Abstract
Individuals with severe psychiatric disorders have a reduced life expectancy compared to the general population. At the biological level, patients with these disorders present features that suggest the involvement of accelerated aging, such as increased circulating inflammatory markers and shorter telomere length (TL). To date, the role of the interplay between inflammation and telomere dynamics in the pathophysiology of severe psychiatric disorders has been scarcely investigated. In this study we measured T-lymphocytes TL with quantitative fluorescent in situ hybridization (Q-FISH) and plasma levels of inflammatory markers in a cohort comprised of 40 patients with bipolar disorder (BD), 41 with schizophrenia (SZ), 37 with major depressive disorder (MDD), and 36 non-psychiatric controls (NPC). TL was shorter in SZ and in MDD compared to NPC, while it was longer in BD (model F6, 137 = 20.128, p = 8.73 × 10−17, effect of diagnosis, F3 = 31.870; p = 1.08 × 10−15). There was no effect of the different classes of psychotropic medications, while duration of treatment with mood stabilizers was associated with longer TL (Partial correlation controlled for age and BMI: correlation coefficient = 0.451; p = 0.001). Levels of high-sensitivity C-Reactive Protein (hsCRP) were higher in SZ compared to NPC (adjusted p = 0.027), and inversely correlated with TL in the whole sample (r = −0.180; p = 0.042). Compared to NPC, patients with treatment resistant (TR) SZ had shorter TL (p = 0.001), while patients with TR MDD had higher levels of tumor necrosis factor-α (TNFα) compared to NPC (p = 0.028) and to non-TR (p = 0.039). Comorbidity with cardio-metabolic disorders did not influence the observed differences in TL, hsCRP, and TNFα among the diagnostic groups. Our study suggests that patients with severe psychiatric disorders present reduced TL and increased inflammation.
Subject terms: Schizophrenia, Diagnostic markers, Bipolar disorder, Depression
Introduction
According to the World Health Organization (WHO) around 450 million people in the world currently suffer from mental disorders [1]. The global burden of mental illness accounts for 21.2–32.4% of years lived with disability (YLDs), causing a considerable socio-economic impact [2]. Among severe mental disorders, depression is the most prevalent, affecting more than 250 million people worldwide, but large proportions of the general population are affected by bipolar disorder (BD) (45 million people) and schizophrenia (SZ) (20 million people), which both significantly contribute to the cumulative high prevalence of mental illness worldwide [3]. Differently from somatic disorders, most of severe mental illnesses have their onset in late adolescence and young adulthood, determining substantial impairment over the remaining lifespan [4, 5]. In addition, severe mental disorders are associated with significant excess mortality as well as decreased life expectancy [6, 7]. Indeed, it has been reported that affected individuals have 10–20 years reduction in life expectancy compared with the general population [8, 9]. A vast body of data suggests that this excess mortality is accounted for by a higher prevalence of comorbid chronic disorders compared to individuals without mental illnesses [10–13]. In particular, age-related disorders with an inflammatory component, such as cardiovascular and metabolic disorders, present significantly higher incidence in patients with mental disorders than in the general population [10, 14, 15]. This evidence has led to the hypothesis that accelerated aging and inflammation may play a central role in the etiopathogenesis and detrimental trajectory of severe mental disorders. This hypothesis is also supported by findings from several studies reporting increased brain age in patients with psychiatric disorders compared to non-psychiatric controls (NPC) [16–20]. Interestingly, other studies showed that individuals affected by severe psychiatric disorders have shorter telomere length (TL) compared to unaffected individuals [21].
Telomere shortening is a hallmark of cellular aging. In humans, telomeric DNA consists of multiple (TTAGGG)n repeats ending in a single stranded-overhang of the G-rich 3’ strand. Together with a number of specific proteins, called shelterins, which directly bind DNA or are associated with chromatin, it contributes to the structure of the chromosome telomeres [22]. This highly conserved complex ensures chromosome stability, preventing chromosome shortening and chromosome end fusion, as well as distinguishing telomeres from double strand breaks, thus avoiding their degradation by the DNA repair machinery. Telomeres physiologically shorten after each cell division in most somatic tissues, whereas early in human development this shortening is counteracted by the enzyme telomerase. After birth, telomerase is active predominately in stem cells and germ cells, while in telomerase-negative somatic cells each DNA replication leads to a loss of ~100 bp of telomeric sequence. This progressive telomere decline impairs the number of cell generations and causes cell senescence, with either growth arrest or activation of apoptotic processes [23]. The cell senescence inevitably impacts on a vast number of physiological processes that influence aging and longevity. A recent study evaluating mortality in more than 64,000 subjects from the general population, showed that short telomeres in peripheral blood leukocytes were associated with high mortality [24]. Some studies have also shown that TL correlates with brain age, with shortest telomeres correlating with older age [25–28], and with reduced hippocampal volume in SZ [29]. While data on telomere shortening in psychiatric disorders have not been all concordant, a recent meta-analysis showed a significant overall effect size for telomere shortening across all psychiatric disorders [30].
Telomere attrition is also caused by several biological insults, including inflammatory processes [31]. Interestingly, it has been largely shown that patients affected by severe psychiatric disorders have increased levels of peripheral inflammatory markers, suggesting an involvement of inflammation in the etiopathogenesis of these disorders [32–34]. This could be enacted through their access into the central nervous system and their impact on neurotransmitters and neural circuits [33, 35–38].
Taken together, these findings strongly suggest a complex interplay of telomere shortening and inflammation in modulating the risk for severe psychiatric disorders. However, while the interaction between increased inflammation and telomere shortening has been largely explored in somatic disorders, there is scant evidence on severe mental disorders. Moreover, the impact of comorbid age-related disorders has been often overlooked in previous telomere studies in mental illness and there is paucity of data on the correlation between treatment with psychotropic medications (in terms of exposure, duration and clinical outcome) and TL. This appears of particular importance, as several studies showed that treatment with lithium correlates with longer telomeres [39–41], and that shorter telomeres predict worse outcome of antidepressants treatment [42, 43].
Here, we performed a study involving three deep-phenotyped samples of patients with severe psychiatric disorders, namely BD, major depressive disorder (MDD), and SZ as well as a sample of NPC, to explore differences and similarities in telomere and inflammation dynamics across the different disorders, and to specifically explore the role of pharmacological treatments and comorbid age-related disorders in the interplay between telomere shortening and inflammation in these disorders.
Methods
Sample
The cohort comprised 40 patients with BD, 37 with MDD, and 41 with SZ recruited from patients followed-up and treated at the community mental health center of the Unit of Psychiatry of the Department of Medical Science and Public Health, University of Cagliari and University Hospital Agency of Cagliari, and the Unit of Clinical Pharmacology, University Hospital Agency, Cagliari, Italy. The recruitment process was based on the inclusion and exclusion criteria described in Manchia et al. [44]. Briefly, the diagnosis was made according to DSM-IV criteria and SADS-L (BD patients), and Structured Clinical Interview for DSM IV-TR Axis I Disorders (SCID) (MD and SZ patients). Exclusion criteria comprised presence of acute infections, chronic autoimmune inflammatory conditions, diagnosis of any eating disorders, post-traumatic stress disorder, substance use disorders, neurological disorders, traumatic brain injury, or severe medical conditions (such as cancer, HIV infection). All patients had been followed up longitudinally with periodic assessments (typically on a monthly basis) of their psychopathological status, which included the use of standard psychometric tools, in certain cases since the illness onset. This accurate clinical depiction of the clinical course was the basis for the definition of treatment resistance (TR) in MDD and SZ patients. Specifically, TR for MDD and SZ patients was defined according to the criteria of Souery et al. [45] and Kane et al. [46], respectively, on the basis of the clinical course and of the assessment of treatment response patterns.
In patients with BD response to lithium was characterized using the Retrospective Criteria of Long-Term Treatment Response in Research Subjects with Bipolar Disorder scale (or Alda scale), as previously described [47–49]. The scale quantifies the degree of improvement during lithium treatment with a score from 0 to 10, adjusting for potential confounders. Patients with a total score ≥7 were considered responders [47, 48]. All BD patients enrolled in the study were under mood stabilizing treatment at time of recruitment, with 38 patients being either responders or non-responders to lithium based on the Alda scale. The characterization of lithium response was also useful to inform on the longitudinal patterns of clinical course of BD patients.
A total of 36 NPC with no personal or familial history of psychiatric disorders in first degree were recruited based on the same exclusion criteria described for patients. NPC were administered the Italian version of the SCID-I/NP 26 to rule out the presence of Axis I psychiatric disorders [50]. For each individual included in the study, information about comorbid conditions related to aging (specifically cardiovascular diseases, diabetes mellitus type 2, and obesity) were collected. The presence of comorbid medical conditions was ascertained through the evaluation of medical records, laboratory tests and presence of pharmacological treatments indicated for the illness.
Patients and controls were all from the same geographical area (the Island of Sardinia, Italy), Caucasians and of Italian origin.
Fasting blood was collected in the morning using tubes containing Eparine for the Q-FISH experiments, or ethylenediamine tetraacetic acid (1.5 mg/ml) for the ELISA experiments. Blood was processed for the different protocols within 1 h from collection, during which it was stored at controlled temperature (4 °C).
At blood drawn, patients with BD and MDD were in euthymic phases with an interval of at least 6 months from the last mood episode meeting diagnostic criteria. Similarly, patients with SZ were sampled after at least 6 months from the last psychotic episode. Clinical and demographic variables collected for the present study are reported in Table 1. The research protocol followed the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the University of Cagliari, Italy (approval number: 348/FC/2013 and PG/2018/11693). All participants signed informed written consent after a detailed description of the study procedures.
Table 1.
Demographic and clinical variables in the four diagnostic groups.
| Variables | BD (n = 40) | SZ (n = 41) | MDD (n = 37) | NPC (n = 36) | Statistics |
|---|---|---|---|---|---|
| Gender (M/F) | 17/23 | 36/5 | 12/25 | 21/15 | X2 = 28.2; p = 0.000003 |
| FH (Y/N/U) | 19/21 | 20/20/1 | 20/13/4 | 6/30 | X2 = 15.4; p = 0.001 |
| Suicide attempt (Y/N/U) | 11/29 | 8/33 | 6/30/1 | 0 | X2 = 1.4; p = 0.484 |
| Smoking (Y/N) | 22/18 | 31/10 | 18/18/1 | 13/23 | X2 = 12.6; p = 0.006 |
| Substance use (Y/N/U) | 0 | 16/23/2 | 1/20/16 | 0 | X2 = 7.0; p = 0.008 |
| Physical Activity (Y/N/U) | 18/22 | 15/26 | 13/23/1 | 25/11 | X2 = 10.9; p = 0.013 |
| Cardio-metabolic disorders (Y/N/U) | 14/26 | 15/26 | 10/26/1 | 8/28 | X2 = 2.4; p = 0.498 |
| Cardiovascular disorders (Y/N) | 6/34 | 5/36 | 6/31 | 7/29 | X2 = 0.844; p = 0.839 |
| Metabolic disorders (Y/N/U) | 13/27 | 13/28 | 7/29/1 | 1/35 | X2 = 12.5; p = 0.006 |
| BMI (mean ± SD) | 27.7 ± 6.7 | 27.2 ± 4.1 | 25.4 ± 5.1 | 23 ± 3.5 | F = 6.5; p = 0.0003 |
| Age (mean ± SD) | 51.6 ± 10.7 | 46.9 ± 11.9 | 51.2 ± 12.9 | 43.3 ± 10.7 | F = 4.3; p = 0.006 |
| Age of father at birth (mean ± SD) | 33.8 ± 5.9 | 33.4 ± 6.6 | 33.7 ± 5.4 | 33.7 ± 5.6 | F = 0.04; p = 0.990 |
| Patients under MS treatment (Y/N) | 15/25 | 11/30 | 7/30 | / | |
| Patients under AP treatment (Y/N) | 19/21 | 41/0 | 8/29 | / | |
| Patients under AD treatment (Y/N) | 7/33 | 11/30 | 29/8 | / | |
| Lithium responders (Y/N/U) | 12/26/2 | / | / | / | |
| MDD, Treatment-resistant (Y/N) | / | / | 10/27 | / | |
| SZ, Treatment-resistant (Y/N) | / | 20/21 | / | / |
Smoking was defined as: Y, current or ex-smokers; N, ever smokers. Physical activity was defined as: Y, any physical activity performed at least twice a week; N, no physical activity.
BD bipolar disorder, SZ schizophrenia, MDD major depressive disorder, NPC non psychiatric controls, M males, F females, FH family history of any psychiatric disorder, Y yes, N no, U unknown, BMI body mass index, SD standard deviation, MS mood stabilizers, AP antipsychotics, AD antidepressants.
Quantitative fluorescence in situ hybridization (Q-FISH)
Quantitative Fluorescent in situ hybridization (Q-FISH) was adopted to quantify the target repetitive hexameric sequences (TTAGGG) located at the distal end of chromosomes. Telomere peptide nucleic acid fluorescence in situ hybridization and appropriate digital image software were used for capture and quantification of fluorescence signals.
Phytohemagglutinin-M (GIBCO, Milan, Italy) stimulated T-lymphocytes chromosomes were obtained from whole blood and short-term cultures set up within 24 h. Metaphase preparations analyses were carried out as previously described [51]. Metaphase chromosomes were hybridized using a Cy3-labeled (CCCTAA)3 PNA probe (DAKO, Glostrup, Denmark) according to the manufacturer’s instructions. Slides with cells from patients from the different subgroups and control cells were randomly distributed in a single experiment. Telomere hybridization signals of 20 complete metaphases per subject, all from a single slide, were randomly selected for microscopic evaluation of FISH signals. Images were captured by a digital image analysis system based on an epifluorescence Olympus BX41 microscope using DAPI and Cy3 filters and charge-coupled device camera (Cohu, San Diego, CA), interfaced with the CytoVysion System software (Applied Imaging). Telomere fluorescence signals were quantified by the ImageJ software, version 1.43 μ (National Institutes of Health, http://rsbweb.nih.gov/) according to the provided guidelines. The software measures fluorescence intensity of individual telomeres expressed as the product of the telomere area and the average gray value within the selected telomere. The method provides excellent quantitation as the fluorescence intensity directly correlates to the length of the telomeres and provides a measure of length of individual telomeres.
The mean fluorescence intensity of 23 pairs of chromosomes (i.e., 92 sister chromatid pairs with a total of 184 telomere ends per cell) from twenty images (i.e., 368,000 telomere ends per individual) was estimated for both subjects of control and for patients of each subgroup. TL was calculated as the mean fluorescence intensity signals for each control subject and each patient of the subgroups.
Day to day variations in fluorescence performance was monitored by fluorescence beads of defined size (4.0 μm in diameter) (MultiSpeck Multispectral Fluorescence Microscopy Standard kit, Thermofisher Scientific, Milan Italy). Briefly, a slide with 10 μl of beads solution was prepared and twelve random beads images were acquired at 100 ms exposure time, before each daily image acquisition. Fluorescent intensity was measured, and an average value of fluorescence was daily obtained. No variation of the mean value along the different experiments was observed. Based on this internal control, the same acquisition time of 800 ms was setup for Cy3 telomere signals, insuring consistent settings for image acquisition across the different subject groups.
Two quality control criteria were set for sample exclusion: the number of metaphases in the harvested cultures and processed cells (<40 available metaphases in the slide), and the appearance of chromosome morphology (fuzzy chromosomes). In total, ten samples (2 MDD, 2 BD, 5 SZ, and 1 control) were excluded, since they did not meet the first parameter.
hsCRP and TNFα
Blood samples were centrifuged (3000 × g, 10 min), hence plasma was aliquoted and stored frozen (below −25 °C). Each relevant aliquot was thawed once, immediately before running the respective ELISA assay.
Plasma levels of the C-reactive protein (CRP) were measured with high sensitivity ELISA sandwich kits (Origene: catalog number EA100881). The monoclonal anti-CRP antibody (CRP Mab) was used immobilized on the microtiter wells. Standards (curve range 0.005- 0.1 mg/L; 10 μl / well in duplicate) and samples (dilutions 1:100, 10 μl / well) were incubated with the second antibody (anti-CRP bound to HRP, 100 μl / well) for 60 min at room temperature.
Measurements were done by incubating the TMB substrate for 15 min at room temperature, stopping the reaction with blocking buffer and measuring optical density at 450 nm in the plate reader (Chameleon: Hidex, Turku, Finland).
Plasma tumor necrosis factor alpha (TNF-α) was measured by commercial UltraSensitive ELISA kit (Invitrogen Corporation, Carlsbad, CA, USA, catalog number KHC3014) following manufacturer’s instructions. Standards (curve range 0.5–32 pg/mL; 50 μl / well in duplicate), samples (50 μl / well) and Hu TNF-α biotinylated detection antibody (50 μl / well) were added to the capture antibody coated wells for 2 h at 37 °C. Streptavidin-HRP (30 min at room temperature) and TMB substrate (30 min at room temperature) were used to reveal the positive labeling. The reaction was stopped with blocking solution and the optical density was measured at 450 nm using a multilabel plate reader (Chameleon: Hidex, Turku, Finland).
All samples were run in four assays, each including similar numbers of samples from each patient group, which were assigned to randomly distributed wells across plates, and across each assay plate. Five samples were assessed in each assay and used for quality control and to normalize for minor differences in values calculated in the different assay runs. For TNFα assays, all sample values fell within the range of the standard curve, while for hsCRP levels equal or higher that 10 mg/L where excluded from the analysis (13 in total), as this threshold is indicative of acute, macro-inflammation [52–54], and this would have biased our study, which aimed to only focus on signatures of low grade inflammation. Moreover, it is likely that psychiatric patients with acute inflammatory states do not self-report their concomitant medical conditions. Linear regression analysis of standard curves yielded R2 values between 0.99 and 1. Inter-assays coefficient of variation of TNFα and hsCRP were 7 and 9% respectively.
Statistics
Differences in demographic and clinical variables among the diagnostic groups were assessed using the Pearson’s Chi-Square test for categorial variables, and the ANOVA or Kruskal-Wallis tests for quantitative variables, depending on the normality of the distribution.
To test the differences in the molecular measures among the four diagnostics groups we used two different models: a simplified model including age, sex and BMI as controlling factors, and a full model, controlling for the effect of all variables showing statistically significant differences among groups as in Table 1.
For TL, we used ANCOVA with TL as dependent variable, diagnosis and sex as factors, and age, and BMI as covariates. Since levels of hsCRP and TNFα were not normally distributed, as resulted from the Shapiro–Wilk test, we performed two independent rank analyses of covariance, one for hsCRP and one for TNFα. Unstandardized residuals were obtained by running a linear regression model with the ranks of the molecular measures as dependent variable and the ranks for age and BMI as independent variables (simplified model). The same approach was used for the full model, but ranks were obtained for all the continuous variables tested. Residuals were then used in a general linear model with diagnosis and sex as grouping variables in the simplified model, while the full model also included all the discrete variables showing statistically significant differences among the diagnostic groups as in Table 1. P values of pairwise comparisons were corrected with Bonferroni. Otherwise, a p value < 0.05 was considered statistically significant. We used partial eta squared as a measure of the proportion of total variation attributable to the tested variables. The analyses were conducted using IBM SPSS Statistics v. 25 (IBM Corporation, Armonk, NY, USA) and Graphpad Prism V. 8 (GraphPad Software, San Diego, CA USA).
Results
Demographic and clinical characteristics of the sample can be found in Table 1. The four diagnostic groups differed for several of the analyzed features (Table 1). These variables (age, sex, BMI, cigarette smoking, physical activity, substance abuse, comorbid metabolic disorders, family history for mental disorder) were therefore included in the full model to test their contribution on the differences in levels of molecular measures among the diagnostic groups.
In this section we report detailed findings only for the simplified model, while the outputs of the full model are reported in Tables S1, S2, and S3.
The extreme studentized deviate test showed that none of the included samples was an outlier for any of the molecular measure tested.
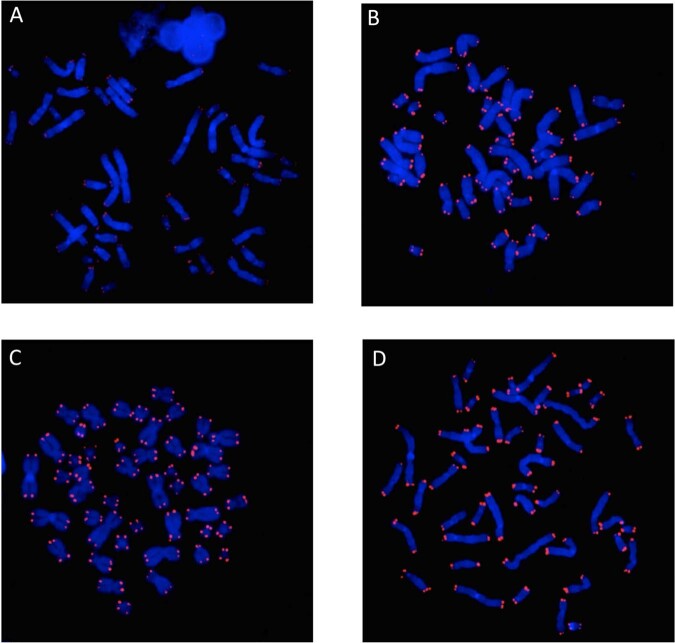
Q-FISH showed different level of fluorescence intensity in the three patient groups versus controls (Fig. 1).
Fig. 1. Fluorescence intensity of telomeres among diagnostic groups.
The figure shows diminished fluorescence intensity of telomeres of a representative partcipant among patients with schizophrenia (a) compared to major depressive disorder (b), bipolar disorder (c) and non-psychiatric controls (d). Telomere probe (red); DAPI-stained metaphases (blue). Note: no evidence of FISH signal randomly observed mainly in SZ chromosomes indicates that telomere shortening reached a size of the exameric sequence that is under the PNA-FISH resolution (200base pairs).
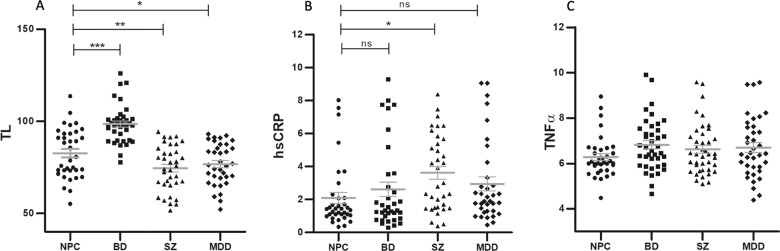
The ANCOVA with age, sex, and BMI as control variables showed that TL was significantly different among the four groups (model F6, 137 = 20.128, p = 8.73 × 10−17, partial eta squared 0.469; effect of diagnosis, F3 = 31.870; p = 1.08 × 10−15; partial eta squared = 0.411; Fig. 2a). There was also a significant contribution of age to the model (F1 = 14.811, p = 0.0001, partial eta squared = 0.098), but diagnosis was the most significant variable explaining the largest proportion of variation. Post-hoc analysis with Bonferroni correction showed that patients with SZ and MDD had significantly shorter TL compared to NPC (SZ versus NPC, p = 0.002; MDD versus NPC, p = 0.039) and to BD (SZ versus BD, p = 1.91 × 10−13; MDD versus BD, p = 4.22 × 10−12). Patients with BD had the longest TL compared to all the other groups (Fig. 2a). This finding could be explained by the effect of exposure to mood stabilizers, as suggested by the significant correlation between duration of treatment with mood stabilizers and TL that we observed in the current study (Partial correlation controlled for age and BMI: correlation coefficient = 0.451; p = 0.001). Indeed, all patients with BD were under mood stabilizing treatment at time of recruitment. The full model was also statistically significant, with diagnosis explaining the largest variance (model F11, 128 = 11.084, p = 9.42 × 10−14, partial eta squared = 0.515; effect of diagnosis, F3 = 20.568, p = 9.71 × 10−11, partial eta squared = 0.349. Table S1). Comorbidity with metabolic disorders alone (Table S1), which showed statistically significant difference in the distribution among the four diagnostics groups, or with any cardio-metabolic disorders had no effect on the model (data not shown).
Fig. 2. Difference in telomere length among diagnostic groups.
a shows the difference in telomere length (TL) among the four diagnostic groups (effect of diagnosis F3 = 31.87, p = 1.08 × 10−15). b shows the difference in levels of high sensitivity C-reactive protein (hsCRP) among the four diagnostic groups (effect of diagnosis F3 = 4.680, p = 0.004). Levels are expressed as mg/L. Figure c shows the difference in levels of tumor necrosis factor alpha (TNFα) among the four diagnostic groups (effect of diagnosis F3 = 1.217, p = 0.306). Levels are expressed as pg/mL. Graphs were obtained using the raw values (unadjusted), while the statistical significance for TL, hsCRP and TNFα is based on post-hoc analysis with Bonferroni correction of the univariate models controlling of age, sex, and BMI as covariates. *p < 0.05; **p < 0.005; ***p < 0.0005; ns, not significant. Bars represent mean and standard errors on the mean. NPC non-psychiatric controls, BD bipolar disorder, SZ schizophrenia, MDD major depressive disorder.
The rank analysis of covariance with age, sex and BMI as controlling variables showed that hsCRP levels were higher in patients with severe psychiatric disorders (model F4, 134 = 4.02; p = 0.004, partial eta squared = 0.107), with diagnosis being the most significant independent variable (F3 = 4.681; p = 0.004; partial eta squared =0.095; contribution of sex, F1 = 5.423, p = 0.021, partial eta squared = 0.039, Fig. 2b). The highest hsCRP levels were observed in patients with SZ (post-hoc analysis: SZ versus NPC, adjusted p = 0.027), with a mean value of 3.62 mg/L (Standard Deviation ± 2.28; mean value in NPC = 2.09, Standard Deviation ± 1.99), suggesting the presence of low grade peripheral inflammation, which is generally defined by hsCRP level ≥3.0 mg/L [55–58]. Moreover, hsCRP levels were inversely correlated with TL when controlling for age and BMI (partial correlation coefficient = −0.180; p = 0.042. Fig. 3). While of moderate magnitude, this finding suggests a potential interplay between shorter TL and low-grade inflammation, which appeared to be stronger in SZ. The full model was not significant, with a trend for significance only for diagnosis (model F9, 125 = 1.886, p = 0.061, partial eta squared = 0.135; effect of diagnosis, F3, = 2.520, p = 0.062, partial eta squared = 0.065. Table S2).
Fig. 3. Correlation between hsCRP and telomere length.
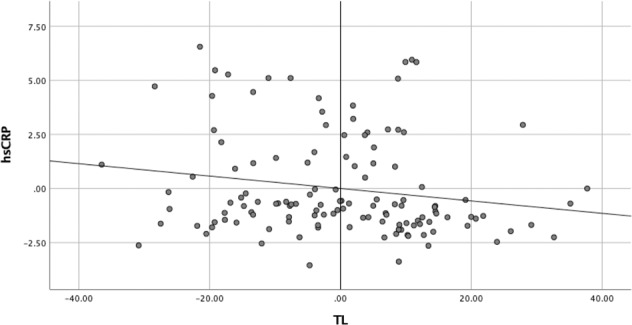
Partial correlation between hsCRP and TL in the whole sample controlled for Body Mass Index (BMI) and age. Correlation coefficient = −0.180, p = 0.042. TL telomere length, hsCRP high sensitivity C-reactive protein.
As for TNFα levels, we found no significant differences among the four diagnostic groups in the simplified model (F4, 148 = 1.099, p = 0.359; Fig. 2c), while the full model was statistically significant (F9, 139 = 2.067, p = 0.037, partial eta squared = 0.131), with the sole significant contribution of diagnosis (F3 = 6.159, p = 0.004, partial eta squared = 0.103. Table S3). The latter finding was driven by the higher levels of TNFα in MDD compared to NPC (Bonferroni corrected p = 0.010).
We also applied the simplified model to test possible differences in molecular measures according to response to antipsychotics in SZ and antidepressants in MDD, characterized as TR or non-TR, and to lithium response in BD, characterized as responders or non-responders.
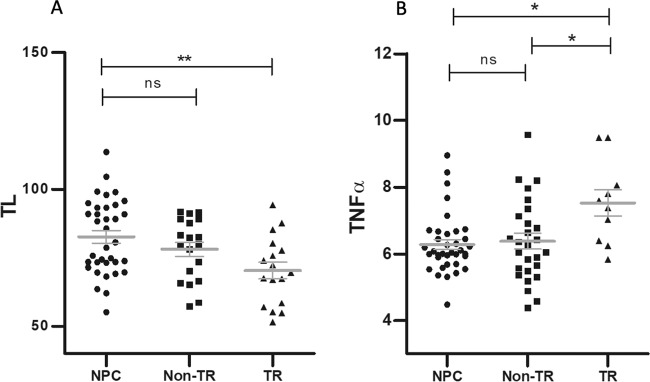
In SZ, the model with age, sex, and BMI as controlling variables showed that TL was significantly different among groups (model F5, 64 = 4.584, p = 0.001, partial eta squared=0.261; effect of diagnosis, F2 = 6.927, p = 0.002, partial eta squared = 0.176), with shorter TL in TR-SZ compared to NPC (adjusted p = 0.001), but not in non-TR compared to NPC (adjusted p = 0.128; Fig. 4a), suggesting that TR SZ might be characterized by more severe molecular impairments. Age (F1 = 7.630, p = 0.007, partial eta squared = 0.105) and sex (F1 = 5.006, p = 0.029, partial eta squared = 0.072) also significantly contributed to the model, but diagnosis was the most significant factor explaining the largest proportion of variation. There was no difference in hsCRP or TNFα levels between TR and non-TR patients.
Fig. 4. Association between telomere length and treatment-resistant schizophrenia or treatment-resistant depression.
a shows the difference in TL among non-psychiatric controls (NPC), patients with treatment-resistant schizophrenia (TR), and patients with non-treatment resistant schizophrenia (non-TR) (effect of diagnosis F2 = 6.927; p = 0.002). b shows the difference in levels of tumor necrosis factor alpha (TNFα) among non-psychiatric controls (NPC), patients with treatment-resistant major depressive disorder (TR), and patients with non-treatment resistant major depressive disorder (non-TR) (effect of diagnosis F2 = 3.998; p = 0.023). Graphs were obtained using the raw values (unadjusted), while the statistical significance for TL, hsCRP and TNFα is based on post-hoc analysis with Bonferroni correction of the univariate models controlling of age, sex, and BMI as covariates. *p < 0.05; **p < 0.005; ns, not significant. Bars represent mean and standard errors on the mean.
In MDD, there was no significant difference in TR, non-TR, or NPC in terms of TL or hsCRP. However, the rank analysis of covariance with age, sex, and BMI as controlling variables showed significant differences in TNFα among TR MDD, non-TR MDD, and NPC (model F3, 68 = 2.722, p = 0.050, partial eta squared=0.107; effect of diagnosis, F2 = 3.998, p = 0.023, partial eta squared 0.105). Post-hoc comparison showed that patients with TR MDD had higher levels of TNFα compared to both non-TR (adjusted p = 0.039) and NPC (adjusted p = 0.028), while non-TR and NPC had similar levels (adjusted p = 1; Fig. 4b) suggesting that TR might be associated with increased inflammation compared to non-TR. As for lithium response, we found no difference for any of the molecular measures tested between BD patients responders or nonresponders (data not shown).
Finally, comorbidity with metabolic disorders (Table S1), which showed statistically significant difference in the distribution among the four diagnostics groups, or with any cardio-metabolic disorders, had no effect on any of the models tested for any of the molecular measures (data not shown).
Discussion
In the present study we compared TL and levels of circulating inflammatory markers among groups of patients with different severe psychiatric disorders (BD, MDD, and SZ) and NPC, and investigated the role of treatment with psychotropic medications and comorbid cardio-metabolic disorders in the possible interplay between telomere shortening, inflammation, and psychiatric disorders.
We showed that patients with SZ and MDD had shorter TL compared to NPC, while BD patients presented the longest telomeres of all groups. Shorter TL has been extensively reported in psychiatric disorders though negative or opposite findings have also been published (for a review see [59]). Concerning SZ, a recent meta-analysis by Rao and colleagues including 11 papers published from 2008 to 2016 [60] showed that TL was significantly shorter in affected individuals compared to controls. A more recent study by Russo and colleagues (2018) further supported the hypothesis of telomere attrition in SZ, showing that a diagnosis of SZ was the most significant variable contributing to shorter leukocyte telomere length (LTL), with a higher weight than gender, age, cigarette smoking or alcohol drinking [61]. Moreover, in this study the authors also performed an updated meta-analysis including eight additional studies compared to Rao et al. (2016), and showed even more robust evidence for decreased LTL in SZ compared to controls. Evidence for shorter telomeres in SZ was also provided by a study from Higgins-Chen and colleagues (2020), who showed a reduced DNA methylation estimator of TL (DNAmTL) in SZ [62]. Leukocyte DNAmTL has been suggested to be more strongly associated with age than LTL [63], and as such, the latter finding is of particular relevance in supporting accelerated aging in SZ. Interestingly, the study by Higgins-Chen also highlighted the complexity of the epigenetic mechanisms underlying the aging process which, overall, appears to be altered in SZ.
In our study we also showed that only patients with TR-SZ and not the non-TR group had significantly shorter telomeres compared to NPC. Shorter telomeres in patients with SZ with poor response to antipsychotics have been previously reported by other studies [60, 63–65]. However, to our knowledge, ours is the first study exploring TL in TR versus non-TR patients. While findings are too sparse to draw definite conclusions, we can hypothesize that short telomeres could be a trait marker of poor response to antipsychotics and possibly TR-SZ.
Another finding of our study was that patients with SZ had higher plasma levels of hsCRP compared to controls and to the other groups of patients with psychiatric disorders, suggesting a state of low-grade systemic inflammation and a putative interaction between inflammatory processes and telomere shortening. This interpretation is supported by our finding of an inverse correlation between TL and hsCRP levels, which was not influenced by age, BMI, or cardio-metabolic disorders. Although inflammation and telomere shortening have been largely studied as independent phenomena in ageing and disease association, their possible interdependent nature has been hypothesized, and a detrimental effect of inflammation on telomere dynamics has been suggested [31]. However, the hypothesis of a role of their interaction in the etiopathogenesis and progression of psychiatric disorders has yet to be fully elucidated. To date several studies showed that telomere attrition and increased inflammation (as well as alterations of other processes involved in aging) could represent molecular signatures of SZ (for a review see [66]), but data so far do not allow to clearly understand whether the correlation between these two processes has causative implications. One of the most valuable findings from longitudinal studies in psychiatric disorders was published by Osler et al. [67], who explored the interplay between stressful life events, clinical features (including the characterization of depressive symptoms) and measures of inflammation and biological stress in a large cohort of Danish men. Results showed that early stressful events were associated with shorter TL in middle-aged men, and that the largest proportion of this association was mediated through depressive mood and CRP. Nevertheless, the number of longitudinal studies exploring TL and inflammation in psychiatric disorders remains limited and more efforts are needed to better elucidate the role of disturbances in these molecular dynamics in mental disorders.
Concerning CRP, increased blood levels have been reported in a number of studies for SZ [56, 58, 68], MDD [69, 70], and BD [58, 69, 71]. In our study, hsCRP levels of patients with BD and MDD were higher than those observed in NPC, but the difference was not statistically significant after correction for multiple comparisons. This finding could be determined by a number of factors that differentiate our study from previous investigations, such as different inclusion and exclusion criteria, exposure to and duration of pharmacological treatments, different methodologies and, most importantly, the limited sample size of the diagnostic groups included. However, while we did not show a significant difference in hsCRP levels between MDD and NPC, we showed significantly higher plasma levels of TNFα in MDD, and especially in TR-MDD when compared to both non-TR MDD and NPC, while levels in non-TR were similar to NPC. This finding suggests that TR-MDD patients might be characterized by increased inflammation, an hypothesis that has been investigated and supported by previous investigations [72–74]. Treatment with antidepressants fails in about 30% of patients, and data suggest that those patients with high inflammation are more prone to be resistant [75, 76]. A role of TNFα in MDD is also supported by the evidence that TNFα inhibitors show antidepressant effects in patients with TR-MDD with higher baseline levels of inflammatory markers [77], that lower baseline levels of TNFa (pretreatment) correlates with better response to electroconvulsive therapy (ECT) in TR-MDD [78], and that ECT reduces plasma levels of TNFα [79, 80].
The importance of modulating levels of TNFα in MDD has also been supported by a recent meta-analysis including 22 studies and 827 patients, which showed that treatment with Selective Serotonin Reuptake Inhibitors (SSRIs) significantly reduces circulating levels of a number of inflammatory markers, including TNFα [81], suggesting that part of the therapeutic effects of SSRIs could be attributable to their anti-inflammatory properties.
The importance of reducing inflammation for better management of psychiatric disorders has been explored not only for MDD but also for BD and SZ. Findings so far are contrasting, but there is a significant number of studies suggesting that reducing inflammation with anti-inflammatory agents might reduce symptoms and severity of psychiatric disorders [82–87].
In our study, MDD patients had shorter telomeres compared to NPC, while there was no significant difference between TR-MDD, TR MDD, and NPC. Shorter TL in MDD has been reported in several studies suggesting that telomere attrition could be a marker of MDD too [21, 88]. A recent meta-analysis including 38 studies and a total of 34,347 cases, showed that MDD was significantly associated with shorter TL [89]. Similar findings were reported by another meta-analysis published in 2016 including 16 studies involving 7207 subjects [90], which reported shorter LTL in patients with MDD compared to controls. Recently, we showed that patients with MDD had shorter LTL compared to controls in an independent sample of 54 MDD patients and 47 NPC [91]. Similar to the present study, our previous work did not show a significant difference in TL between TR and non-TR MDD. While an effect of antidepressants on telomere biology has been suggested in mice [92], only a small number of investigations explored the correlation between TL and response to antidepressants in patients. Hough and coworkers (2016) showed that LTL before treatment with SSRIs was shorter in patients with MDD non-responders to treatment than in responders. Moreover, the authors observed less improvement in negative affect in patients with shorter pre-treatment LTL. Another study published by Wolkowitz et al. [42] showed that MDD patients with lower pretreatment activity of telomerase and greater increase in telomerase activity during antidepressant treatment had better response to antidepressants. Nevertheless, the number of studies exploring the correlation between TL and response to antidepressants remains too scarce to draw definite conclusions and requires further investigation.
In our study, we showed that patients with BD had the longest TL of the tested groups. The latter finding appears in contrast with the hypothesis of telomere attrition in psychiatric disorders, but previous authors reported similar results in BD [40, 93]. This discrepancy between BD and the other groups of psychiatric disorders could be determined by several factors, but our findings suggest that a great role could be played by exposure to mood stabilizing treatments. In fact, TL was significantly positively correlated with duration of treatment with mood stabilizers, and all BD patients included in the study were under treatment with these medications at time of recruitment. A correlation between TL and duration of treatment with lithium, the mood stabilizer of first choice in BD, have been shown by number of previous works [39–41]. While in the present study we were not able to compare patients with BD exposed versus non-exposed to mood stabilizers, our hypothesis is corroborated by our previous study [94] showing that patients with BD exposed to lithium had longer LTL compared to patients never exposed and to healthy controls, thus, suggesting that lithium, and likely other mood stabilizers, might counteract telomere attrition in psychiatric disorders.
We did not show a significant contribution of comorbidity with cardio-metabolic disorders in any of the tested models for TL, hsCRP, and TNFα. Cardio-metabolic disorders have been associated with telomere attrition and increased inflammation [95], but our findings do not support the hypothesis that comorbidity with these disorders may contribute to more prominent molecular alterations in telomere and inflammatory dynamics observed in patients with severe psychiatric disorders.
Overall, our findings suggest that severe psychiatric disorders present altered TL and peripheral levels of inflammatory markers compared to nonpsychiatric controls.
These results need to be interpreted in light of the strengths and limitations of our study. TL was measured using Q-FISH, which compared to other more utilized methods, such as qPCR, provides a more sensitive and accurate tool, especially if performed on metaphases chromosomes rather than on interphase nuclei: the measurements made on individual chromosomes provide indeed an accurate estimation of fluorescence, allowing a precise signal count. On the other hand, being more time consuming and less cost-effective, the choice to use Q-FISH contributed to limiting the sample size included in the present study. However, the number of individuals enrolled is in line with- or larger than previous investigations using FISH to measure TL in severe psychiatric disorders [96–99]. One of the major strengths of our study is the deep phenotypic characterization of the subjects included, which allowed a deeper exploration of the role of a number of potentially relevant features which have been often overlooked in previous investigations, including but not limited to history of pharmacological treatments, cigarette smoking, substance abuse, physical activity, suicidal behavior, family history for psychiatric disorders, and age of father at birth. On the other hand, the main limitation of our study resides in its cross-sectional nature and the lack of patients naïve to treatment with psychotropic medications. It is also possible that the study criteria led to the inclusion of participants more willing to contribute because less affected by the disorders. It should be noted, however, that patients were recruited in primary and tertiary centers of psychiatric care and as such are good representations of real-world clinical populations.
In sum, while we suggest that telomere shortening and increased inflammation might represent signatures of psychiatric disorders (in particular SZ and MDD), our study design did not allow us to explore the causative role of the biological measures investigated. That considered, future studies should try to implement a longitudinal prospective design to clearly elucidate the role of TL, inflammation and aging in severe psychiatric disorders.
Funding and disclosure
This work was supported with a grant funded by Fondazione di Sardegna and Regione Sardegna, Call 2016, Project ID: F72F16003090002, granted to AS. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results. The authors have nothing to declare.
Supplementary information
Author contributions
AS conceived and designed the work, acquired the data, contributed interpreting the results, drafted and revised the manuscript, approved the final version; MM performed and coordinated the recruitment of patients, participated conceiving the study and drafting the manuscript; CP run part of the experiments, drafted the methods and result section, contributed analyzing and interpreting the findings; DC, performed part of the TL experiments and coordinated the activity of the laboratory of pharmacogenomics; AM, performed part of the experiments on telomeres; RA, CA, AB, EC, MG, MAM, AM, PP, FP, GS, contributed recruiting the patients and controls; PC, TD, DVF performed the Q-FISH experiments; MN, RR, VS contributed conceiving the study and interpreting the results; CC, EM, BN performed the ELISA experiments; GLF coordinated the ELISA experiments and contributed interpreting the findings; MDZ and CC coordinated the recruitment of patients and controls at the Unit of Clinical Pharmacology, and contributed designing the study; RV contributed conceiving the study, interpreting findings and finalizing the manuscript; BC coordinated the clinical activity of the Unit of Psychiatry and contributed interpreting the findings and finalizing the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Alessio Squassina, Mirko Manchia, Claudia Pisanu
These authors jointly supervised this work: Caterina Chillotti, Roberta Vanni, Bernardo Carpiniello
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-00844-z).
References
- 1.Who Mental Health. https://www.who.int/mental_health/management/en. Accessed 26 April 2020.
- 2.Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. Lancet Psychiatry. 2016;3:171–8. doi: 10.1016/S2215-0366(15)00505-2. [DOI] [PubMed] [Google Scholar]
- 3.WHO mental disorders. https://www.who.int/news-room/fact-sheets/detail/mental-disorders. Accessed 25 April 2020.
- 4.Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86–97. doi: 10.1016/S0140-6736(15)01121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vieta E, Berk M, Schulze TG, Carvalho AF, Suppes T, Calabrese JR, et al. Bipolar disorders. Nat Rev Dis Prim. 2018;4:18008. doi: 10.1038/nrdp.2018.8. [DOI] [PubMed] [Google Scholar]
- 6.Liu NH, Daumit GL, Dua T, Aquila R, Charlson F, Cuijpers P, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry. 2017;16:30–40. doi: 10.1002/wps.20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordentoft M, Wahlbeck K, Hallgren J, Westman J, Osby U, Alinaghizadeh H, et al. Excess mortality, causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark, Finland and Sweden. PLoS ONE. 2013;8:e55176. doi: 10.1371/journal.pone.0055176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry. 2014;13:153–60. doi: 10.1002/wps.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psychiatr Serv. 2009;60:147–56. doi: 10.1176/ps.2009.60.2.147. [DOI] [PubMed] [Google Scholar]
- 10.De Hert M, Dekker JM, Wood D, Kahl KG, Holt RI, Moller HJ. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC) Eur Psychiatry. 2009;24:412–24. doi: 10.1016/j.eurpsy.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence D, Kisely S, Pais J. The epidemiology of excess mortality in people with mental illness. Can J Psychiatry. 2010;55:752–60. doi: 10.1177/070674371005501202. [DOI] [PubMed] [Google Scholar]
- 12.Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298:1794–6. doi: 10.1001/jama.298.15.1794. [DOI] [PubMed] [Google Scholar]
- 13.Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72:334–41. doi: 10.1001/jamapsychiatry.2014.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodwin RD, Davidson KW, Keyes K. Mental disorders and cardiovascular disease among adults in the United States. J Psychiatr Res. 2009;43:239–46.. doi: 10.1016/j.jpsychires.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO report. http://www.euro.who.int/__data/assets/pdf_file/0009/342297/Comorbidity-report_E-web.pdf. Accessed 25 April 2020.
- 16.Fries GR, Bauer IE, Scaini G, Valvassori SS, Walss-Bass C, Soares JC, et al. Accelerated hippocampal biological aging in bipolar disorder. Bipolar Disord. 2019. 10.1111/bdi.12876 [Online ahead of print]. [DOI] [PubMed]
- 17.Hajek T, Franke K, Kolenic M, Capkova J, Matejka M, Propper L, et al. Brain age in early stages of bipolar disorders or schizophrenia. Schizophr Bull. 2019;45:190–98. doi: 10.1093/schbul/sbx172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nenadic I, Dietzek M, Langbein K, Sauer H, Gaser C. BrainAGE score indicates accelerated brain aging in schizophrenia, but not bipolar disorder. Psychiatry Res Neuroimaging. 2017;266:86–89. doi: 10.1016/j.pscychresns.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Shahab S, Mulsant BH, Levesque ML, Calarco N, Nazeri A, Wheeler AL, et al. Brain structure, cognition, and brain age in schizophrenia, bipolar disorder, and healthy controls. Neuropsychopharmacology. 2019;44:898–906. doi: 10.1038/s41386-018-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Gestel H, Franke K, Petite J, Slaney C, Garnham J, Helmick C, et al. Brain age in bipolar disorders: effects of lithium treatment. Aust N Z J Psychiatry. 2019;53:1179–88. doi: 10.1177/0004867419857814. [DOI] [PubMed] [Google Scholar]
- 21.Squassina A, Pisanu C, Vanni R. Mood disorders, accelerated aging, and inflammation: is the link hidden in telomeres? Cells. 2019;8:52. doi: 10.3390/cells8010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giardini MA, Segatto M, da Silva MS, Nunes VS, Cano MI. Telomere and telomerase biology. Prog Mol Biol Transl Sci. 2014;125:1–40. doi: 10.1016/B978-0-12-397898-1.00001-3. [DOI] [PubMed] [Google Scholar]
- 23.Shay JW. Role of telomeres and telomerase in aging and cancer. Cancer Discov. 2016;6:584–93. doi: 10.1158/2159-8290.CD-16-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rode L, Nordestgaard BG, Bojesen SE. Peripheral blood leukocyte telomere length and mortality among 64,637 individuals from the general population. J Natl Cancer Inst. 2015;107:djv074. doi: 10.1093/jnci/djv074. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs EG, Epel ES, Lin J, Blackburn EH, Rasgon NL. Relationship between leukocyte telomere length, telomerase activity, and hippocampal volume in early aging. JAMA Neurol. 2014;71:921–3. doi: 10.1001/jamaneurol.2014.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King KS, Kozlitina J, Rosenberg RN, Peshock RM, McColl RW, Garcia CK. Effect of leukocyte telomere length on total and regional brain volumes in a large population-based cohort. JAMA Neurol. 2014;71:1247–54. doi: 10.1001/jamaneurol.2014.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staffaroni AM, Tosun D, Lin J, Elahi FM, Casaletto KB, Wynn MJ, et al. Telomere attrition is associated with declines in medial temporal lobe volume and white matter microstructure in functionally independent older adults. Neurobiol Aging. 2018;69:68–75. doi: 10.1016/j.neurobiolaging.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wikgren M, Karlsson T, Soderlund H, Nordin A, Roos G, Nilsson LG, et al. Shorter telomere length is linked to brain atrophy and white matter hyperintensities. Age Ageing. 2014;43:212–7. doi: 10.1093/ageing/aft172. [DOI] [PubMed] [Google Scholar]
- 29.Shivakumar V, Kalmady SV, Rajasekaran A, Chhabra H, Anekal AC, Narayanaswamy JC, et al. Telomere length and its association with hippocampal gray matter volume in antipsychotic-naive/free schizophrenia patients. Psychiatry Res Neuroimaging. 2018;282:11–7. doi: 10.1016/j.pscychresns.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Darrow SM, Verhoeven JE, Revesz D, Lindqvist D, Penninx BW, Delucchi KL, et al. The association between psychiatric disorders and telomere length: a meta-analysis involving 14,827 persons. Psychosom Med. 2016;78:776–87. doi: 10.1097/PSY.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Rane G, Dai X, Shanmugam MK, Arfuso F, Samy RP, et al. Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res Rev. 2016;25:55–69. doi: 10.1016/j.arr.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Bauer ME, Teixeira AL. Inflammation in psychiatric disorders: what comes first? Ann NY Acad Sci. 2019;1437:57–67. doi: 10.1111/nyas.13712. [DOI] [PubMed] [Google Scholar]
- 33.Felger JC. Imaging the role of inflammation in mood and anxiety-related disorders. Curr Neuropharmacol. 2018;16:533–58.. doi: 10.2174/1570159X15666171123201142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfau ML, Menard C, Russo SJ. Inflammatory mediators in mood disorders: therapeutic opportunities. Annu Rev Pharm Toxicol. 2018;58:411–28. doi: 10.1146/annurev-pharmtox-010617-052823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capuron L, Castanon N. Role of inflammation in the development of neuropsychiatric symptom domains: evidence and mechanisms. Curr. Top Behav Neurosci. 2017;31:31–44. doi: 10.1007/7854_2016_14. [DOI] [PubMed] [Google Scholar]
- 36.Felger JC, Haroon E, Patel TA, Goldsmith DR, Wommack EC, Woolwine BJ, et al. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry. 2018;25:1301–11. doi: 10.1038/s41380-018-0096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Felger JC, Treadway MT. Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology. 2017;42:216–41. doi: 10.1038/npp.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haroon E, Miller AH. Inflammation Effects on Glutamate as a Pathway to Neuroprogression in Mood Disorders. Mod Trends Pharmacopsychiatry. 2017;31:37–55. doi: 10.1159/000470805. [DOI] [PubMed] [Google Scholar]
- 39.Coutts F, Palmos AB, Duarte RRR, de Jong S, Lewis CM, Dima D, et al. The polygenic nature of telomere length and the anti-ageing properties of lithium. Neuropsychopharmacology. 2019;44:757–65. doi: 10.1038/s41386-018-0289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinsson L, Wei Y, Xu D, Melas PA, Mathe AA, Schalling M, et al. Long-term lithium treatment in bipolar disorder is associated with longer leukocyte telomeres. Transl Psychiatry. 2013;3:e261. doi: 10.1038/tp.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Squassina A, Pisanu C, Congiu D, Caria P, Frau D, Niola P, et al. Leukocyte telomere length positively correlates with duration of lithium treatment in bipolar disorder patients. Eur Neuropsychopharmacol. 2016;26:1241–7. doi: 10.1016/j.euroneuro.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 42.Wolkowitz OM, Mellon SH, Epel ES, Lin J, Reus VI, Rosser R, et al. Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Mol Psychiatry. 2012;17:164–72. doi: 10.1038/mp.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hough CM, Bersani FS, Mellon SH, Epel ES, Reus VI, Lindqvist D, et al. Leukocyte telomere length predicts SSRI response in major depressive disorder: a preliminary report. Mol Neuropsychiatry. 2016;2:88–96. doi: 10.1159/000446500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manchia M, Paribello P, Arzedi C, Bocchetta A, Caria P, Cocco C, et al. A multidisciplinary approach to mental illness: do inflammation, telomere length and microbiota form a loop? A protocol for a cross-sectional study on the complex relationship between inflammation, telomere length, gut microbiota and psychiatric disorders. BMJ Open. 2020;10:e032513. doi: 10.1136/bmjopen-2019-032513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(Suppl 6):16–22. [PubMed] [Google Scholar]
- 46.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–96. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 47.Grof P, Duffy A, Cavazzoni P, Grof E, Garnham J, MacDougall M, et al. Is response to prophylactic lithium a familial trait? J Clin Psychiatry. 2002;63:942–7. doi: 10.4088/jcp.v63n1013. [DOI] [PubMed] [Google Scholar]
- 48.Manchia M, Adli M, Akula N, Ardau R, Aubry JM, Backlund L, et al. Assessment of response to lithium maintenance treatment in bipolar disorder: a consortium on lithium genetics (ConLiGen) report. PLoS ONE. 2013;8:e65636. doi: 10.1371/journal.pone.0065636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scott J, Etain B, Manchia M, Brichant-Petitjean C, Geoffroy PA, Schulze T, et al. An examination of the quality and performance of the Alda scale for classifying lithium response phenotypes. Bipolar Disord. 2019;22:255–65. [DOI] [PubMed]
- 50.First MB, Spitzer RL, Gibbon M, Williams JBW Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute, 2002.
- 51.Cantara S, Pisu M, Frau DV, Caria P, Dettori T, Capezzone M, et al. Telomere abnormalities and chromosome fragility in patients affected by familial papillary thyroid cancer. J Clin Endocrinol Metab. 2012;97:E1327–31. doi: 10.1210/jc.2011-2096. [DOI] [PubMed] [Google Scholar]
- 52.Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? Am J Med. 2006;119:166 e17–28. doi: 10.1016/j.amjmed.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 53.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–8. [PubMed] [Google Scholar]
- 54.Morley JJ, Kushner I. Serum C-reactive protein levels in disease. Ann NY Acad Sci. 1982;389:406–18. doi: 10.1111/j.1749-6632.1982.tb22153.x. [DOI] [PubMed] [Google Scholar]
- 55.Faugere M, Micoulaud-Franchi JA, Faget-Agius C, Lancon C, Cermolacce M, Richieri R. High C-reactive protein levels are associated with depressive symptoms in schizophrenia. J Affect Disord. 2018;225:671–75. doi: 10.1016/j.jad.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Fond G, Lancon C, Auquier P, Boyer L. C-reactive protein as a peripheral biomarker in schizophrenia. an updated systematic review. Front Psychiatry. 2018;9:392. doi: 10.3389/fpsyt.2018.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sicras-Mainar A, Rejas-Gutierrez J, Navarro-Artieda R, Blanca-Tamayo M. C-reactive protein as a marker of cardiovascular disease in patients with a schizophrenia spectrum disorder treated in routine medical practice. Eur Psychiatry. 2013;28:161–7. doi: 10.1016/j.eurpsy.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 58.Wysokinski A, Margulska A, Strzelecki D, Kloszewska I. Levels of C-reactive protein (CRP) in patients with schizophrenia, unipolar depression and bipolar disorder. Nord J Psychiatry. 2015;69:346–53. doi: 10.3109/08039488.2014.984755. [DOI] [PubMed] [Google Scholar]
- 59.Lindqvist D, Epel ES, Mellon SH, Penninx BW, Revesz D, Verhoeven JE, et al. Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neurosci Biobehav Rev. 2015;55:333–64. doi: 10.1016/j.neubiorev.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rao S, Kota LN, Li Z, Yao Y, Tang J, Mao C, et al. Accelerated leukocyte telomere erosion in schizophrenia: evidence from the present study and a meta-analysis. J Psychiatr Res. 2016;79:50–6. doi: 10.1016/j.jpsychires.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 61.Russo P, Prinzi G, Proietti S, Lamonaca P, Frustaci A, Boccia S, et al. Shorter telomere length in schizophrenia: evidence from a real-world population and meta-analysis of most recent literature. Schizophr Res. 2018;202:37–45. doi: 10.1016/j.schres.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 62.Higgins-Chen AT, Boks MP, Vinkers CH, Kahn RS, Levine ME. Schizophrenia and epigenetic aging biomarkers: increased mortality, reduced cancer risk, and unique clozapine effects. Biol Psychiatry. 2020;88:224–35. doi: 10.1016/j.biopsych.2020.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu AT, Seeboth A, Tsai PC, Sun D, Quach A, Reiner AP, et al. DNA methylation-based estimator of telomere length. Aging (Albany NY) 2019;11:5895–923. doi: 10.18632/aging.102173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Z, Hu M, Zong X, He Y, Wang D, Dai L, et al. Association of telomere length and mitochondrial DNA copy number with risperidone treatment response in first-episode antipsychotic-naive schizophrenia. Sci Rep. 2015;5:18553. doi: 10.1038/srep18553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu WY, Chang HW, Lin CH, Cho CL. Short telomeres in patients with chronic schizophrenia who show a poor response to treatment. J Psychiatry Neurosci. 2008;33:244–7. [PMC free article] [PubMed] [Google Scholar]
- 66.Nguyen TT, Eyler LT, Jeste DV. Systemic biomarkers of accelerated aging in schizophrenia: a critical review and future directions. Schizophr Bull. 2018;44:398–408. doi: 10.1093/schbul/sbx069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Osler M, Bendix L, Rask L, Rod NH. Stressful life events and leucocyte telomere length: do lifestyle factors, somatic and mental health, or low grade inflammation mediate this relationship? Results from a cohort of Danish men born in 1953. Brain Behav Immun. 2016;58:248–53. doi: 10.1016/j.bbi.2016.07.154. [DOI] [PubMed] [Google Scholar]
- 68.Ayari F, Ben Chaaben A, Ben Ammar H, Nefzi R, Ouni N, Mihoub O, et al. Association of high-sensitivity C-reactive protein with susceptibility to Schizophrenia in Tunisian population. Encephale. 2020;46:241–7. doi: 10.1016/j.encep.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 69.Glaus J, von Kanel R, Lasserre AM, Strippoli MF, Vandeleur CL, Castelao E, et al. Mood disorders and circulating levels of inflammatory markers in a longitudinal population-based study. Psychol Med. 2018;48:961–73. doi: 10.1017/S0033291717002744. [DOI] [PubMed] [Google Scholar]
- 70.Leung BMY, Nwoke C. Association between C-reactive protein and mood disorder in a representative sample of the Canadian population: analysis of CHMS data 2013-4. Can J Public Health. 2020. 10.17269/s41997-020-00297-3 [Online ahead of print]. [DOI] [PMC free article] [PubMed]
- 71.Marshe VS, Pira S, Mantere O, Bosche B, Looper KJ, Herrmann N, et al. C-reactive protein and cardiovascular risk in bipolar disorder patients: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:442–51. doi: 10.1016/j.pnpbp.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 72.Chamberlain SR, Cavanagh J, de Boer P, Mondelli V, Jones DNC, Drevets WC, et al. Treatment-resistant depression and peripheral C-reactive protein. Br J Psychiatry. 2019;214:11–9. doi: 10.1192/bjp.2018.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ruland T, Chan MK, Stocki P, Grosse L, Rothermundt M, Cooper JD, et al. Molecular serum signature of treatment resistant depression. Psychopharmacol (Berl). 2016;233:3051–9. doi: 10.1007/s00213-016-4348-0. [DOI] [PubMed] [Google Scholar]
- 74.Strawbridge R, Hodsoll J, Powell TR, Hotopf M, Hatch SL, Breen G, et al. Inflammatory profiles of severe treatment-resistant depression. J Affect Disord. 2019;246:42–51. doi: 10.1016/j.jad.2018.12.037. [DOI] [PubMed] [Google Scholar]
- 75.Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ, et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline ‘predictors’ and longitudinal ‘targets’. Neuropsychopharmacology. 2013;38:377–85. doi: 10.1038/npp.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haroon E, Daguanno AW, Woolwine BJ, Goldsmith DR, Baer WM, Wommack EC, et al. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology. 2018;95:43–9. doi: 10.1016/j.psyneuen.2018.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pinna M, Manchia M, Oppo R, Scano F, Pillai G, Loche AP, et al. Clinical and biological predictors of response to electroconvulsive therapy (ECT): a review. Neurosci Lett. 2018;669:32–42. doi: 10.1016/j.neulet.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 79.Hestad KA, Tonseth S, Stoen CD, Ueland T, Aukrust P. Raised plasma levels of tumor necrosis factor alpha in patients with depression: normalization during electroconvulsive therapy. J ECT. 2003;19:183–8. doi: 10.1097/00124509-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 80.Yrondi A, Sporer M, Peran P, Schmitt L, Arbus C, Sauvaget A. Electroconvulsive therapy, depression, the immune system and inflammation: a systematic review. Brain Stimul. 2018;11:29–51. doi: 10.1016/j.brs.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 81.Wang L, Wang R, Liu L, Qiao D, Baldwin DS, Hou R. Effects of SSRIs on peripheral inflammatory markers in patients with major depressive disorder: a systematic review and meta-analysis. Brain Behav Immun. 2019;79:24–38. doi: 10.1016/j.bbi.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 82.Chang HH, Chen PS. Inflammatory biomarkers for mood disorders–a brief narrative review. Curr Pharm Des. 2020;26:236–43. doi: 10.2174/1381612826666200115100726. [DOI] [PubMed] [Google Scholar]
- 83.Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–91. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 84.Kohler O, Krogh J, Mors O, Benros ME. Inflammation in depression and the potential for anti-inflammatory treatment. Curr Neuropharmacol. 2016;14:732–42. doi: 10.2174/1570159X14666151208113700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller AH, Raison CL. Are anti-inflammatory therapies viable treatments for psychiatric disorders?: where the rubber meets the road. JAMA Psychiatry. 2015;72:527–8. doi: 10.1001/jamapsychiatry.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Muller N. Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr Bull. 2018;44:973–82. doi: 10.1093/schbul/sby024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rosenblat JD, Kakar R, Berk M, Kessing LV, Vinberg M, Baune BT, et al. Anti-inflammatory agents in the treatment of bipolar depression: a systematic review and meta-analysis. Bipolar Disord. 2016;18:89–101. doi: 10.1111/bdi.12373. [DOI] [PubMed] [Google Scholar]
- 88.Muneer A, Minhas FA. Telomere biology in mood disorders: an updated, comprehensive review of the literature. Clin Psychopharmacol Neurosci. 2019;17:343–63. doi: 10.9758/cpn.2019.17.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ridout KK, Ridout SJ, Price LH, Sen S, Tyrka AR. Depression and telomere length: a meta-analysis. J Affect Disord. 2016;191:237–47. doi: 10.1016/j.jad.2015.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin PY, Huang YC, Hung CF. Shortened telomere length in patients with depression: a meta-analytic study. J Psychiatr Res. 2016;76:84–93. doi: 10.1016/j.jpsychires.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 91.Pisanu C, Tsermpini EE, Skokou M, Kordou Z, Gourzis P, Assimakopoulos K, et al. Leukocyte telomere length is reduced in patients with major depressive disorder. Drug Dev Res. 2020;81:268–73. doi: 10.1002/ddr.21612. [DOI] [PubMed] [Google Scholar]
- 92.Zhou QG, Hu Y, Wu DL, Zhu LJ, Chen C, Jin X, et al. Hippocampal telomerase is involved in the modulation of depressive behaviors. J Neurosci. 2011;31:12258–69. doi: 10.1523/JNEUROSCI.0805-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Powell TR, Dima D, Frangou S, Breen G. Telomere length and bipolar disorder. Neuropsychopharmacology. 2018;43:454. doi: 10.1038/npp.2017.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pisanu C, Congiu D, Manchia M, Caria P, Cocco C, Dettori T, et al. Differences in telomere length between patients with bipolar disorder and controls are influenced by lithium treatment. Pharmacogenomics. 2020;21:533–40. doi: 10.2217/pgs-2020-0028. [DOI] [PubMed] [Google Scholar]
- 95.Jose SS, Bendickova K, Kepak T, Krenova Z, Fric J. Chronic inflammation in immune aging: role of pattern recognition receptor crosstalk with the telomere complex? Front Immunol. 2017;8:1078. doi: 10.3389/fimmu.2017.01078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boeck C, Salinas-Manrique J, Calzia E, Radermacher P, von Arnim CAF, Dietrich DE, et al. Targeting the association between telomere length and immuno-cellular bioenergetics in female patients with major depressive disorder. Sci Rep. 2018;8:9419. doi: 10.1038/s41598-018-26867-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elvsashagen T, Vera E, Boen E, Bratlie J, Andreassen OA, Josefsen D, et al. The load of short telomeres is increased and associated with lifetime number of depressive episodes in bipolar II disorder. J Affect Disord. 2011;135:43–50. doi: 10.1016/j.jad.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 98.Karabatsiakis A, Kolassa IT, Kolassa S, Rudolph KL, Dietrich DE. Telomere shortening in leukocyte subpopulations in depression. BMC Psychiatry. 2014;14:192. doi: 10.1186/1471-244X-14-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schutte NS, Malouff JM. The association between depression and leukocyte telomere length: a meta-analysis. Depress Anxiety. 2015;32:229–38. doi: 10.1002/da.22351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.