Abstract
Background
Energy balance-related biomarkers are associated with risk and prognosis of various malignancies. Their relationship to survival in metastatic colorectal cancer (mCRC) requires further study.
Methods
Baseline plasma insulin-like growth factor (IGF)-1, IGF-binding protein (IGFBP)-3, IGFBP-7, C-peptide, and adiponectin were measured at time of trial registration in a prospective cohort of patients with mCRC participating in a National Cancer Institute–sponsored trial of first-line systemic therapy. We used Cox proportional hazards regression to adjust for confounders and examine associations of each biomarker with overall survival (OS) and progression-free survival (PFS). P values are 2-sided.
Results
Median follow-up for 1086 patients was 6.2 years. Compared with patients in the lowest IGFBP-3 quintile, patients in the highest IGFBP-3 quintile experienced an adjusted hazard ratio (HR) for OS of 0.57 (95% confidence interval [CI] = 0.42 to 0.78; Pnonlinearity < .001) and for PFS of 0.61 (95% CI = 0.45 to 0.82; Ptrend = .003). Compared with patients in the lowest IGFBP-7 quintile, patients in the highest IGFBP-7 quintile experienced an adjusted hazard ratio for OS of 1.60 (95% CI = 1.30 to 1.97; Ptrend < .001) and for PFS of 1.38 (95% CI = 1.13 to 1.69; Ptrend < .001). Plasma C-peptide and IGF-1 were not associated with patient outcomes. Adiponectin was not associated with OS; there was a nonlinear U-shaped association between adiponectin and PFS (Pnonlinearity = .03).
Conclusions
Among patients with mCRC, high plasma IGFBP-3 and low IGFBP-7 were associated with longer OS and PFS. Extreme levels of adiponectin were associated with shorter PFS. These findings suggest potential avenues for prognostic and therapeutic innovation.
A growing body of literature demonstrates an association between excess energy balance—denoted by sedentary lifestyle, diabetes, and poor diet—with inferior outcomes in colorectal cancer (CRC), the second leading cause of cancer-related death in the United States (1-4). Although the mechanisms underlying this association are uncertain, clinical and preclinical studies suggest insulin and insulin-like growth factor (IGF) signaling may play a role (2,5). Insulin and IGF-1, which are increased by carbohydrate intake and decreased by fasting, can promote neoplastic growth (5-7). Insulin- and IGF-related biomarkers are associated with risk of various malignancies and cancer patient outcomes and may offer prognostic and mechanistic insights for CRC management (8-14). However, because of inconsistent study results, the clinical significance of these biomarkers in CRC remains unclear.
Therefore, we conducted a prospective cohort study nested within a large National Cancer Institute (NCI)–sponsored clinical trial of systemic therapy examining associations of baseline plasma IGFs, insulin-like growth factor-binding protein (IGFBPs), adiponectin, and C-peptide with outcomes among patients with advanced or metastatic colorectal cancer (mCRC). To avoid an excessive number of hypotheses, we limited our analysis to select members of the IGF and IGFBP families, including IGF-1, the IGF most clearly associated with CRC tumor severity; IGFBP-3, the dominant circulating IGFBP; and IGFBP-7, an IGFBP differentiated from IGFBPs 1 to 6 by its lower affinity for IGF and high affinity for insulin (5,8,10,15). We examined C-peptide, a surrogate for circulating insulin, and adiponectin, an insulin-sensitizing hormone inversely related to obesity, given their relationship to insulin, energy balance, and previous data suggesting prognostic relevance in CRC (11,14). Patients were monitored prospectively for cancer progression and mortality. Data on molecular markers, performance status, treatment, and follow-up were carefully captured in the trial, allowing simultaneous effect of disease characteristics and systemic therapies to be assessed.
Methods
Study Population
Subjects were participants of an NCI-sponsored phase III trial for metastatic colorectal adenocarcinoma of irinotecan, 5-fluorouracil, and leucovorin or oxaliplatin, 5-fluorouracil, and leucovorin combined with either cetuximab, bevacizumab, or cetuximab plus bevacizumab (Cancer and Leukemia Group B [CALGB, now part of the Alliance for Clinical Trials in Oncology]/SWOG 80405; ClinicalTrials.gov identifier NCT00265850). Participants did not receive any systemic therapy for mCRC prior to trial enrollment (16).
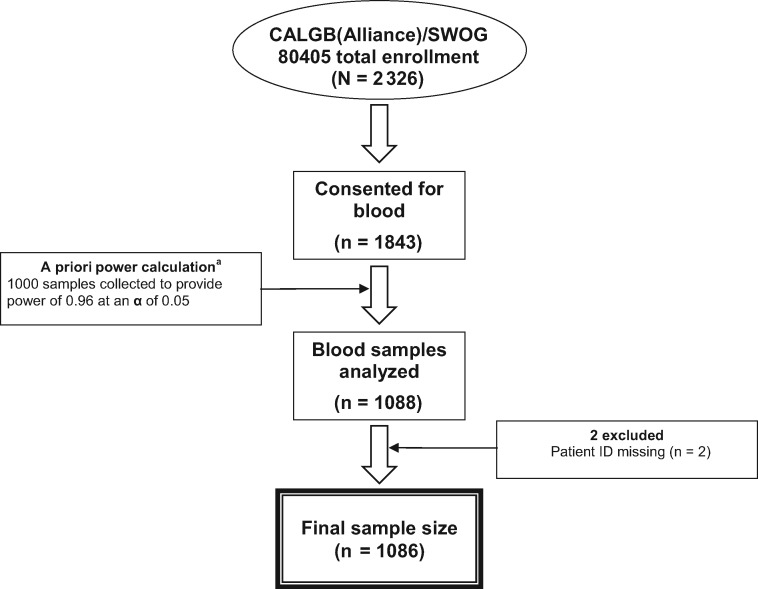
The clinical trial design of CALGB/SWOG 80405 underwent major changes during enrollment because of evolving science in the field of mCRC (Supplementary Methods, available online). Optional research blood collection was open throughout the entire course of the trial. Thus, the cohort in this analysis is derived from patients participating at any point in the trial history; in analyses, we adjust for chemotherapy regimen as well as KRAS status to account for protocol changes. The trial’s primary results demonstrated no statistically significant difference in overall survival (OS) between patients randomized to cetuximab vs bevacizumab, as published previously (16). Patient plasma for biomarker analysis was collected prior to trial chemotherapy initiation. The number of blood samples analyzed was based on a priori power calculations estimating that a sample of 1000 patients would provide a power of 0.96 for testing a null hypothesis of independence of 2-year OS over IGF quintiles at a statistical significance level of .05. Figure 1 demonstrates derivation of the cohort.
Figure 1.
Derivation of the study cohort. aThe number of blood samples analyzed was based on a priori power calculations estimating that a sample of approximately 1000 patients would provide a power of 0.96 for testing the null hypothesis of independence of 2-year overall survival over quintiles of insulin-like growth factor-1 at a statistical significance level of .05. CALGB = Cancer and Leukemia Group B, now Alliance for Clinical Trials in Oncology.
Eligibility for the trial and this companion study required a baseline Eastern Cooperative Oncology Group performance status of 0 to 1 and adequate bone marrow, renal, and hepatic function (17). All patients signed informed consent approved by each site’s institutional review board. The study was performed in accord with an assurance filed with and approved by the US Department of Health and Human Services.
Measurement of Plasma Biomarkers
Ethylenediaminetetraacetic acid plasma samples were collected upon trial registration at the local study site, centrifuged on site for 10-15 minutes, aliquoted and frozen within 3 hours of collection, stored at -80°C (-20°C was acceptable for up to 72 hours), and shipped frozen to the SWOG Solid Tumor Specimen Repository. Biomarker stability during transport has been demonstrated previously (18,19). IGFBP-3, IGFBP-7, adiponectin, C-peptide, and total IGF-1 concentrations were assayed in the laboratory of Dr Michael Pollak, using enzyme-linked immunosorbent assays with reagents from Ansh Labs (Webster, TX). Personnel performing the assays were blinded to patient outcome. Each sample was assayed in duplicate for each analyte, with correlations between replicates greater than 0.98. The mean intrabatch coefficients of variation calculated from the quality-control samples for each assay ranged from 0.2% to 3.1%. For molecular analyses, see the Supplementary Methods (available online).
Study Endpoints
The primary endpoint of the parent study CALGB(Alliance)/SWOG 80405 and this companion study was OS, defined as time from trial registration to death from any cause. Patients without reported deaths were censored at their last known follow-up. We also assessed progression-free survival (PFS), defined as time from trial registration to death from any cause or progression of disease, per Response Evaluation Criteria in Solid Tumors 1.0 (20). Patients alive without documented tumor progression were censored for PFS at the most recent disease assessment.
Statistical Analysis
Hazard ratios (HR) were calculated using Cox proportional hazards regression to compare OS and PFS across quintiles of plasma concentration for each biomarker, adjusting for potential confounders (21). Given potential interactions between IGF-1 and its primary binding protein, IGFBP-3, we also examined associations of patient outcome with the molar ratio of IGF-1 to IGFBP-3. The proportional hazards assumption was verified using time-dependent covariates. Covariates included in adjusted models were chosen a priori. Baseline covariates in adjusted models included sex, age, Eastern Cooperative Oncology Group performance status, planned chemotherapy, prior adjuvant chemotherapy, prior radiation therapy, assigned targeted treatment, KRAS status, tumor sidedness, circulating albumin, diabetes, and body mass index. Given the impact of fasting on C-peptide, C-peptide analyses were further adjusted for fasting state defined as 8 or more hours from last meal (22). Given the possibility of confounding by correlation between IGF-1 and IGFBP-3, we adjusted models examining IGF-1 for IGFBP-3, and adjusted models examining IGFBP-3 for IGF-1. In sensitivity analyses, we further adjusted models examining IGFBP-3 for IGFBP-7 and models examining IGFBP-7 for IGFBP-3 and IGF-1. Sensitivity analyses also included further adjustment for weight change over the 6 months prior to study entry, baseline physical activity (in metabolic equivalent task hours per week), and completion of the voluntary questionnaire used to assess weight change and physical activity. Weight change and physical activity were assessed as previously described (23,24). To reduce the impact of outliers, values for plasma biomarkers and albumin greater than 5 standard deviations from the average were replaced with the maximum or minimum value within 5 standard deviations. For covariates, missing variables were coded with the median if a continuous variable or major category if a categorical variable, or a missing indicator if proportion missing was greater than 5%.
To examine associations of biomarkers with patient outcome, we tested for trends in outcome across concentrations of each biomarker after adjusting for covariates, modeling each biomarker as a continuous variable, consistent with prior studies (14,25). Given the possibility of nonlinear relationships (26,27), we assessed for nonlinear associations between each biomarker and patient outcome nonparametrically with restricted cubic splines (28), excluding the highest and lowest 1% of observations to mitigate effects of outliers. Tests for nonlinearity used the likelihood ratio test, comparing a model with the linear term alone to one with the linear term plus cubic spline terms. If the test for nonlinearity was statistically significant, a nonlinear test for trend was implemented; otherwise, linear tests for trend were used. Linear and cubic spline terms from strongly correlated markers (IGF-1 and IGFBP-3) were also included as covariates. Subgroup analyses were conducted to explore associations of survival with selected biomarkers across strata of known and potential predictors of patient outcome. Data collection was conducted by the Alliance Statistics and Data Center. Data analyses were performed using SAS Version 9.4 (SAS Institute Inc, Cary, NC; RRID: SCR_008567) on a dataset locked January 18, 2018. Data quality was ensured by review by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. P values are 2-sided, statistically significant if less than .05, and not adjusted for multiple comparisons.
Results
Baseline Characteristics
Baseline patient characteristics across quintiles of plasma adiponectin, IGFBP-3, and IGFBP-7 are displayed in Table 1. Differences in baseline characteristics were consistent with known properties of these biomarkers (eg, patients with lower adiponectin tended to be overweight). There were no differences in baseline characteristics between patients with plasma biomarker measurements and other patients enrolled in the trial, except a greater frequency of KRAS testing in individuals who underwent successful plasma biomarker testing (Table 2).
Table 1.
Baseline characteristics by quintiles of plasma adiponectin, IGFBP-3, and IGFBP-7, n = 1086 (median follow-up = 6.2 years)
| Baseline characteristics | Adiponectin |
IGFBP-3 |
IGFBP-7 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 3 | Quintile 5 | Quintile 1 | Quintile 3 | Quintile 5 | Quintile 1 | Quintile 3 | Quintile 5 | |
| (n = 217) | (n = 218) | (n = 217) | (n = 217) | (n = 218) | (n = 217) | (n = 217) | (n = 219) | (n = 217) | |
| Biomarker median, ng/mL | 3511 | 8474 | 16935 | 2111 | 3238 | 4440 | 29 | 39 | 57 |
| Q1-Q3 | 2801-4261 | 7875-9283 | 15 179-20 601 | 1834-2298 | 3116-3352 | 4245-4796 | 28-31 | 38-40 | 54-66 |
| Female, No. (%) | 51 (23.5) | 85 (39.0) | 123 (56.7) | 69 (31.8) | 100 (45.9) | 93 (42.9) | 92 (42.4) | 98 (44.7) | 95 (43.8) |
| Age, median (Q1-Q3), y | 55 (48-63) | 59 (53-68) | 65 (55-73) | 64 (56-72) | 59 (52-67) | 55 (46-63) | 57 (50-65) | 61 (52-67) | 61 (52-69) |
| Race, No. (%) | |||||||||
| White | 166 (76.5) | 189 (86.7) | 201 (92.6) | 186 (85.7) | 184 (84.4) | 181 (83.4) | 186 (85.7) | 187 (85.4) | 183 (84.3) |
| Black | 41 (18.9) | 19 (8.7) | 11 (5.1) | 25 (11.5) | 28 (12.8) | 22 (10.1) | 20 (9.2) | 25 (11.4) | 27 (12.4) |
| Other | 10 (4.6) | 10 (4.6) | 5 (2.3) | 6 (2.8) | 6 (2.8) | 14 (6.5) | 11 (5.1) | 7 (3.2) | 7 (3.2) |
| Performance status, No. (%)a | |||||||||
| ECOG 0 | 137 (63.1) | 147 (67.4) | 116 (53.5) | 105 (48.4) | 136 (62.4) | 152 (70.0) | 149 (68.7) | 140 (63.9) | 107 (49.3) |
| ECOG 1 or 2 | 80 (36.9) | 71 (32.6) | 101 (46.5) | 112 (51.6) | 82 (37.6) | 65 (30.0) | 68 (31.3) | 79 (36.1) | 110 (50.7) |
| Planned chemotherapy, No. (%) | |||||||||
| FOLFIRI | 52 (24.0) | 54 (24.8) | 43 (19.8) | 52 (24.0) | 42 (19.3) | 52 (24.0) | 53 (24.4) | 53 (24.2) | 46 (21.2) |
| mFOLFOX6 | 165 (76.0) | 164 (75.2) | 174 (80.2) | 165 (76.0) | 176 (80.7) | 165 (76.0) | 164 (75.6) | 166 (75.8) | 171 (78.8) |
| Prior adjuvant chemotherapy, No. (%) | 33 (15.2) | 27 (12.4) | 30 (13.8) | 27 (12.4) | 32 (14.7) | 29 (13.4) | 37 (17.1) | 31 (14.2) | 20 (9.2) |
| Prior radiation therapy, No. (%) | 29 (13.4) | 21 (9.6) | 19 (8.8) | 18 (8.3) | 19 (8.7) | 21 (9.7) | 30 (13.8) | 15 (6.8) | 17 (7.8) |
| Assigned treatment arm, No. (%) | |||||||||
| Bevacizumab | 89 (41.0) | 90 (41.3) | 85 (39.2) | 89 (41.0) | 94 (43.1) | 88 (40.6) | 85 (39.2) | 77 (35.2) | 101 (46.5) |
| Cetuximab | 86 (39.6) | 94 (43.1) | 80 (36.9) | 85 (39.2) | 92 (42.2) | 90 (41.5) | 91 (41.9) | 101 (46.1) | 75 (34.6) |
| Bevacizumab & cetuximab | 42 (19.4) | 34 (15.6) | 52 (24.0) | 43 (19.8) | 32 (14.7) | 39 (18.0) | 41 (18.9) | 41 (18.7) | 41 (18.9) |
| KRAS, No. (%) | |||||||||
| Wild-type | 153 (70.5) | 135 (61.9) | 142 (65.4) | 151 (69.6) | 134 (61.5) | 139 (64.1) | 135 (62.2) | 143 (65.3) | 152 (70.0) |
| Mutant | 57 (26.3) | 69 (31.7) | 66 (30.4) | 58 (26.7) | 76 (34.9) | 67 (30.9) | 68 (31.3) | 72 (32.9) | 54 (24.9) |
| Indeterminate | 7 (3.2) | 14 (6.4) | 9 (4.1) | 8 (3.7) | 8 (3.7) | 11 (5.1) | 14 (6.5) | 4 (1.8) | 11 (5.1) |
| Metastases involving multiple regions, No. (%) | 112 (51.6) | 124 (56.9) | 112 (52.3) | 128 (59.5) | 109 (50.5) | 115 (53.2) | 99 (45.6) | 107 (49.3) | 128 (59.5) |
| Tumor sidedness, No. (%) | |||||||||
| Right or transverse colon | 76 (35.0) | 76 (34.9) | 78 (35.9) | 77 (35.5) | 70 (32.1) | 77 (35.5) | 72 (33.2) | 83 (37.9) | 72 (33.2) |
| Left colon or rectum | 131 (60.4) | 131 (60.1) | 122 (56.2) | 123 (56.7) | 137 (62.8) | 127 (58.5) | 134 (61.8) | 127 (58.0) | 132 (60.8) |
| Unknown | 10 (4.6) | 11 (5.0) | 17 (7.8) | 17 (7.8) | 11 (5.0) | 13 (6.0) | 11 (5.1) | 9 (4.1) | 13 (6.0) |
| BMI, median (Q1-Q3), kg/m2 | 30 (27-34) | 27 (24-30) | 24 (21-27) | 27 (24-31) | 27 (24-31) | 27 (25-31) | 27 (24-31) | 27 (24-32) | 28 (24-31) |
| Diet and lifestyle questionnaire available, No. (%)b | 166 (76.5) | 167 (76.6) | 157 (72.4) | 159 (73.3) | 162 (74.3) | 172 (79.3) | 174 (80.2) | 165 (75.3) | 148 (68.2) |
| Physical activity, median (Q1-Q3), MET h/wb | 3.4 (0.8-15.0) | 4.6 (0.5-13.4) | 2.7 (0.4-10.2) | 1.9 (0.2-7.0) | 4.2 (0.7-9.4) | 4.2 (0.9-16.0) | 4.1 (1.0-16.0) | 3.2 (0.6-11.3) | 1.4 (0.2-8.0) |
| Percent weight change over 6 months prior to study, No. (%)b | |||||||||
| Loss ≥5% | 98 (59.0) | 96 (57.5) | 106 (67.5) | 113 (71.1) | 100 (61.7) | 104 (60.5) | 90 (51.7) | 111 (67.3) | 106 (71.6) |
| Change <5% | 63 (38.0) | 65 (38.9) | 45 (28.7) | 38 (23.9) | 59 (36.4) | 65 (37.8) | 77 (44.3) | 46 (27.9) | 39 (26.4) |
| Gain ≥5% | 5 (3.0) | 6 (3.6) | 6 (3.8) | 8 (5.0) | 3 (1.9) | 3 (1.7) | 7 (4.0) | 8 (4.8) | 3 (2.0) |
| Diabetic, No. (%)b | 47 (21.7) | 32 (14.7) | 26 (12.0) | 48 (22.1) | 39 (17.9) | 25 (11.5) | 33 (15.2) | 44 (20.1) | 40 (18.4) |
| Albumin, median (Q1-Q3), g/dL | 3.9 (3.6-4.3) | 3.9 (3.5-4.3) | 3.7 (3.4-4.1) | 3.6 (3.3-3.9) | 3.9 (3.5-4.2) | 4.0 (3.8-4.3) | 4.0 (3.6-4.2) | 3.9 (3.6-4.2) | 3.6 (3.3-4.0) |
| WBC >10x109/L, No. (%) | 31 (14.3) | 43 (19.7) | 36 (16.7) | 67 (31.0) | 30 (13.8) | 30 (13.8) | 28 (12.9) | 33 (15.1) | 60 (27.9) |
| Hgb <11g/L, No. (%) | 183 (84.3) | 183 (83.9) | 166 (77.2) | 154 (71.3) | 170 (78.3) | 194 (89.4) | 199 (91.7) | 180 (82.2) | 144 (67.0) |
| TP53, No. (%) | |||||||||
| Wild-type | 75 (64.7) | 78 (67.2) | 82 (75.2) | 68 (68.0) | 75 (64.7) | 82 (67.8) | 83 (72.2) | 83 (68.0) | 68 (70.8) |
| Mutant | 41 (35.3) | 38 (32.8) | 27 (24.8) | 32 (32.0) | 41 (35.3) | 39 (32.2) | 32 (27.8) | 39 (32.0) | 28 (29.2) |
| APC, No. (%) | |||||||||
| Wild-type | 93 (80.2) | 91 (78.4) | 92 (84.4) | 79 (79.0) | 98 (84.5) | 97 (80.2) | 92 (80.0) | 108 (88.5) | 75 (78.1) |
| Mutant | 23 (19.8) | 25 (21.6) | 17 (15.6) | 21 (21.0) | 18 (15.5) | 24 (19.8) | 23 (20.0) | 14 (11.5) | 21 (21.9) |
| PIK3CA, No. (%) | |||||||||
| Wild-type | 101 (87.1) | 105 (90.5) | 101 (92.7) | 88 (88.0) | 102 (89.5) | 106 (87.6) | 99 (86.1) | 106 (86.9) | 88 (92.6) |
| Mutant | 15 (12.9) | 11 (9.5) | 8 (7.3) | 12 (12.0) | 12 (10.5) | 15 (12.4) | 16 (13.9) | 16 (13.1) | 7 (7.4) |
| BRAF, No. (%) | |||||||||
| Wild-type | 104 (86.7) | 101 (87.1) | 91 (82.7) | 92 (90.2) | 110 (90.9) | 105 (84.7) | 104 (88.9) | 105 (84.0) | 91 (91.0) |
| Mutant | 16 (13.3) | 15 (12.9) | 19 (17.3) | 10 (9.8) | 11 (9.1) | 19 (15.3) | 13 (11.1) | 20 (16.0) | 9 (9.0) |
| MSI, No. (%) | |||||||||
| MSS | 107 (93.0) | 106 (94.6) | 99 (92.5) | 89 (92.7) | 115 (96.6) | 117 (96.7) | 113 (96.6) | 104 (91.2) | 90 (92.8) |
| MSI | 8 (7.0) | 6 (5.4) | 8 (7.5) | 7 (7.3) | 4 (3.4) | 4 (3.3) | 4 (3.4) | 10 (8.8) | 7 (7.2) |
Baseline performance status categories: ECOG 0 is fully active; ECOG 1 is restricted in physically strenuous activity but ambulatory and able to carry out light work; ECOG 2 is ambulatory and capable of all self-care but unable to carry out any work activities, up and about more than 50% of waking hours. Only 1 patient included in the study had an ECOG performance status of 2. BMI = body mass index; ECOG = Eastern Cooperative Oncology Group; FOLFIRI = leucovorin, 5-fluorouracil, and irinotecan; Hgb = hemoglobin; IGFBP-3 = insulin-like growth factor-binding protein-3; IGFBP-7 = insulin-like growth factor-binding protein-7; MET h/w = metabolic equivalent task-hours per week; mFOLFOX6 = leucovorin, 5-fluorouracil, and oxaliplatin; MSI = microsatellite instability; MSS = microsatellite stable; Q = quintile; WBC = white blood cell count.
Data for physical activity, weight change, and diabetes were captured using a voluntary questionnaire completed within 1 month of initiating trial therapy. For patients without questionnaire data, diabetes status was captured using the on-study form.
Table 2.
Baseline characteristics of patients with blood samples collected and analyzed for this companion study compared with all other patients registered in the treatment trial CALGB(Alliance)/SWOG 80405a
| Baseline characteristics | Blood sample analyzed (n = 1086) | No blood sample analyzed (n = 1240) | Entire trial cohort (N = 2326) |
|---|---|---|---|
| No. deaths | 932 | 1041 | 1973 |
| Female, No. (%) | 447 (41.2) | 524 (42.3) | 971 (41.7) |
| Age, median y (Q1-Q3), y | 60 (51-68) | 59 (51-67) | 59 (51-68) |
| Race, No. (%) | |||
| White | 931 (85.7) | 965 (77.8) | 1896 (81.5) |
| Black | 113 (10.4) | 165 (13.3) | 278 (12.0) |
| Other | 42 (3.9) | 110 (8.9) | 152 (6.5) |
| Performance status, No. (%) | |||
| ECOG 0 | 657 (60.5) | 704 (56.8) | 1361 (58.5) |
| ECOG 1 or 2 | 429 (39.5) | 536 (43.2) | 965 (41.5) |
| Planned chemotherapy, No. (%) | |||
| FOLFIRI | 250 (23.0) | 280 (22.6) | 530 (22.8) |
| mFOLFOX6 | 836 (77.0) | 960 (77.4) | 1796 (77.2) |
| Prior adjuvant chemotherapy, No. (%) | 151 (13.9) | 183 (14.8) | 334 (14.4) |
| Prior radiation therapy, No. (%) | 102 (9.4) | 103 (8.3) | 205 (8.8) |
| Assigned treatment arm, No. (%) | |||
| Bevacizumab | 434 (40.0) | 463 (37.3) | 897 (38.6) |
| Cetuximab | 443 (40.8) | 454 (36.6) | 897 (38.6) |
| Bevacizumab + cetuximab | 209 (19.2) | 323 (26.0) | 532 (22.9) |
| KRAS, No. (%) | |||
| Wild-type | 720 (66.3) | 550 (44.4) | 1270 (54.6) |
| Mutant | 319 (29.4) | 139 (11.2) | 458 (19.7) |
| Indeterminate | 47 (4.3) | 551 (44.4) | 598 (25.7) |
| Tumor sidedness, No. (%) | |||
| Right or transverse colon | 380 (35.0) | 405 (32.7) | 785 (33.7) |
| Left colon or rectum | 643 (59.2) | 693 (55.9) | 1336 (57.4) |
| Unknown | 63 (5.8) | 142 (11.5) | 205 (8.8) |
| BMI, median (Q1-Q3), kg/m2 | 27 (24-32) | 27 (24-31) | 27 (24-31) |
| Metastases involving multiple regions, No. (%) | 584 (54.0) | 677 (55.8) | 1261 (55.0) |
| WBC >10x109/L, No. (%) | 198 (18.3) | 252 (20.6) | 450 (19.5) |
| Hgb <11g/L, No. (%) | 879 (81.1) | 986 (80.6) | 1865 (80.8) |
BMI = body mass index; CALGB(Alliance) = Cancer and Leukemia Group B, now Alliance for Clinical Trials in Oncology; ECOG = Eastern Cooperative Oncology Group; FOLFIRI = leucovorin, 5-fluorouracil, and irinotecan; Hgb = hemoglobin; mFOLFOX6 = leucovorin, 5-fluorouracil, and oxaliplatin; Q = quintile; WBC = white blood cell count.
Correlations Between Plasma Biomarkers
Using Spearman coefficients, we examined correlations between plasma levels of the 5 energy-related biomarkers (Table 3). We observed a strong positive correlation (coefficient = 0.75; P < .001) between IGF-1 and IGFBP-3 (36). All other correlations were weak or nonexistent (correlation coefficient < 0.3) (29).
Table 3.
Correlations between energy-related plasma biomarkers (n = 1086)
| Plasma biomarker | Spearman correlation coefficients between biomarkers |
|||
|---|---|---|---|---|
| C-peptide | IGF-1 | IGFBP-3 | IGFBP-7 | |
| Adiponectin | −0.29a | −0.17a | −0.13a | 0.05c |
| C-peptide | — | 0.14a | 0.09b | 0.13a |
| IGF-1 | — | — | 0.75a | −0.18a |
| IGFBP-3 | — | — | — | −0.17a |
P < .001 for Spearman correlation. All P values pertaining to this table are 2-sided. IGF-1 = insulin-like growth factor-I; IGFBP-3 = insulin-like growth factor-binding protein-3; IGFBP-7 = insulin-like growth factor-binding protein-7.
P = .004 for Spearman correlation.
P = .10 for Spearman correlation.
Associations of Biomarkers With Mortality and Disease Progression
The median follow-up from time of trial registration is 6.2 years. During follow-up, 946 of the 1086 patients in this analysis experienced cancer progression; 848 of these patients died. An additional 84 patients died without documented cancer progression.
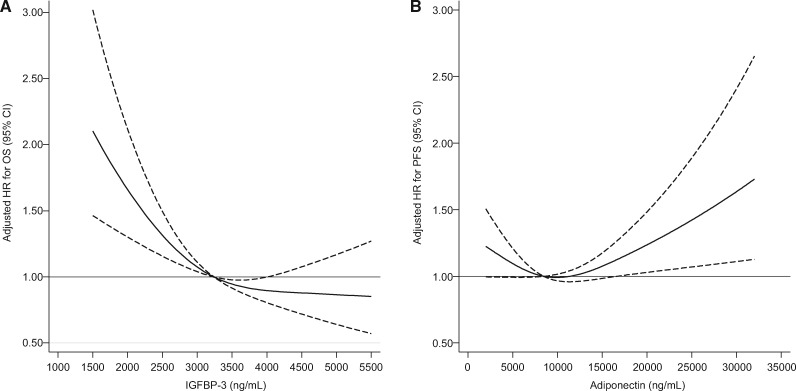
Results from Cox proportional hazards regressions examining associations of plasma biomarkers with patient outcome are displayed in Table 4. Greater plasma IGFBP-3 was associated with reduced risk of mortality and disease progression, even after adjustment for covariates such as plasma IGF-1. The association between IGFBP-3 and OS was nonlinear (Figure 2). Compared with individuals with IGFBP-3 concentrations in the lowest quintile, patients with IGFBP-3 in the highest quintile experienced an adjusted hazard ratio for OS of 0.57 (95% confidence interval [CI] = 0.42 to 0.78; Pnonlinearity < .001) and an adjusted hazard ratio for PFS of 0.61 (95% CI = 0.45 to 0.82, linear Ptrend = .003). In sensitivity analyses, the associations of greater IGFBP-3 with longer OS and PFS remained statistically significant after further adjusting for plasma IGFBP-7, physical activity, and weight change (HR for OS = 0.59, 95% CI = 0.43 to 0.80; Pnonlinearity < .001; HR for PFS = 0.62, 95% CI = 0.46 to 0.84; Ptrend = .007). Despite a known inverse association between IGFBP-3 methylation and microsatellite instability in CpG island methylator phenotype–high CRCs (30), our results were largely unchanged when further adjusted for microsatellite instability status (HR for OS = 0.57, 95% CI = 0.42 to 0.78; Ptrend < .001).
Table 4.
Associations between plasma biomarkers (adiponectin, C-peptide, IGF-1, IGFBP-3, and IGFBP-7) and patient outcome in advanced or metastatic colorectal cancer (n = 1086)a
| Variable | Quintiles |
P trend | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| IGFBP-3 | ||||||
| Median, ng/mL | 2111 | 2749 | 3238 | 3727 | 4440 | — |
| (Min-Max) | (831-2454) | (2459-3008) | (3008-3507) | (3508-4040) | (4044-6767) | |
| (Q1-Q3) | (1834-2298) | (2617-2876) | (3116-3352) | (3608-3861) | (4245-4796) | — |
| OS | ||||||
| Event/N | 203/217 | 194/217 | 178/218 | 176/217 | 181/217 | — |
| Median OS, (95% CI) | 1.79 (1.40 to 2.00) | 2.21 (1.98 to 2.49) | 2.71 (2.39 to 3.01) | 2.66 (2.25 to 3.02) | 2.72 (2.42 to 3.05) | |
| Unadjusted HR (95% CI) | 1 (Referent) | 0.74 (0.60 to 0.90) | 0.53 (0.43 to 0.65) | 0.52 (0.42 to 0.63) | 0.53 (0.43 to 0.64) | <.001b |
| Adjusted HR (95% CI) | 1 (Referent) | 0.80 (0.65 to 1.00) | 0.61 (0.48 to 0.78) | 0.61 (0.47 to 0.81) | 0.57 (0.42 to 0.78) | <.001b |
| PFS | ||||||
| Event/N | 213/217 | 211/217 | 205/218 | 201/217 | 200/217 | — |
| Median PFS, (95% CI) | 0.73 (0.63 to 0.79) | 0.85 (0.77 to 0.97) | 1.01 (0.91 to 1.11) | 0.96 (0.85 to 1.13) | 0.97 (0.91 to 1.09) | |
| Unadjusted HR (95% CI) | 1 (Referent) | 0.81 (0.67 to 0.98) | 0.63 (0.52 to 0.77) | 0.65 (0.54 to 0.79) | 0.63 (0.52 to 0.77) | <.001b |
| Adjusted HR (95% CI) | 1 (Referent) | 0.84 (0.68 to 1.04) | 0.67 (0.53 to 0.86) | 0.71 (0.54 to 0.92) | 0.61 (0.45 to 0.82) | .003 |
| IGFBP-7 | ||||||
| Median, ng/mL | 29 | 34 | 39 | 45 | 57 | — |
| (Min-Max) | (15–32) | (32–36) | (36–41) | (41–50) | (50–126) | |
| (Q1-Q3) | (28-31) | (33–35) | (38–40) | (43–47) | (54–66) | — |
| OS | ||||||
| Event/N | 181/217 | 179/217 | 185/219 | 187/216 | 200/217 | — |
| Median OS, (95% CI) | 2.74 (2.51 to 2.95) | 2.50 (2.25 to 2.81) | 2.60 (2.31 to 2.93) | 2.20 (1.88 to 2.48) | 1.93 (1.58 to 2.13) | |
| Unadjusted HR (95% CI) | 1 (Referent) | 1.08 (0.88 to 1.33) | 1.11 (0.91 to 1.37) | 1.29 (1.05 to 1.58) | 1.71 (1.40 to 2.10) | <.001 |
| Adjusted HR (95% CI) | 1 (Referent) | 1.11 (0.90 to 1.37) | 1.08 (0.87 to 1.33) | 1.21 (0.98 to 1.49) | 1.60 (1.30 to 1.97) | <.001 |
| PFS | ||||||
| Event/N | 205/217 | 202/217 | 205/219 | 208/216 | 210/217 | — |
| Median PFS, (95% CI) | 0.96 (0.85 to 1.08) | 0.96 (0.82 to 1.08) | 0.92 (0.83 to 1.06) | 0.91 (0.80 to 0.97) | 0.79 (0.73 to 0.88) | |
| Unadjusted HR (95% CI) | 1 (Referent) | 0.99 (0.81 to 1.20) | 1.09 (0.89 to 1.32) | 1.12 (0.92 to 1.36) | 1.34 (1.10 to 1.62) | <.001 |
| Adjusted HR (95% CI) | 1 (Referent) | 1.06 (0.87 to 1.29) | 1.11 (0.91 to 1.35) | 1.14 (0.94 to 1.39) | 1.38 (1.13 to 1.69) | <.001 |
| IGF-1 | ||||||
| Median, ng/mL | 68 | 100 | 127 | 155 | 203 | — |
| (Min-Max) | (14–84) | (85–113) | (113–139) | (139–172) | (172-332) | |
| (Q1-Q3) | (56-76) | (92–106) | (120–133) | (146–162) | (187–230) | — |
| OS | ||||||
| Event/N | 203/217 | 192/217 | 177/218 | 177/217 | 183/217 | — |
| Median OS, (95% CI) | 1.84 (1.51 to 2.00) | 2.38 (2.09 to 2.60) | 2.70 (2.28 to 3.23) | 2.68 (2.34 to 2.91) | 2.52 (2.31 to 2.78) | |
| Unadjusted HR (95% CI) | 1 (Referent) | 0.67 (0.55 to 0.81) | 0.54 (0.44 to 0.66) | 0.56 (0.46 to 0.69) | 0.60 (0.49 to 0.73) | <.001b |
| Adjusted HR (95% CI) | 1 (Referent) | 0.97 (0.77 to 1.21) | 0.93 (0.72 to 1.20) | 1.06 (0.80 to 1.41) | 1.20 (0.88 to 1.64) | .30 |
| PFS | ||||||
| Event/N | 212/217 | 211/217 | 201/218 | 200/217 | 206/217 | — |
| Median PFS, (95% CI) | 0.71 (0.64 to 0.82) | 0.86 (0.77 to 0.91) | 1.03 (0.91 to 1.17) | 0.97 (0.85 to 1.09) | 0.96 (0.85 to 1.09) | |
| Unadjusted HR (95% CI) | 1 (Referent) | 0.77 (0.63 to 0.93) | 0.62 (0.51 to 0.75) | 0.63 (0.52 to 0.76) | 0.70 (0.58 to 0.85) | <.001b |
| Adjusted HR (95% CI) | 1 (Referent) | 0.96 (0.77 to 1.19) | 0.85 (0.66 to 1.09) | 0.96 (0.73 to 1.26) | 1.13 (0.84 to 1.54) | .23 |
| C-peptide | ||||||
| Median, ng/mL | 1.6 | 2.9 | 4.2 | 6 | 8.9 | — |
| (Min-Max) | (0.0-2.3) | (2.3-3.6) | (3.6-5.0) | (5.0-7.1) | (7.1-21.8) | |
| (Q1-Q3) | (1.2-2.0) | (2.6-3.2) | (3.9-4.6) | (5.5-6.5) | (7.9-11.4) | — |
| OS | ||||||
| Event/N | 177/217 | 179/217 | 193/218 | 188/217 | 195/217 | — |
| Median OS, (95% CI) | 2.26 (1.96 to 2.60) | 2.24 (1.93 to 2.50) | 2.55 (2.24 to 2.75) | 2.37 (2.04 to 2.60) | 2.52 (2.30 to 2.83) | |
| Unadjusted HR (95% CI) | 1 (Referent) | 1.02 (0.83 to 1.25) | 1.01 (0.82 to 1.23) | 1.03 (0.84 to 1.26) | 1.05 (0.86 to 1.29) | .78 |
| Adjusted HR (95% CI) | 1 (Referent) | 1.05 (0.85 to 1.30) | 1.00 (0.81 to 1.24) | 1.14 (0.92 to 1.41) | 1.13 (0.91 to 1.40) | .28 |
| PFS | ||||||
| Event/N | 202/217 | 200/217 | 211/218 | 207/217 | 210/217 | — |
| Median PFS, (95% CI) | 0.85 (0.77 to 0.97) | 0.83 (0.77 to 0.92) | 0.96 (0.87 to 1.06) | 0.95 (0.86 to 1.08) | 0.91 (0.80 to 0.97) | |
| Unadjusted HR (95% CI) | 1 (Referent) | 0.99 (0.81 to 1.20) | 1.04 (0.86 to 1.27) | 0.98 (0.81 to 1.19) | 1.07 (0.88 to 1.30) | .84 |
| Adjusted HR (95% CI) | 1 (Referent) | 0.99 (0.81 to 1.21) | 1.05 (0.86 to 1.29) | 1.03 (0.84 to 1.26) | 1.11 (0.91 to 1.37) | .41 |
| Adiponectin | ||||||
| Median, ng/mL | 3511 | 5936 | 8474 | 11558 | 16935 | — |
| (Min-Max) | (985-4854) | (4855-7097) | (7102-9988) | (10 040-13 574) | (13 579-40 480) | |
| (Q1-Q3) | (2801-4261) | (5399-6514) | (7875-9283) | (10 780-12 512) | (15 179-20 601) | — |
| OS | ||||||
| Event/N | 189/217 | 185/217 | 179/218 | 189/217 | 190/217 | — |
| Median OS, (95% CI) | 2.66 (2.45 to 3.00) | 2.43 (2.10 to 2.64) | 2.75 (2.41 to 2.98) | 2.21 (1.94 to 2.51) | 1.92 (1.60 to 2.11) | |
| Unadjusted HR (95% CI) | 1 (Referent) | 1.09 (0.89 to 1.34) | 1.00 (0.81 to 1.22) | 1.20 (0.98 to 1.46) | 1.34 (1.10 to 1.64) | .008 |
| Adjusted HR (95% CI) | 1 (Referent) | 1.07 (0.87 to 1.31) | 0.94(0.76 to 1.16) | 1.04 (0.84 to 1.29) | 1.10 (0.87 to 1.39) | .76 |
| PFS | ||||||
| Event/N | 209/217 | 204/217 | 202/218 | 207/217 | 208/217 | — |
| Median PFS, (95% CI) | 0.97 (0.90 to 1.13) | 0.92 (0.79 to 1.03) | 0.96 (0.85 to 1.08) | 0.86 (0.79 to 0.98) | 0.77 (0.70 to 0.88) | |
| Unadjusted HR (95% CI) | 1 (Referent) | 1.02 (0.84 to 1.24) | 0.99 (0.82 to 1.20) | 1.05 (0.87 to 1.28) | 1.29 (1.06 to 1.56) | .01 |
| Adjusted HR (95% CI) | 1 (Referent) | 0.99 (0.81 to 1.20) | 0.90 (0.73 to 1.10) | 0.94 (0.76 to 1.16) | 1.13 (0.90 to 1.40) | .03b |
Adjusting with Cox proportional hazards regression for age (continuous variable), sex (female, male), performance status (ECOG 0 vs 1 or 2), planned chemotherapy (FOLFIRI, mFOLFOX6), prior adjuvant chemotherapy (yes, no), assigned treatment arm (bevacizumab, cetuximab, bevacizumab + cetuximab), KRAS status (wild-type, mutant, indeterminate/missing), tumor sidedness (right or transverse colon vs left colon or rectum), plasma albumin (continuous variable), diabetes (yes, no), and body mass index (<20.9, 21-24.9, 25-29.9, 30-34.9, ≥35 kg/m2). The adjusted model for C-peptide is further adjusted for fasting status. The adjusted model for IGF-1 is further adjusted for IGFBP-3 (continuous and nonlinear term). The adjusted model for IGFBP-3 is further adjusted for IGF-1 (continuous and nonlinear term). CI = confidence interval; ECOG = Eastern Cooperative Oncology Group; FOLFIRI = leucovorin, fluorouracil, and irinotecan; HR = hazard ratio; IGF-1 = insulin-like growth factor-I; IGFBP-3 = insulin-like growth factor-binding protein-3; IGFBP-7 = insulin-like growth factor-binding protein-7; mFOLFOX6 = leucovorin, fluorouracil, and oxaliplatin; OS = overall survival; PFS = progression-free survival; Q = quintile.
P values marked with b reflect statistical significance of a test for nonlinear trend across biomarkers quintiles. All other P values reflect tests for linear trend. Criteria for applying nonlinear vs linear tests for trend are detailed in Methods. All P values are 2-sided.
Figure 2.
Restricted cubic splines depicting hazard ratios for (A) all-cause mortality (OS) as a function of plasma IGFBP-3 concentration and (B) disease progression or mortality (PFS) as function of plasma adiponectin concentration (n = 1086). Hazard ratios treat the median concentrations of adiponectin and IGFBP-3 as reference values. Dashed lines indicate 95% confidence intervals. Models were adjusted for age (continuous variable), sex (female, male), performance status (ECOG 0 vs 1 or 2), planned chemotherapy (FOLFIRI, mFOLFOX6), prior adjuvant chemotherapy (yes, no), assigned treatment arm (bevacizumab, cetuximab, bevacizumab + cetuximab), KRAS status (wild-type, mutant, indeterminate, missing), tumor sidedness (right or transverse colon vs left colon or rectum), plasma albumin (continuous variable), diabetes (yes, no), and body mass index (<20.9, 21-24.9, 25-29.9, 30-34.9, ≥35 kg/m2). The adjusted model for IGFBP-3 is further adjusted for IGF-1 (continuous and nonlinear term). CI = confidence interval; ECOG = Eastern Cooperative Oncology Group; FOLFIRI = leucovorin, fluorouracil, and irinotecan; HR = hazard ratio; IGF-1 = insulin-like growth factor 1; IGFBP-3 = insulin-like growth factor-binding protein-3; mFOLFOX6 = leucovorin, fluorouracil, and oxaliplatin; OS = overall survival; PFS = progression-free survival.
In contrast to IGFBP-3, greater plasma IGFBP-7 was associated with greater risk of mortality and disease progression. Compared with individuals with IGFBP-7 in the lowest quintile, individuals with IGFBP-7 in the highest quintile experienced an adjusted hazard ratio for OS of 1.60 (95% CI = 1 .30 to 1.97; Ptrend < .001) and an adjusted hazard ratio for PFS of 1.38 (95% CI = 1.13 to 1.69; Ptrend < .001). In sensitivity analyses, the association with OS was not substantially altered by further adjustment for plasma IGF-1, IGFBP-3, physical activity, and weight change (HR = 1.48, 95% CI = 1.20 to 1.82; Ptrend < .001), although the association with PFS became statistically non-significant (HR = 1.25, 95% CI = 1.02 to 1.54; Ptrend = .05).
In the univariate model and a model adjusted for patient, disease, and treatment characteristics, increasing IGF-1 demonstrated a nonlinear association with longer OS and PFS (adjusted HR for OS comparing highest IGF-1 quintile with lowest = 0.71, 95% CI = 0.57 to 0.87; Pnonlinearity < .001; adjusted HR for PFS = 0.75, 95% CI = 0.61 to 0.92; Pnonlinearity < .001). However, the associations between IGF-1 and patient outcome became statistically non-significant when further adjusted for IGFBP-3 (HR for OS = 1.20, 95% CI = 0.88 to 1.64; Ptrend = .30; HR for PFS = 1.13, 95% CI = 0.84 to 1.54; Ptrend = .23). Given IGFBP-3’s role as IGF-1’s primary binding protein, we also examined associations of patient outcome with the molar ratio of plasma IGF-1 to plasma IGFBP-3. This ratio was not associated with patient outcome in multivariable models (Ptrend for OS = .99; Ptrend for PFS = .76).
Increasing adiponectin was associated with shorter OS in the unadjusted model (HR = 1.34, 95% CI = 1.10 to 1.64; Ptrend = .008); however, this association was not statistically significant after adjusting for covariates (Ptrend = .76). Regarding PFS, the nonlinear test for trend demonstrated a statistically significant U-shaped association, wherein patients with extreme levels of adiponectin experienced shorter PFS compared with other patients (adjusted Pnonlinearity = .03; Figure 2). This association remained statistically significant after adjusting further for physical activity and weight change (Pnonlinearity = .009). C-peptide was not associated with mortality or disease progression in unadjusted or adjusted models.
Subgroup Analyses by Patient and Tumor Characteristics
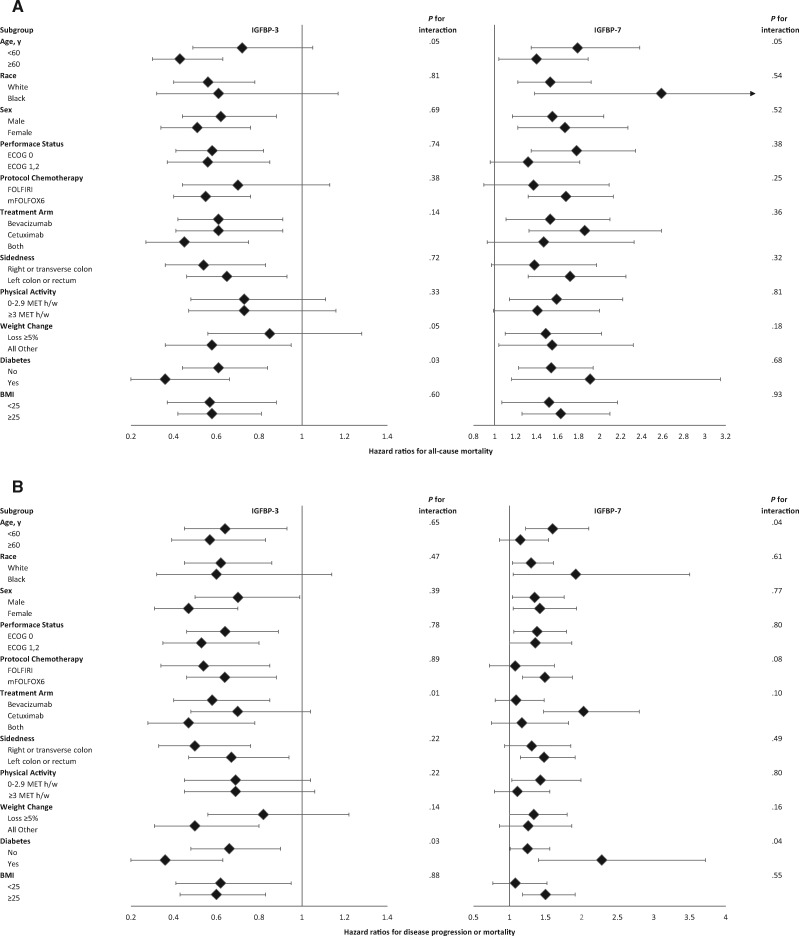
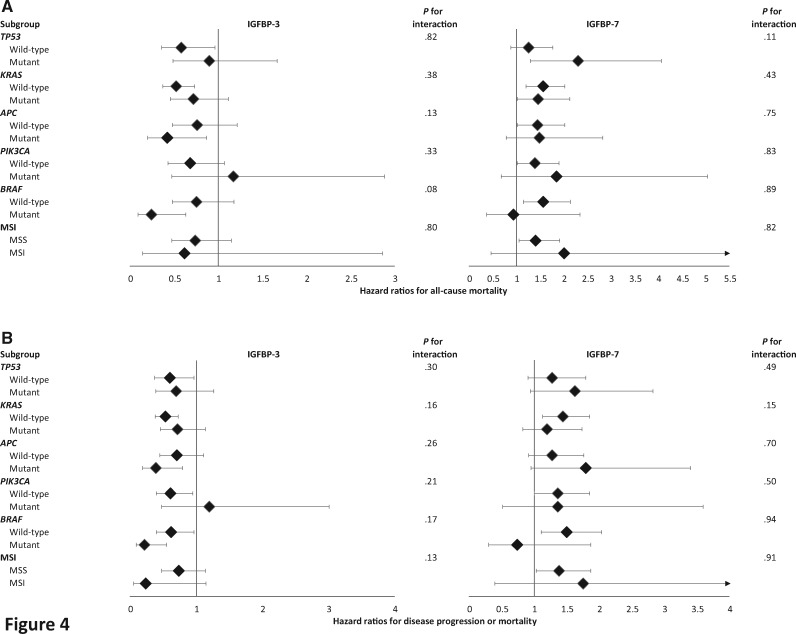
The association of high IGFBP-3 and low IGFBP-7 with longer OS and PFS appeared generally consistent across strata of other predictors (Figures 3 and 4). The subgroup analyses are exploratory and hypothesis generating.
Figure 3.
Multivariate hazard ratios with 95% confidence intervals for (A) all-cause mortality (overall survival) and (B) disease progression or mortality (progression-free survival) in patients with advanced or metastatic colorectal cancer, stratified across various patient, disease, and treatment characteristics. The forest plot represents the hazard ratios of the comparison of the highest quintile of a given marker (eg, IGFBP-3) to the lowest quintile. Adjusting with Cox proportional hazards regression for age (continuous variable), sex (female, male), performance status (ECOG 0 vs 1 or 2), planned chemotherapy (FOLFIRI, mFOLFOX6), prior adjuvant chemotherapy (yes, no), assigned treatment arm (bevacizumab, cetuximab, bevacizumab + cetuximab), KRAS status (wild-type, mutant, indeterminate/missing), tumor sidedness (right or transverse colon vs left colon or rectum), plasma albumin (continuous variable), diabetes (yes, no), and body mass index (<20.9, 21-24.9, 25-29.9, 30-34.9, ≥35 kg/m2). The adjusted model for IGFBP-3 is further adjusted for IGF-1 (continuous and nonlinear term). BMI = body mass index; ECOG = Eastern Cooperative Oncology Group; FOLFIRI = leucovorin, fluorouracil, and irinotecan; IGF-1 = insulin-like growth factor-1; IGFBP-3 = insulin-like growth factor-binding protein-3; IGFBP-7 = insulin-like growth factor-binding protein-7; MET h/w = metabolic equivalent task-hours per week; mFOLFOX6 = leucovorin, fluorouracil, and oxaliplatin.
Figure 4.
Multivariate hazard ratios with 95% confidence intervals for (A) all-cause mortality (overall survival) and (B) disease progression or mortality (progression-free survival) in patients with advanced or metastatic colorectal cancer, stratified across various molecular tumor markers. The forest plot represents the hazard ratios of the comparison of the highest quintile of a given marker (eg, IGFBP-3) to the lowest quintile. Adjusting with Cox proportional hazards regression for age (continuous variable), sex (female, male), performance status (ECOG 0 vs 1 or 2), planned chemotherapy (FOLFIRI, mFOLFOX6), prior adjuvant chemotherapy (yes, no), assigned treatment arm (bevacizumab, cetuximab, bevacizumab + cetuximab), KRAS status (wild-type, mutant, indeterminate/missing), tumor sidedness (right or transverse colon vs left colon or rectum), plasma albumin (continuous variable), diabetes (yes, no), and body mass index (<20.9, 21-24.9, 25-29.9, 30-34.9, ≥35 kg/m2). The adjusted model for IGFBP-3 is further adjusted for IGF-1 (continuous and nonlinear term). ECOG = Eastern Cooperative Oncology Group; FOLFIRI = leucovorin, fluorouracil, and irinotecan; IGF-1 = insulin-like growth factor-1; IGFBP-3 = insulin-like growth factor-binding protein-3; IGFBP-7 = insulin-like growth factor-binding protein-7; mFOLFOX6 = leucovorin, fluorouracil, and oxaliplatin; MSI = microsatellite instability; MSS = microsatellite stable.
Discussion
In this prospective cohort of patients with mCRC enrolled in an NCI-sponsored trial, high baseline plasma IGFBP-3 and low baseline plasma IGFBP-7 were associated with longer OS and PFS after adjusting for potential and known predictors of patient outcome. Plasma adiponectin was not associated with OS, although extreme levels of plasma adiponectin were associated with shorter PFS. In adjusted models, C-peptide and IGF-1 were not associated with patient outcomes.
Aberrant IGF signaling through the PI3K-AKT pathways promotes cellular survival and proliferation and has been implicated in various malignancies (5). IGFBP-3 is IGF-1’s primary binding protein and reduces IGF-1 signaling by preventing IGF-1 from binding the IGF-1 receptor (8). In our study, high IGFBP-3 was associated with longer survival despite the absence of an association between survival and IGF-1. This may be due to tumor suppressor effects of IGFBP-3 that are IGF-1-independent (31). However, our study measured total plasma IGF-1 and cannot exclude associations between mCRC outcome and free plasma IGF-1. As a proxy for free IGF-1, we tested for associations between survival and the ratio of total IGF-1 to IGFBP-3 (18) but found no statistically significant association.
To our knowledge, our study is the first to examine associations of IGFBP-7 with mCRC disease progression and mortality. IGFBP-7 is associated with insulin resistance (32) and is differentiated from other IGFBPs by its relatively low affinity for IGF (33,34). Prior studies suggest that IGFBP-7 acts as a tumor suppressor in several malignancies (35-38), including colon cancer (39), possibly through inhibition of cell growth and survival via IGF-1 and 2 antagonism and blockade of IGF-1 receptor activation (40). Considering such evidence, the association identified in the current study between elevated plasma IGFBP-7 and adverse mCRC outcomes appears paradoxical. However, other evidence suggests that IGFBP-7 may promote tumor progression in malignancies such as glioblastoma, wherein IGFBP-7 was found to promote cancer cell growth and migration and to be associated with decreased patient survival (41). IGFBP-7 has also been reported to be elevated in invasive prostate cancer (42) and colon cancer (43). It has also been associated with aggression in inflammatory breast cancer (44), increased risk of metastasis in sarcoma (45), poor prognosis in multiple myeloma (46), and poor prognosis in colon cancer when expressed by tumor cells (47). IGFBP-7 biology is multifaceted, with effects on tumor vasculature (48) and on anchorage-independent growth that varies depending on tumor cell phenotype (49). Our findings suggest that high plasma IGFBP-7 may be a poor prognostic sign in mCRC and support further investigation of the interplay between IGFBP-7 and CRC tumor biology.
Our findings regarding IGFBP-3, IGF-1, and C-peptide are consistent with prior prospective studies of CRC patients that found high plasma IGFBP-3, but not IGF-1 or C-peptide, to be associated with lower risk of CRC-specific death (50), greater treatment response, and longer time to progression (25). It is unclear whether such findings are generalizable to nonmetastatic CRC. One study of nonmetastatic CRC found no association between prediagnosis IGFBP-3 and patient mortality (14), whereas others identified IGFBP-3 methylation as a predictor of poor disease-free survival (51,52). Notably, our study of associations between CRC survival and circulating IGFBP-3, IGF-1, and C-peptide is the first to control for the presence of diabetes, KRAS status, and tumor sidedness.
Adiponectin is a circulating regulator of insulin sensitivity whose concentration is inversely related to adiposity and CRC incidence (9). One prospective study found high adiponectin to be associated with greater CRC-specific and overall mortality (11), whereas another study found no association (53). We identified a U-shaped association, wherein individuals with low or high adiponectin experienced shorter PFS. The association of high adiponectin with shorter PFS may be attributable to adiponectin elevation during weight loss, because weight loss increases adiponectin and portends a worse prognosis in mCRC (23,54). The association between low adiponectin and shorter PFS may be related to adiponectin’s role as an insulin sensitizer (55), because insulin resistance and hyperinsulinemia may promote CRC progression (2).
Conducting a prospective cohort study nested within an NCI-sponsored clinical trial offers several advantages. All patients had confirmed advanced or metastatic disease at baseline, reducing patient heterogeneity. Treatment and follow-up after biomarker measurement were standardized, allowing disease progression and mortality to be assessed prospectively and accurately. Finally, detailed information on prognostic covariates was collected at baseline, allowing adjustment for potential confounders.
Our study has notable limitations. First, patients in clinical trials may differ from the general population. However, this cohort included patients from community and academic centers throughout North America. Second, our adjustment for weight loss and physical activity was limited because 25% of individuals did not answer the questionnaire for these covariates. Third, our study’s biomarker assessment was limited to baseline measurements. Future studies should aim to measure plasma biomarkers longitudinally. Finally, our study lacks a validation cohort and requires additional studies to confirm our findings. Future directions may include investigation of the adiponectin to leptin ratio, a marker of adipose tissue dysfunction (56).
In summary, this prospective study of patients with mCRC, embedded in a large, phase III trial, demonstrated an association of high baseline plasma IGFBP-3 and low baseline plasma IGFBP-7 with prolonged OS and PFS. Extreme levels of plasma adiponectin were associated with shorter PFS. After adjusting for confounders, there were no statistically significant associations between patient outcome and plasma C-peptide or IGF-1. Although our study’s observational nature precludes inferences of causality, the results further inform CRC biology and suggest potential avenues for prognostic and therapeutic innovation. Future studies should be performed to confirm our findings.
Funding
This work was supported by the National Cancer Institute of the National Institutes of Health (grant numbers U10CA180821 and U10CA180882 to the Alliance for Clinical Trials in Oncology; U10CA180857, U10CA180790, U10CA180791, U10CA180795, U10CA180826, U10CA180836, U10CA180838, U10CA180867, and UG1CA189858; U10CA180820 to ECOG-ACRIN; U10CA180888 and U10CA180830 to SWOG; R01 CA169141 and R01 CA118553 to CSF; R01CA149222 to JAM; R00CA218603 and R25CA203650 to JCB); the National Institute of General Medicine Sciences of the National Institutes of Health (grant number U54GM104940 to JCB); the Stand-Up-to-Cancer Colorectal Dream Team Grant to CSF; the Guo Shu Shi Fund to JAM; the Karen Guo Colon Cancer Research Fund to JAM; the Stone Research Fund to JAM; the Douglas Gray Woodruff Chair Fund to JAM; Eli Lily & Company; Genentech; Pfizer; Sanofi; and supporters of the Alliance for Clinical Trials in Oncology and Alliance Foundation Trials programs, as detailed at https://acknowledgments.alliancefound.org.
Notes
Role of the funder: Government sponsors of the study helped design the study and participated in review of the manuscript. Representatives of Sanofi, Genentech, and Eli Lily & Company were allowed the opportunity to review the manuscript prior to publication. Gene polymerase chain reaction analyses were performed at Genentech. Sponsors of this study played no other role in this companion study’s design; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: BJG has conducted research associated with institutional funding from Bristol-Myers Squibb, Genentech, Eli Lily, Pfizer, and Sanofi and has received honoraria from Medscape. JAM has received institutional research funding from Boston Biomedical and has served as an advisor/consultant to COTA Healthcare and served on a grant review panel for the National Comprehensive Cancer Network funded by Taiho Pharmaceutical. APV has served as an advisor/consultant for Taiho Pharmaceutical, Bayer, Halozyme, and Eisai; has received institutional research funding from Genentech and Bristol-Myers Squibb; has received royalties from Now-UptoDate for authoring and maintaining 2 chapters; and has received travel, accommodations, or other expenses from Genentech, Roche, Halozyme, and Bayer. HJL has served as an advisor/consultant to Merck Serono, Roche, Bayer, and Pfizer; has received honoraria from Merck Serono, Roche, Bayer, and Boehringer Ingelheim; and has received travel, accommodations, or other expenses from Merck Serono, Bayer, and Roche. BHO has been employed by Eli Lilly and has served as an advisor/consultant to Bristol-Myers Squibb and Merck. BNP has received research funding from Merck; has received travel, accommodations, or other expenses from Tapestry Pharmaceuticals; and has other relationships with Gerson Lehrman Group. RMG has served as an advisor/consultant to Merck, Taiho Pharmaceutical, Merck, and Novartis; has received research funding from Bristol-Myers Squibb; has received honoraria from Amgen; and has received travel, accommodations, or other expenses from Merck and Amgen. EOR has received institutional research funding from Genentech, BMS, Halozyme, Celgene, MabVax Therapeutics, ActaBiologica, AstraZeneca, Silenseed, and Polaris and has served as an advisor/consultant to CytomX Therapeutics, BioLineRx, Targovax, Ipsen, Celgene, Bayer, Polaris, Sobi, and Merck. CSF has served in a leadership role for CytomX Therapeutics; has stock or other ownership interests in CytomX Therapeutics and Entrinsic Health; and has served as an advisor/consultant to Eli Lilly, Sanofi, Merck, Entrinsic Health, Agios, Merrimack Pharmaceuticals, Taiho Pharmaceutical, Genentech, CytomX Therapeutics, Unum Therapeutics, Bain Capital, Bayer, Gilead Sciences, Dicerna, Five Prime Therapeutics, KEW, Celgene, and Pfizer. The remaining authors declare no potential conflicts of interest.
Prior presentations: The study’s preliminary findings were shared at ASCO’s annual meeting in 2019.
Author contributions: [Please use CRediT taxonomy and indicate the role of every author.]
Data Availability
The data underlying this article are from the Alliance for Clinical Trials in Oncology. Investigators may request access to this data per Alliance protocol as detailed at https://www.allianceforclinicaltrialsinoncology.org/main/public/standard.xhtml?path=%2FPublic%2FDatasharing.
Supplementary Material
References
- 1. Van Blarigan EL, Meyerhardt JA.. Role of physical activity and diet after colorectal cancer diagnosis. J Clin Oncol. 2015;33(16):1825-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mills KT, Bellows CF, Hoffman AE, Kelly TN, Gagliardi G.. Diabetes mellitus and colorectal cancer prognosis: a meta-analysis. Dis Colon Rectum. 2013;56(11):1304-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown JC, Zhang S, Ou FS, et al. Diabetes and clinical outcome in patients with metastatic colorectal cancer: CALGB 80405 (Alliance). JNCI Cancer Spectr. 2020;4(1):pkz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2017. Bethesda, MD: National Cancer Institute; 2020. [Google Scholar]
- 5. Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12(3):159-169. [DOI] [PubMed] [Google Scholar]
- 6. Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131(11):3109S-3120S. Suppl): [DOI] [PubMed] [Google Scholar]
- 7. Giovannucci E. Nutrition, insulin, insulin-like growth factors and cancer. Horm Metab Res. 2003;35(11-12):694-704. [DOI] [PubMed] [Google Scholar]
- 8. Brahmkhatri VP, Prasanna C, Atreya HS.. Insulin-like growth factor system in cancer: novel targeted therapies. Biomed Res Int. 2015;2015:1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. An W, Bai Y, Deng SX, et al. Adiponectin levels in patients with colorectal cancer and adenoma: a meta-analysis. Eur J Cancer Prev. 2012;21(2):126-133. [DOI] [PubMed] [Google Scholar]
- 10. Sax AT, Jenkins DG, Devin JL, Hughes GI, Bolam KA, Skinner TL.. The insulin-like growth factor axis: a biological mechanism linking physical activity to colorectal cancer survival. Cancer Epidemiol. 2014;38(4):455-459. [DOI] [PubMed] [Google Scholar]
- 11. Chong DQ, Mehta RS, Song M, et al. Prediagnostic plasma adiponectin and survival among patients with colorectal cancer. Cancer Prev Res (Phila). 2015;8(12):1138-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pankaj J, Kumari JR, Kim W, Lee SA.. Insulin-like growth factor-1, IGF-binding protein-3, C-peptide and colorectal cancer: a case-control study. Asian Pac J Cancer Prev. 2015;16(9):3735-3740. [DOI] [PubMed] [Google Scholar]
- 13. Chi F, Wu R, Zeng YC, Xing R, Liu Y.. Circulation insulin-like growth factor peptides and colorectal cancer risk: an updated systematic review and meta-analysis. Mol Biol Rep. 2013;40(5):3583-3590. [DOI] [PubMed] [Google Scholar]
- 14. Wolpin BM, Meyerhardt JA, Chan AT, et al. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol. 2009;27(2):176-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamanaka Y, Wilson EM, Rosenfeld RG, Oh Y.. Inhibition of insulin receptor activation by insulin-like growth factor binding proteins. J Biol Chem. 1997;272(49):30729-30734. [DOI] [PubMed] [Google Scholar]
- 16. Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. 2017;317(23):2392-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zubrod CG, Schneiderman M, Frei IIE, et al. Appraisal of methods for the study of chemotherapy of cancer in man: comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chronic Dis. 1960;11(1):7-33. [Google Scholar]
- 18. Harris TG, Strickler HD, Yu H, et al. Specimen processing time and measurement of total insulin-like growth factor-I (IGF-1), free IGF-1, and IGF binding protein-3 (IGFBP-3). Growth Horm IGF Res. 2006;16(2):86-92. [DOI] [PubMed] [Google Scholar]
- 19. Chan JM, Stampfer MJ, Giovannucci E, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279(5350):563-566. [DOI] [PubMed] [Google Scholar]
- 20. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205-216. [DOI] [PubMed] [Google Scholar]
- 21. Cox DR. Regression models and life tables (with discussion). J R Stat Soc. 1972;34(2):187-220. [Google Scholar]
- 22. Leighton E, Sainsbury CA, Jones GA.. Practical review of C-peptide testing in diabetes. Diabetes Ther. 2017;8(3):475-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guercio BJ, Zhang S, Venook AP, et al. Body mass index and weight loss in metastatic colorectal cancer in CALGB (Alliance)/SWOG 80405. JNCI Cancer Spectrum. 2020; doi: 10.1093/jncics/pkaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guercio BJ, Zhang S, Ou FS, et al. Associations of physical activity with survival and progression in metastatic colorectal cancer: results from cancer and leukemia group B (Alliance)/SWOG 80405. J Clin Oncol. 2019;37(29):2620-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fuchs CS, Goldberg RM, Sargent DJ, et al. Plasma insulin-like growth factors, insulin-like binding protein-3, and outcome in metastatic colorectal cancer: results from intergroup trial N9741. Clin Cancer Res. 2008;14(24):8263-8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kizer JR, Benkeser D, Arnold AM, et al. Associations of total and high-molecular-weight adiponectin with all-cause and cardiovascular mortality in older persons: the Cardiovascular Health Study. Circulation. 2012;126(25):2951-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Svensson J, Carlzon D, Petzold M, et al. Both low and high serum IGF-1 levels associate with cancer mortality in older men. J Clin Endocrinol Metab. 2012;97(12):4623-4630. [DOI] [PubMed] [Google Scholar]
- 28. Durrleman S, Simon R.. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551-561. [DOI] [PubMed] [Google Scholar]
- 29. Cohen J. Statistical Power Analysis for Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 30. Kawasaki T, Nosho K, Ohnishi M, et al. IGFBP3 promoter methylation in colorectal cancer: relationship with microsatellite instability, CpG island methylator phenotype, and p53. Neoplasia. 2007;9(12):1091-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cohen P. Insulin-like growth factor binding protein-3: insulin-like growth factor independence comes of age. Endocrinology. 2006;147(5):2109-2111. [DOI] [PubMed] [Google Scholar]
- 32. Lopez-Bermejo A, Khosravi J, Fernandez-Real JM, et al. Insulin resistance is associated with increased serum concentration of IGF-binding protein-related protein 1 (IGFBP-rP1/MAC25). Diabetes. 2006;55(8):2333-2339. [DOI] [PubMed] [Google Scholar]
- 33. Burren CP, Wilson EM, Hwa V, Oh Y, Rosenfeld RG.. Binding properties and distribution of insulin-like growth factor binding protein-related protein 3 (IGFBP-rP3/NovH), an additional member of the IGFBP Superfamily. J Clin Endocrinol Metab. 1999;84(3):1096-1103. [DOI] [PubMed] [Google Scholar]
- 34. Oh Y, Nagalla SR, Yamanaka Y, Kim HS, Wilson E, Rosenfeld RG.. Synthesis and characterization of insulin-like growth factor-binding protein (IGFBP)-7. Recombinant human mac25 protein specifically binds IGF-1 and -II. J Biol Chem. 1996;271(48):30322-30325. [DOI] [PubMed] [Google Scholar]
- 35. Ruan W, Xu E, Xu F, et al. IGFBP7 plays a potential tumor suppressor role in colorectal carcinogenesis. Cancer Biol Ther. 2007;6(3):354-359. [DOI] [PubMed] [Google Scholar]
- 36. Vizioli MG, Sensi M, Miranda C, et al. IGFBP7: an oncosuppressor gene in thyroid carcinogenesis. Oncogene. 2010;29(26):3835-3844. [DOI] [PubMed] [Google Scholar]
- 37. Tomimaru Y, Eguchi H, Wada H, et al. IGFBP7 downregulation is associated with tumor progression and clinical outcome in hepatocellular carcinoma. Int J Cancer. 2012;130(2):319-327. [DOI] [PubMed] [Google Scholar]
- 38. Ahmed S, Jin X, Yagi M, et al. Identification of membrane-bound serine proteinase matriptase as processing enzyme of insulin-like growth factor binding protein-related protein-1 (IGFBP-rP1/angiomodulin/mac25). FEBS J. 2006;273(3):615-627. [DOI] [PubMed] [Google Scholar]
- 39. Lin J, Lai M, Huang Q, Ruan W, Ma Y, Cui J.. Reactivation of IGFBP7 by DNA demethylation inhibits human colon cancer cell growth in vitro. Cancer Biol Ther. 2008;7(12):1896-1900. [DOI] [PubMed] [Google Scholar]
- 40. Evdokimova V, Tognon CE, Benatar T, et al. IGFBP7 binds to the IGF-1 receptor and blocks its activation by insulin-like growth factors. Sci Signal. 2012;5(255):ra92. [DOI] [PubMed] [Google Scholar]
- 41. Jiang W, Xiang C, Cazacu S, Brodie C, Mikkelsen T.. Insulin-like growth factor binding protein 7 mediates glioma cell growth and migration. Neoplasia. 2008;10(12):1335-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Degeorges A, Wang F, Frierson HF Jr, Seth A, Chung LW, Sikes RA.. Human prostate cancer expresses the low affinity insulin-like growth factor binding protein IGFBP-rP1. Cancer Res. 1999;59(12):2787-2790. [PubMed] [Google Scholar]
- 43. van Beijnum JR, Dings RP, van der Linden E, et al. Gene expression of tumor angiogenesis dissected: specific targeting of colon cancer angiogenic vasculature. Blood. 2006;108(7):2339-2348. [DOI] [PubMed] [Google Scholar]
- 44. Bieche I, Lerebours F, Tozlu S, Espie M, Marty M, Lidereau R.. Molecular profiling of inflammatory breast cancer: identification of a poor-prognosis gene expression signature. Clin Cancer Res. 2004;10(20):6789-6795. [DOI] [PubMed] [Google Scholar]
- 45. Benassi MS, Pazzaglia L, Novello C, et al. Tissue and serum IGFBP7 protein as biomarker in high-grade soft tissue sarcoma. Am J Cancer Res. 2015;5(11):3446-3454. [PMC free article] [PubMed] [Google Scholar]
- 46. Bolomsky A, Hose D, Schreder M, et al. Insulin like growth factor binding protein 7 (IGFBP7) expression is linked to poor prognosis but may protect from bone disease in multiple myeloma. J Hematol Oncol. 2015;8(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Adachi Y, Itoh F, Yamamoto H, et al. Expression of angiomodulin (tumor-derived adhesion factor/mac25) in invading tumor cells correlates with poor prognosis in human colorectal cancer. Int J Cancer. 2001;95(4):216-222. [DOI] [PubMed] [Google Scholar]
- 48. Slater T, Haywood NJ, Matthews C, Cheema H, Wheatcroft SB.. Insulin-like growth factor binding proteins and angiogenesis: From cancer to cardiovascular disease. Cytokine Growth Factor Rev. 2019;46:28-35. [DOI] [PubMed] [Google Scholar]
- 49. Rupp C, Scherzer M, Rudisch A, et al. IGFBP7, a novel tumor stroma marker, with growth-promoting effects in colon cancer through a paracrine tumor-stroma interaction. Oncogene. 2015;34(7):815-825. [DOI] [PubMed] [Google Scholar]
- 50. Haydon AM, Macinnis RJ, English DR, Morris H, Giles GG.. Physical activity, insulin-like growth factor 1, insulin-like growth factor binding protein 3, and survival from colorectal cancer. Gut. 2006;55(5):689-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Perez-Carbonell L, Balaguer F, Toiyama Y, et al. IGFBP3 methylation is a novel diagnostic and predictive biomarker in colorectal cancer. PLoS One. 2014;9(8):e104285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fu T, Pappou EP, Guzzetta AA, et al. IGFBP-3 gene methylation in primary tumor predicts recurrence of stage II colorectal cancers. Ann Surg. 2016;263(2):337-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Volkova E, Willis JA, Wells JE, Robinson BA, Dachs GU, Currie MJ.. Association of angiopoietin-2, C-reactive protein and markers of obesity and insulin resistance with survival outcome in colorectal cancer. Br J Cancer. 2011;104(1):51-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hulver MW, Zheng D, Tanner CJ, et al. Adiponectin is not altered with exercise training despite enhanced insulin action. Am J Physiol Endocrinol Metab. 2002;283(4):E861-E865. [DOI] [PubMed] [Google Scholar]
- 55. Pais R, Silaghi H, Silaghi AC, Rusu ML, Dumitrascu DL.. Metabolic syndrome and risk of subsequent colorectal cancer. World J Gastroenterol. 2009;15(41):5141-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fruhbeck G, Catalan V, Rodriguez A, et al. Involvement of the leptin-adiponectin axis in inflammation and oxidative stress in the metabolic syndrome. Sci Rep. 2017;7(1):6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are from the Alliance for Clinical Trials in Oncology. Investigators may request access to this data per Alliance protocol as detailed at https://www.allianceforclinicaltrialsinoncology.org/main/public/standard.xhtml?path=%2FPublic%2FDatasharing.