Abstract
Ti-6Al-4V ELI is one of the most commonly used dental implant restore function. The solution treatment temperature variation can significantly increase the strength, but it is not yet known the effect of these temperature variations on the alloy's biocompatibility properties. Twelve female Sprague Dawley rats were divided into six groups as follows: the treated group, the control group, and the defect group without implant material. In the treated group, the femur bone defect was implanted with as-cast Ti-6Al-4V ELI, 850°C, 950°C, and 1050°C heat-treated Ti-6Al-4V ELI implant material. The rats were euthanized after 30 days postimplantation and evaluated histologically. The results show that the histological scoring of the specimen for femur defect without implant material is 2 (fibrous union and fibrocartilaginous), score with implant as-cast is 2.5, the sample with 850°C heat treatment material is 2.5, 950°C is 2.5, and the temperature at 1050°C is 2.5. The score of 2.5 is between score 2 and score 3: hemorrhage, fibrous union, fibrocartilaginous microhemorrhage, and mineralized cartilage union. In conclusion, there is no effect of different heat treatment temperatures for Ti-6Al-4V ELI implant material in rat bone regeneration's maturation level.
Key words: Bone regeneration, heat treatment, Ti-6Al-4V ELI, titanium implant
INTRODUCTION
Dental implants need to restore the function of mastication in partial or full dentures. These are recognized as a predictable treatment modality with a high clinical success rate so that this field develops rapidly and dynamically.[1,2] The American Dental Association outlines some acceptance guidelines for dental implants, including the following: evaluation of physical properties that ensure sufficient strength; demonstration of the ease of fabrication and sterilization potential without material degradation; safety and biocompatibility evaluation, including cytotoxicity testing and tissue interface characteristics; and freedom from defects and at least two independent longitudinal prospective clinical studies demonstrating efficacy.[3]
Ti-6Al-4V alloys have been widely used for implant materials due to its good biocompatibility, excellent mechanical properties, and good corrosion resistance.[4] Based on the high demand for medical devices, especially implants in the country and most of them are still filled with imported products, the Center for Material Technology – BPPT Indonesia conducts research and development of Ti-6Al-4V ELI alloy material for medical implant applications. A previous study shows that heat treatment resulted in better mechanical properties of castings.[5]
The price of titanium alloy is quite high, and its production also requires high costs. Therefore, net-shape or near-net-shape technology gets much attention. One type of net-shape or near-net-shape technology is the casting process.[6] To reduce the production costs of implant manufacturing with investment casting, a method of integration of Ti-6Al-4V ELI has been carried out using local raw materials. Increasing the strength of as-cast material is done by solution treatment and the aging process. The solution treatment temperature variation can significantly increase the strength, but it is not yet known the effect of these temperature variations on the alloy's biocompatibility properties. Material production is carried out with local raw materials in vacuum kitchens and investment casting technology. Increasing the hardness of the combined results of Ti-6Al-4V ELI is carried out with a solution to heat treatment and aging to improve microstructure properties. Our previous research results show that the solution treatment temperature variation affects the ELI Ti-6Al-4V alloy's mechanical strength and reduces its corrosion rate.[6]
MATERIALS AND METHODS
This research was conducted according to established animal welfare guidelines and followed protocols approved by the Ethical Committee of Health Research, Faculty of Medicine, Universitas Indonesia, Indonesia (No. 665/UN2.F1/ETIK/VI/2018). Twelve adult female Sprague Dawley rats were used in this experiment. These rats received ad libitum food and drink. These rats were randomly divided into six groups: controls, bone defect controls, implanted with Ti-6Al-4V ELI as-cast alloy, implanted with 850°C heat-treated Ti-6Al-4V ELI, implanted with 950°C heat-treated Ti-6Al-4V ELI, and implanted with 1050°C heat-treated Ti-6Al-4V ELI.
Surgical procedure
The anesthesia consists of 1.5 mL ketamine, and 0.5 mL xylazine was injected by intramuscular injection. The surgical area was sterilized, and the fur was removed until the femur visible and rinse out with povidone-iodine. An incision was made in the femoral condyle area, followed by the soft-tissue's open. A bone defect of 3 mm depth and 1 mm in diameter was made at the femur using bone diamond bur. The defect of treated rats received implant material. The wound was closed using catgut with an interrupted suture technique [Figure 1].
Figure 1.
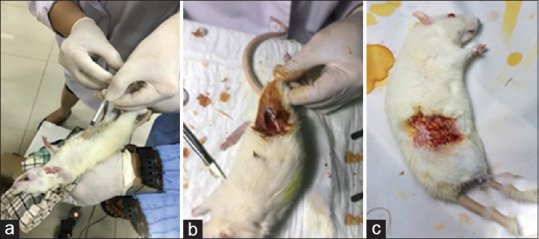
Surgical procedure. (a) Anesthesia; (b) Sample was inserted inside the defect; (c) Wound was closed using catgut
After 4 weeks, the rats were sacrificed, and their femur was taken. The femur was stored in 10% formalin solution (pH 7.2). Then, the femur was immersed in the decalcifying solution for 2 weeks until the bone softened. After that, the femur was neutralized with Na2 SO4 for 24 h before being immersed in a 70% alcohol solution for 24 h and being cut. The sample was immersed in 70%, 80%, 95%, and 100% for 1 h each before the embedding process. The sample then embedded in the paraffin block and cut for microscopic evaluation. The hematoxylin and eosin were evaluated by a microscope at ×20 magnification. Salkeld Histological Scoring was used in order to determine the bone maturation.[7]
RESULTS
The histological evaluation was carried out after 30 days of implantation. We decided to finish the experiment earlier than other previous studies to prevent the animals' infection but still can determine the bone regeneration process by comparing the histological score to the control group. The histological view showed pale purple and dark purple appearances in one of the rat with an implant and two in control group, indicated mineralized bone and osteocytes in these area. In sample 1 and two defect group histological view, bone regeneration just reached fibrous union and fibrocartilage formation. Some lumps of blood were seen in the sample 2 defect group histological view [Figure 2]. The modified Salkeld histological score for this group is 2. A similar result to sample 2 defect group was found in sample 2 as-cast Ti-6Al-4V ELI, sample 2 850°C heat-treated Ti-6Al-4V ELI, sample 1,2 950°C heat-treated Ti-6Al-4V ELI, and sample 2 1050°C heat-treated Ti-6Al-4V ELI. Figure 2 shows, the histological analysis for sample 1 as-cast Ti-6Al-4V ELI, and sample 1 850°C heat-treated Ti-6Al-4V ELI. One of 1050°C heat-treated Ti-6Al-4V ELI sample histological views shows mineralized cartilage and more mature bone formation. The modified Salkeld histological score for this sample is 3 [Figure 3].
Figure 2.
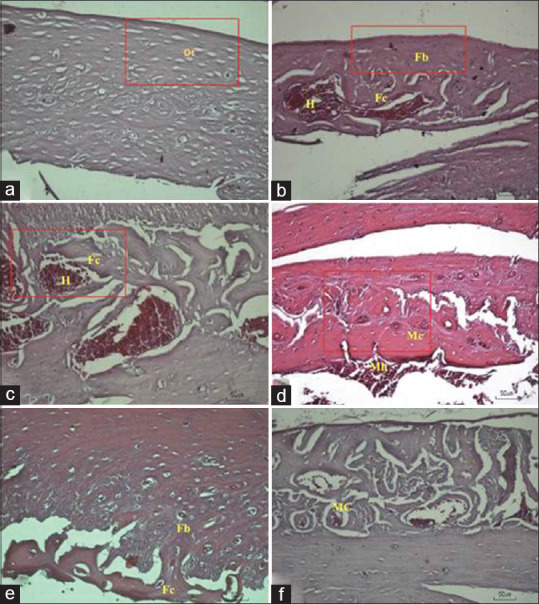
Histological observation at ×20 magnification of sample 1: (a) control; (b) defect; (c) as-cast Ti-6Al-4V ELI; (d) 850°C heat-treated Ti-6Al-4V ELI; (e) 950°C heat-treated Ti-6Al-4V ELI; 1050°C heat-treated Ti-6Al-4V ELI. Bone regeneration component such as fibrous tissue (Fb); fibrocartilage (Fc); (f) mineralized cartilage (Mc), and another component such as hemorrhage (H) or microhemorrhage (Mh) can be seen
Figure 3.
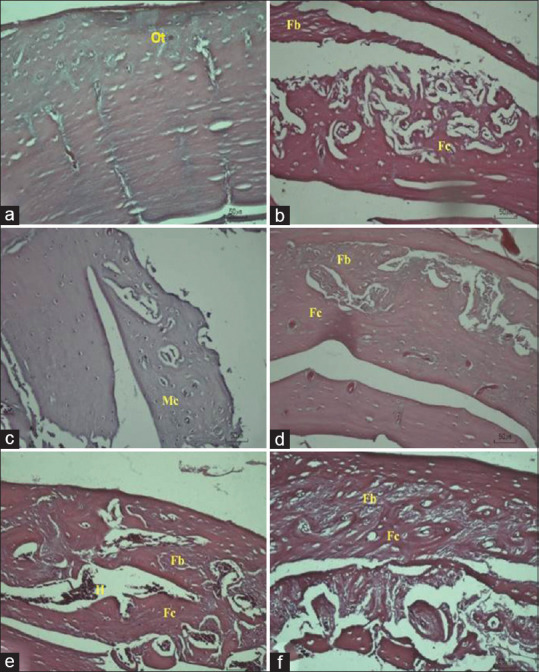
Histological observation at ×20 magnification of sample 2: (a) control; (b) defect; (c) as-cast Ti-6Al-4V ELI; (d) 850°C heat-treated Ti-6Al-4V ELI; (e) 950°C heat-treated Ti-6Al-4V ELI; 1050°C heat-treated Ti-6Al-4V ELI. (f) Bone regeneration component such as fibrous tissue (Fb); fibrocartilage (Fc); mineralized cartilage (Mc), and another component such as hemorrhage (H) or microhemorrhage (Mh) can be seen
The result of the bone healing and regeneration of the animal is shown in Table 1.
Table 1.
Comparison of each sample in the bone regeneration process and its modified Salkeld score
| Sample | Microscopic evaluation in the defect area | Modified Salkeld histological scoring | Average score |
|---|---|---|---|
| Control-1 | No abnormalities | - | Non |
| Control-2 | No abnormalities | - | |
| Defect without implant-1 | Fibrous union and fibrocartilaginous | 2 | 2 |
| Defect without implant-2 | Hemorrhage, fibrous union, and fibrocartilaginous | 2 | |
| Implant as-cast-1 | Microhemorrhage and mineralized cartilage union | 3 | 2.5 |
| Implant as-cast-2 | Hemorrhage, fibrous union, and fibrocartilaginous | 2 | |
| Implant 850-1 | Microhemorrhage and mineralized cartilage union | 3 | 2.5 |
| Implant 850-2 | Hemorrhage, fibrous union, and fibrocartilaginous | 2 | |
| Implant 950-1 | Hemorrhage, fibrous union, and fibrocartilaginous | 2 | 2 |
| Implant 950-2 | Hemorrhage, fibrous union, and fibrocartilaginous | 2 | |
| Implant 1050-1 | Microhemorrhage and mineralized cartilage union | 3 | 2 |
| Implant 1050-2 | Hemorrhage, fibrous union, and fibrocartilaginous | 2 |
DISCUSSION
This study described the evaluation of implantation implant material Ti-6Al-4V ELI with heat treatment 850°C, 950°C, and 1050°C in the femoral bone of Sprague Dawley rats using modified Salkeld histological scoring. There are seen osteocytes, which are mature bone cells that maintain their daily metabolism, such as cell exchange of nutrients.[8]
The histological analysis of the treatment group showed that bone formation reached cartilage mineralization, similar to the research conducted by Berglundh et al. and Abrahamsson et al. This research evaluated the process of bone formation and osseointegration after implant material made of Cp titanium implantation in mandibular dogs, as referred to in the 4th week after the implantation, the large volume of the woven bone had been replaced by lamellar bone that was present in around implant surface and preexisting bone. There are trabeculae of woven bone around the primary bone marrow in the middle part of the defect area and a thin of mineralized tissue.[9,10,11]
The histological analysis of the treatment group based on the modified Salkeld histological scoring showed a different score between sample 1 and sample 2. Many factors influence the process of bone healing.[10,11] Heredity, gender, and age of animals are known to affect fracture healing. Female rats showed delayed bone healing compared with male rats, as did older animals compared to younger ages.[12] Sprague Dawley is rat colonies that are maintained by random breeding. Random mating means that the animals for breeding are chosen without regard to their relationships. So that these colonies have genetics that is more or less heterogeneous.[13]
This study shows that different heat treatment temperature does not affect the level of bone regeneration and maturation. The research conducted by Yi et al. sacrificed the experimental group at the 2 and 6 weeks after titanium implantation in the femur bone of Sprague Dawley rats to evaluate the initial healing status and the osseointegration process. The results showed that after 2 weeks of implant implantation, there is already a process of bone formation. After 6 weeks of implant placement, the procedure of implant processing is continual with more new bone formation and more robust integration of bone into implants' surface.[14]
Research conducted by Berglundh et al. and Abrahamsson et al. observed that there were gradually more mineralized tissues in the observation period of 6 weeks after implant placement.[9,10] In the observation period of 8 weeks and 12 weeks after implant placement, signs of remodeling can be seen in the tissue bone. This hard tissue was surrounded by a bone marrow containing adipocytes, vessels, collagen fibers, and some mononuclear leukocytes. This process indicates that it takes more than 4 weeks to observe until new bones' formation is perfect.[10,11] This study's limitation is the number of animals for the test and the experiment for just 4 weeks after titanium implantation in the femur bone. However, some studies reported that in 4 weeks after the bone defect experiments, the calcium deposition, bone density, and the increase in new bone volume were observed.[15,16,17]
CONCLUSION
Bone regeneration can be found in the surrounding implantation site of the heat-treated Ti-6Al-4V ELI. This condition was indicated by fibrous tissue formation, fibrocartilage, and mineralized cartilage in the surrounding implantation site. Therefore, heat-treated Ti-6Al-4V ELI has the same biocompatibility properties as normal bone regeneration.
Financial support and sponsorship
This study was supported by the grant from the INSINAS program, the Ministry of Research and Higher Education Republic of Indonesia.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors acknowledge to drh. Putri Retno Intan and Suherman for animal study from animal laboratory study, Center for Biomedical and Basic Technology of Health. We also thank drh. Silvia Arin Prabandari, M. Si, APVet from the Primate Animal Study Center, Bogor Agricultural Institute.
REFERENCES
- 1.Zohrabian VM, Sonick M, Hwang D, Abrahams JJ. Dental implants. Semin Ultrasound CT MRI. 2015;36:415–26. doi: 10.1053/j.sult.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Tjikman AP. Straightforward case of dental implant in general Journal of Dentistry Indonesia. 2013;18:81–5. [Google Scholar]
- 3.Ananth H, Kundapur V, Mohammed HS, Anand M, Amarnath GS, Mankar S. A Review on Biomaterials in Dental Implantology. Int J Biomed Sci. 2015;11:113–20. [PMC free article] [PubMed] [Google Scholar]
- 4.Souza JC, Barbosa SL, Ariza EA, Henriques M, Teughels W, Ponthiaux P, et al. How do titanium and Ti6Al4V corrode in fluoridated medium as found in the oral cavity? An in vitro study. Mater Sci Eng C Mater Biol Appl. 2015;47:384–93. doi: 10.1016/j.msec.2014.11.055. [DOI] [PubMed] [Google Scholar]
- 5.Jovanovic MT, Tadic S, Zec S, Miskovic Z, Bobic I. The effect of annealing temperatures and cooling rates on microstucture and mechanical properties of investment cast Ti-6Al-4V alloy. Mater Design. 2006;27:192–9. [Google Scholar]
- 6.Marini D, Cunningham D, Corney JR. Near net shape manufacturing of metal: A review of approaches and their evolutions. Proc Inst Mech Eng B. 2018;L232:650–69. [Google Scholar]
- 7.Wahyudi M, Kamal AF, Siregar NC, Prasetyo M. Effect of extracorporeal irradiation on segmental bone autograft incorporation in Sprague-Dawley rats. Med J Indones. 2014;23:147–53. [Google Scholar]
- 8.Uda Y, Azab E, Sun N, Shi C, Pajevic PD. Osteocyte mechanobiology. Curr Osteoporos Rep. 2017;15:318–25. doi: 10.1007/s11914-017-0373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berglundh T, Abrahamsson I, Lang NP, Lindhe J. De novo alveolar bone formation adjacent to endosseous implants: A model study in the dog. Clin Oral Implants Res. 2003;14:251–62. doi: 10.1034/j.1600-0501.2003.00972.x. [DOI] [PubMed] [Google Scholar]
- 10.Abrahamsson I, Berglundh T, Linder E, Lang NP, Lindhe J. Early bone formation adjacent to rough and turned endosseous implant surfaces. An experimental study in the dog. Clin Oral Implants Res. 2004;15:381–92. doi: 10.1111/j.1600-0501.2004.01082.x. [DOI] [PubMed] [Google Scholar]
- 11.Förster Y, Rentsch C, Schneiders W, Bernhardt R, Simon JC, Worch H, Rammelt S. Surface modification of implants in long bone. Biomatter. 2012;2:149–57. doi: 10.4161/biom.21563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia P, Histing T, Holstein JH, Klein M, Laschke MW, Matthys R, et al. Rodent animal models of delayed bone healing and non-union formation: A comprehensive review. Eur Cell Mater. 2013;26:1–2. doi: 10.22203/ecm.v026a01. [DOI] [PubMed] [Google Scholar]
- 13.McDonald MM, Morse A, Peacock L, Mikulec K, Schindeler A, Little DG. Characterization of the bone phenotype and fracture repair in osteopetrotic incisors absent rats. J Orthop Res. 2011;29:726–33. doi: 10.1002/jor.21293. [DOI] [PubMed] [Google Scholar]
- 14.Yi YA, Park YB, Choi H, Lee KW, Kim SJ, Kim KM, et al. The evaluation of osseointegration of dental implant surface with different Size of TiO2 nanotube in rats? J Nanomater. 2015 doi:http://dx.doi.org/10.1155/2015/581713. [Google Scholar]
- 15.Song SH, Yun YP, Kim HJ, Park K, Kim SE, Song HR. Bone formation in a rat tibial defect model using carboxymethyl cellulose/BioC/bone morphogenic protein-2 hybrid materials. Biomed Res Int. 2014;2014:230152. doi: 10.1155/2014/230152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han D, Han N, Chen Y, Zhang P, Jiang B. Healing of cancellous fracture in a novel mouse model. Am J Transl Res. 2015;7:2279–90. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang D, Gao P, Li Q, Li J, Li X, Liu X, et al. Engineering biomimetic periosteum with β-TCP scaffolds to promote bone formation in calvarial defects of rats. Stem Cell Res Ther. 2017;8:134. doi: 10.1186/s13287-017-0592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


