Abstract
Slc7a11 (xCT) and Slc3a1 (rBAT) are cystine uptake transporters that maintain intracellular concentrations of cysteine, the rate-limiting amino acid in glutathione synthesis. This study was conducted to first determine the tissue distribution of the two transporters in male and female mice. Because Slc3a1 was the primary cystine transporter in liver, its sex-divergent expression, ontogeny, diurnal rhythm and whether its mRNA expression is altered by transcription factors (AhR, CAR, PXR, PPARα, and Nrf2) was also investigated. Slc7a11 was expressed highest in brain and gonads. Slc3a1 was expressed highest in kidney and intestine, followed by liver. Duodenal and hepatic Slc3a1 was higher in females than males. Hepatic Slc3a1 was high during darkness and low during daytime. Hepatic Scl3a1 was lowest pre-birth, increased to near maximal levels at birth, decreased back to pre-birth levels between Days 3–10, and then returned to peak levels by Day 45. Except for CAR, activation of transcription factors did not increase hepatic mRNA expression of Slc3a1. Chemical activation of CAR significantly induced Slc3a1 1.4-fold in wild-type but not CAR-null mice. Slc3a1 mRNA was higher in livers of AhR- and Nrf2-null mice compared to wild-type mice. High doses of diquat but not acetaminophen induced Slc3a1, suggesting Slc3a1 may respond to oxidative stress but not necessarily to GSH depletion. Overall, Slc7a11 is mainly expressed in brain and gonads, whereas Slc3a1 is mainly expressed in kidney, small intestine and liver, and its hepatic expression is regulated by diurnal rhythm and certain xenobiotic treatments.
Keywords: Cystine transporters, Slc3a1, Oxidative stress, Transcription regulation
Introduction
Glutathione is an endogenous chemical that protects against oxidative stress and electrophilic chemicals, and the availability of cysteine is rate limiting for the synthesis of glutathione and maintaining cellular homeostasis of this important endogenous antioxidant [1]. Cystine is absorbed by the intestine and kidney and can be converted into two molecules of cysteine in the cytosol. Therefore, transporting cystine into cells plays an important role in cell survival from oxidative stress and electrophilic/xenobiotic toxicity.
Transport of amino acids across the plasma membrane of mammalian cells is performed by several transport systems with different substrate specificities [2]. Amino transport system b0,+ mediates the exchange of dibasic amino acids including cystine with neutral amino acids, which allows accumulation of cystine inside the cell [3]. This transport system is composed of a heavy chain rBAT (gene name: Slc3a1) and a light chain b0,+AT [4]. Mutations in rBAT cause cystinuria type I, whereas mutations in b0,+AT cause cystinuria non-type I in humans [5]. Reabsorption of cysteine by the kidney correlates with the expression of the rBAT/b0,+AT heterodimer, and cystinuria patients reabsorb very little cystine, indicating that the amino acid transport system b0,+ is the major cystine transport system in kidney [6]. Slc3a1 expression is found in epithelial cells from the S3 segment of the proximal tubule in rat kidney [7], S1 segment in mouse kidney [6], and rabbit kidney cortex [8]. The ontogenic development of Slc3a1 has been reported in rat kidney. Slc3a1 mRNA levels were low in early postnatal life, the onset of its expression is during late fetal life, and full expression is observed after weaning [7].
Amino transport system y+ is the sodium-independent antiporter responsible for the uptake of cystine via a 1:1 exchange with glutamate across the cell membrane [9]. This exchange function is mediated by a heterodimer of a ubiquitous amino acid transporter heavy chain, 4F2hc, and a specific light chain, xCT (gene name: Slc7a11). Transport of cystine via system y+ is an important factor for the uptake of intracellular cystine and maintenance of intracellular concentrations [10]. The expression of Slc7a11, rather than 4F2hc, responds to reactive oxygen species donors in RGC-5 retinal ganglion cells [11] and closely correlates with system y+ activity in macrophages [12], suggesting the importance of Slc7a11 in system y+ function and regulation. Slc7a11 mRNA has been detected in various cell lines and tissues: localized to neurons in the mouse and human brain, brush border membranes in monkey kidney and duodenum [13], and constitutively expressed in the brain, thymus, and spleen in mice [14].
Among the existing studies on the expression and regulation of Slc3a1 and Slc7a11, little has been done on tissue distribution, ontogeny, and mechanism of induction of these genes in mice; however, this work has become of increasing importance because of the existence of knock-out mouse models and their relationship to the study of gene regulation.
Transcription factors are proteins that regulate transcription of genes. The transcription factors Aryl hydrocarbon receptor (AhR), Constitutive androstane receptor (CAR), Pregnane X receptor (PXR), Peroxisome proliferator-activated receptor alpha (PPAR) and Nuclear factor E2-related factor 2 (Nrf2) are involved in the response to xenobiotics, oxidative stress, energy regulation, and nutrition. Even though cystine transporters play an important role in absorption and detoxification, the regulation of these transporters is poorly understood, and little is known about interactions of microsomal enzyme inducers (MEIs) with those cystine transporters.
Therefore, the purpose of the present study was to determine: 1) tissue distribution of Slc3a1 and Slc7a11mRNA in mice, 2) whether there are sex-specific differences in mouse Slc3a1 and Slc7a11 expression, and if so, the mechanisms, 3) the ontogenic patterns Slc3a1 and Slc7a11 in livers of mice, and 4) the inducibility of Slc3a1 and Slc7a11 by transcription factors and oxidative stress stimuli.
Materials and Methods
Chemicals.
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) was a gift from Dr. Karl Rozman (University of Kansas Medical Center, Kansas City, KS). Oltipraz was a gift from Dr. Steven Safe (Texas A&M University, College Station, TX). Rat growth hormone was obtained through Dr. Parlow at the National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases (Torrance, CA). Pellets for subcutaneous (s.c.) release of hormones used in this study, 5α-dihydroxytestosterone (DHT), 17β-estradiol (E2), GH, and placebo, were purchased from Innovative Research of America (Sarasota, FL). All other chemicals were purchased from Sigma-Aldrich Co. (St. Louis, MO).
Animals and Breeding.
C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME) and housed according to the American Animal Association Laboratory Animal Care guidance to study the tissue distribution, ontogeny, diurnal expression, and chemical induction of Slc3a1 and Slc7a11. This study was approved by the Institutional Animal Care and Use Committee at the University of Kansas Medical Center.
Tissue Distribution.
Eight-week-old male and female mice were used (5 mice/sex) and twelve tissues (liver, kidney, lung, stomach, duodenum, jejunum, ileum, colon, heart, brain, testis, and ovary) were collected. All tissues were harvested between 8 am to 11 am. Placenta was removed from pregnant mice on gestation day 17. The tissues were frozen in liquid nitrogen. The intestine was longitudinally dissected, rinsed in saline, and divided into three equal-length sections (referred to as duodenum, jejunum, and ileum), before being frozen in liquid nitrogen. All tissues were stored at −80°C until analysis.
Sex Differences in Duodenum.
Animals and treatment for mechanisms of sex differences were conducted as previously described [15]. Hypophysectomized and gonadectomized C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA); growth-hormone-releasing-hormone-receptor deficient mice (lit-lit mice) were also purchased from Jackson Laboratory. 1) Mice were gonadectomized on day 37 of age, followed by replacement with 5α-dihydroxytestosterone (DHT, 5 mg 21-day-release pellets, s.c. for 10 days), 11β-estradiol (E2, 0.5 mg 21-day-release pellets, s.c. for 10 days), or placebo. 2) Mice were hypophysectomized on day 30 of age, followed by replacement with female-patterned growth hormone (1 mg 21-day-release pellets, s.c. for 10 days), or male-patterned growth hormone (rat growth hormone, 2 × daily, 2.5 mg/kg, i.p. for 10 days); 3) Growth-hormone-releasing-hormone-receptor deficient mice (lit-lit mice) in C57BL/6 background were purchased from The Jackson Laboratory, and were administered a placebo, male-patterned growth hormone, or female-patterned growth hormone. Duodenums were collected from gonadectomized, hypophysectomized, and GHRH receptor-deficient mice between 8 am to 11 am and stored at −80 °C for RNA isolation.
Ontogeny.
Livers from male and female mice were collected at postnatal days −2, 0, 5, 10 15, 22, 30, 35, 40, and 45 (n=5/sex/age) and stored at −80°C for RNA isolation.
Diurnal expression.
Livers from male mice (n = 5/time point) were collected at 2:00, 6:00, and 10:00 AM, as well as 2:00, 6:00, and 10:00 PM. Equal amounts of RNA from individual samples were combined to make a pooled sample for each time point.
Chemical induction.
Male AhR-null mice were obtained from Jackson Laboratories, Inc. (Bar Harbor, ME). The following mice were generous gifts from various laboratories: CAR-null mice from Dr. Ivan Rusyn (University of North Carolina, Chapel Hill, NC), Nrf2-null mice from Dr. Jefferson Chan (University of California, Irvine, Irvine, CA), PPARα-null mice from laboratory of Dr. Frank J. Gonzalez (National Institutes of Health/National Cancer Institute, Bethesda, MD), and PXR-null mice from Dr Jeff L. Staudinger (University of Kansas, Lawrence, Kansas). All mice were backcrossed into C57BL/6J background to >99% congenicity. Mice were treated i.p. with either vehicle (corn oil) or AhR activator 2,3,7,8-tetrachlorodibenzodioxin (TCDD, 40 μg/kg i.p. in corn oil), CAR activator 1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene, 3,3′,5,5′-tetrachloro-1,4-bis(pyridyloxy)benzene (TCPOBOP, 3 mg/kg i.p. in corn oil), PXR activator pregnenolone 16α-carbonitrile (PCN, 200 mg/kg i.p. in corn oil), PPARα activator clofibrate (CLFB, 500 mg/kg, i.p. in saline), or Nrf2 activator oltipraz (OPZ, 150 mg/kg p.o. in corn oil). Mice (5 animals/genotype/treatment) were dosed once daily for 4 consecutive days. Tissues were removed between 8 am to 11 am on day 5, frozen in liquid nitrogen, and stored at −80°C until analysis.
Expression profile of Slc3a1 in Nrf2-null, wild-type, and Keap1-knockdown (kd) mice.
Keap1-kd mice were a generous gift from Dr. Masayuki Yamamoto (University of Tsukuba, Tsukuba, Japan) and were backcrossed into the C57BL/6J background to >99% congenicity. Wild-type, Nrf2-null, and Keap1-kd mice were treated with single dose of either saline (5 ml/kg, i.p.) or diquat dibromide (125 mg/kg, i.p. in saline) between 8 am to 9 am. Livers were collected 6 h after treatment between 2 pm and 3 pm and stored at −80°C [16].
Expression profile of Slc3a1 after acetaminophen treatment.
Wild-type mice were treated with single dose of acetaminophen (300 mg/kg, i.p.) between 8 am to 10 am, and livers were collected at 0, 2, 4, and 6 hours after treatment and stored at −80°C.
RNA Isolation.
Total RNA was isolated using RNAzol B reagent (Tel-Test Inc., Friendswood, TX) according to the manufacturer’s protocol. RNA concentrations were quantified by ultraviolet absorbance at 260 nm.
Branched DNA (bDNA) Signal Amplification Assay.
Mouse Slc3a1 and Slc7a11 mRNA were quantified by the bDNA assay (QuantiGene bDNA signal amplification kit, Panomics, Fremont, CA). Specific oligonucleotide probe sets of Slc3a1 and Slc7a11 were synthesized by QIAGEN Operon (Alameda, CA). The strategy of multiple oligonucleotide probe sets design has been described previously [17]. Probe sets for each mouse Slc3a1 and Slc7a11 (including CEs, capture extenders; LEs, label extenders; and BLs, blockers) are shown in Table 1. 10 μg of total RNA was added to each well of a 96-well plate. Slc3a1 and Slc7a11 mRNA was captured by the probe sets and attached to a branched DNA amplifier. Enzymatic reactions occur upon substrate exposure, and the luminescence for each well is reported as Relative Light Units (RLU).
Table 1.
Oligonucleotide probes generated for analysis of mouse Slc3a1 and Slc7a11 mRNA expression by branched DNA signal amplification assay
| Slc3a1 | |
| Function | Probe Sequence |
| CEa | tcacggcgttgggctgtTTTTTctcttggaaagaaagt |
| CE | gctcctctggagcagggatacTTTTTctcttggaaagaaagt |
| CE | gctccaatgagcaggaacacgTTTTTctcttggaaagaaagt |
| CE | gtatatgggacctgcttgccaTTTTTctcttggaaagaaagt |
| CE | tctcctggatacctttcaggtctTTTTTctcttggaaagaaagt |
| LE | cgcaccgcctttaggtgagTTTTTaggcataggacccgtgtct |
| LE | ggcatccctgcatagggcTTTTTaggcataggacccgtgtct |
| LE | cggtagcgagcctggccTTTTTaggcataggacccgtgtct |
| LE | gatgacgatgatggctatggtgTTTTTaggcataggacccgtgtct |
| LE | ccattcccatccttgtcactgtcTTTTTaggcataggacccgtgtct |
| BL | ggagaactggaagagtacttccttg |
| BL | gaggatctctcggggcacc |
| BL | gaaaccacggtgagccagaa |
| BL | ccagtcaaggcattttggaga |
| BL | cttaaaagacctcgggtagatctg |
| Slc7a11 | |
| CE | gctcataaagagaaagcctgatactTTTTTctcttggaaagaaagt |
| CE | tgcacttttcctaattgatccagTTTTTctcttggaaagaaagt |
| CE | catttcgttcatagtttttatgtaaatcTTTTTctcttggaaagaaagt |
| CE | ccctcttttgcaaattgaattataTTTTTctcttggaaagaaagt |
| CE | ccatttgaatatggctactgttttcTTTTTctcttggaaagaaagt |
| LE | tggagatttatgggcaatgtctcTTTTTaggcataggacccgtgtct |
| LE | gtaattactaacactgcatataaatgggTTTTTaggcataggacccgtgtct |
| LE | tgtcaaaaactcatcaccaggtgTTTTTaggcataggacccgtgtct |
| LE | atagttttaaccagaggcatattttaaTTTTTaggcataggacccgtgtct |
| LE | ttcaatgggtttggtcacatctTTTTTaggcataggacccgtgtct |
| LE | taactctgatttaacatatgtagctagctTTTTTaggcataggacccgtgtct |
| LE | aaaatcccaccattgcattaattTTTTTaggcataggacccgtgtct |
| BL | aaaacagccacaaagtaaaagtga |
| BL | cagaactgtaaagagaatgcacacac |
| BL | cctagattttcacgaagcagtgg |
| BL | actaaagtgaagaaatgactgaaacag |
| BL | gaacaaccgtactgaagactctcc |
CE, capture extender; LE, label extender; BL, blocker
Multiplex Suspension Array.
For diurnal and ontogeny of Slc3a1, mRNA expression Slc3a1 in livers was determined by Panomics 1.0/2.0 QuantiGene plex technology (Panomics Inc., Fremont, CA). Slc3a1 mRNA oligonucleotide probe sets were designed by Thermo Fisher Scientific Inc. Genes and accession numbers are freely available at https://www.thermofisher.com/us/en/home/life-science/gene-expression-analysis-genotyping/quantigene-rna-assays/quantigene-plex-assay/quantigene-plex-assay-ordering.html. Messenger RNA expression is presented as relative light units per 10 μg of total RNA.
Statistics.
Statistical differences between sexes were determined by Student’s t test. Differences between multiple groups were analyzed by one-way ANOVA followed by Duncan’s post-hoc test. Statistical significance was set at p<0.05. Circadian variation was analyzed by the intergroup average cosine method.
Results
Tissue distribution of Slc3a1 and Slc7a11 mRNA.
Messenger RNA expression of Slc3a1 and Slc7a11 were quantified in 13 mouse tissues (Fig.1). Slc3a1 mRNA was highly expressed in kidney and duodenum, moderately expressed in jejunum, ileum, and liver, and lowly expressed in other tissues. Slc3a1 mRNA levels in duodenum and liver were significantly higher (14.5-fold and 1.3-fold, respectively) in females than males.
Fig. 1. Tissue distribution of Slc3a1 and Slc7a11 mRNA.
Total RNA from both male and female C57BL/6 mouse tissues (n = 5/sex) was analyzed by the bDNA assay for expression of Slc3a1 and Slc7a11 mRNA. Data are presented as mean RLU ± S.E.M. Asterisks indicate statistically significant differences between male and female mice (p < 0.05).
Expression of Slc7a11 mRNA was highest in brain and gonads, modest in stomach and lung, and low in other tissues. Slc7a11 exhibited a male predominance in brain, with a 2.3-fold higher mRNA expression in males compared to females. Slc7a11 exhibited a female predominance in gonads, as expression was 1.7-fold significantly higher in the ovaries than in the testes.
Mechanisms of Sex Differences in Duodenum Slc3a1.
Slc3a1 mRNA was highly expressed in duodenum of female mice and markedly lower in males (Fig.1). After surgical gonadectomy, Slc3a1 mRNA increased in males and decreased in female duodenum. Androgen (DHT) and estrogen (E2) replacement increased Slc3a1 mRNA in females but had no effect on mRNA level in males (Fig. 2, upper panel).
Fig. 2. Effects of growth and sex hormones on the sex differences in Slc3a1 mRNA expression in mouse duodenum from naive, gonadectomized (GNX), hypophysectomized (HX), and lit/lit mice.
Total RNA was isolated and analyzed by the bDNA signal amplification assay for Slc3a1 mRNA content. The data are presented as mean RLU ± S.E.M. (n = 6–7). GNX/HX/Lit+Plac (placebo administered to gonadectomized, hypophysectomized, or lit/lit mice), HX/Lit + male-pattern growth hormone (MPGH, rat GH administered twice daily by i.p. injection to hypophysectomized or lit/lit mice mimicking MPGH secretion), HX/Lit + female-pattern growth hormone (FPGH, continuous infusion to hypophysectomized or lit/lit mice via s.c. implanted 21-day-release 1-mg rat GH pellet mimicking FPGH secretion), GNX/HX + DHT (5α-dihydroxytestosterone administered to gonadectomized or hypophysectomized mice), and GNX/HX + E2 (17β-estradiol administered to gonadectomized or hypophysectomized mice). Asterisk (*) represents statistically significant differences (p < 0.05) between male and female mice.
To further investigate the effects of growth hormone secretory patterns on the regulation of mouse Slc3a1, lit/lit mice, which are a genetic model of growth hormone deficiency, were treated with either male-pattern growth hormone or female-pattern growth hormone (Fig. 2, middle panel). Lit/lit mice abolished the female predominant expression of Slc3a1 in duodenum, with an increase in males and no change in females. However, neither male-pattern nor female-pattern growth hormone treatment of the lit/lit mice significantly altered Slc3a1 mRNA expression in males or females. Female-pattern growth hormone treatment tended to increase Slc3a1 mRNA in both males and female mice, but it was not statistically significant.
Hypophysectomized mice were treated with sex and growth hormones to determine the effects of each on the expression of Slc3a1 in the duodenum. In hypophysectomized mice (Fig. 2, bottom panel), Slc3a1 mRNA levels decreased in females but remained the same in males. Male-pattern and female-pattern growth hormone replacement in hypophysectomized mice tended to increase Slc3a1 mRNA level in males, whereas it had no effect in females.
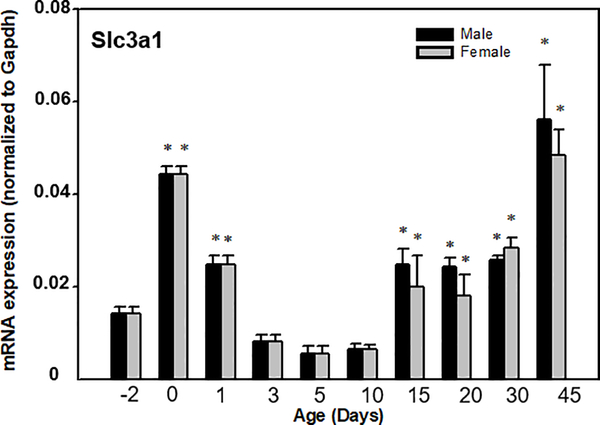
Ontogenic expression of Slc3a1 in livers of male and female mice.
The neonatal and postnatal mRNA expression patterns of the Slc3a1 in male and female mouse livers are depicted in Fig.3. Slc3a1 is moderately expressed in adult liver (Fig.1), and lowly expressed before birth. At birth, the expression increased approximately 3 fold, followed by a dramatic decrease to pre-birth levels by 3 days of age. Slc3a1 mRNA abruptly increased at 15 days of age and then remained relatively constant until 45 days of age when it increased to higher levels. There was no sex difference in the expression of Slc3a1 during this time period. The ontogenic expression of Slc7a11 could not be determined because its mRNA was below the limit of detection in mouse liver (data not shown).
Fig. 3. Ontogenic expression of mouse Slc3a1 mRNA in liver.
Total RNA from C57BL/6 mice at each age (n = 5/sex) was analyzed by the bDNA assay. Data are presented as mean RLU ± S.E.M. Asterisks indicate statistically significant differences compared to day-2 (p < 0.05).
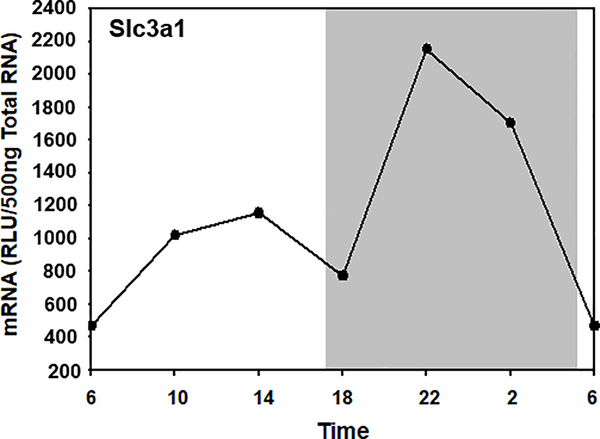
Diurnal variation of Slc3a1 expression in mouse liver.
The transcripts of Slc3a1 were more abundant during the dark phase than during the light phase (Fig. 4). The Slc3a1 mRNA level was low during the light phase, increased rapidly after the dark phase began, and peaked at 10:00 pm. The circadian rhythm adjusted mean was 1253 RLU/500ng total RNA, with peak/trough ratio of 3-fold. The diurnal expression profile of Slc7a11 could not be determined because its mRNA below the limit of detection in mouse liver (data not shown).
Fig. 4. Circadian expression profile of Slc3a1 in mouse liver.
Total RNA from mouse livers (n = 5 per time point) was pooled, and mRNA expression was determined by multiplex suspension array. Data at 6:00 AM were used twice to graph a 24-h cycle of mRNA expression. The shaded area of the figure represents the dark phase, and the white area represents the light phase.
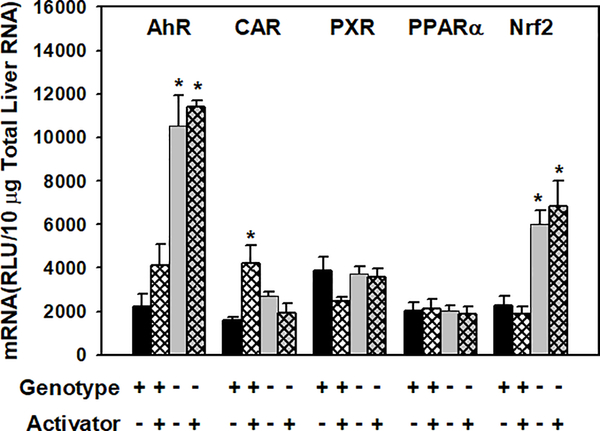
Induction of Slc3a1 by transcription factor chemical activators in wild-type and transcription factor-knockout mice.
To further investigate transcriptional regulation of Slc3a1, male wild-type mice were dosed with prototypical activators of AhR, CAR, PXR, PPARα, and Nrf2. To investigate whether the effects of the activators were transcription factor-dependent, the activators were dosed to their specific transcription factor-knockout mice. As shown in Fig. 5, CAR activator TCPOBOP increased Slc3a1 mRNA 1.4-fold, and this effect was abolished in CAR-null mice. AhR and Nrf2 activators did not alter Slc3a1 mRNA abundance, however, surprisingly, the constitutive expression of Slc3a1 was markedly higher in AhR-null and Nrf2-null mice, with 4-fold and 2.3-fold higher expression than in wild-type mice, respectively. PXR and PPARα activators did not induce Slc3a1 transcription in wild-type mice, and mRNA levels of Slc3a1 in PXR-null and PPARα-null mice remained unchanged compared to wild-type mice.
Figure. 5. Effects of chemical induction on mouse Slc3a1 mRNA expression in liver.
Total liver RNA was isolated and analyzed by the bDNA signal amplification assay for Slc3a1 mRNA content. The dose of chemical treatment was described in the method. Data are presented as RLU ± S.E.M. (n=5). Asterisks indicate statistically significant increase or decrease in mRNA level after treatment compared with the wild-type control group (p<0.05).
Expression profile of Slc3a1 in livers of wild-type, Nrf2-null, and Keap1-kd mice.
Nrf2 is a transcription factor that is sequestered by kelch-like ECH-associated protein 1 (Keap1). Upon activation, Nrf2 promotes GSH biosynthesis and decreases oxidative stress [18]. To further investigate the role of Nrf2 in regulating Slc3a1 transcription, mRNA was quantified in livers of naive wild-type, Nrf2-null, and Keap1-kd mice (with increased Nrf2 activity). As shown in Fig. 6, compared to wild-type mice, Slc3a1 mRNA was markedly higher in Nrf2-null mice than in Keap1-kd mice. To investigate the role of oxidative stress in regulating Slc3a1 transcription, mRNA was quantified in livers of wild-type, Nrf2-null, and Keap1-kd mice treated with diquat dibromide (125 mg/kg, i.p.). Diquat administration caused a significant 1.2-fold induction of Slc3a1 in wild-type mice but did not alter Slc3a1 mRNA abundance in Nrf2-null or Keap1-kd mice. Hepatic Slc7a11 mRNA was not detectable in untreated mice or inducible under oxidative stress (data not shown).
Figure. 6. Expression of profile of Slc3a1 in male Nrf2-null and Keap1-knockdown mice liver.
Nrf2-null mice, wild-type mice, and Keap1-kd mice (with increased Nrf2 activity) were treated with single dose of saline (5 ml/kg, i.p., labeled as -) or diquat dibromide (125 mg/kg, i.p. in saline, labeled as +) and taken down for necropsy after 6 hours. Total liver RNA was isolated and analyzed by the bDNA signal amplification assay for Slc3a1 mRNA content. Data are presented as RLU ± S.E.M. (n=5). Asterisks indicate significant different to vehicle treated group of the same genotype (p<0.05).
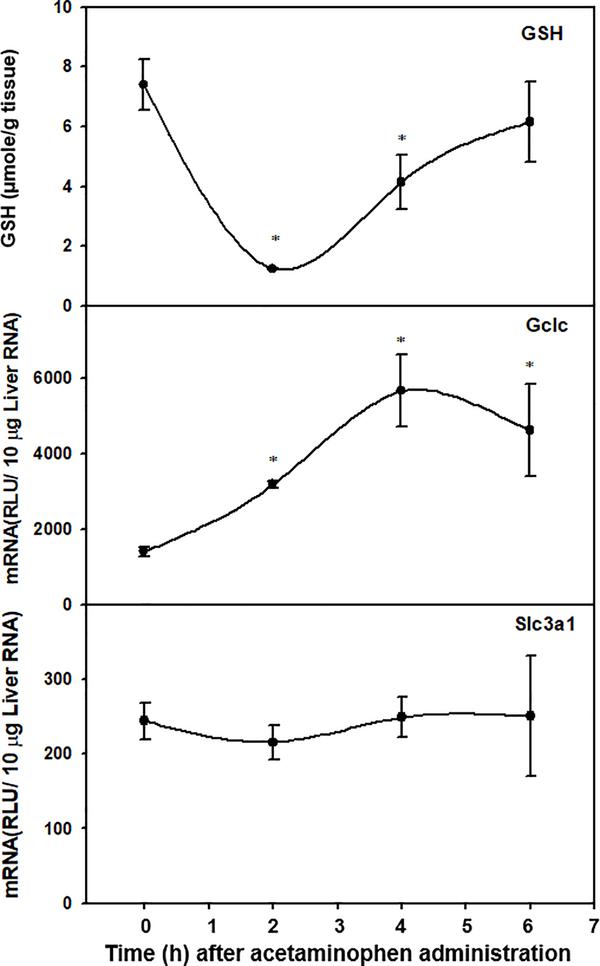
Expression profile of Slc3a1 in livers under GSH depletion.
To further investigate the effect of GSH depletion on the regulation of Slc3a1 transcription, Slc3a1 mRNA was quantified in livers of mice before and up to 6h after acetaminophen (300 mg/kg, i.p.) administration. To confirm the depletion of GSH and activation of GSH biosynthesis, GSH concentrations and abundance of glutamate-cysteine ligase catalytic subunit (Gclc) mRNA were quantified at the same time intervals. As shown in Fig. 7, after acetaminophen treatment, hepatic GSH decreased 83% at the 2 h time point and returned to control levels by 6 h. Acetaminophen treatment significantly increased Gclc mRNA abundance, with 1.2- and 3-fold increases at the 2 h and 4 h time points, respectively. In contrast, Slc3a1 mRNA levels remained unchanged at all time points after acetaminophen treatment. Hepatic Slc7a11 mRNA was not detectable in control mice or inducible after GSH depletion at all time points (data not shown).
Figure. 7. Time course of hepatic GSH concentrations (top), Gclc mRNA levels (middle), and Slc3a1 mRNA levels (bottom) after treatment with acetaminophen (300mg/kg, i.p.).
GSH was extracted from liver and quantified by UPLC-MS/MS as described in the method. Total liver RNA was isolated and analyzed by the bDNA signal amplification assay for Gclc and Slc3a1 mRNA content. Data are presented as mean ± S.E.M μmol/g tissue or RLU ± S.E.M. (n=5) Asterisks indicate statistically significant different to control group (p<0.05).
Discussion
The present study evaluated the constitutive expression of mouse cystine transporters Slc3a1 and Slc7a11 in various tissues, hepatic Slc3a1 expression profile during development and diurnal rhythm, regulatory mechanisms contributing to its sex-predominant expression in duodenum, possible transcription factors that regulate its expression, and effects of oxidative stress as well as glutathione depletion on its gene expression in liver.
The mouse tissues with the highest Slc3a1 mRNA were kidney and duodenum, which indicates its high capability to absorb cystine from the diet and the glomerular filtrate. The human ortholog for mouse Slc3a1 is hBAT1 (gene name SLC3A1), which is highly expressed in kidney, and moderately expressed in liver [19]. Disruption of hBAT1 results in cystinuria type I.
Human SLC7A11 is highly expressed in brain [13] and cancer cell lines, such as esophageal cancer cell lines [20], pancreatic cancer cell lines [21], and hepatoma cell lines [22]. In human cancer cell lines, SLC7A11 expression has been suggested to serve as a predictor of cellular response to glutathione-mediated resistance to chemotherapies [23], and genetic or pharmacological inhibition of SLC7a11 in human malignant brain tumors [24]. Consistent with previous publications, the present study shows that mouse Slc7a11 is highly expressed in brain (Fig.1). Slc7a11 mRNA was barely detectable or inducible in livers.
Slc3a1 exhibited female predominate expression in kidney, liver, duodenum, and colon. Duodenum had the largest difference in Slc3a1 mRNA abundance, with 15-fold higher in females than males (Fig. 1). Although small intestine is not the major organ for GSH synthesis, it plays an important role in cysteine absorption from the diet and represents a major source of amino acids for organ and cellular functions [25]. Sex hormones and growth hormones have been shown to regulate expression of transporters in the gastrointestinal tract and play a role in sex differences in nutrient absorption. For instance, estrogen significantly up-regulates expression of the intestinal cholesterol transporter Npc1l1, and enhances cholesterol absorption [26]. To further explore whether sex hormones are involved in sex differences of intestinal Slc3a1 expression, gonadectomized mice with absence of sex hormones were examined. After gonadectomy, Slc3a1 mRNA markedly decreased in female mice, and estradiol treatment increased Slc3a1 mRNA, indicating a stimulatory effect of estrogen (Fig2, upper panel). To understand the role of growth hormones in sex-divergent Slc3a1 expression, growth hormone was administered with male- and female-pattern (GHM or GHF) to either growth hormone releasing hormone receptor (GHRH)-deficient little (lit/lit) mice or hypophysectomized (HX) mice. Slc3a1 mRNA expression was higher in male lit/lit mice than wild-type mice, indicating an inhibitory role of GHRH. However, neither GHM nor GHF altered mRNA abundance in lit/lit mice or HX mice (Fig. 2). Thus, the role of growth hormones on Slc3a1 mRNA expression is unclear. It should be noted that the tissue distribution of Slc3a1 was assessed in mice under C57BL/J background, whereas gonadectomized and hypophysectomized mice were under C57BL/N background. The differences in genetic background and impact on hormonal responses are not controlled here.
Slc3a1 has an integral role in overall growth and development. Whole body SLC3A1 deficiency causes cystinuria type I in humans, which is characterized by hypotonia at birth and growth retardation [27]. Liver is the primary site for GSH biosynthesis, thus neonatal and postnatal expression of Slc3a1 gene potentially plays an important role in both liver development and defense against electrophiles. The abundance of Slc3a1 mRNA was low before birth and increased markedly at birth (Fig.3). The enhanced Slc3a1 expression may benefit newborns in nutrient acquisition as wells as detoxification of xenobiotics in milk.
Cysteine and cystine concentrations undergo a diurnal variation in mouse liver, and the maximum and minimum values precede the diurnal rhythm for hepatic GSH concentrations [28]. Variations of cellular concentrations of cysteine and GSH, the most abundant thiol resource, are linked to the dietary intake of sulfur amino acids [29]. In the present study, the abundance of Slc3a1 mRNA varied significantly over a 24-hr period (Fig.4), with the maximum level occurring at mealtime. Because the Slc7a11 transporter contribute little to the diurnal variation of cysteine and cystine concentrations [29], Slc3a1 might be the transporter that is responsible for this variation.
Transcription factors are proteins that bind to promoter sites of genes and regulate gene expression by promoting or blocking transcription. Human SLC3A1 transcription can be induced by the proximal tubule transcription factor, PAX8 [30]; however, little is known about the regulation of Slc3a1 gene expression in liver. The aryl hydrocarbon receptor (AhR) is the transcription factor that regulates genes encoding xenobiotic-metabolizing enzymes as well as proteins controlling cell proliferation and differentiation [31]. Constitutive androstane receptor (CAR) and the pregnane X receptor (PXR) are xenosensors that regulate the expression of Phase-I and Phase-II drug-metabolizing enzymes and transporters. In addition, they play a role in modulating hormone, lipid, and energy homeostasis as well as cancer and liver steatosis [32]. Peroxisome proliferator-activated receptor alpha (PPARalpha) regulates expression of enzymes and transporters in adaptive response of lipid metabolism in fasting [33]. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor that promotes transcription of a large battery of cytoprotective genes and is essential for the antioxidative response in the liver [34]. In the present study, however, none of the transcription factor activators induced Slc3a1 transcription markedly (Fig.5). Surprisingly, Slc3a1 mRNA was increased in AhR-null and Nrf2-null mice (6- and 4-fold, respectively), indicating a negative role for AhR and Nrf2 in Slc3a1 transcription. Further experiments need to be performed to fully elucidate the roles of AhR and Nrf2 in Slc3a1 regulation.
It is known that the other cystine transporter, Slc7a11, can be induced through Nrf2 activation [35] and contributes to GSH biosynthesis under oxidative stress in mesenchymal stem cells [36]. However, neither pharmacologic nor genetic activation of Nrf2 induced Slc3a1 transcription in mouse liver (Fig 5 and 6). Diquat is a herbicide that is not metabolized, but undergoes redox cycling and generation of oxidative stress [37]. After diquat treatment, Slc3a1 mRNA was increased, suggesting that Slc3a1 transcription can be induced by oxidative stress through Nrf2-independent mechanisms. The induction of Slc3a1 by diquat was abolished in Keap1-kd mice, suggesting that Nrf2 activation may inhibit the adaptive response of Slc3a1 against oxidative stress.
Acetaminophen is a widely prescribed analgesics and can cause hepatic GSH depletion upon acute overdosing. Acetaminophen is metabolized by cytochrome P450s (Cyps) to N-acetyl-p-benzoquinone imine (NAPQI) in the liver. NAPQI is highly reactive and is conjugated with GSH [38]. In the present study, more than 80% of the hepatic GSH was depleted after acetaminophen treatment (Fig. 6, upper panel), and Gclc, the rate-limiting enzyme for GSH synthesis, was up-regulated (Fig.6, middle panel), indicating activation of a compensative response upon GSH depletion. Surprisingly, Slc3a1 mRNA remained constant at all time points (Fig.6, bottom panel), indicating that there is little to no effect of GSH depletion on Slc3a1 gene regulation.
In conclusion, the cystine transporter Slc7a11 is highly expressed in brain and lowly expressed in liver. Hepatic Slc7a11 cannot be detected during development and is not induced under conditions of oxidative stress or GSH depletion. The cystine transporter Slc3a1 is highly expressed in kidney and moderately expressed in liver. The abundance of hepatic Slc3a1 mRNA varies with developmental age and time of the day. Slc3a1 transcription can be induced under oxidative stress, and the transcription factor Nrf2 inhibits Slc3a1 transcription under basal as well as inducible conditions.
Acknowledgments
Footnotes: This work was supported by NIH grants ES-013714, ES-09716, ES-09649, and RR021940.
Reference
- [1].Orrenius S, Ormstad K, Thor H, Jewell SA. Turnover and functions of glutathione studied with isolated hepatic and renal cells. Fed Proc 1983;42(15):3177–88. [PubMed] [Google Scholar]
- [2].Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev 1990;70(1):43–77. [DOI] [PubMed] [Google Scholar]
- [3].Chillaron J, Estevez R, Mora C, Wagner CA, Suessbrich H, Lang F, Gelpi JL, Testar X, Busch AE, Zorzano A and others. Obligatory amino acid exchange via systems bo,+-like and y+L-like. A tertiary active transport mechanism for renal reabsorption of cystine and dibasic amino acids. J Biol Chem 1996;271(30):17761–70. [DOI] [PubMed] [Google Scholar]
- [4].Chairoungdua A, Segawa H, Kim JY, Miyamoto K, Haga H, Fukui Y, Mizoguchi K, Ito H, Takeda E, Endou H and others. Identification of an amino acid transporter associated with the cystinuria-related type II membrane glycoprotein. J Biol Chem 1999;274(41):28845–8. [DOI] [PubMed] [Google Scholar]
- [5].Calonge MJ, Volpini V, Bisceglia L, Rousaud F, de Sanctis L, Beccia E, Zelante L, Testar X, Zorzano A, Estivill X and others. Genetic heterogeneity in cystinuria: the SLC3A1 gene is linked to type I but not to type III cystinuria. Proc Natl Acad Sci U S A 1995;92(21):9667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fernandez E, Carrascal M, Rousaud F, Abian J, Zorzano A, Palacin M, Chillaron J. rBAT-b(0,+)AT heterodimer is the main apical reabsorption system for cystine in the kidney. Am J Physiol Renal Physiol 2002;283(3):F540–8. [DOI] [PubMed] [Google Scholar]
- [7].Furriols M, Chillaron J, Mora C, Castello A, Bertran J, Camps M, Testar X, Vilaro S, Zorzano A, Palacin M. rBAT, related to L-cysteine transport, is localized to the microvilli of proximal straight tubules, and its expression is regulated in kidney by development. J Biol Chem 1993;268(36):27060–8. [PubMed] [Google Scholar]
- [8].Bertran J, Werner A, Moore ML, Stange G, Markovich D, Biber J, Testar X, Zorzano A, Palacin M, Murer H. Expression cloning of a cDNA from rabbit kidney cortex that induces a single transport system for cystine and dibasic and neutral amino acids. Proc Natl Acad Sci U S A 1992;89(12):5601–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bannai S, Kitamura E. Transport interaction of L-cystine and L-glutamate in human diploid fibroblasts in culture. J Biol Chem 1980;255(6):2372–6. [PubMed] [Google Scholar]
- [10].Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc. Antioxid Redox Signal 2000;2(4):665–71. [DOI] [PubMed] [Google Scholar]
- [11].Dun Y, Mysona B, Van Ells T, Amarnath L, Ola MS, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (xc-) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006;324(2):189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sato H, Kuriyama-Matsumura K, Hashimoto T, Sasaki H, Wang H, Ishii T, Mann GE, Bannai S. Effect of oxygen on induction of the cystine transporter by bacterial lipopolysaccharide in mouse peritoneal macrophages. J Biol Chem 2001;276(13):10407–12. [DOI] [PubMed] [Google Scholar]
- [13].Burdo J, Dargusch R, Schubert D. Distribution of the cystine/glutamate antiporter system xc- in the brain, kidney, and duodenum. J Histochem Cytochem 2006;54(5):549–57. [DOI] [PubMed] [Google Scholar]
- [14].Taguchi K, Tamba M, Bannai S, Sato H. Induction of cystine/glutamate transporter in bacterial lipopolysaccharide induced endotoxemia in mice. J Inflamm (Lond) 2007;4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Buckley DB, Klaassen CD. Mechanism of gender-divergent UDP-glucuronosyltransferase mRNA expression in mouse liver and kidney. Drug Metab Dispos 2009;37(4):834–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wu KC, Zhang Y, Klaassen CD. Nrf2 protects against diquat-induced liver and lung injury. Free Radic Res 2012;46(10):1220–9. [DOI] [PubMed] [Google Scholar]
- [17].Hartley DP, Klaassen CD. Detection of chemical-induced differential expression of rat hepatic cytochrome P450 mRNA transcripts using branched DNA signal amplification technology. Drug Metab Dispos 2000;28(5):608–16. [PubMed] [Google Scholar]
- [18].Cuadrado A, Moreno-Murciano P, Pedraza-Chaverri J. The transcription factor Nrf2 as a new therapeutic target in Parkinson’s disease. Expert Opin Ther Targets 2009;13(3):319–29. [DOI] [PubMed] [Google Scholar]
- [19].Mizoguchi K, Cha SH, Chairoungdua A, Kim DK, Shigeta Y, Matsuo H, Fukushima J, Awa Y, Akakura K, Goya T and others. Human cystinuria-related transporter: localization and functional characterization. Kidney Int 2001;59(5):1821–33. [DOI] [PubMed] [Google Scholar]
- [20].Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX and others. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009;28(4):599–609. [DOI] [PubMed] [Google Scholar]
- [21].Lo M, Ling V, Wang YZ, Gout PW. The xc- cystine/glutamate antiporter: a mediator of pancreatic cancer growth with a role in drug resistance. Br J Cancer 2008;99(3):464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Srivastava S, Chan C. Application of metabolic flux analysis to identify the mechanisms of free fatty acid toxicity to human hepatoma cell line. Biotechnol Bioeng 2008;99(2):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Huang Y, Dai Z, Barbacioru C, Sadee W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance. Cancer Res 2005;65(16):7446–54. [DOI] [PubMed] [Google Scholar]
- [24].Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Eyupoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14(6):629–32. [DOI] [PubMed] [Google Scholar]
- [25].Dave MH, Schulz N, Zecevic M, Wagner CA, Verrey F. Expression of heteromeric amino acid transporters along the murine intestine. J Physiol 2004;558(Pt 2):597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Duan LP, Wang HH, Ohashi A, Wang DQ. Role of intestinal sterol transporters Abcg5, Abcg8, and Npc1l1 in cholesterol absorption in mice: gender and age effects. Am J Physiol Gastrointest Liver Physiol 2006;290(2):G269–76. [DOI] [PubMed] [Google Scholar]
- [27].Martens K, Jaeken J, Matthijs G, Creemers JW. Multi-system disorder syndromes associated with cystinuria type I. Curr Mol Med 2008;8(6):544–50. [DOI] [PubMed] [Google Scholar]
- [28].Neuschwander-Tetri BA, Rozin T. Diurnal variability of cysteine and glutathione content in the pancreas and liver of the mouse. Comp Biochem Physiol B Biochem Mol Biol 1996;114(1):91–5. [DOI] [PubMed] [Google Scholar]
- [29].Blanco RA, Ziegler TR, Carlson BA, Cheng PY, Park Y, Cotsonis GA, Accardi CJ, Jones DP. Diurnal variation in glutathione and cysteine redox states in human plasma. Am J Clin Nutr 2007;86(4):1016–23. [DOI] [PubMed] [Google Scholar]
- [30].Boutros M, Vicanek C, Rozen R, Goodyer P. Transient neonatal cystinuria. Kidney Int 2005;67(2):443–8. [DOI] [PubMed] [Google Scholar]
- [31].Bock KW, Kohle C. The mammalian aryl hydrocarbon (Ah) receptor: from mediator of dioxin toxicity toward physiological functions in skin and liver. Biol Chem 2009;390(12):1225–35. [DOI] [PubMed] [Google Scholar]
- [32].di Masi A, De Marinis E, Ascenzi P, Marino M. Nuclear receptors CAR and PXR: Molecular, functional, and biomedical aspects. Mol Aspects Med 2009;30(5):297–343. [DOI] [PubMed] [Google Scholar]
- [33].Eder K, Ringseis R. The role of peroxisome proliferator-activated receptor alpha in transcriptional regulation of novel organic cation transporters. Eur J Pharmacol;628(1–3):1–5. [DOI] [PubMed] [Google Scholar]
- [34].Klaassen CD, Reisman SA. Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicol Appl Pharmacol;244(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002;277(47):44765–71. [DOI] [PubMed] [Google Scholar]
- [36].Takahata Y, Takarada T, Iemata M, Yamamoto T, Nakamura Y, Kodama A, Yoneda Y. Functional expression of beta2 adrenergic receptors responsible for protection against oxidative stress through promotion of glutathione synthesis after Nrf2 upregulation in undifferentiated mesenchymal C3H10T1/2 stem cells. J Cell Physiol 2009;218(2):268–75. [DOI] [PubMed] [Google Scholar]
- [37].Drechsel DA, Patel M. Differential contribution of the mitochondrial respiratory chain complexes to reactive oxygen species production by redox cycling agents implicated in parkinsonism. Toxicol Sci 2009;112(2):427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol 2010(196):369–405. [DOI] [PMC free article] [PubMed] [Google Scholar]