Abstract
Purpose
To demonstrate the marginal corneal vascular remodelling using optical coherence tomography angiography (OCTA) after pterygium surgery.
Methods
Twenty-two eyes of 19 patients (8 males, 11 females; age, 58.68 ± 0.34 years) with primary grade-T3 nasal pterygium were enroled in this study. The eyes underwent excision of the pterygium followed by a free limbal-conjunctival autograft. OCTA was performed in the nasal limbal area before surgery and at 10 days, 1 month, and 3 months after surgery. The scans were analyzed in terms of postoperative vascular remodelling of the autograft and marginal corneal vascular arcades (MCAs).
Results
Preoperatively, the pterygium presented as abnormal centripetal vascular growth in OCTA scans. The conjunctival vessel density in the nasal quadrant was 29.26% ± 1.00%, 15.80% ± 0.83%, 19.80% ± 0.88%, and 20.26% ± 0.89% before and 10 days, 1 month, and 3 months, respectively, after surgery (F = 1.55, P < 0.01). The vessel density of MCAs was 28.33% ± 0.88%, 42.09% ± 0.41%, and 42.46% ± 0.31% 10 days, 1 month, and 3 months, respectively, after surgery (F = 188.2, P < 0.01).
Conclusions
We describe a new application of OCTA for MCA vasculature imaging. Vascular remodelling of the graft and MCAs appeared at 1 month and continued for 3 months after surgery.
Subject terms: Conjunctival diseases, Corneal diseases
Introduction
Optical coherence tomography (OCT) has shown rapid development and gained widespread usage for in vivo imaging of the retina since its introduction in 1991 [1]. Based on sequential OCT cross-sectional B-scan, the OCT angiography (OCTA) was developed and demonstrated on the human eye for the first time in 2006 [2]. More recently, in 2014, the first commercial OCTA was introduced to delineate blood vessels by comparing phase speckle contrast, changes in intensity, or a variation of the full OCT signal between consecutive B-scans [3, 4]. Currently, OCTA plays an important role in the imaging of retinal diseases, including diabetic retinopathy, retinal vein/artery occlusion, age-related macular degeneration, choroidal neovascularization, etc [5]. Although current commercial OCTA systems are not specifically designed for the anterior segment, several studies have been conducted and have recognised its future potential [6]: malignant iris melanomas and benign iris lesions [7], corneal neovascularization [8], pharmaceutical effect on perilimbal vasculature [9], graft reperfusion after conjunctival autograft transplantation [10] have been successfully observed using OCTA. We here applied OCTA in visualisation of marginal corneal vascular remodelling following pterygium surgery with limbal-conjunctival autograft.
Pterygium is a wing-shaped fibrovascular growth of bulbar conjunctiva that extends toward the cornea. The prevalence of pterygium is as high as 9.84% in China [11], and the condition causes signs of ocular irritation, visual disturbances, etc. This common chronic inflammatory ocular surface disease is characterised by proliferation, angiogenesis, and extracellular matrix remodelling in the lesion [12]. The pathogenesis of pterygium is still controversial. It is thought to originate from limbal stem cells (LSCs) [13] that have been altered by chronic ultraviolet light exposure [14, 15]. Recent studies have also demonstrated that overexpression of angiogenic factors such as von-Willebrand factor and VEGF [16, 17] and decreased expression of angiogenesis inhibitors such as thrombospondin-1 [17] were involved in the pathogenic mechanism of pterygium.
Surgical techniques are the preferred option to treat pterygium, but bare scleral excision of the pterygium [18] results in recurrence rates as high as 88% after surgical excision in certain populations [19]. Pterygium excision followed by limbal-conjunctival autograft has been confirmed to reduce pterygium recurrence for both primary and recurrent cases [20, 21]. However, failure of vascular reperfusion of the autograft can lead to recurrent pterygium [22]. The marginal corneal vascular arcades (MCAs) were defined as complex vascular loops and projections in the corneal limbus internal to the Vogt palisades extending into the corneal margin [23–25]. Kim et al. recently reported deteriorated MCAs in the affected limbus in pterygium and reconstruction of MCAs after surgery [22] by using indocyanine green angiography (ICGA). Nevertheless, little is known about the angiographic features or roles of MCAs during the vascular remodelling after pterygium surgery.
Since OCTA can serve as a novel non-invasive angiographic technology, this study was designed with objectives to explore the angiographic features of pterygium and vascular reperfusion of autograft and particularly, to better understand the hemangiogenesis in terms of MCAs remodelling on 3-month follow-up after pterygium surgery.
Methods
This prospective observational clinical study included 22 eyes of 19 patients (8 males, 11 females; age, 58.68 ± 0.34 years) with nasal primary pterygium at the Department of Ophthalmology in Shanghai Ninth People’s Hospital (Shanghai, China) from January 2018 to December 2018. Patients with a history of ocular surgery, chronic topical medication over 3 months, ocular trauma, contact lens wear, conjunctivitis, corneal scarring, glaucoma, systemic diseases as thyroidopathy, or medication as immunosuppressants that would change vascular appearance were excluded from the study.
Pterygium was graded according to the following system described by Tan [26]: Grade T1, in which episcleral vessels were unobscured by the body of pterygium; grade-T2, which contained partially obscured episcleral vessels; and grade-T3, in which episcleral vessels were obscured by the body of pterygium.
This study followed the tenets of the Declaration of Helsinki and was in accordance with the Health Insurance Portability and Accountability Act of 1996. The study was approved by the Investigational Review Board of Shanghai Ninth People’s Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China (Approval number: 2018-21-T21). All subjects enrolled were informed about the aim of this study, and informed consent was obtained from 19 grade-T3 patients, 1 grade-T2 patient, 1 grade T1 patient, and another healthy volunteer.
Surgical procedures
The surgeries were performed by the same team by using the same technique: excision of the pterygium followed by a free limbal-conjunctival autograft, taken from a superior position. After surgery, all patients received an identical regimen of topical levofloxacin eye drops (Santen Pharmaceutical, Japan), 0.1% fluorometholone eye drops (Santen Pharmaceutical, Japan), and Solcoseryl (Shengyang Sinqi Pharmaceutical, China), which were tapered off over 1 month. Nylon sutures were removed at 10 days after surgery. Clinical measurements (described below) were obtained before surgery and 10 days, 1 month, and 3 months after surgery.
Slit-lamp biomicroscopy
Colour images of each eye with pterygium were acquired using a slit-lamp–mounted digital camera system (Topcon SL-D Digital Slit-Lamp; Topcon, Tokyo, Japan). Images at ×16 and ×25 magnifications were taken using a 45-degree angled beam of white light projected through a diffusion filter.
Imaging technique
All scans were performed by the same physician (Z. Z.) using a swept-source DRI OCTA unit (Triton, Topcon, Tokyo, Japan), which uses a wavelength-sweeping laser with a centre wavelength of 1050 nm up to a depth of 2.6 mm and a tuning range of ~100 nm. Patients were asked to open their eyes as wide as possible and fixate on the temporal 45-degree angled green indicator light for about 10 s. The “OCT Macular Angiography” function was chosen to obtain 4.5 × 4.5 mm (336 × 336 pixels) scans, where 100,000 A-scans are acquired per second with optical axial resolution of 8 μm and lateral resolution of 20 μm. The appropriate focus was achieved by using forehead shield and adjusting the dioptre compensation lens selector to (+) without any anterior segment lens.
Image analysis
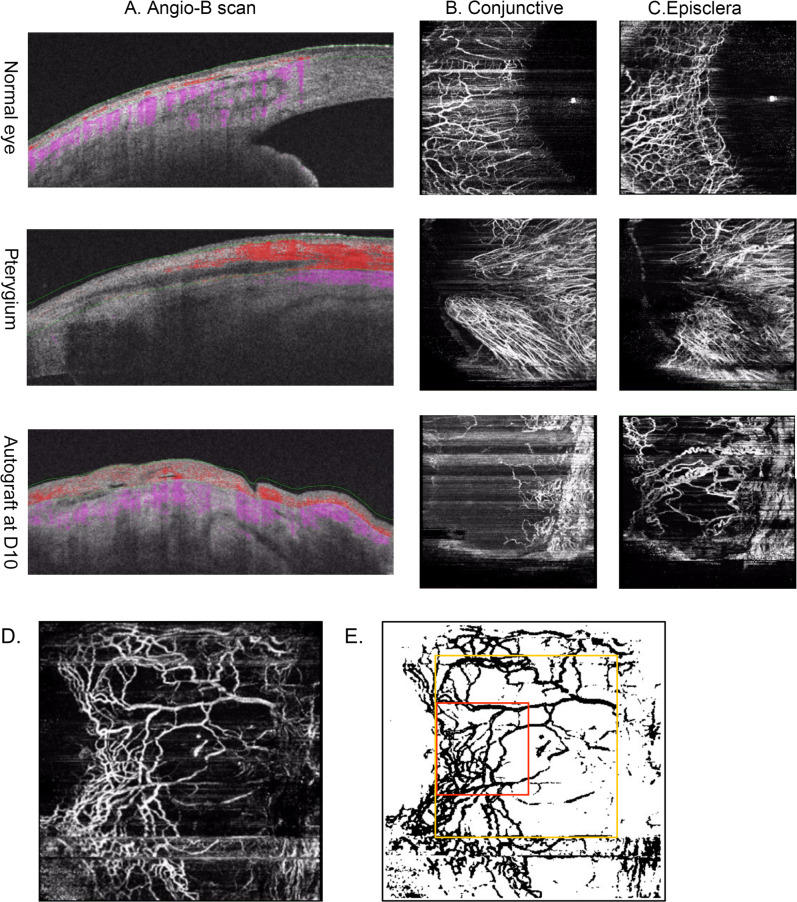
As the conjunctival layer and episcleral layer are laminar with corresponding stratification of blood supply [27], delineation of OCTA scans into specific layers allows en face visualisation of their respective vasculature [28]. All OCTA scans were processed automatically to reduce motion artefacts such as transverse saccadic and residual axial motion by the internal software (IMAGEnet6, version 1.22; Topcon, Tokyo, Japan). Next, the conjunctival and episcleral layers of OCTA scans were delineated manually based on B-scans (the depth of conjunctive layer is based on the depth of pterygium infiltration before surgery or distinct margin of autograft after surgery) and optimised based on en face vascular images. As shown in Fig. 1, the thickness between conjunctival epithelium and scleral boundary was much thicker than healthy conjunctiva due to pterygium overgrowth and its scleral boundary between conjunctiva and episclera was more distinct. The autograft could be identified on the operative margin and showed more irregularity after surgery. The thickness of conjunctival layer of each scan was noted as the distance from conjunctival epithelium to scleral boundary. For the purpose of this study, only 0–150 μm under scleral boundary on OCTA scans was analysed as episcleral layer in our study.
Fig. 1. Demonstration of OCTA scans on anterior segment and image processing.
Demonstration of Angio-B scan (a), conjunctival (b), and episcleral (c) vasculature in the nasal quadrant of normal conjunctiva, pterygium and autograft area and demonstration of processing scans and defining regions of interest. An episcleral scan was adjusted to the best contrast (d) and blood vessels were binarized (e). A 224 × 224 pixels square at 3 o’clock perpendicular to the corneal limbus was analysed for episcleral vessel density (yellow frame) and a 112 × 112 pixels square at 3 o’clock perpendicular to the corneal limbus was analysed for MCA density (red frame).
Representative conjunctival and episcleral images at each time point were selected for analysis, considering criteria such as best focus, good contrast, and fewest motion artefacts with TopQ image quality above 70. We firstly enhanced the OCTA image by histogram equalisation and median filtering. Then a binary image was obtained from adaptive threshold binarization and morphological process to measure vessel density with MATLAB R2010a software (The MathWorks, Inc, Natick, MA). Vessel density was presented as the ratio of the area occupied by the vessels divided by the total area using the previously described method [8] in regions of interest: a 224 × 224 pixels square at 3 o’clock perpendicular to the corneal limbus for conjunctival and episcleral vessel density (Fig. 1e yellow frame), and a 112 × 112 pixels square at 3 o’clock perpendicular to the corneal limbus in scans of episcleral layer for MCA density (Fig. 1e red frame).
OCTA scans of T1, T2, and T3 pterygium were demonstrated in Fig. 2. OCTA Angio B-scans showed good vascular signals under conjunctiva in corneal limbus in T1 pterygium while very poor vascular signals under conjunctiva in the corneal limbus in T3 pterygium (Fig. 2b). Only scans from grade-T3 patients were analysed for the aim of this study.
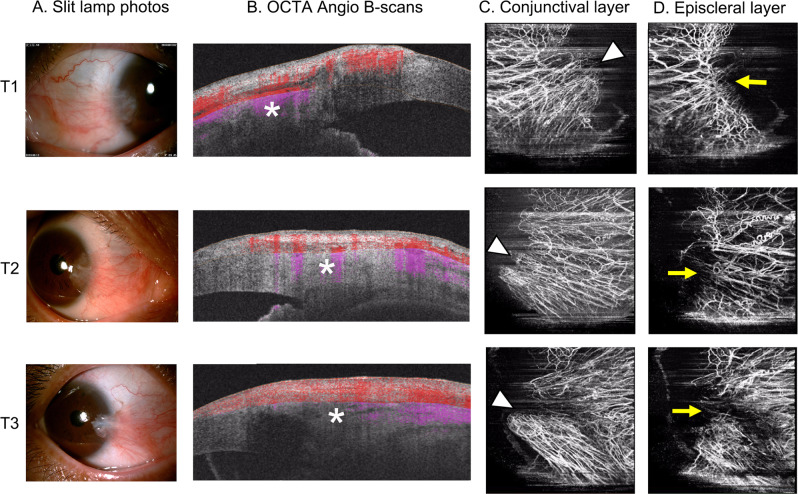
Fig. 2. Demonstration of slit-lamp images and OCTA scans in patients with pterygium.
Demonstration of pterygium in a slit-lamp image (x16, a) and the respective OCTA B-scans (b) in the conjunctival layer (c) and episcleral layer (d). Note the growing limbal episcleral avascularity (stars), pterygium head (arrow heads) and deteriorated or indistinctive vascular loops (arrows) in T1, T2, and T3 pterygium.
Statistical analysis
A blinded investigator analysed all scan images and compared the average of the OCTA scans pre- and post-surgery at the 3-month follow-up. A P value < 0.05 (one-way repeated-measures ANOVA test, GraphPad Prism version 6.00 for mac) was considered statistically significant for comparisons among data of vessel density in regions of interest before surgery and 10 days, 1 month, and 3 months after surgery. Data were expressed as means ± standard error of mean (SEM).
Results
Twenty-two grade-T3 pterygium patients were examined by the slit lamp and OCTA imagining technique.
Demonstration of pterygium on OCTA scans
Pterygium presented as abnormal centripetal vascular growth on OCTA scans (Fig. 2). The head of the pterygium trespassed the corneal limbus corresponding to slit lamp photos (Fig. 2a, c). The characteristic vascular loops of MCAs could not be distinguished on episcleral layer in preoperative grade-T3 pterygium (Fig. 2d), implying deteriorated MCAs around the affected corneal limbus. As we focused on the postoperative vascular remodelling of MCAs, statistical analysis was performed with the results from grade-T3 patients in this study. The conjunctival thickness analysed in this study was, respectively, 369.9 ± 51.26 μm, 345.5 ± 56.96 μm, 256 ± 54.22 μm, and 199.7 ± 43.21 μm before, 10 days, 1 month, and 3 months after surgery (F = 78.65, P < 0.01), which corresponded to preoperative conjunctival overgrowth of pterygium, early postoperative autograft oedema, and remodelling of autograft. The preoperative conjunctival vessel density of the nasal quadrant was 29.26% ± 1.00% because of the abnormal fibrovascular growth of pterygium.
Vessel density analysis of limbal conjunctiva and episclera
The quantification of OCTA scans demonstrated the reperfusion of MCAs and autograft during the 3-month follow-up after surgery.
At 10 days after surgery, tortuous capillaries from the conjunctival vessels appeared on the borders of the autograft and engorged vessels began to arise from the adjacent episcleral vessels (Fig. 3, 4a). The vascularity in the conjunctiva became finer and more complex at 1 month postoperatively, covering a greater part of the autograft (Fig. 4b). At 3 months after surgery, the conjunctival vascularity appeared to be completely reconstructed and homogeneous in the autograft (Fig. 4c).
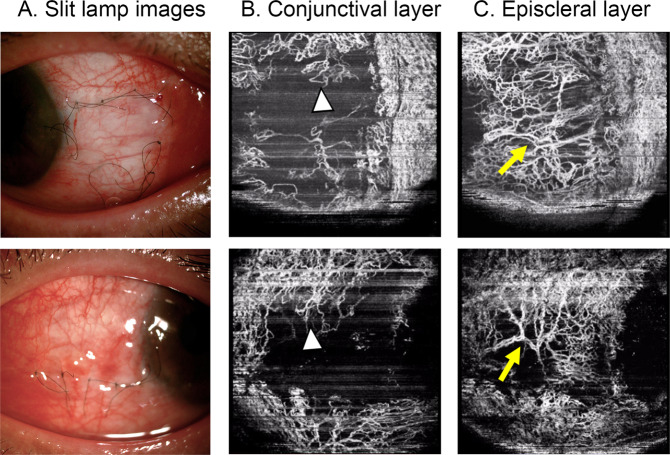
Fig. 3. Demonstration of OCTA scans of operative area at 10 days after surgery.
Demonstration of the operative area in a slit-lamp image (×25, a) and the respective OCTA scans in the conjunctival layer (b) and episcleral layer (c) at 10 days after surgery. Note the tortuous capillaries on the borders of the autograft in the conjunctival layer (arrow heads) and the engorged reperfusion vessels in the episcleral layer (arrows).
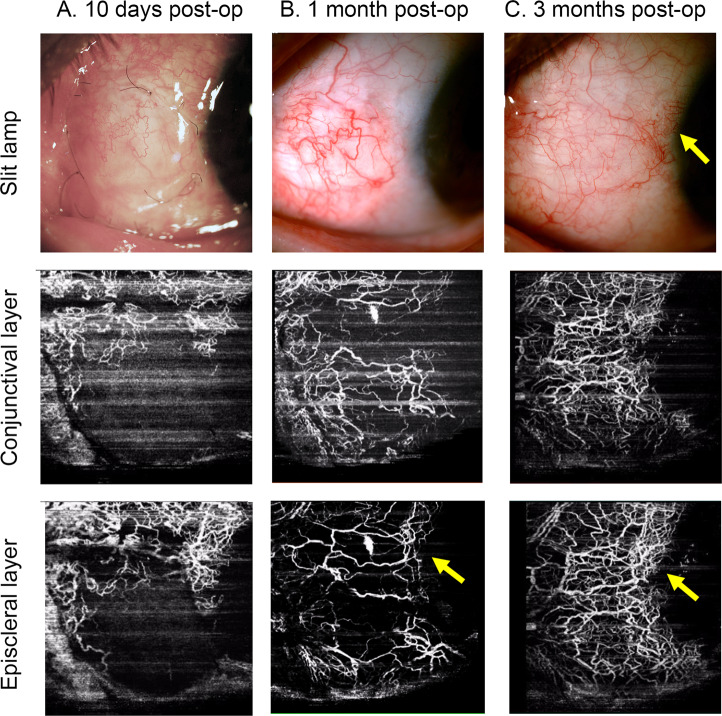
Fig. 4. Demonstration of the OCTA scans of the autograft after surgery.
Demonstration of vascular remodelling of the autograft in the episclera at 10 days (a), 1 month (b), and 3 months (c) after surgery from the same patients. Note the fine tortuous capillaries (arrow heads) at 10 days after surgery and reconstruction of the MCA (arrows) 1 and 3 months after surgery.
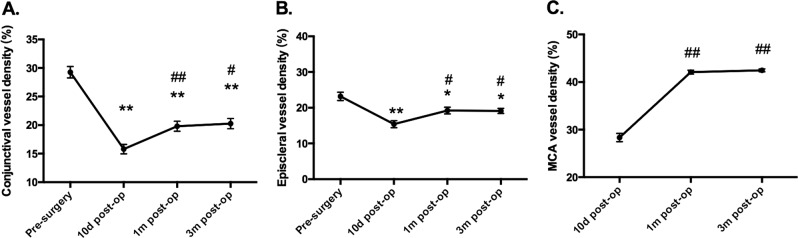
Quantitative analysis of conjunctival vessel density showed a significant drop in the operated area at 10 days after surgery compared with the preoperative data (15.80% ± 0.83%, P < 0.01), since the surgery removed the abnormal fibrovascular growth of pterygium. Vascular density in the conjunctival layer of the graft increased during the time course after surgery (F = 1.55, P < 0.01; Fig. 5a). The conjunctival vessel density increased with the time course and reached 19.80% ± 0.88%, and 20.26% ± 0.89% 1 and 3 months after surgery, but remained much lower than the preoperative value (P < 0.01).
Fig. 5. Quantification of vessel density on the nasal limbal area.
Vessel density in the conjunctival layer (a) and episcleral layer (b) and vessel density of the MCA (c) on the nasal limbal area. Data are presented as mean ± SEM. *Compared with presurgery data, P < 0.05; **compared with presurgery data, P < 0.01; #compared with 10-day post-op, P < 0.05; ##compared with 10-day post-op, P < 0.01.
Meanwhile, the episcleral vessel density in the nasal quadrant was 23.18% ± 1.16%, 15.40% ± 0.99%, 19.22% ± 0.92%, and 19.11% ± 0.70% before, 10 days, 1 month, and 3 months after surgery (F = 13.26, P < 0.01; Fig. 5b). These findings are in agreement with those of a previous study using angiography with fluorescein or indocyanine green (FA and ICGA, respectively) [22], indicating that the vessel remodelling continued for 3 months after surgery.
Reconstruction of limbal vasculature in the operative area
Vascular loops were rarely observed in the postoperative area at 10 days after surgery. The reconstruction of the limbus vasculature began between 10 days and 1 month after surgery. At 3 months, the distinct limbal vascular loops were detected in all eyes, suggesting vascular remodelling of MCAs in the affected limbus and the subsequent reconstruction.
Preoperative MCAs not quantified as distinctive vascular loops in the affected cornea limbus could not be detected due to pterygium. Quantification of MCA vessel density in the operative area showed an increase with the time course (F = 188.2, P < 0.01; Fig. 5c). The MCA vessel density was 28.33% ± 0.88% at 10 days. It gradually raised to 42.09% ± 0.41% (P < 0.01) at 1 month and 42.46% ± 0.31% (P < 0.01) at 3 months after surgery compared with 10 days postoperative data. No significant difference in MCA vessel density was found between the data at 1 and at 3 months.
Discussion
OCTA provides three-dimensional images, making it specific for assessment of the depth of a lesion or abnormal vasculature with an optical axial resolution of 8 μm and lateral resolution of 20 μm. Before the advent of OCTA, FA, and ICGA could also be performed for imaging of the anterior segment after intravenous administration of dye. However, because of the risk of adverse reactions to the dyes and the long time needed for the examination, these techniques are rarely used. Furthermore, OCTA has a great potential use for monitoring changes in corneal vascular areas. Previous studies have demonstrated that OCTA had a good correlation with ICGA angiography in a rabbit model when detecting corneal vascularisation [29, 30]. Similarly, Cai et al. observed pronounced regression of corneal neovascularization in patients with herpetic keratitis after subconjunctival bevacizumab injection using OCTA [31]. Moreover, the Topcon swept-source OCTA makes the distinction of the conjunctival and episcleral layer possible, based on a long wavelength of 1050 nm which penetrates light-retaining tissues such as the sclera and limbus. We thus applied the commercial swept-source OCTA to explore the limbal vasculature before and after pterygium surgery.
Slit-lamp photos and OCTA conjunctive scans in our study showed that the T3 pterygium head trespassed the cornea limbus, and OCTA episcleral scans confirmed the deteriorated vascular loops in the affected cornea limbus beyond the head of the pterygium. These findings support the idea that the pterygium originates from altered LSCs [13] and the blood supply of the head of the pterygium originates from the episcleral vessels, in agreement with previous studies [17, 22]. The literature has demonstrated that altered LSCs disrupt the Bowman’s layer and migrate to the cornea [32, 33]. The migrated LSCs produce various cytokines and growth factors, including VEGF [17], resulting in neovascularization and remodelling of the MCAs in the affected limbus.
In the era of limbal-conjunctival autografts, successful treatment of pterygium depends on the complete removal of altered LSCs and fibrovascular tissue and successful vascular reperfusion of the autograft [20, 21]. A recent study has applied OCTA using a wavelength of 840 nm on evaluating prognosis of conjunctival graft following pterygium excision [10]. They found reperfusion of the graft began at 1 month but the surgery had no impact on the episcleral bed at harvested region. Meanwhile, we have demonstrated the presence of engorged vessels in the adjacent episclera at 10 days after surgery during the vascular remodelling of the graft, indicating that the tight connection between the graft and episcleral tissue plays a role in early reperfusion of the graft. The difference between our results and theirs may be due to the long wavelength of OCTA we used to better penetrate conjunctival and episcleral structures. We also observed that the reconstruction of limbal vascular loops began before 1 month after surgery and continued for 3 months after surgery. The increased vessel density of MCAs observed during the time course after surgery supports the hypothesis that healthy MCAs play a role in better protecting the ocular surface. MCAs were formed by the recurrent conjunctival and palisadal vessels [23, 34], comprising a network of branched interlinked elliptical loops supporting circumferential blood flow in the corneal limbus [24]. A previous in vivo study has demonstrated that the loss of the limbal microvascular net blood supply led to a breakdown of limbal function, and the limbal function was restored by blood reperfusion of the limbal autograft [35]. As the vascular microenvironment is essential for homoeostasis of the corneal limbus, successful reconstruction of MCAs accounts for healthy limbal function and prevents pterygium recurrence. Excessive cauterisation of the episcleral bed should be avoided as it would delay reperfusion of the graft and therefore result in graft failure [36]. In addition, intraoperative mitomycin C should be taken great caution as increased concentration and duration of exposure to mitomycin C were reported of conjunctival avascularity and delayed wound healing [20].
To our knowledge, this is a pilot clinical study to use OCTA to evaluate the affected limbal vascularisation in eyes with pterygium, suggesting that OCTA may serve as a promising non-invasive imaging alternative to FA or ICGA. Second, our work successfully demonstrated the angiographic features of pterygium and the vascular remodelling of the autograft and MCAs after pterygium excision. The reconstruction of MCAs continued for up to 3 months, consistent with previous findings. A delayed reconstruction of MCAs might result in failure of the graft and then recurrence of pterygium. As shown in our results, pterygium has a characteristic centripetal pattern of vascular overgrowth which trespasses corneal limbus, while MCAs are a vessel network consisted of complex anastomoses in the corneal limbus. We believe that OCTA could therefore be used for the postoperative follow-up of pterygium and early detection of recurrent pterygium. However, anterior OCTA scans also had some limitations: OCTA cannot demonstrate vessel leakage and the sequence of flow, has a limited field of view, and is more prone to artefacts than FA or ICGA [37]. Future improvements in motion correction and image processing are needed for further application of OCTA in the ocular anterior segment. In conclusion, our study implies the clinical usefulness of OCTA in in vivo investigation of MCA alterations or limbal neovascularization in various anterior segment diseases.
Summary
What was known before
OCTA is used normally for retinal diseases; marginal corneal vascular arcades play a role in sustaining corneal limbal microenvironment.
What this study adds
OCTA was used here for observing pterygium and marginal corneal vascular arcades; marginal corneal vascular arcades recovered at 3 months after pterygium surgery, in concordance with clinical recovery.
Acknowledgments
Funding
This work was supported by the Science and Technology Commission of Shanghai (17411963800, 19JC1411703), Joint Research Project of the Emerging Cutting-Edge Technology of Shanghai Shen-Kang Hospital Development Center (SHDC12018110), and Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20161421).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Zhanlin Zhao, Yu Yue, Siyi Zhang
Contributor Information
Xianqun Fan, Email: fanxq@sjtu.edu.cn.
Fu Yao, Email: drfuyaofy@sina.com.
References
- 1.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makita S, Hong Y, Yamanari M, Yatagai T, Yasuno Y. Optical coherence angiography. Opt Express. 2006;14:7821–40. doi: 10.1364/OE.14.007821. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz DM, Fingler J, Kim DY, Zawadzki RJ, Morse LS, Park SS, et al. Phase-variance optical coherence tomography: a technique for noninvasive angiography. Ophthalmology. 2014;121:180–7. doi: 10.1016/j.ophtha.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CL, Wang RK. Optical coherence tomography based angiography [Invited] Biomed Opt Express. 2017;8:1056–82. doi: 10.1364/BOE.8.001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55. doi: 10.1016/j.preteyeres.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang M, Baskaran M, Werkmeister RM, Chua J, Schmidl D, Aranha Dos Santos V, et al. Anterior segment optical coherence tomography. Prog Retin Eye Res. 2018;66:132–56. doi: 10.1016/j.preteyeres.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Skalet AH, Li Y, Lu CD, Jia Y, Lee B, Husvogt L, et al. Optical coherence tomography angiography characteristics of iris melanocytic tumors. Ophthalmology. 2017;124:197–204. doi: 10.1016/j.ophtha.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ang M, Cai Y, Tan ACS. Swept source optical coherence tomography angiography for contact lens-related corneal vascularization. J Ophthalmol. 2016;2016:3. doi: 10.1155/2016/9685297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel CN, Antony AK, Kommula H, Shah S, Singh V, Basu S. Optical coherence tomography angiography of perilimbal vasculature: validation of a standardised imaging algorithm. Br J Ophthalmol. 2019. 10.1136/bjophthalmol-2019-314030. [DOI] [PubMed]
- 10.Liu YC, Devarajan K, Tan TE, Ang M, Mehta JS. Optical coherence tomography angiography for evaluation of reperfusion after pterygium surgery. Am J Ophthalmol. 2019;207:151–8. doi: 10.1016/j.ajo.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Song P, Chang X, Wang M, An L. Variations of pterygium prevalence by age, gender and geographic characteristics in China: A systematic review and meta-analysis. PLoS ONE. 2017;12:e0174587. doi: 10.1371/journal.pone.0174587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chui J, Di Girolamo N, Wakefield D, Coroneo MT. The pathogenesis of pterygium: current concepts and their therapeutic implications. Ocul Surf. 2008;6:24–43. doi: 10.1016/S1542-0124(12)70103-9. [DOI] [PubMed] [Google Scholar]
- 13.Chui J, Coroneo MT, Tat LT, Crouch R, Wakefield D, Di Girolamo N. Ophthalmic pterygium: a stem cell disorder with premalignant features. Am J Pathol. 2011;178:817–27.. doi: 10.1016/j.ajpath.2010.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moran DJ, Hollows FC. Pterygium and ultraviolet radiation: a positive correlation. Br J Ophthalmol. 1984;68:343–6. doi: 10.1136/bjo.68.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coroneo MT. Pterygium as an early indicator of ultraviolet insolation: a hypothesis. Br J Ophthalmol. 1993;77:734–9. doi: 10.1136/bjo.77.11.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kria L, Ohira A, Amemiya T. Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-beta and tumor necrosis factor-alpha in the pterygium. Acta Histochem. 1996;98:195–201. doi: 10.1016/S0065-1281(96)80038-9. [DOI] [PubMed] [Google Scholar]
- 17.Aspiotis M, Tsanou E, Gorezis S, Ioachim E, Skyrlas A, Stefaniotou M, et al. Angiogenesis in pterygium: study of microvessel density, vascular endothelial growth factor, and thrombospondin-1. Eye. 2007;21:1095–101. doi: 10.1038/sj.eye.6702495. [DOI] [PubMed] [Google Scholar]
- 18.D’ombrain A. The surgical treatment of pterygium. Br J Ophthalmol. 1948;32:65–71. doi: 10.1136/bjo.32.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen PP, Ariyasu RG, Kaza V, Labree LD, Mcdonnell PJ. A randomized trial comparing mitomycin C and conjunctival autograft after excision of primary pterygium. Am J Ophthalmol. 1995;120:151–60. doi: 10.1016/S0002-9394(14)72602-9. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman SC, Jacobs DS, Lee WB, Deng SX, Rosenblatt MI, Shtein RM. Options and adjuvants in surgery for pterygium: a report by the American Academy of Ophthalmology. Ophthalmology. 2013;120:201–8. doi: 10.1016/j.ophtha.2012.06.066. [DOI] [PubMed] [Google Scholar]
- 21.Mutlu FM, Sobaci G, Tatar T, Yildirim E. A comparative study of recurrent pterygium surgery: limbal conjunctival autograft transplantation versus mitomycin C with conjunctival flap. Ophthalmology. 1999;106:817–21. doi: 10.1016/S0161-6420(99)90172-0. [DOI] [PubMed] [Google Scholar]
- 22.Kim YJ, Yoo SH, Chung JK. Reconstruction of the limbal vasculature after limbal-conjunctival autograft transplantation in pterygium surgery: an angiography study. Investig Ophthalmol Vis Sci. 2014;55:7925–33.. doi: 10.1167/iovs.14-15288. [DOI] [PubMed] [Google Scholar]
- 23.Graves B. Certain clinical features of the normal limbus. Br J Ophthalmol. 1934;18:305–41. doi: 10.1136/bjo.18.6.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y, Kaye AE, Boker A, Stewart RK, Tey A, Ahmad S, et al. Marginal corneal vascular arcades. Investig Ophthalmol Vis Sci. 2013;54:7470–7. doi: 10.1167/iovs.13-12614. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg MF, Bron AJ. Limbal palisades of Vogt. Trans Am Ophthalmol Soc. 1982;80:155–71. [PMC free article] [PubMed] [Google Scholar]
- 26.Tan DT, Chee SP, Dear KB, Lim AS. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol. 1997;115:1235–40. doi: 10.1001/archopht.1997.01100160405001. [DOI] [PubMed] [Google Scholar]
- 27.Weinhaus RS, Burke JM, Delori FC, Snodderly DM. Comparison of fluorescein angiography with microvascular anatomy of macaque retinas. Exp Eye Res. 1995;61:1–16. doi: 10.1016/S0014-4835(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 28.Spaide RF, Klancnik JM, Jr., Cooney MJ. Retinal vascular layers in macular telangiectasia type 2 imaged by optical coherence tomographic angiography. JAMA Ophthalmol. 2015;133:66–73. doi: 10.1001/jamaophthalmol.2014.3950. [DOI] [PubMed] [Google Scholar]
- 29.Devarajan K, Di Lee W, Ong HS, Lwin NC, Chua J, Schmetterer L, et al. Vessel density and En-face segmentation of optical coherence tomography angiography to analyse corneal vascularisation in an animal model. Eye Vis. 2019;6:2. doi: 10.1186/s40662-018-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanzel TP, Devarajan K, Lwin NC, Yam GH, Schmetterer L, Mehta JS, et al. Comparison of optical coherence tomography angiography to indocyanine green angiography and slit lamp photography for corneal vascularization in an animal model. Sci Rep. 2018;8:11493. doi: 10.1038/s41598-018-29752-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai Y, Alio Del Barrio JL, Wilkins MR, Ang M. Serial optical coherence tomography angiography for corneal vascularization. Graefe’s Arch Clin Exp Ophthalmol. 2017;255:135–9. doi: 10.1007/s00417-016-3505-9. [DOI] [PubMed] [Google Scholar]
- 32.Dushku N, John MK, Schultz GS, Reid TW. Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol. 2001;119:695–706. doi: 10.1001/archopht.119.5.695. [DOI] [PubMed] [Google Scholar]
- 33.Notara M, Lentzsch A, Coroneo M, Cursiefen C. The role of limbal epithelial stem cells in regulating corneal (lymph)angiogenic privilege and the micromilieu of the limbal niche following UV exposure. Stem Cells Int. 2018;2018:8620172. doi: 10.1155/2018/8620172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talusan ED, Schwartz B. Fluorescein angiography. Demonstration of flow pattern of anterior ciliary arteries. Arch Ophthalmol. 1981;99:1074–80. doi: 10.1001/archopht.1981.03930011074018. [DOI] [PubMed] [Google Scholar]
- 35.Huang M, Wang B, Wan P, Liang X, Wang X, Liu Y, et al. Roles of limbal microvascular net and limbal stroma in regulating maintenance of limbal epithelial stem cells. Cell Tissue Res. 2015;359:547–63. doi: 10.1007/s00441-014-2032-4. [DOI] [PubMed] [Google Scholar]
- 36.Chan CM, Chew PT, Alsagoff Z, Wong JS, Tan DT. Vascular patterns in pterygium and conjunctival autografting: a pilot study using indocyanine green anterior segment angiography. Br J Ophthalmol. 2001;85:350–3. doi: 10.1136/bjo.85.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ang M, Cai Y, Macphee B, Sim DA, Keane PA, Sng CC, et al. Optical coherence tomography angiography and indocyanine green angiography for corneal vascularisation. Br J Ophthalmol. 2016;100:1557–63. doi: 10.1136/bjophthalmol-2015-307706. [DOI] [PubMed] [Google Scholar]