Abstract
Lime peel contains metabolic compounds that have lethal effects of bacterial cells, but its effect as an antibacterial modulate innate immunity pathways, especially toll-like receptor 4 (TLR-4) signaling pathway, is unclear. This study examined the effects of lime peel extract (LPE) on the activity of TLR 4 in Balb/c mice induced by Salmonella typhi. Mice were induced intraperitoneally and then 3 days after induction, LPE was given orally on two doses (510 and 750 mg/kg BW). The number of bacterial colonization was counted using peritoneal fluid samples by the method of plate count agar. Intervention LPE for 5 days can degrade TLR-4 and the number of colonies of S. typhi. On day 3 after was induced S. typhi, TLR-4 gene expression of Balb/c mice is increased. Postintervention LPE for 5 days, the expression of TLR-4 gene decreased, significantly different at a dose of 750 mg/kg BW (P = 0.04). There was a positive correlation between the expression of TLR-4 gene by the number of bacterial colonization, decreasing gene expression of TLR-4, the number of bacterial colonization is also getting smaller (P = 0.013, r = 0.408). LPE can modulate the TLR-4 signaling pathway in host immunity so that the gene TLR-4 is expressed fewer in numbers. This mechanism causes the bacterial colony number to decrease, not even growth.
Key words: Bacterial colonization, Citrus aurantifolia, lime peel extract, Salmonella typhi, toll-like receptors 4
INTRODUCTION
Many herbal medicines have been explored as antioxidant, antibacterial, and immunomodulator.[1,2,3,4,5,6] Typhoid fever is an acute gastrointestinal infection caused by Salmonella typhi and still a major problem since endemic in several countries.[7] In addition to genetic factors that can affect the multiplication of S. Typhi, several immune factors that determine the severity of typhoid fever can also affect S. Typhi's multiplication.[8]
When the body is infected with S. typhi, the body will hold out as a response to the reaction of the innate immunity. Special marker molecule of a Gram-negative bacterial is lipopolysaccharide (LPS), which will be instantly recognizable by protein receptors on the host that is toll-like receptor 4 (TLR-4) receptor, TLR-4 binds to LPS.[9] The body's defense mechanism is elaborated and discussed in this study. The reaction when S. typhi first enters the body, the bacteria are destroyed by macrophages because the bacteria are recognized by TLR-4 is located on the surface of phagocytes.[10] TLRs belong to the pattern-recognition receptors and represent the first line of defense against pathogens, playing a pivotal role in both innate and adaptive immunities. Among components present in the diet, flavonoids have been suggested as antioxidant dietary factors able to modulate TLR-mediated signaling pathways.[11]
Preliminary studies, through qualitative and quantitative phytochemical screening, the metabolic compounds obtained from lime peel extract (LPE) are phenols, flavonoids, tannins, alkaloids, and triterpenoid.[12] Studies of LPE interventions on TLR4 gene mRNA expression activity in S. typhi-infected hosts have not been conducted so far. Therefore, the aim of this study was to know the effect of LPE to mechanism of the host's body defense against S. typhi infection through the TLR-4 signaling pathway relationship with the presence of bacterial cell death.
SUBJECTS AND METHODS
Study area and design
This research is a true experimental pre–posttest design and approved by the Research Ethics Committee of Medical Health in the Faculty of Medicine, University of Hasanuddin (Makassar, Indonesia) with registration number 900/H4.8.4.5.31/PP36-KOMETIK/2018 on October 31, 2018.
Lime peel extract
Lime selected with homogeneity, according to the similarity in color and size lemon (diameter 3-5 cm). A lime was separated between the peel and the fruit. One kilogram lime produces approximately 300 g of lime peel. The lime peel separated from the fruit, dried using oven with a temperature 55°0C to speed up the drying process until the water content decreases and reaches 10%. And then inserted into a glass container to do the maceration process, dissolved with ethanol 96%. Followed by a filtration process, after macerated and then filtered by vacuum filtration method using Buchner Funnels. Results Extraction if the average weight of 1 lime is 100 g, it can produce ± 30 g of lime peel and has the potential to produce 0.1 g of LPE.
Experimental animals
Balb/c mice (age: 8–12 weeks, weighing: 30–40 g, n = 20) placed in a cage made of wire with a floor area size of 30 cm × 50 cm × 15 cm. Adaptation procedures were carried out for 7 days. Further randomization, all the mice were divided into four groups (n = 5): Group 1 LPE510 (a group of mice which intervened LPE dose of 510 mg/kgBW), Group 2: LPE750 (a group of mice which intervened LPE dose of 750 mg/kgBW), Group 3: positive control (group of mice was given Levofloxacin 1,95 mg/kgBW, and Group 4 negative control (group of mice placebo). Animal interventions were carried out for 5 days.
Induction of Salmonella typhi bacteria
On day 0 after adaptation, all the mice do baseline blood sampling, and then day 1, mice were induced by the bacteria S. typhi as much as 3 × 103 CFU/mL strain thy1 by intraperitoneal injection. On day 2 to day 4, mice were observed for the occurrence of infection process.
Peritoneal fluid intake and investigation bacteria colonies
Peritoneal fluid was take in the positioning body of mice was supine, the abdomen was cleaned with 70% alcohol and NaCl injected as much as 0.8–1 mL into the peritoneal cavity. Then allowed for 1 min while the abdomen was shaken it slowly. Peritoneal fluid removed 0.5 mL is aspirated from the peritoneal cavity of the supine position. Peritoneal fluid collection and examinations were conducted Samples of peritoneal fluid were taken 3 times, pre-intervention (day 5), post-intervention (day 10) and 20 days after intervention (day 30).
Count the number of bacterial colonies using the plate count agar method. 0.5 mL of sample is put into 4.5 ml of saline (0.9% NaCl), then diluted 3 times. 1 ml of suspension is poured into a sterile petri dish, followed by pouring warm sterile nutrient agar (45°C) and then tightly closed and incubated for 24-48 hours at 370C in an upside-down position. Calculation the number of bacterial colonies based on the growth colonies per ml is equal to the number of colonies per cup divided by dilution factor.[13]
Toll-like receptor-4
Examination of expression mRNA gene TLR-4 was performed four times: at baseline (day 0), day 5 preintervention, the 10th-day postintervention, and the 30th day after the maintenance of 20 days postintervention. RNA extraction was done using previous methods and stored at a temperature of −80°C. Real-time polymerase chain reaction (PCR) program was run using CFX Connect system, Bio-Rad Laboratories, real-time PCR 96 wells, 0.1 mL, USA.
Primer for mice mRNA TLR4 is TLR4-forward: TGACAGGAAACCCTATCCAGAGTT and TLR4-reverse: TCTCCACAGCCACCAGATTCT.[14]
The parameter thermal cycle was 30 s at a temperature of 95°C and 40 cycles of denaturation at a temperature of 95°C for 10 s and then annealing at 60°C for 15 s and extension at a temperature of 72°C for 40 s. All PCRs were repeated three times, and the data were analyzed by the instrument detection system of CFX Connect Real-Time PCR Bio-Rad, California, USA using the comparative threshold cycle method. The standard curve was constructed and is an indication of good amplification efficiency (90%–100%).
RESULTS
Bacterial colonies
Analysis of the effect of LPE on the amount of bacterial colonization S. typhi, analyzed by using paired t-test to assess the dynamics of change in the number of bacterial colonies according to observation time for each group [Table 1]. There was a significant decrease in the intervention group LPE510 and LPE750. The number of bacterial colonies on day 30, kept a decline even in the intervention group LPE, and antibiotics had no bacterial growth. It can be assumed that the effect of LPE can be as bactericidal or can kill the bacteria.
Table 1.
Effects of lime peel extract on bacterial colonies based on the time of observation before intervention (day 5), after intervention (day 10), and maintenance after intervention (day 30)
| Group | Bacterial colonies S. typhi (CFU/mL) |
|||||
|---|---|---|---|---|---|---|
| Day 5 | Day 10 | P | Day 10 | Day 30 | P | |
| LPE 510 | 24.60±3.85 | 3.00±2.92 | 0.001 | 3.00±2.92 | 0.00±0.00 | 0.830 |
| LPE 750 | 17.20±2.28 | 0.00±0.00 | 0.000 | 0.00±0.00 | 0.00±0.00 | -a |
| Control (+) | 30.40±7.40 | 1.60±1.36 | 0.001 | 1.60±1.36 | 0.00±0.00 | 0.306 |
| Control (−) | 22.60±6.07 | 6.00±2.83 | 0.009 | 6.00±2.83 | 1.20±0.58 | 0.014 |
aThe correlation and t cannot be computed because the standard error of the difference is 0. LPE: Lime peel extract, CFU: Colony-forming units
MRNA gene toll-like receptors 4
Figure 1 shows that there are dynamic changes in gene expression of TLR-4 at four times the observation. The expression mRNA gene TLR-4 on day 5, expressed more than on day 1 (before injected bacteria). There are statistically significant differences in all groups: LPE510 (P = 0.002), LE750 (P = 0.001), positive controls (P = 0.000), and negative controls (P = 0.003).
Figure 1.
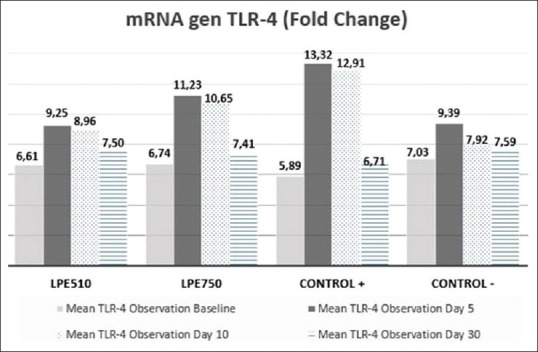
Dynamics changes of toll-like receptor 4 on the time of observation before the intervention (day 5), after the intervention (day 10), and maintenance after the intervention (day 30)
Correlation of toll-like receptors 4 with colonization bacteria
Table 2 shows that there is a linear correlation or relationship between the expression of TLR-4 gene mRNA by the number of bacterial colonization, the strength of the correlation is sufficient, and the direction of a positive correlation means that the lower the gene mRNA expression of TLR-4, the smaller the amount of bacterial colonization.
Table 2.
Correlation of toll-like receptor-4 and bacterial colonization
| Variable | Bacterial colonies (CFU/mL) Bivariate correlation |
|
|---|---|---|
| r | P | |
| mRNA gen TLR-4 (fold change) | 0.408 | 0.013 |
TLR: Toll-like receptor, CFU: Colony-forming unit
DISCUSSION
Typhoid fever begins with the entry of germs through contaminated food and drink through the oral–fecal pathway, which then the body will carry out defense mechanisms through several processes of an immune response. The latest study showed that S. Typhi targets human-specific pathways by inducing host transcriptional changes. These pathways include cytoskeletal rearrangement, polarized cytokine release, and hampering host immune defense system. Salmonella is still a dangerous threat to human health without adequate therapeutic options.[15]
S. typhi infection stimulates macrophage activation.[16] The content of LPS in the cell wall of S. typhi is a signal for macrophages to activate. TLR-4 protein serves as a receptor on the surface of phagocytic cells to recognize LPS Salmonella sp.[17] The TLR-4 receptor protein on the surface of phagocytic cells will capture of LPS of Salmonella sp. that managed to penetrate the intestinal wall and intestinal mucosa.
In this study, it was found that TLR-4 at the baseline examination is lower than the TLR-4 after the host bacteria injected, and this indicates the homeostatic condition.[18] However, if there is inflammation, TLR expression increased and triggered the cytokine pro-inflammation.[19] In this study, day 5 post injection, TLR-4 mRNA expression in all groups of mice increased. This may explain that LPS which is a marker of S. typhi bacteria that are in the cell walls of bacteria, it can stimulate the activation of the receptor TLR-4 on the surface of the host cell.[17]
Decreased expression of TLR-4 gene mRNA showed a decrease in the amount of LPS in the blood of mice and marked the healing process of S. typhi infection in the body of mice. However, this needs to be done further research on the pathomechanism of the number of bacterial colonization, LPS, and TLR4. Previous studies state that if there is a stimulation of LPS Salmonella, it can increase the TLR4 signaling pathway.[20,21,22] The factor that is important in the nonspecific stimulation of the immune response against LPS is the activity of TLR-4. The presence of LPS stimulates TLR-4 then causes the nuclear translocation of nuclear factor-κB and cytokines tumor necrosis factor-α and inducible nitric oxide synthase.[23]
In this study, we assumed that LPE can modulate the TLR-4 signaling pathway in host immunity so that the gene TLR-4 is expressed less and less. TLR-4 recognizes and binds ligand LPS of S. typhi. Metabolic compounds contained in the extract can kill the bacteria,[24,25,26] LPS is nowhere so that expression of the gene TLR-4 is decreased. This mechanism causes the bacterial colony number to decrease, not even growth. It is recommended for further research to ascertain what compounds are contained in LPE that play a role in the modulation of TLR-4 signaling pathways.
CONCLUSIONS
The LPEs have metabolic compounds such as flavonoids, saponins, tannins, triterpenoids, and alkaloids that can kill the bacteria directly as an antibacterial, decreased the number of bacterial colonization, and decreased the mRNA expression TLR-4 gene.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Markus, Rommy, Mus, and Wani (Molecular Biology and Immunology Laboratory for Infection Diseases, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia), who helped in the implementation of our research activities. This research was supported by the Ministry of Research and Technology, Indonesia, or Educational Fund Management Institutions.
REFERENCES
- 1.Simanjuntak TP, Hatta M, Tahir AM, Sirait RH, Karo MB, Tambaib T, et al. Analysis of anti-toxoplasma immunoglobulin G and immunoglobulin M antibody levels after intervention with curcuma longa extract on early pregnant mice with acute toxoplasmosis. J Glob Infect Dis. 2019;11:25–9. doi: 10.4103/jgid.jgid_28_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simanjunta TP, Hatta M, Rauf S, Prabandari SA, Siagian C, Dwiyanti R. Tumor necrosis factor-alpha levels and histopathology finding after intervention with Curcuma Longa extract. J Med Sci. 2018;18:56–62. [Google Scholar]
- 3.Idrus HH, Hatta M, Febriza A, Kasim VN. Antibacterial activities of Sapodilla fruit extract inhibiting Salmonella typhi on mice Balb/c. Int J Appl Pharm. 2019:121–6. [Google Scholar]
- 4.Khairi AU, Hatta M, Tahir H, Alam G, Asmawati A. Expression of IL-10 in a. actinomycetemcomitans induced rat treated by purple Miana leaves. Biomed Pharmacol J. 2019;12:2099–104. [Google Scholar]
- 5.Karo M, Hatta M, Salma W, Patellongi I, Natzir R. Effects of Miana (Coleus scutellariodes (L) Benth) to expression of mRNA IL-37 in Balb/c Mice infected Candida albicans. Pharmacogn J. 2017;10:16–9. [Google Scholar]
- 6.Syamsuri F, Hatta M, Natzir R, Alam G, Massi MN, Dwiyanti R, et al. A review: worldwide medicinal plants for typhoid fever. Indian J Public Heal Res Dev. 2018;9:1461. [Google Scholar]
- 7.Dwiyanti R, Hatta M, Natzir R, Pratiwi S, Sabir M, Yasir Y, et al. Seroprevalence rates of typhoid fever among children in endemic areas, South Sulawesi, Indonesia. Indian J Public Heal Res Dev. 2019;10:1130. [Google Scholar]
- 8.Dwiyanti R, Hatta M, Natzir R, Pratiwi S, Sabir M, Yasir Y, et al. Association of typhoid fever severity with polymorphisms NOD2, VDR and NRAMP1 genes in endemic area, Indonesia. J Med Sci. 2017;17:133–9. [Google Scholar]
- 9.Abbas AK, Lichtman AH, Pillai S. Celluler and Moleculer Immunology. Philadelphia: W. B. Saunders Company; 2014. Innate immunity; pp. 63–6. [Google Scholar]
- 10.Hurley D, McCusker MP, Fanning S, Martins M. Salmonella-host interactions-Modulation of the host innate immune system. Front Immunol. 2014;5:481. doi: 10.3389/fimmu.2014.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez-Cano FJ, Massot-Cladera M, Rodríguez-Lagunas MJ, Castell M. Flavonoids affect host-microbiota crosstalk through TLR modulation. Antioxidants (Basel) 2014;3:649–70. doi: 10.3390/antiox3040649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasim VN, Hatta M, Febriza A, Idrus HH, Hadju V. Lime Peel Extract Effects in Decreasing Levels of Inteleukin 6 in Mice Infected with Salmonella Typhi. Proceeding of International Conference on BioMedical Sciences ICBMS; Istanbul, Turkey: Topcuoglu, Bulent. 2019. [Google Scholar]
- 13.Harley J, Prescott L. The Cell and Moleculer Biology. Laboratory Exercises in Microbiology. Lab Exerc Microbiol. New York City, USA: McGraw-Hill; 2002. Bacterial Count of a Food Product; pp. 117–24. [Google Scholar]
- 14.Syamsuri F, Hatta M, Natzir R, Alam G, Massi MN, Bahar B, et al. Expression of TLR-4 in Salmonella typhi-induced Balb/c mice treated by Miana Leaves (Coleus scutellaroides (L) Benth) Indian J Public Heal Res Dev. 2018;9:1449. [Google Scholar]
- 15.Nickerson KP, Senger S, Zhang Y, Lima R, Patel S, Ingano L, et al. Salmonella typhi colonization provokes extensive transcriptional changes aimed at evading host mucosal immune defense during early infection of human intestinal tissue. EBioMedicine. 2018;31:92–109. doi: 10.1016/j.ebiom.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurtz JR, Goggins JA, McLachlan JB. Salmonella infection: Interplay between the bacteria and host immune system. Immunol Lett. 2017;190:42–50. doi: 10.1016/j.imlet.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosadini CV, Kagan JC. Early innate immune responses to bacterial LPS. Curr Opin Immunol. 2017;44:14–9. doi: 10.1016/j.coi.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seibert SA, Mex P, Köhler A, Kaufmann SH, Mittrücker HW. TLR2-, TLR4- and Myd88-independent acquired humoral and cellular immunity against Salmonella enterica serovar Typhimurium. Immunol Lett. 2010;127:126–34. doi: 10.1016/j.imlet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Vijay K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int Immunopharmacol. 2018;59:391–412. doi: 10.1016/j.intimp.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li N, Ansari AR, Sun Z, Huang H, Cui L, Hu Y, et al. Toll like receptor 4 signaling pathway participated in Salmonella lipopolysaccharide-induced spleen injury in young chicks. Microb Pathog. 2017;112:288–94. doi: 10.1016/j.micpath.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Cawthraw S, Pennings JL, Hodemaekers HM, de Jonge R, Havelaar AH, Hoebee B, et al. Gene expression profiles induced by Salmonella infection in resistant and susceptible mice. Microbes Infect. 2011;13:383–93. doi: 10.1016/j.micinf.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Ansari AR, Arshad M, Masood S, Huang HB, Zhao X, Li N, et al. Salmonella infection may alter the expression of toll like receptor 4 and immune related cells in chicken bursa of Fabricius. Microb Pathog. 2018;121:59–64. doi: 10.1016/j.micpath.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter S, O'Neill LA. How important are toll-like receptors for antimicrobial responses? Cell Microbiol. 2007;9:1891–901. doi: 10.1111/j.1462-5822.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 24.Pathan R khan, Gali PR, Pathan P, Gowtham T, Pasupuleti S. In vitro antimicrobial activity of Citrus aurantifolia and its phytochemical screening. Asian Pacific J Trop Dis. 2012;2(Suppl 1):S328–31. [Google Scholar]
- 25.Cushnie TP, Cushnie B, Lamb AJ. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int J Antimicrob Agents. 2014;44:377–86. doi: 10.1016/j.ijantimicag.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Ghasemi K, Sciences SA, Ghasemi Y, Ebrahimzadeh MA. Antioxidant activity, phenol and flavonoid contents of 13 Citrus species peels and tissues. Pak J Pharm Sci. 2009;22:277–81. [PubMed] [Google Scholar]


