CD32a contributes to inflammation by interacting with CD137L to induce inflammatory dendritic cell differentiation.
CD137 ligand (CD137L) is a costimulatory ligand that is expressed on antigen-presenting cells (APCs) and is important for enhancing T cell activation by crosslinking CD137 on activated T cells.1–3 While CD137 triggers a costimulatory signal in T cells, CD137L has been reported to trigger reverse signaling in APCs.4 In human monocytes, reverse CD137L signaling leads to the differentiation of dendritic cells (DCs), which are termed CD137L-DCs.5 CD137L-DCs are a novel type of DC and a potent candidate for DC-based cancer immunotherapy. CD137L-DCs are effective at polarizing Th1 and Tc1 responses and induce stronger cytotoxic T cell responses than those of monocyte-derived DCs generated with the more commonly used granulocyte–macrophage colony-stimulating factor and interleukin-4 (IL-4).5–7
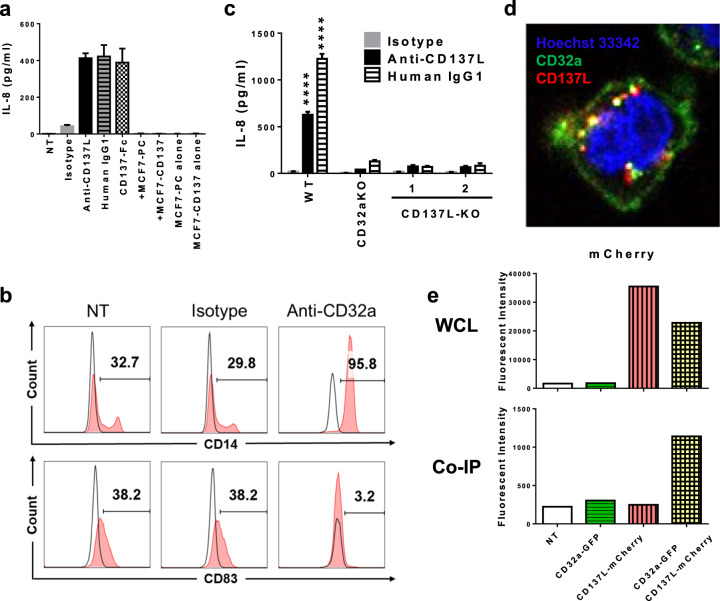
The differentiation of monocytes into CD137L-DCs by reverse CD137L signaling was thought to be mediated solely by CD137L, since neither the isolated Fc domain of human immunoglobulin G (IgG) nor isotype controls for anti-CD137L antibodies induced CD137L-DC differentiation.5 The absence of CD137L-DCs in mice is consistent with the fact that human CD137L and mouse CD137L share little homology.8 However, all studies relied on the use of immobilized CD137-Fc protein or anti-CD137L antibodies to crosslink CD137L. MCF-7 cells that were transfected to express CD137 failed to activate monocytic THP-1 cells to secrete IL-8 (Fig. 1a), indicating that CD137L alone is not enough to trigger reverse CD137L signaling.
Fig. 1.
CD137 ligand interacts with CD32a to trigger reverse CD137 ligand signaling. a A total of 105 THP-1 cells were cultured in 96-well plates coated with protein or seeded with 2.5 × 104 MCF-7 cells for 24 h. IL-8 in supernatants was measured. PC: parental control. b Primary monocytes were preblocked with NT, 5 μg/ml isotype, or anti-CD32a antibody (IV.3) for 30 min before seeding onto an anti-CD137L antibody-coated plate. CD14 and CD83 expression was measured on day 7 by flow cytometry. c WT or knockout (KO) THP-1 cells were cultured on antibody-coated plates for 1 day before IL-8 in supernatants was measured. ****P < 0.0001 (two-tailed Student’s t test) versus other KO THP-1 cells. d Confocal images of surface-stained CD137L mRNA-electroporated CD137L-KO THP-1 cells. Yellow dots indicate cointernalized anti-CD32a and anti-CD137L antibodies. e WT HEK-Blue cells were transfected with CD32a-GFPSpark and/or CD137L-mCherry plasmids. Cell lysates were coimmunoprecipitated by IV.3-crosslinked agarose. The fluorescent intensity of mCherry in whole-cell lysate (WCL) and coimmunoprecipitation (co-IP) samples were measured. Representative data from at least two independent experiments are shown. NT: nontreated
Both the anti-CD137L antibody (clone 5F4) and CD137-Fc can crosslink not only CD137L but also Fc gamma receptors (FcγRs). Moreover, THP-1 cells were activated by a monoclonal human IgG1 antibody, even though this antibody does not recognize CD137L (Fig. 1a). The involvement of FcγRs in reverse CD137L signaling was further confirmed using two pairs of antibodies in which the wild-type (WT) but not the LALA-mutated antibodies, which cannot bind FcγRs, induced CD137L-DC differentiation (Supplementary Fig. 1a). We narrowed down the candidates to low-affinity activating FcγRs, such as CD16 and CD32a, since only immobilized antibodies triggered reverse CD137L signaling. Both, THP-1 cells and negatively-isolated primary monocytes expressed CD32a but not CD16 (Supplementary Fig. 1b, c). By pretreating the monocytes with a CD32a blocking antibody (clone IV.3), differentiations of monocytes to CD137L-DCs was completely blocked, as evidenced by the absence of CD14 downregulation or CD83 upregulation (Fig. 1b). These data show that CD32a is indispensable for reverse CD137L signaling.
Further evidence comes from the genetic manipulation of the expression of CD32a and CD137L. When CD32a was specifically knocked down by a cocktail of three CD32a-specific small interfering RNAs (Supplementary Fig. 1d), the differentiation of CD137L-DCs was significantly inhibited, as evidenced by the absence of the typical CD137L-DC morphology, the high expression of CD14, and the low expression of CD83 (Supplementary Fig. 1e, f). Moreover, activation of THP-1 cells by anti-CD137L antibody was almost completely abrogated in CD32a- and CD137L-knockout THP-1 cells (Fig. 1c). Surprisingly, activation of THP-1 cells by monoclonal IgG1, which does not target CD137L, was suppressed when CD137L was absent, indicating that CD137L and CD32a might interact to trigger reverse CD137L signaling.
Indeed, cointernalization of CD32a and CD137L was observed in CD137L-reconstituted but not in CD32a-electroporated CD137L-knockout THP-1 cells (Fig. 1d). When anti-CD32a-crosslinked protein A/G agarose was used for coimmunoprecipitation, CD137L-mCherry was found in precipitates of CD32a-GFPSpark- and CD137L-mCherry-transfected HEK-Blue cells but not in the controls (Fig. 1e). Taken together, these data clearly demonstrate an interaction between CD137L and CD32a. Based on these observations, we hypothesize that CD32a, CD137L, and some other molecules form a signaling complex whose activation is dependent on the total activation strength (Supplementary Fig. 2).
Human CD137L has low homology with mouse CD137L.9 CD32a is expressed in humans but not in mice. These species differences may explain why CD137L-DCs are only found in humans but not in mice. This species difference hinders in vivo studies of CD137L-DCs. Moreover, CD137L-DCs lack a specific marker for their unequivocal identification in tissues. However, CMAP analysis demonstrated that in vivo inflammatory DCs and CD137L-DCs are mutually enriched in similar gene signatures, indicating a close relationship.10 It is possible that CD137L-DCs contribute to the pool of inflammatory DCs in vivo and that ligation of CD32a and CD137L by IgGs and CD137 induces CD137L-DC differentiation during inflammation, such as infections, treatments with therapeutic antitumor antibodies, and autoimmune diseases. With a better understanding of CD137L-DC differentiation, we may take advantage of the potent proinflammatory function of CD137L-DCs for cancer immunotherapy.
Supplementary information
Acknowledgements
This research was supported by a grant (NMRC/BnB/018b/2015) from the National Medical Research Council of Singapore. We thank the LSI core facility, Dr. Paul MacAry for the donation of WT and LALA-mutated antibodies, and the YLLSOM Confocal Microscopy Unit.
Competing interests
The authors declare no competing interests.
Supplementary information
The online version of this article (10.1038/s41423-020-0370-6) contains supplementary material.
References
- 1.Mbanwi AN, Watts TH. Costimulatory TNFR family members in control of viral infection: outstanding questions. Sem. Immunol. 2014;26:210–219. doi: 10.1016/j.smim.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Zapata JM, et al. CD137 (4-1BB) signalosome: complexity is a matter of TRAFs. Front. Immunol. 2018;9:2618. doi: 10.3389/fimmu.2018.02618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SW, Croft M. 4-1BB as a therapeutic target for human disease. Adv. Exp. Med. Biol. 2009;647:120–129. doi: 10.1007/978-0-387-89520-8_8. [DOI] [PubMed] [Google Scholar]
- 4.Shao Z, Schwarz H. CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. J. Leukoc. Biol. 2011;89:21–29. doi: 10.1189/jlb.0510315. [DOI] [PubMed] [Google Scholar]
- 5.Kwajah MMS, Schwarz H. CD137 ligand signaling induces human monocyte to dendritic cell differentiation. Eur. J. Immunol. 2010;40:1938–1949. doi: 10.1002/eji.200940105. [DOI] [PubMed] [Google Scholar]
- 6.Harfuddin Z, et al. CD137L-stimulated dendritic cells are more potent than conventional dendritic cells at eliciting cytotoxic T-cell responses. Oncoimmunol. 2013;2:e26859. doi: 10.4161/onci.26859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dharmadhikari B, et al. CD137L dendritic cells induce potent response against cancer-associated viruses and polarize human CD8(+) T cells to Tc1 phenotype. Cancer Immunol. Immunother. 2018;67:893–905. doi: 10.1007/s00262-018-2144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Q, Jiang D, Shao Z, Martinez Gomez JM, Schwarz H. Species difference of CD137 ligand signaling in human and murine monocytes. PLoS ONE. 2011;6:e16129. doi: 10.1371/journal.pone.0016129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alderson MR, et al. Molecular and biological characterization of human 4-1BB and its ligand. Eur. J. Immunol. 1994;24:2219–2227. doi: 10.1002/eji.1830240943. [DOI] [PubMed] [Google Scholar]
- 10.Harfuddin Z, et al. Transcriptional and functional characterization of CD137L-dendritic cells identifies a novel dendritic cell phenotype. Sci. Rep. 2016;6:29712. doi: 10.1038/srep29712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.