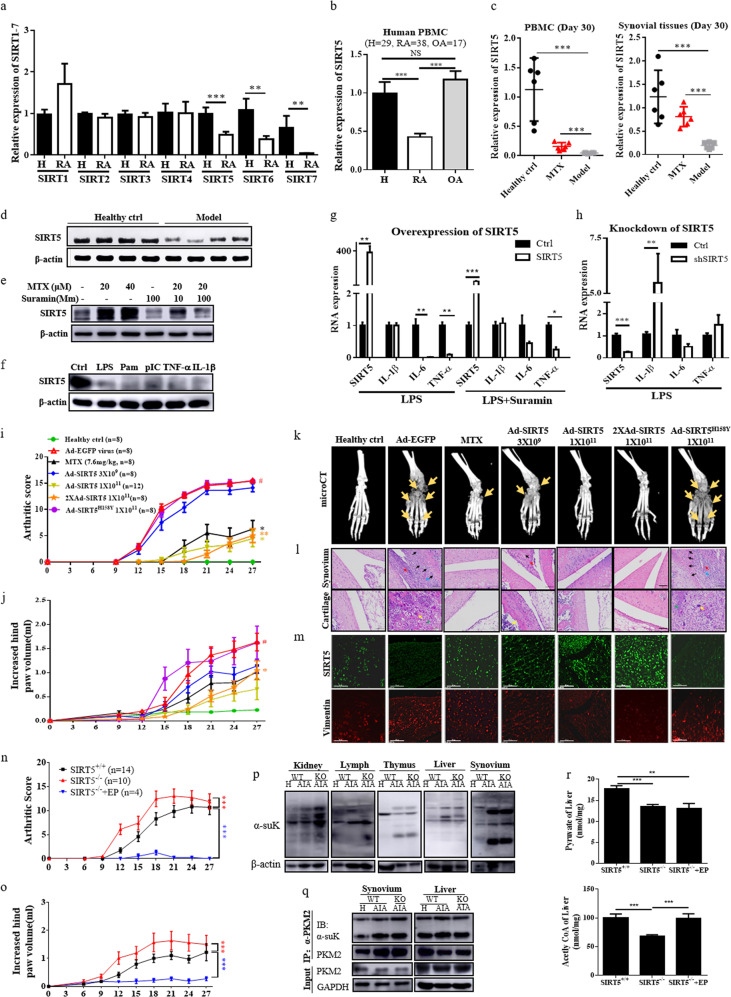
Sirtuin (SIRT) 1 and 6 are known to be involved in the pathogenesis of rheumatoid arthritis (RA)1. Recent reports showed that SIRT5 prevents dextran sulfate sodium (DSS)-induced colitis in mice2 and recovered the innate inflammatory response of endotoxin-tolerant macrophages by blocking SIRT2-mediated deacetylation of p65;3 however, the role of SIRT5 remains unclear. Here, we demonstrated that SIRT5 mRNA was significantly downregulated in RA patients compared with that of healthy controls (Fig. 1a). Real-time PCR analysis showed more than twofold lower SIRT5 mRNA levels in RA patients compared with those of healthy controls or patients with osteoarthritis (OA) (P < 0.001) (Fig. 1b). Alternatively, both mRNA and protein levels of SIRT5 were significantly reduced in PBMCs and synovial tissues from AIA rats compared with those of healthy control animals (Fig. 1c, d), whereas SIRT5 mRNA expression was partially recovered in methotrexate (MTX)-treated AIA rats (Fig. 1c). Immunofluorescence staining of synovial tissues from AIA rats revealed that SIRT5 expression (green) was markedly suppressed in the region containing high levels of activated monocytes/macrophages (CD68+/red) and proliferating synovial fibroblasts (vimentin/red) (Supplementary Fig. S1A), suggesting that SIRT5 is closely associated with the pathogenesis of AIA and human RA.
Fig. 1. The anti-inflammatory role of SIRT5 in RA.
a RNA expression of SIRT1–7 in PBMCs from healthy controls and RA patients (n = 5–20). b SIRT5 gene expression in healthy volunteers (n = 29) and patients with RA (n = 38) or osteoarthritis (n = 17). Unpaired t-test, *P < 0.05, **P < 0.01, and ***P < 0.001. c SIRT5 mRNA levels in PBMCs and synovial tissues of AIA rats (day 30, n = 6). d SIRT5 protein expression in rat synovial tissues (day 30, n = 4). e Effect of SIRT5 expression in MTX- and SIRT5 inhibitor suramin-treated RASFs. f Effect of SIRT5 expression in proinflammatory cytokine-treated RASFs. g Production of proinflammatory cytokines in human CD14+CD16− monocytes with overexpression or h knockdown of SIRT5 in the presence or absence of suramin. *P < 0.05, **P < 0.01, and ***P < 0.001 vs untransfected controls. i The arthritic scores and j hind paw volumes of Ad-SIRT5 overexpression in AIA rats (n = 8–12). #P < 0.05 vs healthy ctrl; *P < 0.05 and **P < 0.01 vs Ad-EGFP vehicle ctrl. k Micro-CT radiographic images showing Ad-SIRT5 overexpression in AIA rats. l Histopathological sections (scale bar: 100 μm) and m immunofluorescence images (scale bar: 50 μm) showing synovial tissue from Ad-SIRT5-treated AIA rats. n Arthritic scores and o increased hind paw volumes of SIRT5+/+, SIRT5−/−, and SIRT5−/− + EP AIA rats. p Characterization of succinylation distribution in different tissues and q the hypersuccinylation of PKM2 in SIRT5+/+ and SIRT5−/− SD rats. r Detection of acetyl CoA and pyruvate in livers of SIRT5+/+, SIRT5−/−, and SIRT5−/− + EP rats. **P < 0.01 and ***P < 0.001, by one-way ANOVA. The data shown are the mean ± SEM.
The influence of inflammatory stimuli on SIRT5 expression was further examined by using RA patient-derived synovial fibroblasts (RASFs) in the presence of MTX, suramin (a SIRT5 inhibitor), Toll-like receptor ligands such as lipopolysaccharides (LPS), Pam, and poly (I:C), and the proinflammatory cytokines TNF-α and IL-1β. As shown in Fig. 1e, suramin suppressed SIRT5 expression and markedly abolished MTX-stimulated expression of SIRT5 in RASFs, while RASFs treated with Toll-like receptor ligands and proinflammatory cytokines downregulated SIRT5 expression (Fig. 1f). In purified human CD14+CD16− monocytes, overexpression of SIRT5 abolished LPS-mediated release of IL-6 and TNF-α, whereas the addition of suramin partially blocked the inhibitory effect of SIRT5 (Fig. 1g). In contrast, knockdown of SIRT5 significantly enhanced the LPS-mediated release of IL-1β and TNF-α in CD14+CD16− monocytes (Fig. 1h). The anti-inflammatory role of SIRT5 was further characterized by injection of adenovirus-SIRT5 (Ad-SIRT5) into the ankle joints of AIA rats. While severe swelling, erythema and joint rigidity developed in the hind paws of AIA rats that were injected with Ad-EGFP virus (vehicle control), the arthritic scores and hind paw volumes were significantly attenuated in rats that were injected with either a single dose or double doses of Ad-SIRT5 (1 × 1011 PFU) (Fig. 1i–l). However, the SIRT5 mutant SIRT5H158Y,4 failed to attenuate the arthritic condition in AIA rats, suggesting that the deacetylation and desuccinylation activity of SIRT5 contributes to the anti-inflammatory effects of SIRT5 in RA. Notably, AIA rats that were injected with double doses of Ad-SIRT5 (1 × 1011 PFU) exhibited significant recovery in the masses of the spleen and thymus, indicating the suppression of spleen enlargement and prevention of thymus shrinkage, compared with those of vehicle-treated animals (Supplementary Fig. S1B–D). These findings suggest that the amelioration of inflammatory conditions in AIA rats is closely associated with the recovery of immune organ functions in animals.
Microcomputed tomography (micro-CT) and histopathological analysis further indicated the typical symptoms of swollen joints, bone destruction, synovial membrane hyperplasia with severe inflammatory cell infiltration, and pannus formation in joint tissues of Ad-EGFP vehicle and Ad-SIRT5H158Y mutant rats (Fig. 1k, l). Notably, infiltration of inflammatory cells and hyperplasia of articular cartridge were ameliorated in AIA rats that were injected with low-dose (3 × 109 PFU), single-dose, and double-dose Ad-SIRT5 (1 × 1011 PFU) or were treated with MTX (Fig. 1k, l), whereas immunofluorescence staining of the synovial tissues further showed the increasing trend in SIRT5 expression and the reduced level of vimentin, representing the low proliferative rate of synovial fibroblasts5 (Fig. 1m). Micro-CT analysis of bone mineral density, bone volume fraction, cortical mineral density (TMD), trabecular number, and total porosity of Ad-SIRT5-treated AIA rats showed that the mean micro-CT score increased significantly from 0.26 (Ad-EGFP vehicle control) to 0.70 [Ad-SIRT5 (1 × 1011 PFU)] (Supplementary Fig. S2A, B), and the mean radiological score dropped from 4.0 to 0.42 for these same groups (Supplementary Fig. S2C). In addition, the animals that were treated with MTX and high-dose Ad-SIRT5 showed significant suppression of the proinflammatory cytokines monocyte chemoattractant protein-1 (MCP-1), TNF-α, IL-1β, and IL-6, with a concomitant reduction in erythrocyte sedimentation rate and C-reactive protein levels in blood serum (Supplementary Fig. S3A). Collectively, these results validated the potential anti-inflammatory role of SIRT5 in AIA rats.
We finally characterized the anti-inflammatory role of SIRT5 in RA using CRISPR/Cas9-generated SIRT5-deficient SD rats compared with the effects in SIRT5+/+ rats with AIA, SIRT5−/− rats developed more rapid and severe arthritic inflammation, as shown by the early increases in arthritic scores and hind paw volumes (Fig. 1n, o and Supplementary Fig. S3B). The mRNA expression of TNF-α and secretion of IL-1β, IL-6, and MCP-1 were significantly upregulated in PBMCs and serum of SIRT5−/− rats in comparison to those of SIRT5+/+ rats (Supplementary Fig. S3C, D). Thus, SIRT5 slowed the pathogenic progression of RA.
SIRT5 regulates energy metabolic networks through the desuccinylation of SIRT5-target proteins, participating in fatty acid β-oxidation, the tricarboxylic acid (TCA) cycle, and glycolysis6. Notably, protein succinylation was increased in the kidney, lymphonodus, thymus, liver, and synovium in SIRT5−/− rats (Fig. 1p). Pyruvate kinase M2 (PKM2) is one of the desuccinylation substrates of SIRT5 that catalyzes the rate-limiting step of glycolysis7. The immunoprecipitation results showed that hypersuccinylation of PKM2 was found in the synovium and liver of SIRT5+/+ AIA rats compared with those of the healthy control, whereas the succinylation of PKM2 was further enhanced only in the synovium of SIRT5−/− AIA rats (Fig. 1q). Alternatively, pyruvate, a key metabolic intermediate in glycolysis and the TCA cycle, exhibited a protective effect against excessive inflammation8, whereas ethyl pyruvate (EP), a form of pyruvate with improved bioavailability, was recently reported to ameliorate inflammatory arthritis in mice9, supporting a new therapeutic strategy for RA via the manipulation of cellular bioenergetics10. Here, we revealed that SIRT5−/− AIA rats that were treated with EP exhibited complete elimination of the arthritic condition (Fig. 1n, o), accompanied by a reduction in proinflammatory cytokines (Supplementary Fig. S3C, D). However, EP supplementation increased the production of acetyl CoA but not pyruvate (Fig. 1r). These results indicated that the energy metabolism aerobic respiration recovered after supplementation of the energy metabolite EP in SIRT5−/− rats with AIA. In conclusion, these findings provide a strategy for manipulating SIRT5-related energy metabolism for RA intervention.
Supplementary information
Supplementary Methods and Figure Legends
Acknowledgments
Funding
This work was supported by a FDCT grant from the Macao Science and Technology Development Fund (Project code: 0048/2018/A2), FDCT grant from the Macao Science and Technology Development Fund (Project code:0003/2019/AKP), and the Foshan Medicine Dengfeng Project of China (2019–2021).
Competing interests
The authors declare no competing interests.
Ethics, consent, and permissions
Blood samples were collected from patients with RA and OA and healthy volunteers who provided signed informed and voluntary consent in Guangdong General Hospital, Guangdong Academy of Medical Sciences, Guangzhou (China), and with the approval of the Research Ethics Committee (GDREC 2015391H). Animal care and experimental procedures were approved and conducted under the guide for the Laboratory Animal Research Committee Guidelines of Guangzhou, University of Chinese Medicine, and ethics approval by the Macau University of Science and Technology, Medical Ethics Committee, Macau (China).
Contributor Information
Vincent Kam Wai Wong, Email: bowaiwong@gmail.com.
Liang Liu, Email: lliu@must.edu.mo.
Supplementary information
The online version of this article (10.1038/s41423-020-0380-4) contains supplementary material.
References
- 1.Niederer F, et al. SIRT1 overexpression in the rheumatoid arthritis synovium contributes to proinflammatory cytokine production and apoptosis resistance. Ann. Rheum. Dis. 2011;70:1866–1873. doi: 10.1136/ard.2010.148957. [DOI] [PubMed] [Google Scholar]
- 2.Wang F, et al. SIRT5 desuccinylates and activates pyruvate kinase M2 to block macrophage IL-1 beta production and to prevent DSS-induced colitis in mice. Cell Rep. 2017;19:2331–2344. doi: 10.1016/j.celrep.2017.05.065. [DOI] [PubMed] [Google Scholar]
- 3.Qin KW, et al. NAD(+) dependent deacetylase Sirtuin 5 rescues the innate inflammatory response of endotoxin tolerant macrophages by promoting acetylation of p65. J. Autoimmun. 2017;81:120–129. doi: 10.1016/j.jaut.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137:560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunological Rev. 2010;233:233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rardin MJ, et al. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 2013;18:920–933. doi: 10.1016/j.cmet.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat. Rev. Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 8.Ulloa L, et al. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc. Natl Acad. Sci. USA. 2002;99:12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung SM, et al. Ethyl pyruvate ameliorates inflammatory arthritis in mice. Int. Immunopharmacol. 2017;52:333–341. doi: 10.1016/j.intimp.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 10.Veras FP, et al. Fructose 1,6-bisphosphate, a high-energy intermediate of glycolysis, attenuates experimental arthritis by activating anti-inflammatory adenosinergic pathway. Sci. Rep. 2015;5:15171. doi: 10.1038/srep15171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods and Figure Legends