Abstract
Acanthopanax trifoliatus has been used as both traditional plant food and medicinal plant in Thailand. This study aimed to evaluate proximate, vitamin, and mineral compositions of A. trifoliatus leaf samples together with antioxidant and anticancer activities of ethanolic leaf extract of A. trifoliatus. For leaf samples, quantitative determination of proximate composition was evaluated comprising moisture, crude protein, total fat, total carbohydrate, dietary fiber, ash, as well as energy. Quantitative determination of vitamin and mineral composition including Vitamin A, Vitamin B1, Vitamin B2, Vitamin C, calcium, sodium, and iron was also assessed. For ethanolic leaf extract, antioxidant activity was investigated using oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) methods. Anticancer activity was determined against human ductal, bronchogenic, liver, gastric, and colon cancer cell lines using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide method. Proximate composition of A. trifoliatus was found to be 74.62 ± 0.38, 5.01 ± 0.05, 0.95 ± 0.04, 16.47 ± 0.40, 8.54 ± 0.06, and 2.95 ± 0.04 g/100 g sample, respectively, and energy was found to be 94.48 ± 1.30 kcal/100 g sample. Vitamin and mineral composition was found to be 428.47 ± 3.00 μg/100 g sample, 0.41 ± 0.01, 0.17 ± 0.00, 11.95 ± 0.86, 675.35 ± 46.57, 13.46 ± 0.95, and 4.79 ± 0.15 mg/100 g sample, respectively. A. trifoliatus ethanolic leaf extract revealed antioxidant capacity with ORAC value of 9057.29 ± 43.08 μmol TE/100 g and FRAP value of 1230.88 ± 19.51 μmol TE/100 g. Its extract also showed cytotoxic potential against all tested cancer cell lines. A. trifoliatus leaf is a good source of essential nutrients, which had antioxidant and anticancer potential.
Key words: Anticancer, antioxidant, mineral, nutrition, proximate, vitamin
INTRODUCTION
Phak-pam (Acanthopanax trifoliatus), a medium-sized shrub that commonly distributes in Asia, belongs to the Araliaceae family. It has been used as both traditional plant food and medicinal plant.[1,2,3,4,5,6] As plant food, people of Thailand consume Phak-pam as a vegetable. As a medicinal plant, Phak-pam has been reported as ginseng-like plant.[5] It also possessed pharmacological effects, for instance, anti-inflammatory, antioxidant, and anticancer properties.[1,2,3,4,5,6] Plant food provides nutrients that help maintain the body functions. Nowadays, nutritional information is increasingly concern for many reasons, for example, to meet nutritional requirements or to prevent nutritional deficiency disease.[7,8] Nutrients can be divided into two groups based on the requirement in amounts per day: macronutrients include proteins, fats, and carbohydrates; in opposition, micronutrients include vitamins and minerals.[8,9]
Medicinal plant is also used to promote good health. Plant and its compounds have biological capability, such as antioxidant and anticancer.[10] Some bioactive compounds in the food plant has a natural antioxidant capacity, especially polyphenols, to scavenge free radicals.[3,10,11] Oxygen radical absorbance capacity (ORAC) measures the effectiveness of chain-breaking antioxidants, while ferric reducing antioxidant power (FRAP) measures the action of the electron donation from antioxidants (the reducing capacity of antioxidants).[10,11,12,13,14] Cancer is a disease differentiated by the uncontrolled growth and spread of abnormal cells.[15,16] 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay can be used for estimating the quantitative of antiproliferative and anticancer potential.[15] There is a necessity to find drug or medicinal plant with antioxidant and anticancer capability. This study covered evaluations of proximate, vitamin, and mineral compositions of leaf samples together with antioxidant capacity and anticancer activity of ethanolic leaf extract of A. trifoliatus.
MATERIALS AND METHODS
Materials and chemicals
A. trifoliatus leaf samples were collected in Thailand. They were authenticated at Queen Sirikit Botanic Garden, Thailand. Voucher specimens were deposited at School of Integrative Medicine, Mae Fah Luang University, Thailand. Leaf samples were washed and then dried at 50°C. The dried leaf samples were stored at room temperature. For antioxidant and anticancer activities, the dried leaf samples were pulverized and exhaustively extracted using Soxhlet apparatus. The extract was filtered, evaporated, and then stored at −20°C. All chemicals were analytical grade.
Proximate analysis
For quantitative determination of proximate composition of A. trifoliatus leaf samples, moisture was determined using hot air oven (Association of Official Analytical Chemists; AOAC 952.08, 2016),[17] dried leaf samples at 105°C, until constant weight. Crude protein was determined according to the Kjeldahl method (AOAC 992.23, 2016);[17] total nitrogen was multiplied by a protein factor of 6.25. Total fat was determined according to the acid hydrolysis method (AOAC 948.15, 2016),[17] using Soxhlet extractor at 60°C, until constant weight. Dietary fiber was determined according to the enzymatic gravimetric method (AOAC 985.29, 2016),[17] digested leaf samples with heat using alpha-amylase, protease, and amyloglucosidase at 60°C, respectively, then added ethanol to samples to precipitate fiber. Ash was determined according to gravimetric method (AOAC 930.30, 2016),[17] incinerated leaf samples at 550°C, until constant weight. Total carbohydrate was determined according to the difference method by calculation. Energy was determined by calculation based on the contents of carbohydrate, protein, and lipid multiplied by a factor of 4, 4, and 9 respectively, and then added the results together.
Mineral analysis
For quantitative determination of mineral composition of A. trifoliatus leaf samples, Vitamin A as alpha-carotene was determined using high-performance liquid chromatography (HPLC),[18] then measured absorbance at 445 nm, and calculated as follows:
Vitamin A = 1/6 (alpha-carotene) +1/12 (remaining carotenoids)
Vitamin C was determined using ultraviolet-HPLC methods[19] and then measured absorbance at 245 nm. Vitamin B1 and Vitamin B2 were determined by fluorometric method according to the AOAC 942.23 and 970.65, 2016,[17] respectively, and then measured emission at 365 nm and excitation at 435 nm. Calcium and sodium were determined by atomic absorption spectrophotometer (AAS) (AOAC 985.35, 2016),[17] dried leaf samples at 450°C then measured AAS absorbance at 425 and 590 nm, respectively. Iron was determined by AAS (AOAC 984.27, 2016)[17] and then measured absorbance at 250 nm.
Antioxidant analysis
For antioxidant capacity of A. trifoliatus, the ethanolic leaf extract was determined using ORAC, according to the method reported by Ou et al. (2001),[12] using fluorescein as the fluorescent probe. For FRAP, according to the method reported by Benzie and Strain (1996).[14]
Anticancer analysis
Cell cultures
The human cancer cell lines – ductal carcinoma (BT474, ATCC HTB-20), bronchogenic carcinoma (Chago K1, ATCC HTB-168TB), liver hepatoblastoma (Hep G2, ATCC HB-8065), gastric carcinoma (Kato III, ATCC HTB-103), and colon adenocarcinoma (SW 620, ATCC CCL-227) – were obtained from the Institute of Biotechnology and Genetic Engineering, Chulalongkorn University, Thailand.
For anticancer activity of A. trifoliatus ethanolic leaf extract, cell viability was determined using MTT (Sigma-Aldrich, USA) methods[15] with minor modifications. Each human cancer cell line in 198 μl of RPMI 1640 culture medium was incubated with 5% CO2 atmosphere at 37°C for 24 h. Either 2 μl of A. trifoliatus leaf extract or dimethyl sulfoxide (DMSO) (as negative control) was added and then incubated at 37°C for 48 h. 10 μl of MTT solution (5 mg/ml) was added, then incubated at 37°C for 4 h, and removed all media. 150 μl of DMSO and 25 μl of glycine (0.1 mol/l) were added, mixed, and then measured absorbance at 540 nm.
Statistical analysis
For proximate composition, vitamin and mineral composition, and antioxidant capacity, the data were expressed as mean ± standard deviation (SD). All tests were carried out in triplicates. For anticancer activity, the data were expressed as mean ± SD. This test was carried out in quadruplicates.
RESULTS
Proximate analysis
Proximate composition of A.trifoliatus leaf samples including moisture, crude protein, total fat, total carbohydrate, dietary fiber, ash; as well as, energy were found to be 74.62±0.38, 5.01±0.05, 0.95±0.04, 16.47±0.40, 8.54±0.06, 2.95±0.04 g/100 g sample respectively, and energy was found to be 94.48±1.30 kcal/100 g sample.
Mineral analysis
Vitamin and mineral composition of A.trifoliatus leaf samples comprising vitamin A, vitamin B1, vitamin B2, vitamin C, calcium, sodium and iron were found to be 428.47±3.00 μg/100 g sample, 0.41±0.01, 0.17±0.00, 11.95±0.86, 675.35±46.57, 13.46±0.95 and 4.79±0.15 mg/100 g sample respectively.
Antioxidant analysis
A. trifoliatus ethanolic leaf extract showed antioxidant capacity with ORAC value of 9,057.29±43.08 μmoles TE/100 g and FRAP value of 1,230.88±19.51 μmoles TE/100 g.
Anticancer analysis
A. trifoliatus ethanolic leaf extract possessed cytotoxic effects against all tested human cancer cell lines against gastric and colon human cancer cell lines with the IC50 of 72.9 and 73.4 μg/ml, respectively; against ductal, bronchogenic and liver human cancer cell lines with the IC50 at ≥100 μg/ml.
DISCUSSION
Proximate analysis is used for estimation of the quantitative of food and food substance including moisture, crude protein, total fat, total carbohydrate, and dietary fiber.[7,20,21] Proximate composition of A. trifoliatus leaf samples is shown in Table 1. Moisture content is the amount of loss on drying of water and volatile substances.[7,20,21] Moisture is sometime used for estimation of the qualitative of food. However, the amount of moisture content is one of the main factors in storage, due to the proliferation of microorganisms, such as fungi and mold.[7,20] When fresh A. trifoliatus leaf samples dried until constant weight, moisture content was found to be 74.62 ± 0.38 g/100 g sample. Crude protein is the amount of total nitrogen multiplied by protein factors. Total nitrogen consisted of protein nitrogen and a few nonprotein nitrogens. Different types of food had different protein factors.[7,20,21] In this study, A. trifoliatus leaf samples used protein factor of 6.25 to convert nitrogen to protein; then, crude protein in the leaf samples was found to be 5.01 ± 0.05 g/100 g sample. Total fat, ether extract, is the amount of fat including fatty acid, oil-soluble dyes, fat-soluble vitamins, and steroids.[7,20,21] Total fat of A. trifoliatus leaf samples was found to be 0.95 ± 0.04 g/100 g sample. Total carbohydrate is the amount of carbohydrate, which is one of the main components of structural materials in plants.[7,20,21] In this study, we calculated that based on difference method, the total carbohydrate of A. trifoliatus leaf samples was found to be 16.47 ± 0.40 g/100 g sample. Dietary fiber is the amount of total dietary fiber.[7,20,21] Dietary fiber of A. trifoliatus leaf samples was found to be 8.54 ± 0.06 g/100 g sample. Ash content is the amount of total mineral residue left after incinerated leaf samples until constant weight.[7,20,21] Ash content of A. trifoliatus leaf samples was found to be 2.95 ± 0.04 g/100 g sample. Protein, lipid, and carbohydrate each contribute to the total energy composition. Energy of A. trifoliatus leaf samples was found to be 94.48 ± 1.30 kcal/100 g sample.
Table 1.
Proximate composition of Acanthopanax trifoliatus leaf samples (per 100 g sample)
| Proximate analyses | Compositions* |
|---|---|
| Energy (kcal) | 94.48±1.30 |
| Moisture (g) | 74.62±0.38 |
| Crude protein (g) | 5.01±0.05 |
| Total fat (g) | 0.95±0.04 |
| Total carbohydrate (g) | 16.47±0.40 |
| Dietary fiber (g) | 8.54±0.06 |
| Ash (g) | 2.95 ± 0.04 |
Vitamin and mineral analyses are used for estimation of the quantitative of mineral in food. Vitamins are arranged into two groups based on solubility including water-soluble vitamins and fat-soluble vitamins.[8,9,22,23] In this study, we determined the content of Vitamin A, fat-soluble vitamins, calculated as retinal equivalent (1 μg of retinol = 6 μg of alpha-carotene or 12 μg of mixed carotenoids), according to the WHO.[18,24] The content was found to be 428.47 ± 3.00 μg/100 g sample. Vitamin B1, Vitamin B2 and Vitamin C, and water-soluble vitamins were determined. Their contents were found to be 0.41 ± 0.01, 0.17 ± 0.00, and 11.95 ± 0.86 mg/100 g sample, respectively. Minerals perform an essential role as a catalyst of biochemical reaction in the plant. Minerals are grouped into two groups based on human diets in amounts. Macroelements or major minerals are required >100 mg, whereas microelements or trace minerals are required <100 mg.[8,9,22,23] In this study, we determined calcium and sodium, macroelements, and iron, a microelement. Calcium, sodium, and iron contents of A. trifoliatus leaf samples were found to be 675.35 ± 46.57, 13.46 ± 0.95, and 4.79 ± 0.15 mg/100 g sample, respectively. The vitamin and mineral compositions of A. trifoliatus leaf samples are shown in Table 2.
Table 2.
Vitamin and mineral compositions of Acanthopanax trifoliatus leaves (per 100 g sample)
| Mineral analyses | Compositions* |
|---|---|
| Vitamin A as alpha-carotene (µg) | 428.47±3.00 |
| Vitamin B1 (mg) | 0.41±0.01 |
| Vitamin B2 (mg) | 0.17±0.00 |
| Vitamin C (mg) | 11.95±0.86 |
| Calcium (mg) | 675.35±46.57 |
| Sodium (mg) | 13.46±0.95 |
| Iron (mg) | 4.79 ± 0.15 |
In this study, ethanolic leaf extract of A. trifoliatus had antioxidant capacity, which is shown in Table 3. ORAC quantified antioxidant capacity, measured fluorescence quenching, based on the production of peroxyl free radicals generated interacted with fluorescent probe.[11,12,13]A. trifoliatus leaves' extracts had an ORAC value of 9057.29 ± 43.08 μmol TE/100 g. FRAP quantified antioxidant power, measured directly reducing capacity of antioxidants (ferric to ferrous ion reduction).[11,14,25] Ethanolic leaf extract of A. trifoliatus had an FRAP value of 1230.88 ± 19.51 μmol TE/100 g. However, from previous study, A. trifoliatus leaf extracts had also been reported with strong antioxidant capacity. For DPPH assay, leaves extracts had antioxidant capacity with EC50 value of 100.81 ± 5.05 μg/ml (leaf maceration extract), 72.20 ± 9.61 μg/ml (leaf refluxing extract), and 14.50 ± 1.04 μg/ml (leaf decoction extract).[5] For FRAP assay, leaf extract had antioxidant capacity with 930 μmol Fe (II)/g extract.
Table 3.
Antioxidant capacity of ethanolic leaf extract of Acanthopanax trifoliatus
| Parameter | Antioxidant capacity* |
|---|---|
| ORAC (µmol TE) | 9057.29±43.08 |
| FRAP (µmol TE) | 1230.88 ± 19.51 |
ORAC: Oxygen radical absorbance capacity, FRAP: Ferric reducing antioxidant power, µmol TE: Micromoles Trolox Equivalents
From previous study, stem and leaf extracts from A. trifoliatus were found anticancer effects on human cancer cell lines, including prostate cancer (PC-3 cells), central nervous system tumor (SF-268), breast adenocarcinoma (MCF-7), hepatoma cell (Huh-7), liver hepatoblastoma (Hep-G2), lung cancer (A 549), nonsmall-cell lung carcinoma (NCL-H460) and fibrosarcoma cell (HT1080).[4,6,16] In this study, ethanolic leaf extract of A. trifoliatus also possessed cytotoxic activities against all tested cancer cell lines, especially gastric carcinoma (Kato-III) and colon adenocarcinoma (SW 620) with IC50 value of 72.9 and 73.4 μg/ml, respectively. However, its extract also had cytotoxic activities against ductal carcinoma (BT474), bronchogenic carcinoma (Chago K-1), and liver hepatoblastoma (Hep-G2) cell lines at high dose (IC50>100 μg/ml). The anticancer analysis of ethanolic leaf extract of A. trifoliatus is shown in Table 4 and Figure 1.
Table 4.
Anticancer analysis of ethanolic leaf extract of Acanthopanax trifoliatus
| Cancer cell lines | IC50 (µg/ml) |
|---|---|
| Ductal carcinoma (µg/ml) | ˃100 |
| Bronchogenic carcinoma (µg/ml) | ˃100 |
| Liver hepatoblastoma (µg/ml) | ˃100 |
| Gastric carcinoma (µg/ml) | 72.9 |
| Colon adenocarcinoma (µg/ml) | 73.4 |
IC50: Inhibitory concentration
Figure 1.
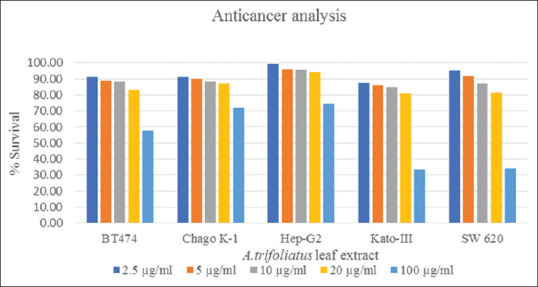
Inhibition of cancer cells growth by ethanolic Acanthopanax trifoliatus leaf extract
CONCLUSION
A. trifoliatus leaf is a good source of essential nutrients including protein, carbohydrate, dietary fiber, and fat. It also contains health-promoting compounds including vitamins and minerals, especially calcium. Moreover, ethanolic A. trifoliatus leaf extract had antioxidant and anticancer potentials. For antioxidant capacity, ORAC value and FRAP value were found to be 9057.29 ± 43.08 and 1230.88 ± 19.51 μmol TE/100 g, respectively. For cytotoxic activity, its leaves' extract possessed cytotoxic activities against all tested cancer cell lines.
Financial support and sponsorship
This research was supported by Mae Fah Luang University.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors are grateful to Mae Fah Luang University for funding the research. The authors are also grateful to Medicinal Plant Innovation Center of Mae Fah Luang University for necessary assistance and laboratory facilities.
REFERENCES
- 1.Li DL, Zheng X, Chen YC, Jiang S, Zhang Y, Zhang WM, et al. Terpenoid composition and the anticancer activity of Acanthopanax trifoliatus. Arch Pharm Res. 2016;39:51–8. doi: 10.1007/s12272-015-0655-y. [DOI] [PubMed] [Google Scholar]
- 2.Chien TM, Hsieh PC, Huang SS, Deng JS, Ho YL, Chang YS, et al. Acanthopanax trifoliatus inhibits lipopolysaccharide-induced inflammatory response in vitro and in vivo. Kaohsiung J Med Sci. 2015;31:499–509. doi: 10.1016/j.kjms.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Li D, Du Z, Huang M, Cui X, Lu Y, et al. Antioxidant and anti-inflammatory properties of Chinese ilicifolius vegetable (Acanthopanax trifoliatus (L) Merr) and its reference compounds. Food Sci Biotechnol. 2015;24:1131–8. [Google Scholar]
- 4.Wang HQ, Li DL, Lu YJ, Cui XX, Zhou XF, Lin WP, et al. Anticancer activity of Acanthopanax trifoliatus (L) Merr extracts is associated with inhibition of NF-kB activity and decreased Erk1/2 and Akt phosphorylation. Asian Pac J Cancer Prev. 2014;15:9341–6. doi: 10.7314/apjcp.2014.15.21.9341. [DOI] [PubMed] [Google Scholar]
- 5.Sithisarn P, Jarikasem S. Antioxidant activity of Acanthopanax trifoliatus. Med Princ Pract. 2009;18:393–8. doi: 10.1159/000226294. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen BT, Trinh NT, Do TH, Nguyen MK, Tran VH, Tran TH, et al. Screening of Vietnamese medicinal plants for cytotoxic activity. Nat Prod Sci. 2010;16:43–9. [Google Scholar]
- 7.Thangaraj P. Rainsford KD, editor. Pharmacological assays of plant-based natural products. Springer Nature. 2016;71:21–9. [Google Scholar]
- 8.Kotue T, Marlyne JM, Wirba L, Amalene S, Nkenmeni D, Kwuimgoin DW, et al. Nutritional properties and nutrients chemical analysis of common beans seed. MOJ Biol Med. 2018;3:41–7. [Google Scholar]
- 9.Naik P. Biochemistry. New Delhi: Jaypee Brothers Medical Publishers; 2015. [Google Scholar]
- 10.Wilson DW, Nash P, Buttar HS, Griffiths K, Singh R, De Meester F, et al. The role of food antioxidants, benefits of functional foods, and influence of feeding habits on the health of the older person: An overview. Antioxidants (Basel) 2017;6:81. doi: 10.3390/antiox6040081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu DP, Li Y, Meng X, Zhou T, Zhou Y, Zheng J, et al. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int J Mol Sci. 2017;18:1–32. doi: 10.3390/ijms18010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–26. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 13.Litescu SC, Eremia SAV, Tache A, Vasilescu I, Radu G. The Use of Oxygen Radical Absorbance Capacity (ORAC) and Trolox Equivalent Antioxidant Capacity (TEAC) Assays in the Assessment of Beverages' Antioxidant Properties: Academic Place. 2014 [Google Scholar]
- 14.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 16.Iqbal J, Abbasi BA, Mahmood T, Kanwal S, Ali B, Shah SA, et al. Plant-derived anticancer agents: A green anticancer approach. Asian Pac J Trop Biomed. 2017;7:1129–50. [Google Scholar]
- 17.AOAC International. Official Methods of Analysis of AOAC International. 20th ed. Gaithersburg, MD, USA: AOAC International; 2016. p. 3172. [Google Scholar]
- 18.Speek AJ, Temalilwa CR, Schrijver J. Determination of beta-carotene content and Vitamin A activity of vegetables by high-performance liquid chromatography and spectrophotometry. Food Chem. 1986;19:65–74. [Google Scholar]
- 19.Odriozola-Serrano I, Hernandez-Jover T, Martin-Belloso O. Comparative evaluation of UV-HPLC methods and reducing agents to determine Vitamin C in fruit. Food Chem. 2007;105:1151–8. [Google Scholar]
- 20.Food and Agricultural Materials Inspection Center. Proximate Composition and Detergent Fiber. 2019. [Last accessed on 2020 May 01]. Available from: http://www.famic.go.jp/english/index.html .
- 21.Puwastien P, Siong TE, Kantasubrata J, Craven G, Feliciano RR, Judprasong K. ASEAN Manual of Food Analysis. Institute of Nutrition, Mahidol University. 2011 [Google Scholar]
- 22.Dasgupta A, Klein K. Antioxidants in Food, Vitamins and Supplements. San Diego, CA, USA: Elsevier; 2014. p. 360. [Google Scholar]
- 23.Asiedu M, Nilsen R, Lie O, Lied E. Effect of processing (sprouting and/or fermentation) on sorghum and maize. I: Proximate composition, minerals and fatty acids. Food Chem. 1993;46:351–3. [Google Scholar]
- 24.Requirements of Vitamin A, Thiamine, Riboflavine and Niacin: Report of a Joint FAO/WHO Expert Group. Geneva: World Health Organization; 1967. World Health Organization & Food and Agriculture Organization of the United Nations; p. 86. [Google Scholar]
- 25.Nanasombat S, Yansodthee K, Jongjaited I. Evaluation of antidiabetic, antioxidant and other phytochemical properties of Thai fruits, vegetables and some local food plants. Walailak J Sci Tech. 2019;16:851–66. [Google Scholar]


