Abstract
Subject terms: Translational immunology, Inflammation
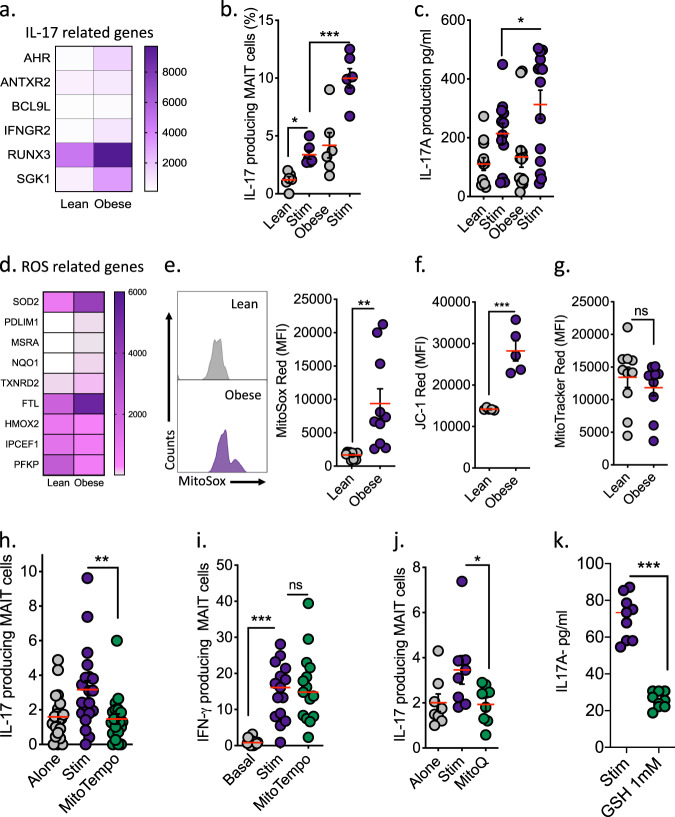
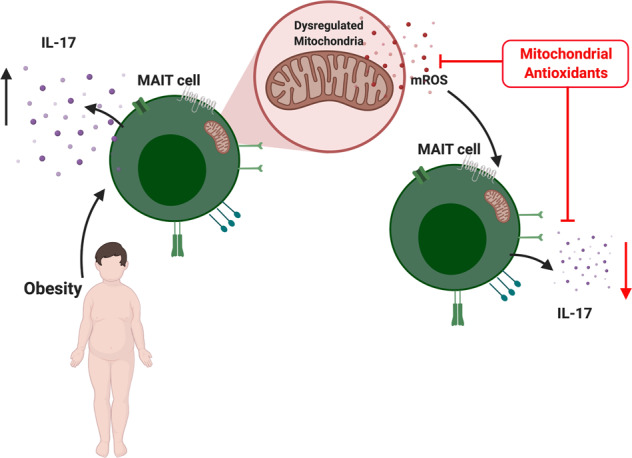
Mucosal-associated invariant T (MAIT) cells are a population of evolutionarily conserved “innate” T cells that express the invariant T-cell receptor (TCR) α-chain Vα7.2-Jα33. MAIT cells are capable of rapidly producing several cytokines, including interferon gamma (IFNy), tumour necrosis factor alpha (TNFa) and interleukin 17 (IL-17).1 MAIT cells, in particular IL-17-producing subsets, have been implicated in numerous chronic inflammatory diseases including rheumatoid arthritis, ankylosing spondylitis and psoriasis.2 We have previously described alterations in MAIT cell cytokine profiles in obesity, including reduced IFNy production and elevated IL-17 production.3 More recently, we have reported alterations in MAIT cell metabolism in patients with obesity that underpin the loss of IFNy production by MAIT cells.4 The mechanism(s) driving the increase in type-17 MAIT cells in obesity is not fully understood. A pair of recent studies have linked type-17 inflammation to dysfunctional mitochondria; Zhang et al.5 demonstrated that reactive oxygen species (ROS) from the mitochondria (mROS) could be linked to the initiation of type-17 inflammation,5 while Nicholas et al. linked changes in mitochondrial metabolism to Th17 inflammation in type 2 diabetes patients.6 The role of dysregulated mitochondria in IL-17-producing MAIT cells is currently unknown and may represent a novel therapeutic target in obesity and beyond. To test this hypothesis, we first confirmed the presence of a type-17 MAIT cell phenotype using RNA sequencing, intracellular staining for flow cytometry and enzyme-linked immunosorbent assay in a cohort of patients with severe obesity (body mass index >40). MAIT cells isolated from patients with obesity displayed an IL-17-related gene signature (Fig. 1a) and increases in both the percentage of MAIT cells producing IL-17 and the quantity of IL-17 produced (Fig. 1b, c). Using our sequencing data, we next investigated mROS-related gene expression and showed an elevated mROS signature in MAIT cells from patients with obesity (Fig. 1d). To confirm these observations, we measured mROS levels in MAIT cells from the patients with obesity using the specific mROS dye MitoSox. We observed elevated mROS levels in the MAIT cells from patients with obesity when compared with MAIT cells from healthy age and sex-matched controls (Fig. 1e). We also demonstrated an elevated mitochondrial membrane potential in obese MAIT cells using JC-1 staining but did not find a difference in mitochondrial mass (Fig. 1f, g), further supporting the concept of dysregulated mitochondria, as a high membrane potential has been linked to elevated ROS levels and dysfunction in conventional T cells.7 In chronic hepatitis B infection, targeting mitochondrial dysfunction with antioxidants restores the antiviral activity of exhausted CD8+ T cells.8 With the elevated levels of mROS noted in the MAIT cells from patients with obesity, we next utilised the mitochondria-targeted antioxidant MitoTEMPO and asked whether the mitochondrial dysfunction of obese MAIT cells could be reversed. We showed reduced IL-17 production by MitoTEMPO-treated MAIT cells from obese patients (Fig. 1h). However, we did not observe any changes in IFNy production (Fig. 1i). To verify this reduction in the IL-17-producing MAIT cell frequency following treatment with MitoTEMPO, we investigated a second mitochondrial antioxidant, mitoquinone (MitoQ),9 and similar to MitoTEMPO, MitoQ induced a reduction in the IL-17-producing MAIT cell frequency (Fig. 1j). Finally, we investigated the impact of the endogenous antioxidant glutathione (GSH), which is crucial in the maintenance of the intracellular redox balance, and demonstrated a robust reduction in IL-17 production by MAIT cells from patients with obesity (Fig. 1k). Type-17 MAIT cells are linked to the pathogenesis of many chronic conditions ranging from obesity and liver fibrosis to arthritis and even cancer,2,10 highlighting the therapeutic potential of targeting type-17 MAIT cells. Collectively, our data link dysregulated mitochondria to IL-17 production in MAIT cells in patients with obesity and highlight a potential novel therapeutic strategy using mitochondria-targeted antioxidants in chronic inflammatory conditions where type-17 MAIT cells are implicated in the pathogenesis.
Fig. 1.
Targeting mitochondrial ROS in obese MAIT cells reduces IL-17 levels. a Heatmap displaying the mean counts of IL-17-related genes in MAIT cells isolated from lean (n = 5) or obese (n = 4) adults. b Scatter plot displaying the frequencies of IL-17-producing MAIT cells (resting or stimulated with TCR beads and 50 ng/ml IL-12/IL-18) in lean and obese cohorts. c Scatter plot displaying the levels of IL-17 produced by MAIT cells (resting or stimulated with TCR beads and 50 ng/ml IL-12/IL-18) from lean and obese cohorts. d Heatmap displaying the mean counts of ROS-related genes in MAIT cells isolated from lean (n = 5) or obese (n = 4) adults. e Representative histograms and a scatter plot displaying the expression of mROS (MitoSox) in MAIT cells from lean and obese cohorts. f Scatter plot displaying the mitochondrial membrane potential (JC-1) of MAIT cells from lean and obese cohorts. g Scatter plot displaying the mitochondrial mass (MitoTracker) of MAIT cells from lean and obese cohorts. h, i Scatter plot displaying the impact of MitoTEMPO treatment on the frequencies of IL-17- and IFNy-producing MAIT cells (stimulated with TCR beads and 50 ng/ml IL-12/IL-18) in patients with obesity (n = 20). j Scatter plot displaying the impact of MitoQ treatment on the frequency of IL-17-producing MAIT cells (stimulated with TCR beads and 50 ng/ml IL-12/IL-18) in patients with obesity (n = 8). k Scatter plot displaying the impact of glutathione (GSH) treatment on IL-17 production by MAIT cells from patients with obesity (stimulated with TCR beads and 50 ng/ml IL-12/IL-18) (n = 3). Data are representative of a minimum of three independent experiments unless otherwise stated. Significant differences are indicated by *p < 0.05, **p < 0.01 and ***p < 0.001
Acknowledgements
A.E.H. is supported by the National Children’s Research Centre.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Donal O’Shea, Andrew E. Hogan
References
- 1.Godfrey DI, Koay HF, McCluskey J, Gherardin NA. The biology and functional importance of MAIT cells. Nat. Immunol. 2019;20:1110–1128. doi: 10.1038/s41590-019-0444-8. [DOI] [PubMed] [Google Scholar]
- 2.Toubal A, Nel I, Lotersztajn S, Lehuen A. Mucosal-associated invariant T cells and disease. Nat. Rev. Immunol. 2019;19:643–657. doi: 10.1038/s41577-019-0191-y. [DOI] [PubMed] [Google Scholar]
- 3.Carolan E, et al. Altered distribution and increased IL-17 production by mucosal-associated invariant T cells in adult and childhood obesity. J. Immunol. 2015;194:5775–5780. doi: 10.4049/jimmunol.1402945. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien A, et al. Obesity Reduces mTORC1 activity in mucosal-associated invariant T Cells, driving defective metabolic and functional responses. J. Immunol. 2019;202:3404–3411. doi: 10.4049/jimmunol.1801600. [DOI] [PubMed] [Google Scholar]
- 5.Zhang D, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51:671–681.e5. doi: 10.1016/j.immuni.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholas DA, et al. Fatty acid metabolites combine with reduced β oxidation to activate Th17 inflammation in human type 2 diabetes. Cell Metab. 2019;30:447–461.e5. doi: 10.1016/j.cmet.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sukumar M, et al. Mitochondrial membrane potential identifies cells with enhanced stemness for cellular therapy. Cell Metab. 2016;23:63–76. doi: 10.1016/j.cmet.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisicaro P, et al. Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat. Med. 2017;23:327–336. doi: 10.1038/nm.4275. [DOI] [PubMed] [Google Scholar]
- 9.Smith RA, et al. Mitochondria-targeted antioxidants in the treatment of disease. Ann. N. Y. Acad. Sci. 2008;1147:105–111. doi: 10.1196/annals.1427.003. [DOI] [PubMed] [Google Scholar]
- 10.Hegde P, et al. Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nat. Commun. 2018;9:2146. doi: 10.1038/s41467-018-04450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]