Infection with influenza A virus (IAV) results in an acute respiratory disease with a highly variable pathogenesis. Viral infection triggers various inflammatory mechanisms that promote viral clearance on the one hand but can have deleterious effects on pulmonary tissue integrity on the other hand. A detailed knowledge of the mechanisms involved in the antiviral immune responses to IAV is key for a better understanding the concept of host susceptibility and eventually for the design of future therapeutic treatment strategies. In their latest publication in Mucosal Immunology, Yang et al. highlight a pivotal role of the transcription factor nuclear matrix protein 4 (NMP4) in driving inflammatory recruitment of monocytes and neutrophils and increasing disease severity during IAV infection.1
The pathogenesis of IAV is dependent on both the viral strain2 and host-intrinsic factors (e.g., genetic variants,3 immune and/or nutritional status4). During IAV infection, the immune system is acutely challenged with balancing the tasks “limiting pathogen spread” vs. “limiting tissue damage”, and severe courses of disease are often the result of inadequate performance of one or both of these tasks.5 Consistent with this view, innate leukocytes such as neutrophils6 and inflammatory monocytes7,8 were associated with adverse disease outcomes in mice and humans during influenza virus infection.
The transcription factor NMP4 was previously shown to promote Il1b transcription and the development of arthritis,9 indicating its role in driving immunopathology, but the implications of Nmp4-mediated signaling for host defense and immune regulation during infection are unknown.
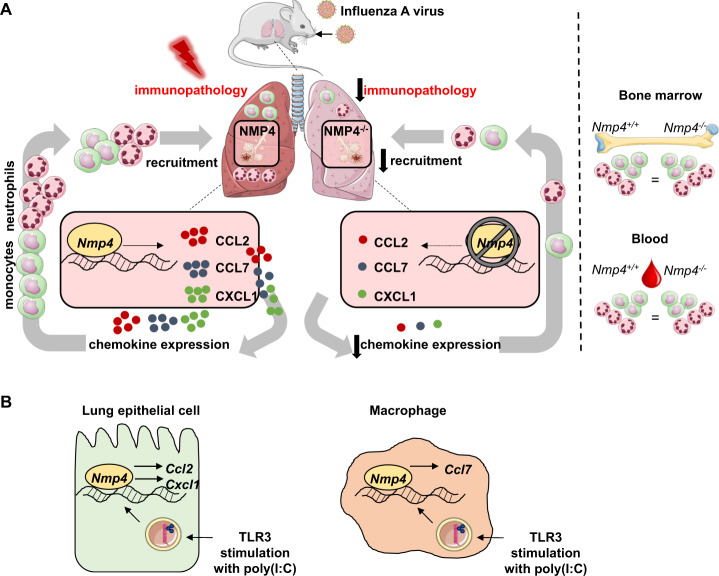
By utilizing Nmp4-deficient mice, Yang et al. showed that genetic loss of Nmp4 reduces disease severity during IAV infection, which was not associated with altered viral clearance. While T- and B-cell responses during infection were comparable between the Nmp4+/+ and Nmp4−/− mice, early gene (Il1b, Il6) and protein expression (IL-6) of several innate proinflammatory mediators was diminished in the lungs of the Nmp4−/− mice. Consistent with the decreased protein (CCL2, CXCL1) and transcript levels (Ccl2, Cxcl1, Ccl7) of hallmark monocyte and neutrophil attractants in the airways, substantially reduced numbers of monocytes and neutrophils were observed in the lungs of the Nmp4−/− mice during the acute phase of IAV infection (Fig. 1a). Importantly, the monocyte and neutrophil levels in the blood and bone marrow were not different between the Nmp4+/+ and Nmp4−/−mice, thus highlighting the role of the NMP4 protein in regulating chemokine-driven intrapulmonary leukocyte recruitment rather than hematopoiesis or mobilization during IAV infection. By using bone marrow chimeric mice, Yang et al. showed that NMP4-driven monocyte and neutrophil infiltration into the lung tissue during influenza infection is equally orchestrated by structural and hematopoietic cells. To determine the role of NMP4 in antiviral immune responses in lung epithelial cells, the authors used guide RNA and CRISPR-Cas9-mediated Nmp4 knockdown in a murine lung epithelial cell line (MLE-12 cells). Here, Yang et al. found that Nmp4 silencing decreased the expression of Cxcl1 and Ccl2 upon stimulation with the Toll-like receptor 3 agonist poly(I:C). In contrast, poly(I:C) stimulation of bone marrow-derived macrophages (BMDMs) from the Nmp4+/+ vs. Nmp4−/− mice revealed that BMDMs respond to a viral trigger with Ccl7 induction in an NMP4-dependent fashion (Fig. 1b). In addition to providing robust evidence for the cell-specific effects of NMP4 on chemokine expression during IAV infection, the authors correlated target gene regulation with the NMP4/DNA interaction by employing a chromatin immunoprecipitation assay, demonstrating a direct effect of NMP4 on inflammatory mediator expression in lung structural and hematopoietic cells.
Fig. 1.
a Lung-specific effects of NMP4 during IAV infection. Local induction of Ccl2, Ccl7, and Cxcl1, intrapulmonary recruitment of monocytes and neutrophils and subsequent development of immunopathology during IAV infection are driven by Nmp4. Development and mobilization of monocytes and neutrophils were not affected by Nmp4 deficiency, which was characterized by comparable levels of monocytes and neutrophils in the bone marrow and blood. b Cell-specific effects of NMP4 on viral ligand stimulation. Nmp4 drives induction of Ccl2 and Cxcl1 in murine lung epithelial cells and induction of Ccl7 in murine bone marrow-derived macrophages upon poly(I:C)-mediated agonistic stimulation of Toll-like receptor (TLR) 3
In conclusion, the present study by Yang et al. identified a hitherto unknown role of the transcription factor Nmp4 in antiviral immune responses. Nmp4 exerts pleiotropic and cell-type-specific effects during influenza infection and increases host susceptibility most likely by triggering excessive inflammation. Pulmonary epithelial cells10 as well as myeloid cells, e.g., alveolar macrophages,11 shape early inflammatory immune responses upon influenza infection. Consistent with this finding, this study reveals that inflammatory immune cell recruitment is the net result of NMP4-driven signaling in structural and myeloid cells. Next, to determine the cell-type-specific contribution of NMP4 to influenza-induced inflammation, researchers must determine the individual roles of Nmp4-dependent gene products in antiviral defense and immunopathology to increase our knowledge of the etiology of influenza and the role of NMP4 herein.
Competing interests
The authors declare no competing interests.
References
- 1.Yang, S. et al. Mucosal Immunol. 204 (2020).
- 2.Garigliany MM, et al. Emerg. Infect. Dis. 2010;16:595–603. doi: 10.3201/eid1604.091061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Everitt AR, et al. Nature. 2012;484:519–523. doi: 10.1038/nature10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karlsson EA, et al. Influenza Other Respir. Viruses. 2012;6:449–460. doi: 10.1111/j.1750-2659.2012.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwasaki A, et al. Nat. Rev. Immunol. 2014;14:315–328. doi: 10.1038/nri3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang BM, et al. Nat. Commun. 2019;10:3422. doi: 10.1038/s41467-019-11249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coates BM, et al. J. Immunol. 2018;200:2391–2404. doi: 10.4049/jimmunol.1701543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole SL, et al. JCI Insight. 2017;2:e91868. doi: 10.1172/jci.insight.91868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamoto T, et al. J. Cell Biochem. 2016;117:970–977. doi: 10.1002/jcb.25382. [DOI] [PubMed] [Google Scholar]
- 10.Stegemann-Koniszewski S, et al. mBio. 2016;7:e00276–16. doi: 10.1128/mBio.00276-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, et al. PLoS ONE. 2012;7:e29879. doi: 10.1371/journal.pone.0029879. [DOI] [PMC free article] [PubMed] [Google Scholar]