Abstract
To date, IgG in the tumor microenvironment (TME) has been considered a product of B cells and serves as an antitumor antibody. However, in this study, using a monoclonal antibody against cancer-derived IgG (Cancer-IgG), we found that cancer cells could secrete IgG into the TME. Furthermore, Cancer-IgG, which carries an abnormal sialic acid modification in the CH1 domain, directly inhibited effector T-cell proliferation and significantly promoted tumor growth by reducing CD4+ and CD8+ T-cell infiltration into tumor tissues. Mechanistic studies showed that the immunosuppressive effect of sialylated Cancer-IgG is dependent on its sialylation and binding to sialic acid-binding immunoglobulin-type lectins (Siglecs) on effector CD4+ and CD8+ T cells. Importantly, we show that several Siglecs are overexpressed on effector T cells from cancer patients, but not those from healthy donors. These findings suggest that sialylated Cancer-IgG may be a ligand for Siglecs, which may serve as potential checkpoint proteins and mediate tumor immune evasion.
Keywords: IgG, Cancer-derived IgG, tumor microenvironment, sialylation, tumor immune escape
Subject terms: Tumour immunology, Cancer microenvironment
Introduction
Although elevated immunoglobulin (Ig) levels in patients with cancer are believed to be the result of increased expression of B cell-derived antitumor antibodies, a growing body of evidence indicates that IgG in the tumor microenvironment (TME) usually has procancer activity by blocking either T-cell-mediated tumor cytotoxicity (once believed to be mediated by a molecule named “blocking factor”) or proinflammatory activity.1–3 Thus, B cells have been considered to play either antitumor or “rebel” roles in antitumor immunity. In fact, increasing evidence from our group and others indicates that IgG is overexpressed in many cancer cells.4–7 Moreover, cancer-derived IgG (Cancer-IgG) that displays growth factor-like activity and promotes the progression of cancer cells can be produced.4,8–11 These findings suggest that IgG in the TME includes Cancer-IgG and B cell-derived IgG (B-IgG) and that the “rebel” IgG may be derived from cancer cells.
Recently, we used a monoclonal antibody, RP215, which was developed by the Lee group in 1987, and found that RP215 can distinguish Cancer-IgG from B-IgG.12,13 Unexpectedly, the epitope recognized by RP215 carries a unique sialic acid modification localized to a new N-glycosylation site, Asn162, in the CH1 domain of the IgG heavy chain14 rather than the classic Asn297 site.15 Cancer-derived sialylated IgG (SIA-CIgG) can directly promote cancer progression by binding to integrin α6β4 and activating integrin-FAK signaling. Evidently, RP215 shows strong anticancer activity.14
In recent years, it has been found that Ig can negatively regulate the T-cell-mediated immune response. IgG and IgA in colostrum can maintain immune homeostasis in the neonatal intestinal mucosa by inhibiting T-cell activation;16 in addition, sialylated IgM has immunomodulatory effects on effector T cells.17 Importantly, intravenous immunoglobulin (IVIG), which is widely used in the clinic, has anti-inflammatory effects. Mechanistic studies suggest that the anti-inflammatory effect of IVIG is dependent on a small fraction of IgG, in which the IgG Fc domain is modified by sialic acid.15,18 However, the detailed mechanism needs to be investigated further.19 Sialylated IgG in IVIG can indirectly inhibit dendritic cell (DC)- and macrophage-mediated CD4+ T-cell activation by binding to the sialic acid receptor dendritic cell-specific ICAM-3 grabbing nonintegrin (DC-SIGN) on DCs.20–22 Siglecs are a family of sialic acid receptors that comprise 15 members in humans, and most of these receptors mediate immune suppression. Inhibitory Siglecs are frequently expressed on some immune cells, such as myeloid cells, monocytes, DCs, NK cells, and B cells, and inhibit the activation of these immune cells.23,24 Recently, growing evidence has shown that Siglecs, including Siglec-3, Siglec-7, Siglec-9, and Siglec-10, are also expressed on effector T cells.25–32 In addition, mouse Siglec-E, Siglec-F, and Siglec-G have also been reported to be expressed on T cells.30,33 Based on these observations, we hypothesized that SIA-CIgG is involved in tumor immune escape by binding to sialic acid receptors on immune cells.
In this study, we first found that SIA-CIgG could inhibit T-cell proliferation and promote tumor growth by inducing reductions in CD4+ and CD8+ T-cell frequencies in tumor tissues. Mechanistically, SIA-CIgG, which depends on sialylation of the novel CH1 domain but not the classic CH2 domain, directly bound to Siglecs, such as Siglec-7 and Siglec-10, on effector CD4+ and CD8+ T cells, inhibiting T-cell proliferation and promoting tumor growth. Importantly, we found that Siglecs, such as Siglec-3, Siglec-6, Siglec-7, and Siglec-10, were expressed at significantly higher levels on CD4+ and CD8+ T cells from patients with cancer than on those from healthy donors. These findings reveal that SIA-CIgG may be a novel ligand for sialic acid receptors, and that the Siglecs expressed on effector T cells are potential immune checkpoint molecules.
Results
IgG containing Cancer-IgG in the TME can directly suppress CD4+ and CD8+ T-cell proliferation
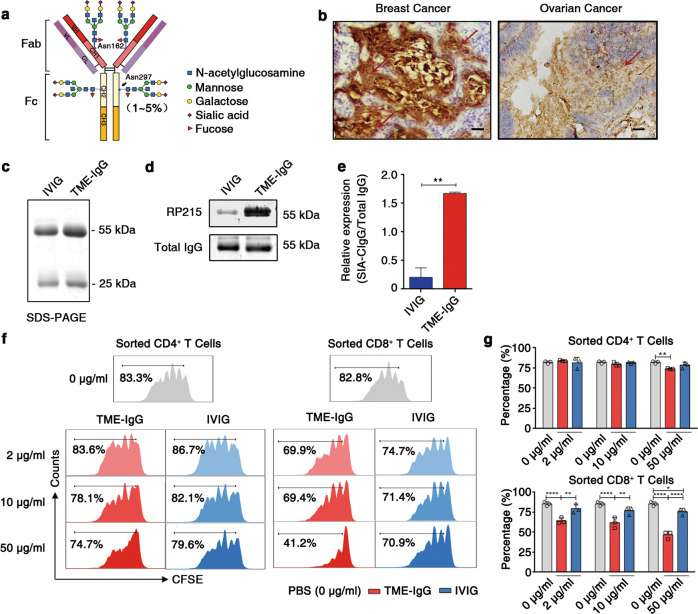
RP215 recognizes Cancer-IgG, which was found to be widely expressed in epithelial cancer cells, such as breast, liver, lung, and colon cancer cells, especially in the cancer stem cell populations.4,14,34,35 The expression of RP215-recognized Cancer-IgG directly indicates a poor prognosis in patients with one of several epithelial carcinomas, such as breast cancer,35 bladder cancer, salivary gland cystadenocarcinoma,36 non-small cell lung cancer,14,37 and renal cell carcinoma.38 Moreover, recognition by RP215 depends on a novel N-glycosylation site-related sialylated epitope located in CH1 (Asn162) of the Cancer-IgG heavy chain,14 which is distinct from the classic N-glycosylation site located in CH2 (Asn297)15 (Fig. 1a). Therefore, we first identified SIA-CIgG in the TME of various cancers, including breast cancer and ovarian cancer, by immunohistochemistry using RP215 and found a large amount of SIA-CIgG deposition in the TMEs (Fig. 1b). Next, we purified IgG with protein G and found a high proportion of RP215-recognized IgG in the IgG purified from the TME (TME-IgG) by Western blotting. In contrast, a commercial anti-human IgG antibody, which mainly recognizes circulating IgG, recognized both TME-IgG and IVIG (Fig. 1c–e). These results suggest that there is a large amount of SIA-CIgG in the TME. Furthermore, SIA-CIgG carries a unique sialic acid modification; therefore, RP215 can be used to distinguish SIA-CIgG from B-IgG.
Fig. 1.
Purified TME-IgG containing abundant SIA-CIgG can directly inhibit T-cell proliferation. a The structure of the sugar moiety that is linked to the Fc fragment of intravenous immunoglobulin (IVIG) with enhanced anti-inflammatory activity. The structures of sialylated N-glycans located at the Asn162 site of SIA-CIgG Fab fragments and classic sialylated N-glycans attached to Asn297 in IgG Fc fragments are shown. Variable residues such as N-acetylglucosamine (blue square), mannose (green circle), galactose (yellow circle), sialic acid (purple diamonds), and fucose (red triangle) are shown. The classic sialylated N-glycans attached to Asn297 in IgG were present in ~1–5% of the total serum IgG pool. b Immunohistochemical staining for SIA-CIgG in the TME of human breast and ovarian cancer tissue samples using RP215. Scale bars, 100 μm. c Identification of purified TME-IgG using SDS-PAGE. IVIG was used as a control. d SIA-CIgG in TME-IgG detected by western blotting using RP215, and total IgG estimated by a commercial anti-IgG antibody. e Expression of SIA-CIgG relative to that of the total IgG evaluated as in (d) and calculated with the average gray value of three measurements for each band. f Proliferation of PBS-, TME-IgG-, and IVIG-treated CD4+ and CD8+ T cells. The doses of TME-IgG or IVIG were 0 μg/ml, 10 μg/ml, and 50 μg/ml. Cells were sorted by flow cytometry, labeled with carboxyfluorescein succinimidyl ester (CFSE), and then activated for 72 h using precoated 3 μg/ml anti-CD3 and 1 μg/ml anti-CD28 mAbs. g Proportion and statistical significance of proliferating CD4+ and CD8+ T cells sorted by flow cytometry and treated with TME-IgG or IVIG as in (f) (n = 3 each group). IVIG intravenous immunoglobulin, TME-IgG tumor microenvironment IgG. Small horizontal lines (e, g) indicate the mean ( ± s.d.). *P ≤ 0.05, **P ≤ 0.01, and ****P ≤ 0.0001 (two-tailed Student’s t test for unpaired data). Data are from one experiment that was representative of three (c–g) independent experiments with similar results. Also see Supplementary Fig. S1.
Currently, IVIG is widely used in the treatment of autoimmune diseases and many inflammatory diseases. Mechanistically, IVIG, which is dependent on its 1–5% sialylated IgG content,15,39,40 can directly bind to the sialic acid receptor DC-SIGN on DCs or macrophages, decreasing the activity of the DCs or macrophages and thus indirectly inhibiting CD4+ T-cell activation mediated by the DCs or macrophages.20–22,41 To confirm the immunomodulatory effect of TME-IgG, which contains a high proportion of SIA-CIgG, TME-IgG was first purified by protein G and then used to treat peripheral blood mononuclear cells (PBMCs), including lymphocytes, monocytes, and DCs, isolated from healthy donors. These cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and stimulated with anti-CD3 and anti-CD28 mAbs, and the proliferation of CD4+ and CD8+ T cells was measured after 72 h. As expected, the proliferation of CD4+ and CD8+ T cells was markedly inhibited by TME-IgG in a dose-dependent manner. However, IVIG showed a weaker immunomodulatory effect on T-cell proliferation than TME-IgG (Supplementary Fig. S1a). To determine whether the inhibitory effect of TME-IgG is myeloid cell dependent, we treated peripheral blood lymphocytes (PBLs) with TME-IgG or IVIG and stimulated these cells with anti-CD3 and anti-CD28 mAbs in the absence of myeloid cells. Unlike those of previous reports,20–22,41 the results in this study clearly showed that TME-IgG retained its inhibitory effects on T-cell proliferation; similarly, the inhibitory effect of TME-IgG was stronger than that of IVIG (Supplementary Fig. S1b). Accordingly, the levels of the Th1 cytokines IL-2, IFN-γ, and TNF-α and the Th2 cytokines IL-4 and IL-10 were significantly decreased in culture supernatants after treatment with 50 μg/ml TME-IgG (Supplementary Fig. S1c), which suggested that TME-IgG could directly inhibit T-cell proliferation. IL-6 levels were not changed by the addition of TME-IgG or IVIG. Subsequently, CD4+ and CD8+ T cells were sorted by flow cytometry. As expected, TME-IgG exhibited a significant inhibitory effect on the proliferation of sorted CD4+ or especially CD8+ T cells that was dose dependent (Fig. 1f, g). It is worth noting that no obvious inhibitory effect or dose-dependent effect of TME-IgG on the proliferation of sorted B cells stimulated by staphylococcal protein A was observed (Supplementary Fig. S1d). These results demonstrate that IgG in a TME containing Cancer-IgG can directly suppress CD4+ and CD8+ T-cell proliferation.
TME-IgG containing SIA-CIgG promotes tumor growth in mice in a manner dependent on reducing T-cell frequencies
To perform the necessary in vivo experiments, we first evaluated the immunosuppressive effects of both mouse TME-IgG and human TME-IgG on mouse T cells. We purified mouse TME-IgG from B16 and EMT-6 cancer tissue samples that were grown in B and T-cell-deficient RAG1−/− or NOD SCID mice. We found that both mouse TME-IgG and human TME-IgG significantly suppressed the proliferation of mouse T cells activated with anti-CD3 and anti-CD28 mAbs or ConA (Supplementary Fig. S2a–c). These results suggest that both human and mouse TME-IgG have immunosuppressive effects on mouse T-cell proliferation. Importantly, human TME-IgG has cross-species immunosuppressive effects on mouse T cells; thus, it is reasonable that human TME-IgG can be used in place of mouse TME-IgG.
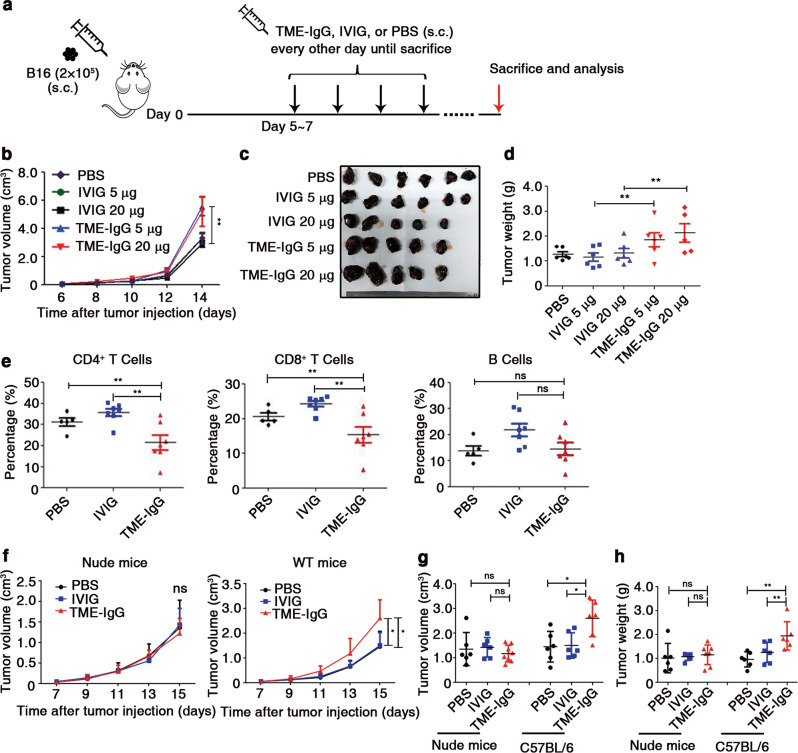
Next, we first established a mouse melanoma model in C57BL/6 mice (Fig. 2a), in which human TME-IgG or IVIG (as a control) was injected subcutaneously into the peritumoral area, and the progrowth effects of these treatments were monitored by measuring tumor size. TME-IgG significantly promoted tumor growth, whereas IVIG had no influence on tumor growth (Fig. 2b–d). Notably, the numbers of CD4+ and CD8+ T cells in the draining lymph nodes (DLNs) of the mice significantly decreased after treatment with TME-IgG (Fig. 2e).
Fig. 2.
TME-IgG promotes tumor growth by decreasing the proportions of both CD4+ and CD8+ T cells. a Establishment of mouse models used in (b–h). b On the 6th day after subcutaneous (s.c.) injection of B16 cells, 5 μg or 20 μg TME-IgG dissolved in 100 μl PBS (n = 5 per group), IVIG (n = 6 (5 μg), and n = 5 (20 μg)), or 100 μl PBS (n = 6 per group) was injected subcutaneously into the peritumoral area, and the tumor volume of each group was measured. Images of the tumors were taken (c), and tumor weights (d) were measured on day 14 in mice, as in (b). e Proportions of T and B lymphocytes in the draining lymph nodes (DLNs) of each group (TME-IgG, n = 7 (four samples from the 20-μg group and three samples from the 5-μg group); IVIG, n = 7 (four samples from the 20-μg group and three samples from the 5-μg group); and PBS, n = 5). Tumor growth curves of nude mice (TME-IgG, n = 8; IVIG and PBS, n = 6) or wild-type C57BL/6 mice (n = 6 for each group) treated with 20 μg TME-IgG or IVIG dissolved in PBS or the same volume of PBS (control) are shown in (f). Tumor volume (g) and weight (h) were measured on the 17th day after B16 implantation. Each symbol represents an individual tumor sample; small horizontal lines indicate the mean ( ± s.e.m. in b, d, and e; ± s.d. in f, g, and h). ns, not significant (P ≥ 0.05); *P ≤ 0.05; and **P ≤ 0.01 (one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparisons test (b, d, e–h)). Data are from one experiment that was representative of four (b–e) independent experiments with similar results. Also see Supplementary Fig. S2.
To determine whether the protumor effect of TME-IgG was dependent on T cells, we performed the same experiments in T-cell-deficient nude mice. The results showed that the TME-IgG-treated mice exhibited no protumor effect compared with wild-type mice (Fig. 2f–h). To analyze whether TME-IgG can directly promote the proliferation of B16 cells, TME-IgG or IVIG was used to treat B16 cells in vitro for 3 days, and the proliferation of the B16 cells was assessed by a CCK8 assay. The results suggested that neither TME-IgG nor IVIG directly promoted the proliferation of B16 cells in vitro in the absence of immune cells (Supplementary Fig. S2d). Thus, both the in vivo and in vitro results provide evidence that the protumor effect of TME-IgG is dependent on a reduction in the effector T-cell frequency induced by TME-IgG.
SIA-CIgG but not B-IgG can directly inhibit proliferation and reduce effector T-cell frequencies in vitro and in vivo
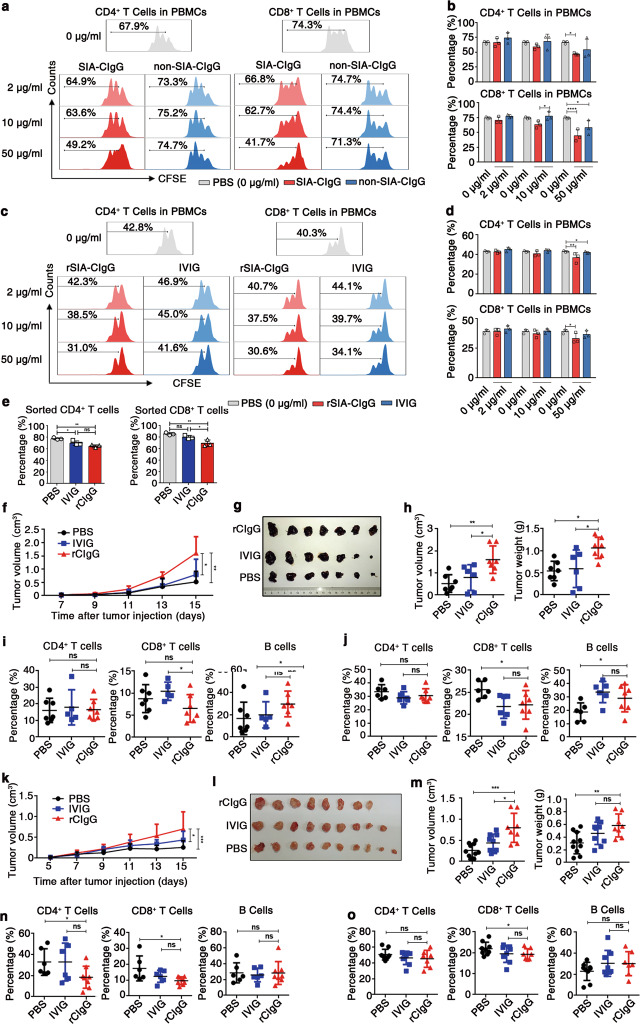
To exclude the possibility that TME-IgG contains B-IgG, we utilized an affinity column coupled with RP215 to purify SIA-CIgG from TME-IgG, as described previously,14 and the inhibitory effect of SIA-CIgG on T-cell proliferation was also measured, as described above. Compared with the nonbinding IgG fractions, the SIA-CIgG fraction significantly inhibited T-cell proliferation, especially CD8+ T-cell proliferation (Fig. 3a, b).
Fig. 3.
Inhibition of proliferation and promotion of tumor immune escape mediated by Cancer-IgG. a SIA-CIgG was purified from TME-IgG by an RP215 affinity column, then PBS, SIA-CIgG, or non-SIA-CIgG (the fraction of TME-IgG that did not bind to the RP215 affinity column) was added to peripheral blood mononuclear cells (PBMCs) that were activated as described in Fig. 1f. The proliferation of CFSE-labeled CD4+ and CD8+ T cells was measured after 72 h. The doses of SIA-CIgG or non-SIA-CIgG were 2 μg/ml, 10 μg/ml, and 50 μg/ml. b Proportions and statistical significance of proliferating CD4+ and CD8+ T cells among all PBMCs treated by SIA-CIgG or non-SIA-CIgG as described in (a) (n = 3 each group), as evaluated by flow cytometry. c Proliferation of activated CFSE-labeled CD4+ and CD8+ T cells treated with rSIA-CIgG or IVIG in the PBMC culture system. The doses of rSIA-CIgG or IVIG were 2 μg/ml, 10 μg/ml, and 50 μg/ml. d Proportions and statistical significance of proliferating CD4+ and CD8+ T cells among all PBMCs treated with rSIA-CIgG or IVIG as described in (c) (n = 3 each group), as determined by flow cytometry. e Proportions of proliferating CD4+ and CD8+ T cells sorted by flow cytometry and treated with rCIgG (rSIA-CIgG) or IVIG at a dose of 50 μg/ml (n = 3 each group). Small horizontal lines (b, d, and e) indicate the mean ( ± s.d.). ns, not significant (P ≥ 0.05); *P ≤ 0.05; **P ≤ 0.01; and ****P ≤ 0.0001 (two-tailed Student’s t test for unpaired data). The protumor effect of subcutaneously injected rCIgG (20 μg, n = 7), IVIG (20 μg, n = 6), or PBS (n = 7) in C57BL/6 mice was measured by evaluating tumor growth (f), tumor images (g), and tumor volume and weight (h) on the 15th day after B16 injection. The proportions of lymphocytes in tumor tissue (i) and DLNs (j) were measured by flow cytometry. Tumor growth curves (k) of rCIgG- (20 μg, n = 8), IVIG- (20 μg, n = 9), and PBS-treated (n = 10) BALB/c mice, tumor images (l), and tumor volume and weight (m) were determined on the 15th day after injection of EMT-6 cells. n The proportions of lymphocytes in tumor tissue. o The proportions of lymphocytes in DLNs (rCIgG, n = 8; IVIG and PBS, n = 6). Each symbol represents an individual tumor sample; small horizontal lines indicate the mean ( ± s.d.). ns, not significant (P ≥ 0.05); *P ≤ 0.05; **P ≤ 0.01; and ***P ≤ 0.001 (one-way ANOVA followed by Tukey’s multiple-comparisons test). Data are from one experiment that was representative of three (a) or six (c) independent experiments with similar results. Also see Supplementary Fig. S3a.
Subsequently, in accordance with our previous findings, we produced recombinant Cancer-IgG with VH5-51/D3-9/JH4 (GenBank: AY270190.1) heavy chains and Vκ4-1/Jκ3 (GenBank: AY505537.1) light chains, which are widely expressed by many epithelial cancer cells, such as lung cancer, breast cancer, colon cancer, and oral carcinoma cells.4 As expected, our results showed that recombinant Cancer-IgG expressed in CHO cells could be recognized by RP215 (Supplementary Fig. S3a). More importantly, this recombinant SIA-CIgG (rSIA-CIgG) also had significant inhibitory effects on the proliferation of CD4+ and CD8+ T cells with or without myeloid cells (Fig. 3c–e).
To further examine the inhibitory effect of rSIA-CIgG in vivo, we also established B16 tumor model in C57BL/6 mice, and tumor growth and the proportion of immune cells in the tumor tissue or DLNs were evaluated. Similar to TME-IgG, rSIA-CIgG significantly promoted tumor growth (Fig. 3f–h) and markedly decreased CD8+ T-cell frequencies in both tumor tissue (Fig. 3i) and the DLNs (Fig. 3j). Next, we established a mouse breast cancer EMT-6 model in BALB/c mice and treated these mice with rSIA-CIgG. As seen in the B16 model mice, rSIA-CIgG showed significant protumor effects (Fig. 3k–m) and decreased CD4+ and CD8+ T-cell frequencies in both tumor tissue (Fig. 3n) and the DLNs (Fig. 3o). Together, these results suggest that the majority of TME-IgG is produced by cancer cells; moreover, SIA-CIgG can be involved in tumor immune escape by reducing the proportions of effector CD4+ and CD8+ T cells.
Inhibitory effect of SIA-CIgG on effector T cells is dependent on sialylation
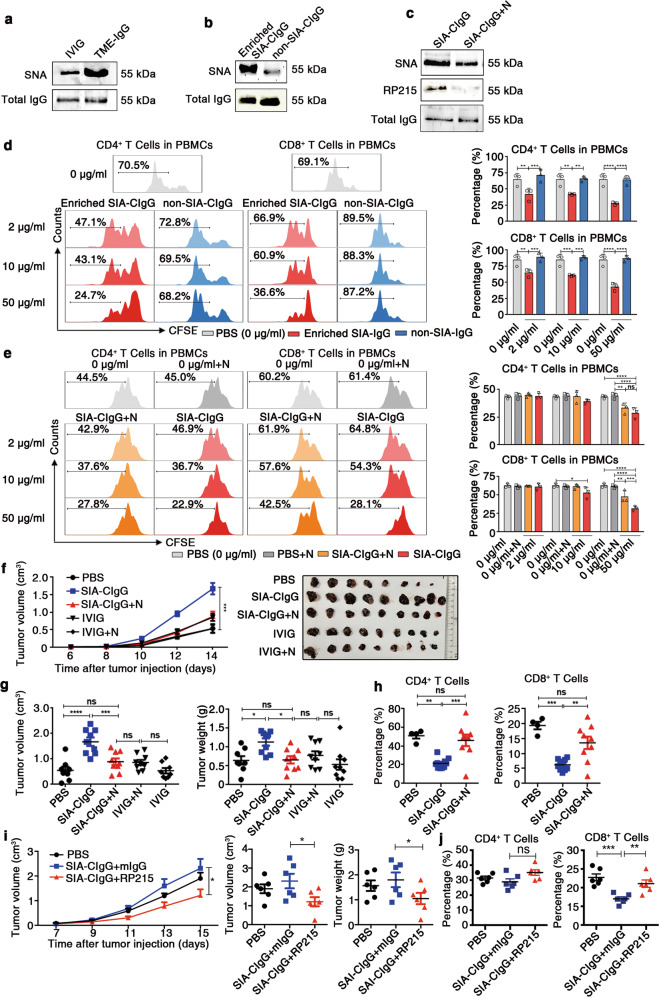
To analyze the modification of SIA-CIgG with sialic acid, Sambucus nigra lectin (SNA), a lectin specific for α2,6 sialic acids, was used to determine the sialylation state of SIA-CIgG. Nearly 1–5% of IVIG was previously reported to be sialylated, and IVIG was used as a control.18,39,40 As expected, TME-IgG displayed a higher level of sialylation than IVIG by western blotting using the lectin SNA (Fig. 4a). In addition, Maackia amurensis lectin II (MAL II), a lectin specific for α2,3 sialic acids, was also used to determine the sialylation state of SIA-CIgG. We used α2,3 neuraminidase to remove α2,3 terminal sialic acids on SIA-CIgG, and the result showed that the signal for RP215 remained unchanged (Supplementary Fig. S3b). Next, we investigated whether the inhibitory effect of SIA-CIgG on T cells was sialylation dependent. Utilizing an SNA affinity column, we first enriched sialylated IgG from total TME-IgG (Fig. 4b) or used neuraminidase to remove terminal sialic acids from SIA-CIgG (Fig. 4c). The Cancer-IgG samples in which sialic acids had been enriched or removed were then used to treat sorted T cells that were stimulated with anti-CD3 and anti-CD28 mAbs. The results showed that the enriched SIA-CIgG fractions, but not the nonbinding fractions of IgG, displayed strong inhibitory effects on CD4+ and CD8+ T cells (Fig. 4d). In contrast, the inhibitory effect of SIA-CIgG on T cells, especially CD8+ T cells, was significantly reduced after the sialic acids were removed (Fig. 4e).
Fig. 4.
Inhibitory effect of Cancer-IgG on T cells is sialylation dependent. a Comparison of the sialylation state between TME-IgG and IVIG. The sialylation state was identified by SNA lectin blotting (upper panel), and total IgG was estimated with a commercial anti-IgG antibody by western blotting (lower panel). b SIA-CIgG was enriched by an SNA affinity column. The sialylation state of SIA-CIgG and nonsialylated Cancer-IgG (the nonbinding fractions) was determined by SNA lectin blotting (upper panel) and western blotting (lower panel). c Sialic acid residues in SIA-CIgG were digested with neuraminidase, and SIA-CIgG was identified by SNA lectin, RP215, or a commercial anti-IgG antibody. N, neuraminidase. d Proliferation (left) and proportions (right) of CFSE-labeled CD4+ and CD8+ T cells treated with Enriched SIA-CIgG or non-SIA-CIgG. The doses of Enriched SIA-CIgG and non-SIA-CIgG were 2 μg/ml, 10 μg/ml, and 50 μg/ml. e Proliferation (left) and proportions (right) of CFSE-labeled CD4+ and CD8+ T cells treated with PBS, neuraminidase, SIA-CIgG, or sialic acid-removed Cancer-IgG. The doses of SIA-CIgG or sialic acid-removed Cancer-IgG were 2 μg/ml, 10 μg/ml, and 50 μg/ml. Small horizontal lines (d and e right) indicate the mean ( ± s.d.). ns, not significant (P ≥ 0.05); *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; and ****P ≤ 0.0001 (two-tailed Student’s t test for unpaired data). Tumor growth curves, tumor images (f), and tumor volume and weight (g) were determined on the 14th day after injection of B16 cells into C57BL/6 mice (PBS, n = 8; SIA-CIgG, SIA-CIgG digested with neuraminidase, IVIG, and IVIG digested with neuraminidase, n = 9 per group). Key to (f). h Proportions of lymphocytes in the DLNs of mice treated with SIA-CIgG (n = 9), SIA-CIgG digested with neuraminidase (n = 9) or PBS (n = 4). Key to (f). i Tumor growth curves and tumor volume and weight were determined on the 15th day after injection of B16 cells into C57BL/6 mice and treatment with 5 μg SIA-CIgG and 5 μg RP215 (or mouse IgG or PBS) (n = 6). j Proportions of T cells in DLNs. Each symbol represents an individual tumor sample; small horizontal lines indicate the mean ( ± s.e.m.). ns, not significant (P ≥ 0.05); *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; and ****P ≤ 0.0001 (one-way ANOVA followed by Tukey’s multiple-comparisons test). Data are from one experiment that was representative of three (a–c) or six (d, e) independent experiments with similar results. Also see Fig. S3c–i.
To determine if the protumor effect of SIA-CIgG depended on its sialylation, we used neuraminidase to remove sialic acids from SIA-CIgG and treated B16 model mice as described above. We found that after removing the sialic acids, the protumor effect of SIA-CIgG was significantly reduced (Fig. 4f, g). Correspondingly, the populations of effector CD4+ and CD8+ T cells in the DLNs were evidently enhanced (Fig. 4h). We also removed sialic acids from rSIA-CIgG and treated EMT-6 model mice in vivo with the treated rSIA-CIgG. Similarly, the protumor effect of rSIA-CIgG was significantly reduced (Supplementary Fig. S3c–e), and the proportion of effector CD8+ T cells in the tumor tissue was enhanced (Supplementary Fig. S3f). To exclude the direct inhibitory effects of neuraminidase on EMT-6 cancer cells, we used neuraminidase to treat EMT-6 model mice in vivo, and the results showed that there was no significant difference in the in vivo protumor effect between neuraminidase and a PBS control (Supplementary Fig. S3g–i). Since the monoclonal antibody RP215 only recognizes the sialylated epitope of Cancer-IgG,14 we used RP215 to bind the sialylated epitope of SIA-CIgG before SIA-CIgG was used to treat B16 model mice in vivo. As expected, RP215 treatment significantly decreased the protumor effect of SIA-CIgG and reversed the reductions in CD4+ and CD8+ T-cell frequencies in the DLNs (Fig. 4i, j). Collectively, these results demonstrate that the inhibitory effect of SIA-CIgG on effector T cells is dependent on SIA-CIgG sialylation.
Fab fragments of SIA-CIgG containing the CH1 domain that are recognized by RP215 display a strong inhibitory effect on T-cell proliferation
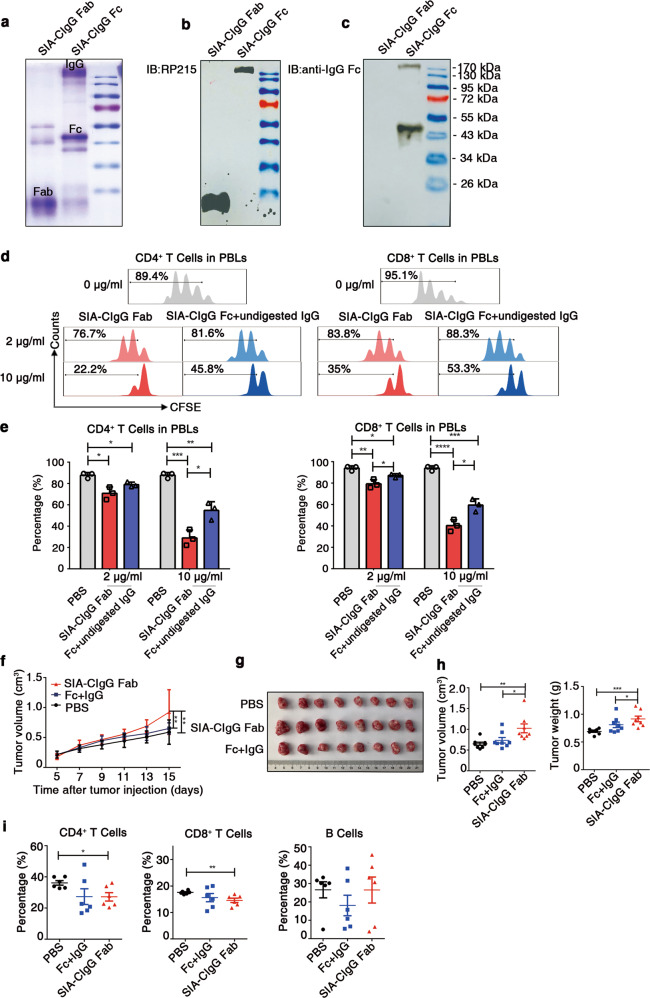
To further explore whether the inhibitory effect of SIA-CIgG is dependent on the CH1 domain recognized by RP215, papain was used for digestion to obtain Fab and Fc fragments (Fig. 5a). As is shown, RP215 only recognized Fab fragments and undigested IgG, not Fc fragments (Fig. 5b, c).
Fig. 5.
Fab fragments of SIA-CIgG containing the CH1 domain that are recognized by RP215 display a strong inhibitory effect on T-cell proliferation. a Coomassie light blue staining of SIA-CIgG Fab and SIA-CIgG Fc fragments digested by papain. The sialylation site was identified with RP215 (b) and a commercial anti-human-IgG Fc antibody (c) by western blotting. d SIA-CIgG Fab and SIA-CIgG Fc + undigested IgG fragments were digested by papain. Then, PBS, the SIA-CIgG Fab fragments or the SIA-CIgG Fc + undigested IgG fragments were added to peripheral blood lymphocytes (PBLs) that were activated as described in Fig. 1f. The proliferation of CFSE-labeled CD4+ and CD8+ T cells was measured after 72 h. The doses of SIA-CIgG Fab fragments or SIA-CIgG Fc + undigested IgG fragments were 0 μg/ml, 2 μg/ml, and 10 μg/ml. e Proportions and statistical significance of proliferating CD4+ and CD8+ T cells among all PBLs treated with PBS, SIA-CIgG Fab fragments or SIA-CIgG Fc + undigested IgG fragments (Fc + undigested IgG) as described in (d) (n = 3 each group), as determined by flow cytometry. Small horizontal lines (e) indicate the mean (± s.d.). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001 (two-tailed Student’s t test for unpaired data). Tumor growth curves (f), tumor images (g), and tumor volume and weight (h) were determined on the 15th day after injection of EMT-6 cells into BALB/c mice (PBS, n = 8; SIA-CIgG Fab, 20 μg, n = 8; and SIA-CIgG Fc + undigested IgG, 20 μg, n = 8). i Proportions of lymphocytes in the tumors of mice treated with PBS (n = 8), SIA-CIgG Fab fragments (n = 8), or SIA-CIgG Fc + undigested IgG fragments (Fc + IgG) (n = 8). Each symbol represents an individual tumor sample; small horizontal lines indicate the mean ( ± s.e.m.). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001 (one-way ANOVA followed by Tukey’s multiple-comparisons test). Data are from one experiment that was representative of three (a–d) or two (f–i) independent experiments with similar results.
Next, we addressed whether the RP215-recognized Fab fragments of SIA-CIgG containing the CH1 domain display a strong inhibitory effect on T-cell proliferation. Fab fragments were used to treat sorted human T cells stimulated with anti-CD3 and anti-CD28 mAbs in vitro, and Fc fragments (containing some undigested SIA-CIgG) were used as controls. Although both the Fab fragments and the control (Fc fragments and undigested SIA-CIgG) displayed strong inhibitory effects on CD4+ and CD8+ T cells that were dose dependent, the Fab fragments showed stronger inhibitory effects than the Fc fragments (Fig. 5d, e).
Next, Fab fragments were used to treat EMT-6 model mice, as described above. As expected, we found that Fab fragments but not controls, including PBS and Fc fragments mixed with undigested SIA-CIgG, showed a significant protumor effect (Fig. 5f–h). Moreover, effector CD4+ and CD8+ T-cell infiltration into tumor tissue was decreased (Fig. 5i). Thus, these data demonstrate that RP215-recognized Fab fragments of SIA-CIgG containing the CH1 domain have a strong inhibitory effect on T-cell proliferation.
Siglecs mediate the suppression of T-cell proliferation induced by SIA-CIgG
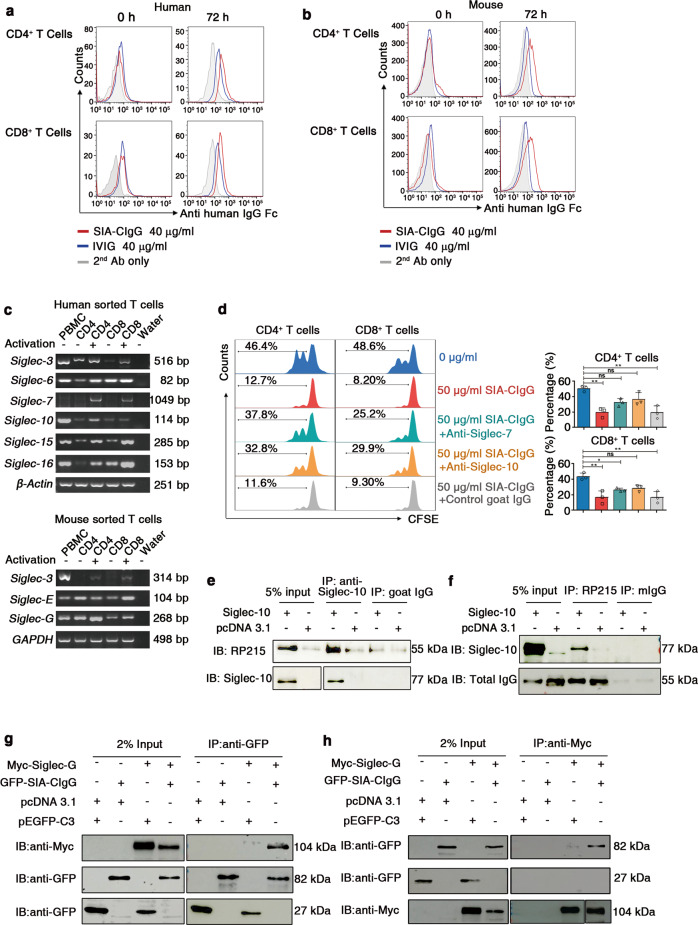
As shown above, SIA-CIgG directly suppressed T-cell proliferation. Hence, we hypothesized that SIA-CIgG binds to some suppressive sialic acid receptors on the surface of T cells. We first determined whether SIA-CIgG directly binds to the T-cell surface of cells in either the resting or activated state. The results indicated that SIA-CIgG could bind to resting CD8+ T cells, but hardly bound to resting CD4+ T cells. However, after the T cells were stimulated by phytohemagglutinin (PHA) or anti-CD3 and anti-CD28 mAbs, the binding of SIA-CIgG to CD4+ or CD8+ T cells significantly increased (Fig. 6a). Correspondingly, after mouse T cells were stimulated by anti-CD3 and anti-CD28 mAbs, the binding of human SIA-CIgG to the mouse CD4+ or CD8+ T cells also increased (Fig. 6b). These results suggested that there were some unidentified receptors for SIA-CIgG on activated CD4+ and CD8+ T cells.
Fig. 6.
T-cell suppression induced by SIA-CIgG is mediated by Siglecs. a Resting human PBMCs activated by 20 μg/ml phytohemagglutinin (PHA) or anti-CD3 and anti-CD28 mAbs for 72 h were incubated with 40 μg/ml SIA-CIgG that was enriched by an RP215 affinity column, 40 μg/ml IVIG or PBS (2nd Ab) for 45 min; washed; and incubated with phycoerythrin (PE)-labeled anti-human IgG. The binding of SIA-CIgG and IVIG was detected by flow cytometry. b Resting mouse lymph node-derived CD4+ and CD8+ T cells activated by anti-CD3 and anti-CD28 mAbs for 48 h were incubated with 40 μg/ml SIA-CIgG that was enriched by an RP215 affinity column, 40 μg/ml IVIG or PBS (2nd Ab) for 45 min; washed and incubated with PE-labeled anti-human IgG. The binding of SIA-CIgG and IVIG was detected by flow cytometry. c RT-PCR detection of human (upper) and mouse (lower) Siglec gene transcripts in CD4+ and CD8+ T cells sorted by flow cytometry and activated with anti-CD3 and anti-CD28 mAbs or left unstimulated. The PCR mixture without templates was used as a negative control (water), and PBMCs were used as a positive control. d Proliferation (left), proportions, and statistical significance (right) of 50 μg/ml SIA-CIgG-treated PBLs blocked with 20 μg/ml goat anti-human Siglec-7 antibody or anti-human Siglec-10 antibody. Nonspecific goat IgG was used as a control. The blocking antibodies were added 2 h prior to the addition of SIA-CIgG. Small horizontal lines (d right) indicate the mean ( ± s.d.). ns, not significant (P ≥ 0.05); *P ≤ 0.05; and **P ≤ 0.01, (two-tailed Student’s t test for unpaired data). e, f Interaction between endogenous SIA-CIgG and exogenous Siglec-10 in the human lung cancer cell line NCI-H520, as detected by Co-IP. mIgG, isotype mouse IgG. g, h Interaction between exogenous Myc-Siglec-G and GFP-SIA-CIgG in the cell line 293 T by Co-IP. The plasmids pcDNA 3.1 and pEGFP-C3 were used as controls. Data are from one experiment that was representative of three (a–h) independent experiments with similar results. Also see Supplementary Figs. S4 and S5.
Siglecs have been reported to be sialic acid-binding receptors, and the Siglec family includes 15 members in humans and 9 members in mice.42 Siglecs can transmit either inhibitory (human: Siglec-2, Siglec-3, Siglec-5, Siglec-6, Siglec-7, Siglec-8, Siglec-9, Siglec-10, and Siglec-11; mouse: Siglec-2, Siglec-3, Siglec-E, Siglec-F, and Siglec-G) or activating (human: Siglec-14, Siglec-15, and Siglec-16; mouse: Siglec-H and Siglec-15) signals depending on the presence of immunoreceptor tyrosine-based inhibitory motifs (ITIMs) or immunoreceptor tyrosine-based activation motifs (ITAMs), respectively, in the cytosolic region. Notably, most Siglecs provide an inhibitory signal to immune cells.23,28,29,43 Because the inhibitory effect of SIA-CIgG is dependent on sialic acid modification, we tried to identify inhibitory Siglecs that can be expressed on T cells. We first used the ImmuSort website (http://immusort.bjmu.edu.cn)44 to analyze the gene expression of all Siglec members with ITIMs from 433 datasets containing 10,422 human samples in the GEO database and found that the genes encoding Siglec-6, Siglec-7, and Siglec-10 were expressed at significant levels in human CD4+ and CD8+ T cells (Supplementary Fig. S4a). In addition, from 455 datasets containing 3929 mouse samples, we found that the mouse homologs of the human Siglec-3 and Siglec-10 genes, named the Siglec-3 (CD33) and Siglec-G (Siglec-10) genes, were expressed at significant levels in mouse CD4+ and CD8+ T cells (Supplementary Fig. S4b). Next, we determined whether Siglec transcripts are expressed in activated or resting CD4+ or CD8+ T cells sorted from humans or mice. We found that human Siglecs, such as Siglec-3, Siglec-6, Siglec-10, Siglec-15, and Siglec-16, were expressed in both resting and activated CD4+ and CD8+ T cells. However, Siglec-7 was only detected in activated CD4+ and CD8+ T cells. Mouse Siglec-E and Siglec-G were expressed in both resting and activated CD4+ and CD8+ T cells. However, mouse Siglec-3 was only detected in activated CD4+ and CD8+ T cells (Fig. 6c). The transcripts of other human Siglecs were not detected in T cells (Supplementary Fig. S5a). Subsequently, Siglec-7 and Siglec-10 expression was found on both CD4+ and CD8+ T cells by flow cytometry (Supplementary Fig. S5b). Moreover, mouse Siglec-G was determined to be expressed on both CD4+ and CD8+ T cells (Supplementary Fig. S5c). Importantly, neutralizing antibodies for either Siglec-7 or Siglec-10 significantly blocked the SIA-CIgG-mediated inhibition of CD4+ and CD8+ T-cell proliferation (Fig. 6d).
We next investigated whether Siglecs interact with SIA-CIgG by overexpressing human Siglec-10 in the lung cancer cell line NCI-H520. We found that endogenous SIA-CIgG interacted with exogenous Siglec-10 by coimmunoprecipitation (Co-IP) (Fig. 6e, f). Correspondingly, we coexpressed one of three mouse inhibitory Siglecs (Siglec-3, Siglec-E, and Siglec-G) and human SIA-CIgG in the cell line 293T, and found that human GFP-SIA-CIgG interacted with exogenous Myc-Siglec-G and Myc-Siglec-E by Co-IP (Fig. 6g, h; Supplementary Fig. S5d, e). In contrast, exogenous His-Siglec-3 (CD33) did not interact with GFP-SIA-CIgG (Supplementary Fig. S5f).
Siglecs are highly expressed in effector T cells from patients with cancer but not those from healthy donors
Since SIA-CIgG inhibited T-cell proliferation by interacting with Siglecs, we examined whether Siglecs with ITIMs are expressed on effector T cells in the peripheral blood or TME of cancer patients. We first analyzed the transcripts of Siglec family members in tumor-specific CD8+ T cells from patients with melanoma or head and neck cancer, CD8+ T cells in the TME of patients with lung cancer and CD4+ T cells in the peripheral blood of patients with breast cancer in microarray or RNA-Seq datasets from the GEO database. The results for 18 melanoma patients (GSE24536) revealed that there was a significant increase in Siglec-10 expression in tumor-specific CD8+ T cells compared to naive CD8+ T cells (Supplementary Fig. S6a). Similarly, compared with CD8+ T cells that infiltrated adjacent tissues, CD8+ T cells in the non-small cell lung carcinoma (NSCLC; adenocarcinoma or squamous carcinoma) TME (GSE90728) expressed higher levels of Siglec-10 (Supplementary Fig. S6b). Siglec-7 and Siglec-10 were also overexpressed in tumor-specific CD8+ T cells compared with nonspecific CD8+ T cells in head and neck cancer (squamous cell carcinoma) (GSE114944) (Supplementary Fig. S6c). Similarly, the expression of Siglec-3, Siglec-7, Siglec-9, and Siglec-10 was also higher in the peripheral blood CD4+ T cells of patients with breast cancer (invasive ductal carcinoma) (GSE36765) than in those of healthy donors, but the difference was not significant (Supplementary Fig. S6d). In addition, Kwok-Kin Wong’s group showed that treating lung tumor model mice with the CDK4/6 inhibitor G1T28 enhanced T-cell activity, contributing to antitumor effects in vivo.45 By analyzing mouse RNA-Seq datasets in the GEO database (GSE89477) to examine the expression of suppressive Siglecs in individual tumor-infiltrating T cells in mouse lung cancer, we found significant increases in Siglec-3 and Siglec-G expression in activated T cells (treated with G1T28) compared with control T cells (Supplementary Fig. S7e).
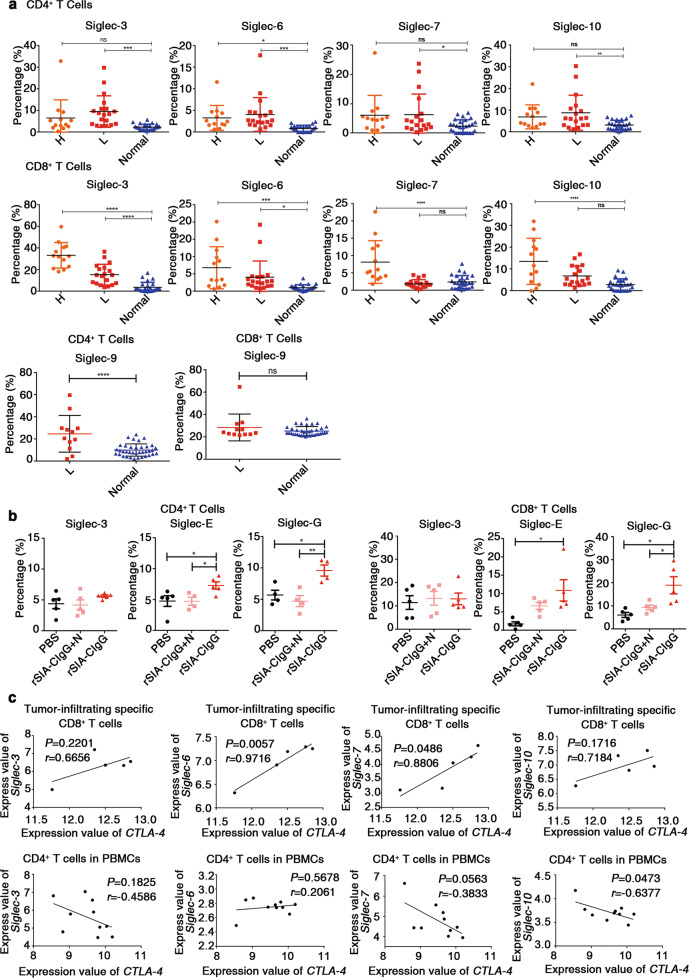
Subsequently, we analyzed the expression of Siglec-3, Siglec-6, Siglec-7, and Siglec-10 on effector T cells in the peripheral blood of 13 patients with head and neck cancer, 19 patients with lung cancer, and 26 control healthy donors by flow cytometry (Siglec-9: 19 patients with lung cancer and 37 healthy donors). We found that Siglec-3, Siglec-6, Siglec-7, Siglec-9, and Siglec-10 levels in the CD4+ T cells, and Siglec-3 and Siglec-6 levels in the CD8+ T cells of the patients with lung cancer were significantly elevated compared to those in the corresponding cells of the healthy donors. In addition, the expression of Siglec-6 in CD4+ T cells and that of Siglec-3, Siglec-6, Siglec-7, and Siglec-10 in CD8+ T cells were also significantly higher in the patients with head and neck cancer (Fig. 7a) than in the healthy donors. Correspondingly, we isolated tumor-infiltrating T cells from EMT-6 tumor model mice that were treated with rSIA-CIgG with or without sialic acid and analyzed the expression of Siglec-3, Siglec-E, and Siglec-G on the T cells by flow cytometry. The results showed that the Siglec-G and Siglec-E expression in the T cells treated with rSIA-CIgG was significantly elevated compared with that in the T cells from control mice (Fig. 7b). These results provide indications that Siglecs, especially Siglec-10, may serve as potential immune checkpoint molecules that facilitate tumor immune escape by interacting with SIA-CIgG.
Fig. 7.
Expression of Siglecs in effector T cells is elevated in patients with cancers, but not in healthy donors. a Frequencies of Siglec+ cells among CD4+ or CD8+ T cells from the PBMCs of patients with cancer (n = 13 (head and neck cancer) and n = 19 (lung cancer)) or healthy donors (n = 26), as determined by flow cytometry. Frequencies of Siglec-9+ cells among CD4+ or CD8+ T cells from the PBMCs of patients with cancer (n = 19 (lung cancer)) or healthy donors (n = 37), as determined by flow cytometry. H, head and neck cancer; L, lung cancer. b Proportions of Siglec-3-, Siglec-E-, and Siglec-G-expressing CD4+ or CD8+ T cells in EMT-6 tumors in mice treated with PBS, rSIA-CIgG, or rSIA-CIgG + N (20 μg, n = 5). N, neuraminidase. rSIA-CIgG, recombinant SIA-CIgG. c Correlations between the expression of Siglec and CTLA-4 in GSE114944 (upper panel) and GSE36765 (lower panel). Each symbol represents an individual sample; small horizontal lines (a, b) indicate the mean ( ± s.d.). ns, not significant (P ≥ 0.05); *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; and ****P ≤ 0.0001 (one-way ANOVA followed by Tukey’s multiple-comparisons test (a, b); two-tailed Pearson correlation for unpaired data (c)). Also see Supplementary Fig. S6.
In addition, we also compared the expression levels of Siglecs with those of two well-known checkpoint molecules, PD-1 and CTLA-4, in the CD4+ and CD8+ T cells of cancer patients, as described above. We found similar expression levels between most Siglecs and PD-1 or CTLA-4 in the CD4+ and CD8+ T cells of head and neck cancer patients (Supplementary Fig. S6f, g); however, no or little coexpression was observed between Siglecs and either PD-1 or CTLA-4 in the CD4+ and CD8+ T cells analyzed in this study (Supplementary Fig. S6h). However, Siglec-6 and Siglec-7 showed significant coexpression with CTLA-4 in the CD8+ T cells of five patients with head and neck cancer (Fig. 7c).
Discussion
To date, IgG deposits in the TME have been believed to be produced by B cells and to generally have antitumor activity. However, in this study, we found that IgG deposits in the TME contained a large portion of cancer-derived IgG. Importantly, we found for the first time that Cancer-IgG could be involved in tumor immune evasion by directly inhibiting proliferation and reducing the proportions of effector CD4+ and CD8+ T cells. Mechanistic research showed that Cancer-IgG could bind to the sialic acid receptors called Siglecs expressed on effector T cells via a unique sialylation of the CH1 domain and could promote the maintenance of the immunosuppressive TME.
Historically, only B cells were believed to produce Igs, but a growing body of evidence has shown that most non-B cells can also express Igs and that cancer cells usually overexpress IgG.4–8,11,34,46,47 However, for a long time, we could not distinguish Cancer-IgG from B-IgG with the commercial anti-IgG antibody that we previously used. Recently, using mAb-RP215, which mainly recognizes Cancer-IgG but not B-IgG, we identified a unique sialic acid modification on Cancer-IgG that occurs as an N-glycosylation-related sialylation modification at the Asn162 site but not the classic Asn297 site.14 Therefore, the unique sialylation modification recognized by RP215 can distinguish Cancer-IgG from B-IgG.
Cancer-IgG has been shown to promote tumorigenesis and metastasis via various mechanisms that are different from those of IgG antibodies.8,10,11,48 Cancer-IgG overexpression is indicative of a high metastatic ability and poor prognosis;11,37,38,49 however, the protumor effect of Cancer-IgG is not fully understood. Recently, using RP215, we found that SIA-CIgG, which carries a unique sialic acid modification, was overexpressed in many epithelial cancer cells, especially in the corresponding cancer stem cells. Importantly, SIA-CIgG maintains the biological behaviors of cancer stem cells, such as self-renewal, drug resistance and metastasis.35 Mechanistic research has indicated that Cancer-IgG can activate the FAK pathway by binding to integrin a6β4 on cancer stem cells.14 However, whether SIA-CIgG is involved in tumor immune escape remains unclear.
Given that the sialylated IgM and sialylated IgG in IVIG have immunomodulatory effects on effector T cells,17 we hypothesized that SIA-CIgG may be involved in tumor immune escape by inhibiting T-cell activity. In this study, we found that unlike the sialylated IgG in IVIG, which was previously reported to indirectly inhibit T-cell activity by directly suppressing myeloid cells, surprisingly, SIA-CIgG could directly inhibit the proliferation of effector T cells, especially CD8+ T cells, in vitro. More importantly, SIA-CIgG could significantly promote tumor progression and reduce effector T-cell frequencies in vivo. However, in T-cell-deficient mice, SIA-CIgG did not have a protumor effect. Notably, the protumor effect of SIA-CIgG was T-cell dependent. Moreover, unlike the sialylated IgG in IVIG, for which the inhibitory effect on myeloid cells depends on the classic Asn297 sialylation epitope in the Fc region, SIA-CIgG mediated its inhibitory effect on effector T cells via the distinctive Asn162 sialylation epitope, which is specifically recognized by RP215.14
Increased levels of sialic acid are pervasive in cancer, and a growing body of evidence demonstrates how hypersialylation is advantageous to cancer cells, particularly from the perspective of modulating immune responses. Sialic acid-binding receptors, such as Siglecs, are well positioned to be exploited by cancer hypersialylation. Mounting evidence also shows that Siglecs modulate key immune cell types in the TME.50 Siglecs have been frequently found to be expressed on innate immune cells, such as tumor-associated neutrophils, tumor-associated macrophages,31,50,51 NK cells, dendritic cells, and B cells.43 However, the results for whether Siglecs are expressed on T cells have not been consistent; in fact, as early as 1999, Siglec-7 was found on a minor subset of CD8+ T cells,26 and then siglec-9 was observed on minor subsets of CD8+ and CD4+ T cells.27 In recent years, a growing body of evidence has proven that several Siglecs, such as Siglec-3, Siglec-7, Siglec-9, and Siglec-10, are found on normal and tumor-infiltrating T cells25–32, and that the Siglecs expressed on T cells can suppress T-cell activation and proliferation.29,30,32 In particular, Siglec-9 expression has been found to be upregulated on tumor-infiltrating T cells from patients with NSCLC, colorectal cancer, or ovarian cancer. Siglec-9 was identified as a potential target for improving T-cell activation by immunotherapy.30 Therefore, we hypothesized that SIA-CIgG can also bind T cells via Siglecs.
We first observed that SIA-CIgG could bind to the cell surface of CD4+ and CD8+ T cells, by RT-PCR and flow cytometry, found that Siglec-3, Siglec-6, Siglec-7, and Siglec-10 were expressed on resting and activated CD8+ T cells and activated CD4+ T cells. Furthermore, neutralizing antibodies against either Siglec-7 or Siglec-10 significantly blocked the SIA-CIgG-mediated inhibition of CD4+ and CD8+ T-cell proliferation. Importantly, we found that SIA-CIgG interacted with Siglec-10 by Co-IP. It is important to note that like mouse TME-IgG, human SIA-CIgG has an immunosuppressive effect on mouse T-cell proliferation that involves binding to mouse Siglecs. These results suggest that Siglecs on mouse T cells can also recognize human N-acetylneuraminic acid (Neu5Ac, the same sialic acid of mouse Neu5Ac) and mediate immunosuppressive effects.
We then addressed whether T cells in patients with cancer widely express Siglecs. In this study, we first mined the GEO database and found that normal and malignant CD4+ and CD8+ T cells could express Siglec-3, Siglec-6, Siglec-7, and Siglec-10, which contain ITIMs in their cytoplasmic tail.43 By analyzing microarray or RNA-Seq datasets in the GEO database to examine the expression of Siglec family members, we found a significant increase in Siglec-10 expression in the tumor-infiltrating CD8+ T cells in patients with NSCLC, and in tumor-specific CD8+ T cells of patients with head and neck cancer or melanoma. Moreover, Siglec-2, Siglec-3, Siglec-7, Siglec-9, and Siglec-10 were also more highly expressed in the peripheral blood CD4+ T cells of patients with breast cancer than in those of healthy donors. Next, we collected peripheral blood from patients with NSCLC or head and neck cancer and healthy donors, which were used as controls, and found that CD4+ and CD8+ T cells could express Siglec-3, Siglec-6, Siglec-7, Siglec-9, and Siglec-10. Importantly, most Siglecs detected in this study were overexpressed on the CD4+ or CD8+ T cells of patients with cancer compared with those of healthy donors, which suggests that Siglecs may serve as potential immune checkpoint molecules and that SIA-CIgG may be a potential ligand of Siglecs.
In addition, we also analyzed whether Siglecs were coexpressed with two well-known immune checkpoint molecules, PD-1 and CTLA-4, in any GEO database or the patients with cancer included in our experiment. We found that Siglecs were not coexpressed with PD-1 on effector T cells, which suggested that Siglec+ T cells were not exhausted T cells. However, Siglec-6 and Siglec-7 were coexpressed with CTLA-4, a checkpoint molecule and a marker of the later phase of T-cell activation, in CD8+ T cells from patients with head and neck cancer. Similarly, our results also indicated that Siglecs were expressed at high levels on activated T cells. These results suggest that the expression of Siglecs may be related to T-cell activation.
In summary, we first found that SIA-CIgG, as a potential ligand of Siglecs, could be involved in tumor immune escape via the inhibition of activated effector T cells. In other words, we found a pair of potential checkpoint molecules, SIA-CIgG/Siglec, similar to PD-1/PD-L1. However, unlike PD-1/PD-L1, which mainly suppress activated T cells, SIA-CIgG does not only affect T cells; our results indicate that although effector T cells are the major targets of SIA-CIgG, the NK cell frequency in tumor tissue was significantly decreased, and the frequencies of M2 macrophages and myeloid-derived suppressor cells (MDSCs), two major suppressive populations in the TME, were increased by SIA-CIgG in vivo. Together, these results suggest that SIA-CIgG/Siglec may have broad immunosuppressive activity against different immune cells. However, the detailed mechanism needs to be clarified in further studies.
Materials and methods
Mice
C57BL/6 female mice, BALB/c female mice, female nude mice, NOD SCID mice, and RAG1−/− mice were purchased from Beijing Vital River Laboratory Animal Technology Company and used at 6–8 weeks of age. All mice were housed in a pathogen-free facility at the Peking University Health Science Center (reference number for the ethic offices: LA2019091).
Tissue and peripheral blood samples
Ovarian cancer tissue samples for IgG purification were obtained from Peking University People’s Hospital. Human peripheral blood from healthy donors was provided by the Beijing Red Cross Blood Center. Peripheral blood from lung cancer and head and neck cancer patients was provided by the specimen bank of Peking University third hospital. The study was conducted according to an institutional review board-approved protocol.
Cell culture and reagents
The 293T cell line, human lung squamous carcinoma cell line NCI-H520, mouse melanoma cell line B16, and mouse breast cancer cell line EMT-6 were obtained from the American Type Culture Collection (ATCC), and maintained by the Peking University Center for Human Disease Genomics. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density gradient centrifugation. Peripheral blood lymphocytes (PBLs) were isolated from nonadherent PBMCs after 4 h of culture. T and B cells were sorted from PBMCs using the FACSAria II platform (BD Biosciences). Splenic or lymph node CD4+ T cells and CD8+ T cells from C57BL/6 mice were sorted using the FACSAria II platform (BD Biosciences).
PBMCs, PBLs, sorted T and B cells, and NCI-H520 cells were cultured in RPMI-1640 medium (Thermo Fisher Scientific), 293T, B16 and EMT-6 cells were maintained in Dulbecco’s modified Eagle’s medium (Thermo Fisher Scientific); all media were supplemented with 10% fetal bovine serum (FBS; HyClone) and a 1% penicillin–streptomycin solution (SV30010, HyClone), and cells were cultured in a humidified atmosphere of 5% CO2 at 37 °C. All cell lines used in this study were regularly authenticated by morphological observation and tested for the absence of mycoplasma contamination.
Purification of IgG from the TME
Ovarian cancer tissue samples or EMT-6 tumors implanted in NOD SCID mice were minced in liquid nitrogen and lysed in RIPA lysis buffer (10 mM Tris-HCL, pH 7.2; 1% Triton X-100; 1% sodium deoxycholate; 0.1% sodium dodecyl sulfate; and 0.15 M NaCl) containing a protease inhibitor cocktail (04693159001, Roche) at 4 °C for 30 min. The lysates were then centrifuged at 13,000 rpm for 30 min at 4 °C. The supernatants were collected, diluted with PBS and loaded onto a Protein G SepharoseTM 4 Fast Flow (17-0618-01, GE Healthcare) column, followed by incubation, washing and elution according to the manufacturer’s recommendations. The eluted IgG from the TME was preserved in PBS with 10% maltose.
Purification of cancer-derived sialylated IgG
Cancer-derived sialylated IgG (SIA-CIgG) was purified either with agarose-bound Sambucus nigra Lectin (SNA) or RP215-coupled sepharose. IgG from the TME was loaded onto a SNA (AL-1303, Vector Laboratories) column to obtain sialylated Cancer-IgG according to the manufacturer’s recommendations. The SIA-CIgG-specific antibody RP215 (generated by Prof. Lee) was coupled to CNBr-activated Sepharose™ 4B (71-7086-00 AF, GE Healthcare) according to the manufacturer’s recommendations. IgG from the TME was incubated with the RP215-coupled CNBr-activated sepharose column for RP215 recognition-based SIA-CIgG purification.
Preparation of recombinant Cancer-IgG
Recombinant Cancer-IgG was constructed according to the predominant VHDJH sequence VH5-51/D3-16/JH4 (GenBank: AY270190.1) and the VκJκ sequence Vκ4-1/Jκ3 (GenBank: AY505537.1) in human epithelial cancer cells, expressed in the CHO-K1 cell expression system and purified by Beijing Sino Biological Inc.
Neuraminidase digestion
IgG from the TME was combined with H2O in a total reaction volume of 9 μl, and then 2 μl 10 × GlycoBuffer and 2 μl neuraminidase (P0720, New England Biolabs) or α2-3 neuraminidase (P0743S, New England Biolabs) were added and incubated at 37 °C for 1 h. For the control group, only GlycoBuffer was added.
Preparation of Fab and Fc fragments
Purified SIA-CIgG was treated according to the Pierce™ Fab Preparation Kit (44985 Pierce) recommendations. The Thermo Scientific™ Pierce™ Fab Preparation Kit enables efficient Fab generation from IgG. This kit uses papain, a nonspecific thiol endopeptidase, immobilized on agarose resin. Enzyme immobilization is advantageous because digestion can be immediately stopped by simply removing the IgG solution from the resin, resulting in a digest that is enzyme-free.
SDS-PAGE and western blotting
Purified IgG was denatured with heat, analyzed by 12.5% SDS-polyacrylamide gel electrophoresis (PAGE), and stained with Coomassie Brilliant Blue. For western blot analysis, proteins were transferred to nitrocellulose membranes. RP215, a goat anti-human IgG (gamma chain specific) polyclonal antibody (sc-34665, Santa Cruz), a goat anti-human Siglec-10 antibody (AF-2130, R&D), a rabbit anti-GFP antibody (50430-2-AP, Proteintech), a rabbit anti-Myc antibody (16286-1-AP, Proteintech), a mouse anti-His antibody (66005-1-Ig, Proteintech), and a rabbit anti-human IgG (Fc)-HRP antibody (SE205, Solarbio) were used as primary antibodies. After an incubation with secondary antibodies, including HRP-conjugated goat anti-mouse or rabbit anti-goat IgG antibodies (ZB-2301, ZB-2305, Beijing Zhongshan Golden Bridge Biotechnology), the blots were visualized by ECL chemiluminescence (32106, Pierce and RPN2235, GE). For sialylated IgG detection, the membranes were incubated with biotinylated SNA (B-1305, Vector Laboratories), MAL II (B-1265-1, Vector Laboratories) and IRDye 800-conjugated streptavidin (926-32230, LI-COR Biosciences) or horseradish peroxidase-labeled streptomycin (ZB-2404, Beijing Zhongshan Golden Bridge Biotechnology), and the signal was detected by the Odyssey Imaging System (LI-COR Bioscience).
Immunohistochemistry
Antigen retrieval was conducted by immersing slides in Tris-EDTA buffer (pH 9.0) at 120 °C for 2 min. The sections were incubated with 3% H2O2 for 10 min and blocked in 10% normal goat serum (ZLI-9056, Beijing Zhongshan Golden Bridge Biotechnology) at room temperature for 10 min. The sections were then incubated with the primary antibody RP215 overnight at 4 °C. After thorough washing, the sections were incubated with horseradish peroxidase (HRP)-conjugated anti-mouse IgG (GK500705, Dako) at room temperature for 20 min. The bound antibodies were detected using 0.05% DAB (Dako).
Cell proliferation
PBMCs, PBLs, and sorted B and T cells were suspended at a concentration of 5 × 106 cells/ml in PBS containing 5% FBS. Then, mouse T cells were blocked with anti-mouse CD16/CD32 antibodies (14-0161-82, eBioscience), and the cells were stained with CellTrace™ CFSE (C34554, Invitrogen) at a concentration of 5 μM and incubated at room temperature for 5 min. Staining was quenched by the addition of 10 ml PBS, and the cells were centrifuged at 1800 rpm for 5 min at 4 °C. The cells were washed twice in PBS, added to a 96-well plate, and stimulated by either 20 μg/ml PHA (00-4977-93, Thermo Fisher Scientific), precoated 3 μg/ml anti-CD3 antibody (OKT3; 317303, BioLegend) and 1 μg/ml anti-CD28 antibody (302913, BioLegend), or 20 μg/ml staphylococcal protein A (P7155 Sigma) or left unstimulated. Mouse T cells were stimulated with 5 μg/ml anti-CD3e antibody (145-2c11, 553057 BD Biosciences) and 2 μg/ml anti-CD28 antibody (37.51, 553294 BD Biosciences) or left unstimulated. IgG from the TME, IVIG or the same volume of PBS was then added to the culture medium. In blocking experiments, 20 μg/ml anti-Siglec-7 (AF-1138, R&D), anti-Siglec-10 (AF-2130, R&D), or goat IgG antibody (ZDR-5005, Beijing Zhongshan Golden Bridge Biotechnology) was added 2 h before SIA-CIgG addition.
CCK8 assay
B16 cells were seeded in a 96-well plate at a density of 2×104 cells/well in 100 μL of culture medium with PBS, hTME-IgG, or IVIG. The cells were cultured in a CO2 incubator at 37 °C for 0, 24, 48, or 72 h in the incubator. In total, 10 μL of CCK8 solution was added to each well of the plate using a repeating pipettor. The plate was incubated for 2 h in the incubator. The absorbance at 450 nm was measured using a microplate reader after testing cell proliferation.
Flow cytometry
Human PBMCs and mouse T cells were prepared as previously described and stained for 30 min at 4 °C with the following fluorophore-conjugated monoclonal antibodies obtained from eBioscience: anti-human CD4 (45-0048-41), CD8a (17-0087-41), CD19 (11-0199-41), Siglec-3 (12-0338-41), and Siglec-10 (347603) from BioLegend. Anti-mouse CD16/CD32 (14-0161-82, eBioscience), mouse IgG1K Isotype Control APC (17-4714-82, eBioscience), anti-mouse Siglec-G (17-5833-80, eBioscience), anti-mouse Siglec-E (677111, BioLegend), and anti-mouse Siglec-3 (CD33) (12-0331-80, eBioscience) were also obtained. For sialylated IgG binding detection, 40 μg/ml sialylated IgG was incubated with cells for 45 min at 4 °C, and the secondary antibody anti-human IgG Fc (12-4998-82, eBioscience) was added for 30 min at 4 °C. For Siglec-6, Siglec-7 and siglec-9 detection, anti-human Siglec-6 (MAB2859-SP, R&D Systems), Siglec-7 (AF-1138, R&D Systems), and Siglec-9 (1529365, R&D Systems) antibodies were incubated with cells for 45 min at 4 °C, and FITC-labeled goat anti-mouse IgG or rabbit anti-goat IgG antibodies (ZF-0312 or ZF-0314, Beijing Zhongshan Golden Bridge Biotech) were used as secondary antibodies and incubated for 30 min at 4 °C. Immune cells isolated from mouse draining lymph nodes and tumors with Percoll (P8370, Solarbio Life Science) were stained with anti-mouse CD4 (45-0042-80), CD8 (12-0081-81), CD19 (11-0191-82), CD11b (45-0112-82), CD45 (17-0451-82), Siglec-G (17-5833-80, eBioscience), Siglec-E (677111, BioLegend), and Siglec-3 (CD33) (12-0331-80, eBioscience) antibodies. Cells were collected on a FACSVerse or FACSCanto plus flow cytometer (BD Biosciences) and analyzed using FlowJo software.
RNA isolation and RT-PCR
The total RNA was extracted from PBMCs and mouse spleen or lymph node, sorted and stimulated CD4+ and CD8+ T cells using Trizol Reagent (15596018, Life Technology) or the Rapure Total RNA Mini Kit (R4011-02, Magen). Reverse transcription (RT) was carried out with the RevertAid First Stand cDNA Synthesis Kit (K1622, Thermo Fisher Scientific) according to the manufacturer’s protocol. After the cDNA was obtained, specific primers were used to amplify the Siglec gene using the conditions described in Supplementary Tables S1 and S2.
GAPDH: Sense primer: CAAGGTCATCCATGACAACTTTG; and anti-sense primer: GTCCACCACCCTGTTGCTGTAG.
Coimmunoprecipitation analysis
The human Siglec-10 gene in the pcDNA 3.1 plasmid (gifted by Professor Yu) or a control vector was transfected into NCI-H520 cells using Lipofectamine 3000 Reagent (L3000001, Thermo Fisher Scientific). The cells were suspended in a lysis buffer containing 50 mM Tris-HCl, pH 8.0; 120 mM NaCl; 1% nonidet P-40 (NP-40); and a protease inhibitor cocktail. The lysates were incubated with antibodies at 4 °C overnight and with protein G agarose beads (17-0618-01, GE Healthcare) for 4 h. The antibodies used for immunoprecipitation were an anti-Siglec-10 antibody and RP215. A matched isotype IgG (goat IgG, ZDR-5005; mouse IgG, ZDR-5006, Beijing Zhongshan Golden Bridge Biotechnology) was used as a negative control. The mouse Siglec-E/G genes in the pcDNA 3.1 plasmid (gifted by Professor Yu), mouse Siglec-3 (CD33) gene in the pCMV3 plasmid (MG50172-CH, Sino Biological), human SIA-CIgG pEGFP-C3 plasmid (from Tang Jingshu) or a control vector was transfected into 293 T cells using PEI Reagent (23966, Polysciences, Inc). The cells (1 × 107) were washed two times with PBS and resuspended with 1 mL of ice-cold lysis buffer (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; and 0.05% NP-40) containing appropriate protease inhibitors. The tube was centrifuged at 12,000 × g for 5 min at 4 °C, and the supernatant was transferred to another tube. Anti-Myc-tag mAb magnetic beads or Anti-GFP-tag mAb magnetic beads (M047-11, MBL; D153-11, MBL) were added as suggested into the supernatant prepared earlier. The sample was mixed well and incubated with gentle agitation for 1 h at 4 °C. The tube was placed on a magnetic rack (MBL; code no. 3190) for a few seconds. The supernatant was removed, 1 mL of cold wash buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.05% NP-40) was added, and the magnetic beads were resuspended. The tube was then placed on the magnetic rack for a few seconds, and the supernatant was removed. This process was repeated three times. The magnetic beads were resuspended in 30 μL of loading buffer and boiled for 10 min, and then the tube was placed on the magnetic rack for a few seconds. Then, the samples were analyzed by 12.5% SDS-polyacrylamide gel electrophoresis (PAGE) and western blotting.
In vivo experiments
C57BL/6 mice or nude mice were injected with 2 × 105 B16 cells, and BALB/c mice were injected with 2 × 105 EMT-6 cells subcutaneously in the armpits of both forelimbs. After 5–7 days, tumor volume was measured, and the mice were randomly allocated into three groups. Each group was injected subcutaneously around the tumors with 5 or 20 μg IgG TME or IVIG, 20 μg recombinant SIA-CIgG, 20 μg enriched SIA-CIgG, or SIA-CIgG digested with neuraminidase or papain, PBS or neuraminidase every other day. The mice were then killed and analyzed for tumor volume and weight and the proportions of immune cells in the draining lymph nodes (DLN) or tumor tissue.
Dataset collection and GEO analysis
Siglec gene expression was evaluated in 433 datasets containing 10,422 Affymetrix Human Genome U133 Plus 2.0 array samples from the Gene Expression Omnibus (GEO) database and in 455 datasets containing 3,929 mouse genome 430 2.0 arrays on the ImmuSort website (http://immusort.bjmu.edu.cn). An RNA-Seq dataset of tumor-infiltrated T cells in lung cancer patients in the GEO database (GSE90728) was downloaded, and Siglec expression was evaluated by calculating the Transcripts Per Kilobase Million (TPM) score. Siglec gene expression in arrays of peripheral blood T cells of patients with melanoma (GSE24536) or breast cancer (GSE36765), tumor-infiltrating T cells of patients with head and neck cancer (GSE114944), or tumor-infiltrating T cells of mice (GSE89477) were analyzed by GEO2R.
Statistics
All data were analyzed with GraphPad Prism software and are presented as the mean ± s.d. or s.e.m. Statistical significance was determined by a two-tailed paired or unpaired Student’s t test or one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparisons test, with significance levels of *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001; and ns, not significant (P ≥ 0.05).
Supplementary information
Acknowledgements
We would like to thank G. Lee (Andrology Lab, University of British Columbia Centre for Reproductive Health, Vancouver, BC V5Z 4H4, Canada) for developing RP215. We thank Y. Yu (National Key Laboratory of Medical Immunology & Institute of Immunology, Second Military Medical University) for the human and mouse Siglec-10 plasmids. This work was supported by research grants to X. Qiu from the key support projects of the National Natural Science Foundation’s major research program (91642206), major international cooperation projects of the National Natural Science Foundation (81320108020), research institute fund of the NHC Key Laboratory of Medical Immunology, Peking University (BMU2018JDJS010), the Science Technology and Innovation Committee of Shenzhen Municipality (JCYJ20170413141047772) and nonprofit central research institute fund of the Chinese Academy of Medical Sciences (2018PT31039).
Author contributions
Y.Z. and X.Q. initiated and designed the research; Z.W., Z.G., W.S., E.L., and L.X. performed the experiments and analyzed and interpreted the results; J.Z. and J.T. contributed to testing the specificity of RP215 and confirmed the epitope recognized by RP215; L.Z. and X.Y. purified SIA-CIgG; W.P. carried out the GEO database mining and analysis; X.S. performed cell sorting by flow cytometry; W.X. produced and purified RP215; Z.W., Z.G., and X.Q. wrote the paper; and L.X., H.C. and X.C. provided clinical specimens and clinical and pathological information.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Zihan Wang, Zihan Geng.
Contributor Information
Xiaohong Chang, Email: changxiaohong@pkuph.edu.cn.
Xiaoyan Qiu, Email: qiuxy@bjmu.edu.cn.
Supplementary information
The online version of this article (10.1038/s41423-019-0327-9) contains supplementary material.
References
- 1.Nand S, Molokie R. Therapeutic plasmapheresis and protein A immunoadsorption in malignancy: a brief review. J. Clin. Apher. 1990;5:206–212. doi: 10.1002/jca.2920050408. [DOI] [PubMed] [Google Scholar]
- 2.Veltri RW, Kikta VA, Wainwright WH, Sprinkle PM. Biologic and molecular characterization of the IgG serum blocking factor (SBF-IgG) isolated from sera of patients with EBV-induced infectious mononucleosis. J. Immunol. 1981;127:320–328. [PubMed] [Google Scholar]
- 3.Affara NI, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25:809–821. doi: 10.1016/j.ccr.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu X, et al. Human epithelial cancers secrete immunoglobulin g with unidentified specificity to promote growth and survival of tumor cells. Cancer Res. 2003;63:6488–6495. [PubMed] [Google Scholar]
- 5.Babbage G, Ottensmeier CH, Blaydes J, Stevenson FK, Sahota SS. Immunoglobulin heavy chain locus events and expression of activation-induced cytidine deaminase in epithelial breast cancer cell lines. Cancer Res. 2006;66:3996–4000. doi: 10.1158/0008-5472.CAN-05-3704. [DOI] [PubMed] [Google Scholar]
- 6.Geng LY, et al. Expression of SNC73, a transcript of the immunoglobulin alpha-1 gene, in human epithelial carcinomas. World J. Gastroenterol. 2007;13:2305–2311. doi: 10.3748/wjg.v13.i16.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu X, et al. Distinct regulatory mechanism of immunoglobulin gene transcription in epithelial cancer cells. Cell Mol. Immunol. 2010;7:279–286. doi: 10.1038/cmi.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M, et al. Promotion of cell proliferation and inhibition of ADCC by cancerous immunoglobulin expressed in cancer cell lines. Cell Mol. Immunol. 2012;9:54–61. doi: 10.1038/cmi.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, et al. The presence of IGHG1 in human pancreatic carcinomas is associated with immune evasion mechanisms. Pancreas. 2011;40:753–761. doi: 10.1097/MPA.0b013e318213d51b. [DOI] [PubMed] [Google Scholar]
- 10.Liang PY, et al. Overexpression of immunoglobulin G prompts cell proliferation and inhibits cell apoptosis in human urothelial carcinoma. Tumour Biol. 2013;34:1783–1791. doi: 10.1007/s13277-013-0717-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang C, et al. Immunoglobulin G expression in lung cancer and its effects on metastasis. PloS One. 2014;9:e97359. doi: 10.1371/journal.pone.0097359. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Lee G, Laflamme E, Chien CH, Ting HH. Molecular identity of a pan cancer marker, CA215. Cancer Biol. Ther. 2008;7:2007–2014. doi: 10.4161/cbt.7.12.6984. [DOI] [PubMed] [Google Scholar]
- 13.Lee G. Cancer cell-expressed immunoglobulins: CA215 as a pan cancer marker and its diagnostic applications. Cancer Biomark. 2009;5:137–142. doi: 10.3233/CBM-2009-0610. [DOI] [PubMed] [Google Scholar]
- 14.Tang J, et al. Lung squamous cell carcinoma cells express non-canonically glycosylated IgG that activates integrin-FAK signaling. Cancer Lett. 2018;430:148–159. doi: 10.1016/j.canlet.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 16.Koch MA, et al. Maternal IgG and IgA antibodies dampen mucosal T helper cell responses in early life. Cell. 2016;165:827–841. doi: 10.1016/j.cell.2016.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colucci M, et al. Sialylation of N-linked glycans influences the immunomodulatory effects of IgM on T cells. J. Immunol. 2015;194:151–157. doi: 10.4049/jimmunol.1402025. [DOI] [PubMed] [Google Scholar]
- 18.Nimmerjahn F, Ravetch JV. The antiinflammatory activity of IgG: the intravenous IgG paradox. J. Exp. Med. 2007;204:11–15. doi: 10.1084/jem.20061788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Gunten S, et al. IVIG pluripotency and the concept of Fc-sialylation: challenges to the scientist. Nat. Rev. Immunol. 2014;14:349. doi: 10.1038/nri3401-c1. [DOI] [PubMed] [Google Scholar]
- 20.Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat. Rev. Immunol. 2013;13:176–189. doi: 10.1038/nri3401. [DOI] [PubMed] [Google Scholar]
- 21.Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29:491–498. doi: 10.1093/intimm/dxx039. [DOI] [PubMed] [Google Scholar]
- 22.Padet L, Bazin R. IVIg prevents the in vitro activation of T cells by neutralizing the T cell activators. Immunol. Lett. 2013;150:54–60. doi: 10.1016/j.imlet.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Bull C, Heise T, Adema GJ, Boltje TJ. Sialic acid mimetics to target the sialic acid-siglec axis. Trends Biochem. Sci. 2016;41:519–531. doi: 10.1016/j.tibs.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura Y, et al. Expression of CD33 antigen on normal human activated T lymphocytes [letter] Blood. 1994;83:1442–1443. [PubMed] [Google Scholar]
- 26.Nicoll G, et al. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J. Biol. Chem. 1999;274:34089–34095. doi: 10.1074/jbc.274.48.34089. [DOI] [PubMed] [Google Scholar]
- 27.Zhang JQ, Nicoll G, Jones C, Crocker PR. Siglec-9, a novel sialic acid binding member of the immunoglobulin superfamily expressed broadly on human blood leukocytes. J. Biol. Chem. 2000;275:22121–22126. doi: 10.1074/jbc.M002788200. [DOI] [PubMed] [Google Scholar]
- 28.Ikehara Y, Ikehara SK, Paulson JC. Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9. J. Biol. Chem. 2004;279:43117–43125. doi: 10.1074/jbc.M403538200. [DOI] [PubMed] [Google Scholar]
- 29.Bandala-Sanchez E, et al. T cell regulation mediated by interaction of soluble CD52 with the inhibitory receptor Siglec-10. Nat. Immunol. 2013;14:741–748. doi: 10.1038/ni.2610. [DOI] [PubMed] [Google Scholar]
- 30.Stanczak MA, et al. Self-associated molecular patterns mediate cancer immune evasion by engaging Siglecs on T cells. J. Clin. Invest. 2018;128:4912–4923. doi: 10.1172/JCI120612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barkal AA, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572:392–396. doi: 10.1038/s41586-019-1456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, et al. Malignant ascite-derived extracellular vesicles inhibit T cell activity by upregulating Siglec-10 expression. Cancer Manag. Res. 2019;11:7123–7134. doi: 10.2147/CMAR.S210568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toubai T, et al. Siglec-G represses DAMP-mediated effects on T cells. JCI Insight. 2017;2:92293. doi: 10.1172/jci.insight.92293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang B, et al. Correlation of immunoglobulin G expression and histological subtype and stage in breast cancer. PloS One. 2013;8:e58706. doi: 10.1371/journal.pone.0058706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao Q, et al. Aberrant high expression of immunoglobulin G in epithelial stem/progenitor-like cells contributes to tumor initiation and metastasis. Oncotarget. 2015;6:40081–40094. doi: 10.18632/oncotarget.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheng Z, et al. Involvement of cancer-derived IgG in the proliferation, migration and invasion of bladder cancer cells. Oncol. Lett. 2016;12:5113–5121. doi: 10.3892/ol.2016.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, et al. Binding of the monoclonal antibody RP215 to immunoglobulin G in metastatic lung adenocarcinomas is correlated with poor prognosis. Histopathology. 2015;67:645–653. doi: 10.1111/his.12686. [DOI] [PubMed] [Google Scholar]
- 38.Sheng Z, et al. IgG is involved in the migration and invasion of clear cell renal cell carcinoma. J. Clin. Pathol. 2016;69:497–504. doi: 10.1136/jclinpath-2015-202881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prabagar MG, Choi HJ, Park JY, Loh S, Kang YS. Intravenous immunoglobulin-mediated immunosuppression and the development of an IVIG substitute. Clin. Exp. Med. 2014;14:361–373. doi: 10.1007/s10238-013-0255-4. [DOI] [PubMed] [Google Scholar]
- 40.van de Bovenkamp FS, Hafkenscheid L, Rispens T, Rombouts Y. The Emerging Importance of IgG Fab Glycosylation in Immunity. J. Immunol. 2016;196:1435–1441. doi: 10.4049/jimmunol.1502136. [DOI] [PubMed] [Google Scholar]
- 41.Bayry J, et al. Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin. Blood. 2003;101:758–765. doi: 10.1182/blood-2002-05-1447. [DOI] [PubMed] [Google Scholar]
- 42.Zhou JY, Oswald DM, Oliva KD, Kreisman LSC, Cobb BA. The glycoscience of immunity. Trends Immunol. 2018;39:523–535. doi: 10.1016/j.it.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 2014;14:653–666. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang P, Yang Y, Han W, Ma D. ImmuSort, a database on gene plasticity and electronic sorting for immune cells. Sci. Rep. 2015;5:10370. doi: 10.1038/srep10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng J, et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Disco. 2018;8:216–233. doi: 10.1158/2159-8290.CD-17-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kimoto Y. Expression of heavy-chain constant region of immunoglobulin and T-cell receptor gene transcripts in human non-hematopoietic tumor cell lines. Genes Chromosomes Cancer. 1998;22:83–86. doi: 10.1002/(sici)1098-2264(1998)22:1<83::aid-gcc12>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 47.Zheng J, et al. Immunoglobulin gene transcripts have distinct VHDJH recombination characteristics in human epithelial cancer cells. J. Biol. Chem. 2009;284:13610–13619. doi: 10.1074/jbc.M809524200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lv WQ, et al. Expression of cancer cell-derived IgG and extra domain A-containing fibronectin in salivary adenoid cystic carcinoma. Arch. Oral. Biol. 2017;81:15–20. doi: 10.1016/j.archoralbio.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 49.Ji F, et al. Prognostic value and characterization of the ovarian cancer-specific antigen CA166-9. Int J. Oncol. 2015;47:1405–1415. doi: 10.3892/ijo.2015.3115. [DOI] [PubMed] [Google Scholar]
- 50.Rodrigues E, Macauley MS. Hypersialylation in cancer: modulation of inflammation and therapeutic opportunities. Cancers. 2018;10:E207. doi: 10.3390/cancers10060207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat. Med. 2019;25:656–666. doi: 10.1038/s41591-019-0374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.