Abstract
Purpose:
Sedentary time relates to higher anxiety and more negative affect in children. This study assessed whether interrupting sitting over 3 hours is sufficient to influence state anxiety, positive affect, or negative affect, and tested weight status as a moderator.
Methods:
Analyses were the second (preplanned) purpose of a larger study. Children (N = 61; age: mean [SD] = 9.5 [1.3]; 43% healthy weight) completed 2 experimental conditions: continuous sitting for 3 hours and sitting for 3 hours interrupted with walking for 3 minutes in every 30 minutes. State anxiety, positive affect, and negative affect were reported at pretest and posttest. Multilevel models for repeated measures assessed whether experimental condition predicted posttest scores.
Results:
Experimental condition was unrelated to posttest state anxiety or positive affect. Weight status moderated how experimental condition influenced posttest negative affect (P = .003). Negative affect was lower in the children of healthy weight after interrupted sitting (vs continuous sitting; β = −0.8; 95% confidence interval, −1.5 to 0.0, P = .05), but it was higher in the children with overweight/obesity after interrupted sitting (vs continuous sitting; β = 0.6; 95% confidence interval, 0.0 to 1.2, P = .06).
Conclusions:
Interrupting sitting acutely reduced negative affect in children of healthy weight, but not in children with overweight. Further research is needed to better understand the potential emotional benefits of sitting interruptions in youth.
Keywords: pediatrics, sedentary behavior, mood, experimental research
Childhood overweight and obesity remain prevalent (31) and is associated with an increased risk for adult obesity (19), certain cancers (24), and cardiometabolic morbidity later in life (44). Pediatric obesity is also associated with numerous detrimental mental health outcomes (42). Children with obesity are twice as likely to suffer from anxiety compared with their healthy weight peers (53). Furthermore, a recent longitudinal study indicated that compared with their healthy weight counterparts, children with overweight and obesity are more likely to demonstrate elevated negative affect and decreased positive affect during adolescence (23).
Emerging epidemiological evidence suggests that sedentary behavior (SB), a constellation of low-energy behaviors performed in the sitting or lying position, such as time spent for watching television or computer screens, is associated with increased cardiometabolic risk (10,11,25) and poorer emotional health (51,59) independent of physical activity. Observational studies indicate that SB is positively associated with risk of anxiety (54), and that increases in screen time are linked to increases in anxiety throughout adolescence (22). Furthermore, high levels of SB have been found to have an association with negative affective states related to depression (52), and adolescents who are more sedentary at a given time in the day compared with their typical level of SB are at greater risk for subsequent decreased positive affect (57). Thus, SB may have detrimental effects on both anxiety and affective states, which could increase the risk for depression in children. Subsequently, even more concerning, youth with greater depressive symptoms compared with their peers are more likely to engage in additional detrimental health behaviors (2,12), further increasing health risk.
One proposed strategy to ameliorate the negative consequences of excessive SB is to interrupt sitting time with short activity breaks (3,6,9,35). Findings from randomized controlled trials show that long-term physical activity interventions (eg, multiple days per week, for at least 4 wk) can reduce anxiety in youth, regardless of exercise intensity (29). Acutely, short bouts of exercise have been shown to be an effective method for increasing positive affect (43); however, a recent review of the acute effects of exercise on affective states across all ages highlights the heterogeneity of exercise protocols (intensity and duration), making it difficult to determine the minimum or optimal dose of activity needed to achieve affective improvements (4). The opponent-process theory postulates that a stressor invokes an initial psychological response, followed by a compensatory psychological response of the opposite valence (eg, pleasure/displeasure [47]); this theory has been applied to the context of postexercise improvements in affective states in adults (33,41). Studies of affective responses to exercise in youth are suggestive that this theory may also be generalized to younger ages; affective states decline during bouts of both moderate and vigorous exercise, followed by rapid improvements in affect immediately following the conclusion of physical activity (46,50).
Although changes in anxiety and affect are observed after physical activity, it is currently unknown whether interrupting SB with short periods of moderate-intensity activity will have similar beneficial effects in children. To our knowledge, the extant literature on in-lab interruptions of SB with short activity bouts and subsequent affective outcomes is limited to adults (8); which may be attributed to the potential applicability to the workplace environment (13,32). However, with more than half of the school day comprised of sitting (1), children also spend a large portion of their time being sedentary (40). Therefore, there is a need for a greater understanding of the effects of interrupting sitting on affective outcomes in youth. Thus, the goal of this analysis is to examine the effects of prolonged sitting time versus interrupting sitting time with short activity bouts on state anxiety, positive affect, and negative affect in children aged 7–11 years in an in-lab randomized crossover study. In partial alignment with the opponent-process theory, we hypothesized that after the interrupted sitting condition, participants would experience less state anxiety, more positive affect, and less negative affect, compared with after the prolonged sitting condition. We also investigated whether weight status moderated the relationship between experimental condition and the anxiety and affective outcomes.
Methods
Study Overview
This analysis was the second (preplanned) purpose of a larger study; therefore, data in this report are from participants in a randomized crossover trial examining the effects of interrupting children’s sitting time on metabolic outcomes, which was carried out in 2 phases (data collected first from children of healthy weight and then from children with overweight or obesity) (6,9) (ClinicalTrials.gov registration no. NCT01888939). Participants were seen at the National Institutes of Health (NIH) Clinical Center for a baseline screening visit to assess participant eligibility. Children who were eligible provided assent, whereas their parents provided informed consent. They then returned to the NIH Clinical Center on 2 more occasions to complete both experimental conditions (uninterrupted sitting and interrupted sitting with walking) in random order with a washout period of 7 to 30 days in between experimental visits; this washout time frame was originally established to minimize any metabolic carry over effects of the experimental condition, according to previous studies in children and adults (14,49). Furthermore, a recent study of the effects of interrupting sitting on affective outcomes in adults also utilized a 7-day minimum washout period (8). Randomization was stratified by sex and participants were assigned 1:1 to uninterrupted sitting or interrupted sitting with walking at their first experimental visit using random permutations (www.randomization.com). Study team members assigned the participants to the experimental conditions and were, therefore, not blinded to the experimental order. Once the anticipated number of participants was reached, the trial concluded. This trial was approved by the Eunice Kennedy Shriver National Institute of Child Health and human development institutional review board.
Participants
Children (aged 7–11 y) in good general health were recruited between June 2013 and January 2017 via flyers, listservs, word-of-mouth, and social media in the surrounding geographical region.
Baseline Screening Visit
As determined with a standard history and physical, children with cardiac or pulmonary disease, evidence of type 2 diabetes, presence of endocrinologic disorders leading to obesity, or taking medications for attention-deficit/hyperactivity disorder were excluded. Body composition (via dual-energy X-ray absorptiometry) measures were then taken. Participants were classified as having healthy weight (body mass index below the 85th percentile) or overweight/obesity (body mass index ≥85th percentile), according to Centers for Disease Control and Prevention standards (28). Children completed a VO2max fitness test using a modified Balke continuous ramp protocol. Ventilatory threshold was estimated by gas exchange using the V-slope method and dual criteria graphs (5). The fitness test results were then utilized to determine the individualized moderate-intensity walking speed and treadmill grade for each participant during the interrupted sitting condition.
Experimental Visits
The experimental conditions were:
Uninterrupted sitting (SIT): Participants were seated for 3 hours with limited movement, and only rising for bathroom use.
Sitting interrupted with walking (SIT+WALK): Participants walked on a treadmill at their individualized walking speed and treadmill grade (80% of ventilatory threshold) for 3 minutes every 30 minutes (eg, minutes 27–30, 57–60, etc). The participants walked a total of 18 minutes in the SIT+WALK condition. The SIT+WALK protocol was designed for the larger study of the metabolic effects of interrupting sitting, the activity dose and timing was based on previous work in adults (14), is consistent with other experimental studies of the benefits of breaking up prolonged sitting time (7), and has the potential for extrapolation into real-world interventions.
Participants reported to the NIH Hatfield Clinical Center Metabolic Unit at approximately 7:30 AM for experimental visits. At pretest, participants completed the affect and state anxiety questionnaires. Children then completed the experimental condition (SIT or SIT+WALK) assigned for that test visit. Immediately following the 3-hour condition (eg, within 10 min), the affect and state anxiety questionnaires were again completed. The affect and state anxiety questionnaires were obtained at both test visits such that each child had pretest and posttest measures of affect and state anxiety for each experimental condition. The research team was present throughout the experimental visits to assist participants.
Measures
Affective States.
Positive affect and negative affect were measured with the 10-item Positive and Negative Affect Scale-Child, which has been validated for use in children as young as 6 years old (17,30). The Positive and Negative Affect Scale-Child contains 5 items to measure positive affect (happy, cheerful, etc) and 5 items to measure negative affect (sad, miserable, etc). Participants were asked to report the extent to which they were currently feeling each item, with response options ranging from 1 (very slightly to not at all) to 5 (extremely). Thus, scores can range from 5 to 25 for each affect subscale (positive affect: Cronbach α = .91; negative affect: Cronbach α = .72).
State Anxiety.
The State Trait Anxiety Inventory for Children was used to measure state anxiety (48). The State Trait Anxiety Inventory for Children has demonstrated favorable psychometric properties in children as young as kindergarten age (39). The State Trait Anxiety Inventory for Children consists of two 20-item scales, one intended to measure state anxiety and the other to measure trait anxiety; however, participants only reported on state anxiety in this study. Participants were asked to report the extent to which they felt each item (nervous, jittery, scared, etc) at that very moment. Responses ranged from 1 (not) to 3 (very) (eg, not nervous, nervous, very nervous). Scores on this scale can range from 20 to 60, with higher scores indicating more state anxiety (Cronbach α = .83).
Statistical Analysis
Frequencies and/or means were calculated for all variables. Correlations assessed the association between posttest affective and state anxiety measures. All correlations were run within each experimental condition (SIT or SIT+WALK). Independent samples t tests assessed differences in posttest affective and state anxiety measures by weight status, stratified by experimental condition. Mean (SE) differences in posttest variables (SIT − SIT+WALK) were also calculated and plotted by weight status such that a positive value indicated a higher posttest score in the SIT condition (compared with the SIT+WALK condition), whereas a negative value indicated a higher posttest score in the SIT+WALK condition (compared with the SIT condition).
Separate multilevel models for repeated measures examined the association between experimental condition and each affective outcome. Multilevel models for repeated measures are often used in psychophysiological research where multiple measures are taken within participants (eg, affective states and state anxiety for both experimental conditions) for their ability to characterize individual-level (rather than group level) effects (27); therefore, these models do not assume that the average group level affective and state anxiety responses to each experimental condition are representative of each individual response. A priori covariates included randomization order and pretest scores. Sex, age, and race (white vs non-white) were tested as confounders one at a time by using the change in estimate criterion of 15% (56). Only race demonstrated significant confounding and was, therefore, included in all final models. Weight status (healthy weight vs overweight/obese) was tested as a moderator by further entering the main effect of weight status in addition to the interaction term between weight status and experimental condition into each model. The main effect of weight status and the interaction term between weight status and experimental condition were only retained in the final model if the interaction term was significant. In addition, analyses were stratified by weight status following a significant interaction term at the P < .05 level. Finally, Cohen f2, a measure of effect size appropriate for multilevel models, was calculated using established methods outlined elsewhere (45). All analyses were conducted using SAS (version 9.4; SAS Institute, Cary, NC).
Results
Figure 1 presents a diagram of the participant flow. The sample (N = 61) was 55.7% male and 47.5% non-Hispanic white. The mean participant age was 9.5 years old (range: 7–11 y). About 43% (N = 26) of the sample had healthy weight, whereas the remaining 57% (N = 35) had overweight/obesity. Participants wore a triaxial accelerometer (ActiGraph GT3X+; ActiGraph, Pensacola, FL) on the nondominant wrist for 7 to 10 days prior to each study visit to measure habitual, free-living activity, and sleep. Data were recorded from the 3 axes at a rate of 80 Hz and the raw accelerometer data were processed with the GGIR R package (55). On average, participants accumulated 25.3 (±14.8) minutes per day of moderate to vigorous physical activity during the free-living observational period prior to each visit. As there were 2 measures (state anxiety and affective states for both experimental conditions) for each of the 61 participants, our analytic sample consisted of 122 observations (level 1) clustered within 61 individuals (level 2).
Figure 1 —
Participant flow diagram.
Among all participants, posttest state anxiety was strongly inversely correlated with posttest positive affect (r = −.66, P < .001) in the SIT condition and in the SIT+WALK condition (r = −.65, P < .001). There were very weak correlations between posttest state anxiety and posttest negative affect in the SIT (r = −.003, P = .98) or the SIT+WALK condition (r = .03, P = .79). Posttest positive affect was very weakly correlated with posttest negative affect in the SIT (r = .09, P = .49) and the SIT+WALK condition (r = .08, P = .56).
State Anxiety
The mean pretest state anxiety score for the SIT condition was 29.2 (range: 20–41) and was 30.4 (range: 21–48) for the SIT+WALK condition (P = .23); the mean posttest state anxiety score for the SIT condition was 28.3 (range: 20–37) and was 28.7 (range: 20–37) for the SIT+WALK condition (P = .53). Mean posttest state anxiety scores in the SIT and SIT+WALK conditions did not differ by weight status (Table 1). Individual pretest and posttest state anxiety scores in the SIT+WALK condition by weight status are plotted in Supplementary Figure 1 (available online). The multilevel model results (Table 2) indicate that experimental condition did not predict posttest state anxiety scores (β = −0.2; 95% confidence interval [CI], −1.1 to 0.7, P = .65, f2 = 0.002). Weight status did not moderate this relationship (β = 0.6; 95% CI, −1.1 to 2.3, P = .51).
Table 1.
Mean Posttest Score in Each Condition by Weight Status (N = 61)
| SIT |
SIT+WALK |
|||||
|---|---|---|---|---|---|---|
| Condition | Healthy weight | Overweight | P value | Healthy weight | Overweight | P value |
| Anxiety | 28.6 | 28.1 | .64 | 28.7 | 28.7 | .97 |
| Positive affect | 14.8 | 15.3 | .73 | 15.2 | 15.4 | .87 |
| Negative affect | 6.0 | 5.1 | .02 | 5.2 | 5.6 | .23 |
Abbreviations: SIT, uninterrupted sitting; SIT+WALK, sitting interrupted with walking. Note: P values derived from independent samples t tests.
Table 2.
Multilevel Model for Repeated-Measures Estimates and 95% CIs for Visit Type Predicting 3 Mood State Outcomes (Level 1: N = 122, Level 2: N = 61)
| State anxiety |
Positive affect |
Negative affect |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Estimate | 95% CI | F value | Estimate | 95% CI | F value | Estimate | 95% CI | F value |
| Visit type | −0.2 | −1.1 to 0.7 | 0.2 | 0.2 | −1.0 to 1.3 | 0.1 | 0.6 | 0.0 to 1.2 | 3.7 |
| Randomization order | −0.1 | −1.4 to 1.1 | 0.1 | 0.2 | −1.1 to 1.6 | 0.1 | −0.3 | −0.8 to 0.2 | 1.1 |
| Pretest variable | 0.5*** | 0.4 to 0.6 | 74.6 | 0.9*** | 0.7 to 1.0 | 221.4 | 0.0 | −0.1 to 0.1 | 0.3 |
| Race | 0.2 | −1.1 to 1.5 | 0.1 | −1.0 | −1.4 to 1.2 | 0.03 | 0.3 | −0.2 to 0.8 | 1.2 |
| Weight status | N/A | N/A | N/A | N/A | N/A | N/A | 0.3 | −0.4 to 1.0 | 0.9 |
| Visit type × weight status | N/A | N/A | N/A | N/A | N/A | N/A | −1.4 ** | −2.3 to −0.5 | 9.4 |
Abbreviations: CIs, confidence intervals; N/A, not applicable; SIT, uninterrupted sitting. Note: SIT visit type is the reference. Predictors marked with “N/A” were not included in the final model due to nonsignificance. Visit type, randomization order, and pretest variable are level 1 predictors. Race and weight status are level 2 predictors. Visit type × weight status is a cross-level interaction.
P < .01.
P < .001.
Positive Affect
The mean pretest positive affect score for the SIT and SIT+WALK conditions was 14.2 (range: 5–25) (P = .97); the mean posttest positive affect score for the SIT condition was 15.1 (range: 5–25) and 15.3 (range: 5–25) for the SIT+WALK condition (P = .88). Mean posttest positive affect scores in the SIT and SIT+WALK conditions did not differ by weight status (Table 1). Individual pretest and posttest positive affect scores in the SIT+WALK condition by weight status are plotted in Supplementary Figure 1 (available online). Experimental condition was not related to posttest positive affect scores (β = 0.2; 95% CI, −1.0 to 1.3, P = .79, f2 = 0.001; Table 2). Weight status did not moderate this relationship (β = 0.3; 95% CI, −1.9 to 2.6, P = .77).
Negative Affect
The mean pretest negative affect score for the SIT condition was 5.5 (range: 4–10) and was 5.9 (range: 4–20) for the SIT+WALK condition (P = .29); the mean posttest score for negative affect for the SIT condition was 5.4 (range: 4–12) and 5.4 (range: 3–10) for the SIT+WALK condition (P = 1.00). The mean posttest score for the children with healthy weight in the SIT condition was 6.0 (range: 4–12), which was significantly higher than the mean score of 5.1 (range: 4–7) in the children with overweight/obesity in the same condition (P = .02). Mean posttest negative affect did not differ by weight status in the SIT+WALK condition (Table 1). Supplementary Figure 1 (available online) shows plots of the individual pretest and posttest negative affect scores in the SIT+WALK condition by weight status. Participant weight status moderated the relationship between experimental condition and posttest negative affect (β = −1.4; 95% CI, −2.3 to −0.5, P = .003; Table 2). The SIT+WALK condition was associated with decreased posttest negative affect in the healthy weight children, compared with the SIT condition (β = −0.8; 95% CI, −1.5 to 0.0, P = .05, f2 = 0.08). Alternatively, the SIT +WALK condition was associated with marginally increased posttest negative affect in the overweight children compared with the SIT condition (β = 0.6; 95% CI, 0.0 to 1.2, P = .06, f2 = 0.04; Figure 2).
Figure 2 —
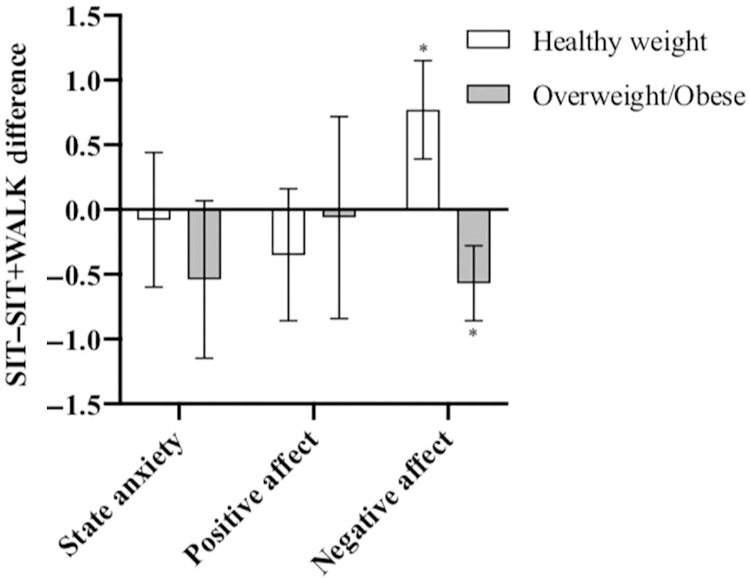
SIT versus SIT+WALK mean posttest score differences, stratified by weight status (N = 61). Note: Mean and SE are presented. The negative values indicate that the posttest score was higher in the SIT +WALK condition compared with the SIT condition. SIT indicates uninterrupted sitting; SIT+WALK, sitting interrupted with walking. *P < .01 for independent samples t test.
Discussion
The aim of this study was to assess the effects of reducing children’s sedentary time via brief walking breaks on subsequent affective and state anxiety outcomes in an in-lab randomized controlled trial. A similar randomized trial conducted in adults has demonstrated the efficacy of interrupting prolonged sitting for improving self-rated mood (8). Although interrupting sitting with minibouts of walking did not influence subsequent state anxiety and positive affect scores in our study, it was associated with decreased negative affect in the children with healthy weight. Interestingly, there was a significant interaction between weight status and treatment group, such that interrupting sitting was associated with higher levels of negative affect among the children with overweight/obesity. It is worth noting that although statistically significant, the effect sizes of the experimental condition on negative affect were small.
A recent review indicates that there is likely an association between sitting time and symptoms of anxiety, such that increasing sitting time may increase risk for anxiety (54). Longitudinally, more screen time in adolescence is associated with greater anxiety up to 11 years later, suggesting that behaviors developed in childhood can have lasting detrimental effects in adulthood (22). The results of our study do not indicate the efficacy of interrupting sedentary time as means of reducing state anxiety; however, we may not have seen significant effects in our trial because the SIT condition was only 3 hours in duration and participants were not selected based on the presence of elevated state anxiety. The existing literature base typically addresses the relationship between overall daily or weekly SB levels and anxiety, and a longer experimental condition may be needed to have an effect on state anxiety via minibouts of walking. In addition, it is possible that the walking dose was too low (18 min in total) in the SIT+WALK condition to influence state anxiety outcomes. Long-term aerobic exercise interventions have been shown to be efficacious in reducing symptoms in youth with anxiety disorders; evidence suggests that interventions comprised of 120 minutes of aerobic exercise per week is sufficient to achieve reduced anxiety (38). Thus, there may conceivably be potential for reducing state anxiety ratings by engaging in higher intensity or longer bouts of walking for sedentary breaks across the week.
Other research suggests that SBs can influence affective outcomes. Observational studies in youth demonstrate consistent associations between SB and negative affect (21,52). In a recent free-living study, Endrighi et al (18) randomly assigned participants to a sedentary condition lasting 2 weeks and found that negative mood ratings significantly increased in the study sample. Our findings are consistent with those of Endrighi et al, as we observed higher ratings of negative affect following the prolonged sitting condition in the children of healthy weight, although our trial was of much shorter duration. Similarly, the reductions in negative affect in the participants with healthy weight following the interrupted sitting condition provide support for the opponent-process theory, as hypothesized. Our findings suggest that negative affect may be more malleable and acutely influenced by manipulations of sedentary time compared with other markers of mood. Because this is, to our knowledge, the first study of interruptions in sitting time with moderate-paced walking in relation to affective states in youth, future work should focus on replicating our findings prior to the development of intervention strategies.
Addressing negative affective states is critical, because adolescents with higher negative mood have been shown to engage in detrimental health behaviors, such as being less physically active (37), which in turn can, increase risk for cardiometabolic diseases. Minibouts of moderate-paced walking to reduce sedentary time may be an effective approach to reducing negative affect; a predictor of obesogenic behaviors. Although our findings are promising, it is important to note that interrupting sitting only reduced negative affect among the children with healthy weight in our sample; and that negative affect appeared to increase marginally as a result of interrupting sitting among the youth with overweight. It is conceivable that those with overweight could have needed a longer amount of time postintervention to experience the reductions in negative affect that their healthy weight counterparts reported. Populations with overweight/obesity are more likely to experience various emotional health detriments, including depressed mood (26,42) and acute exercise can reduce negative affect, even in previously clinically depressed populations (34). Therefore, it may be that a different dose of activity is needed to influence the negative affect ratings of children with overweight/obesity; perhaps a repetitive, sustained intervention is required to demonstrate an effect on negative affect. However, more data are needed to understand if a stronger or weaker intensity of sitting interruptions (compared with our moderate intensity) may be more effective at achieving affective improvements in children with overweight/obesity.
The short activity breaks in our trial did not influence positive affect scores. In contrast, an ecological momentary assessment study of children aged 9–13 years found that positive affect was higher in the 30 minutes following moderate to vigorous physical activity (15). Moreover, a study in young adults using similar methods found that positive affect increased directly following a physical activity bout, and this affective change lasted up to 180 minutes (58). In addition, physical activity has been linked to affective improvements in a number of other populations in various settings (16,20), and even low-intensity physical activity such as walking has been shown to increase positive affect (36). Our findings may be inconsistent with this literature base due to the generally high positive affect scores within our sample. Of the 3 mood state outcomes measured in this study, the children scored highest on positive affect. Therefore, there may have been a ceiling effect resulting in little potential for increased positive affect after the SIT+WALK condition. It is also possible that positive affect was not influenced by prolonged sitting because the children were permitted to watch movies during the 3-hour visits, which may have increased feelings of positive affect. Moreover, self-selected activity may yield different affective experiences compared with the prescribed activity in the present study.
Limitations and Strengths
There are several limitations that warrant discussion. First, the study was originally powered to detect changes in metabolic biomarkers (6,9), therefore, we may have lacked sufficient power to detect changes in affective outcomes; post hoc power calculations indicated that the current sample size (N = 61) was powered to detect only a relatively large effect size (0.87). Having a larger sample with a broader distribution of affective outcome and state anxiety scores may have yielded different results. In addition, measures were only taken at the pretest and posttest time points; measuring affective and anxiety states throughout the duration of each experimental condition (eg, every 30 min) or at later time points may provide greater insight into the acute effects of interrupting sitting on mood states. We also did not collect data on each participant’s comfort level while walking at their individualized treadmill speed and grade; however, evidence in youth indicates that prescribed activity intensity at 80% of ventilatory threshold is comparable with that of self-selected activity (46). Future researchers should collect data on participants’ comfort and perceived exertion during sitting interruptions and investigate how this influences affective outcomes. Finally, participants were permitted to view age-appropriate movies (eg, Cars, Frozen) during the experimental conditions, therefore, it is plausible that our findings may be explained by the combination of both the experimental conditions themselves, and the movies viewed during the conditions. However, if this is the case, then our findings may be more generalizable to the real-world setting, where participants would likely be engaged in screen-based leisure time SB while being prompted to interrupt sitting. Despite the study limitations, our findings do suggest that interrupting prolonged sitting with short bouts of walking may influence affective outcomes, at least among children with healthy weight; future research should aim to replicate and extend these findings using rigorous experimental designs. Strengths of this study include the controlled experimental conditions and the use of previously validated instruments for assessing mood state outcomes. In addition, the range of weight statuses is a strength of the current study, as little research has examined differential effects of interrupting sitting time on mood states among both, children with healthy weight and overweight.
Conclusions
Although the experimental condition did not significantly relate to changes in 2 of the 3 mood state outcomes of interest, it did relate to negative affect within the children with healthy weight. Future research should attempt to gain a greater understanding of the effects of interrupted sitting on affective outcomes in youth by assessing how different “dosages” of walking and different types of activities performed while sedentary may differentially influence affective outcomes. Additional research replicating and extending our findings using rigorous controlled experimental designs can ultimately contribute to a larger body of evidence aimed at informing intervention strategies for reducing negative affect as means of increasing health-promoting behaviors.
Supplementary Material
Acknowledgments
The authors thank the patients and their families for their participation, as well as the National Institutes of Health Clinical Center Staff and the Metabolic Unit staff. B.R.B, D.A.B., and J.A.Y. thank Dr Mai Chin A. Paw for her assistance with power calculations for the study. This study was supported by the Intramural Research Programs of the National Cancer Institute (NCI) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (ZIA-HD-00641; to J.A.Y.) at NIH with supplemental funding from the NIH Office of Disease Prevention, NIH (to J.A.Y.). J.Z. was supported by the Keck/Graduate School Fellowship Award and the NCI T32 Predoctoral Fellowship Award. B.R.B. was supported by a postdoctoral training award from the NCI Cancer Prevention Fellowship Program in the Division of Cancer Prevention. D.A.B. was supported by the NCI, Division of Cancer Control and Population Sciences. M.M.B., S.M.B., R.J.B., K.P.S., D.R.R., P.L.W., A.B.C., S.B.B., and K.Y.C. are federal employees supported by the Intramural Research Program of the NIH. I.L.T. was supported by the NIH Office of the Director. The funding agencies played no role in the decision to submit the article. J.A.Y. and B.E.D. are Commissioned Officers in the US Public Health Service. The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the US Public Health Service.
Footnotes
The authors have no conflicts of interest to report.
Contributor Information
Jennifer Zink, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
David A. Berrigan, Division of Cancer Control & Population Sciences, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
Miranda M. Broadney, Section on Growth and Obesity, Program in Endocrinology, Metabolism and Genetics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA
Faizah Shareef, Section on Growth and Obesity, Program in Endocrinology, Metabolism and Genetics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA.
Alexia Papachristopoulou, Section on Growth and Obesity, Program in Endocrinology, Metabolism and Genetics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA.
Sheila M. Brady, Section on Growth and Obesity, Program in Endocrinology, Metabolism and Genetics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA
Shanna B. Bernstein, Nutrition Department, Clinical Center, National Institutes of Health, Bethesda, MD, USA
Robert J. Brychta, Diabetes, Endocrinology, and Obesity Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA
Jacob D. Hattenbach, Diabetes, Endocrinology, and Obesity Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA
Ira L. Tigner, Jr., Section on Growth and Obesity, Program in Endocrinology, Metabolism and Genetics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA
Amber B. Courville, Nutrition Department, Clinical Center, National Institutes of Health, Bethesda, MD, USA
Bart E. Drinkard, Rehabilitation Medicine Department, Mark O. Hatfield Clinical Research Center, National Institutes of Health, Bethesda, MD, USA
Kevin P. Smith, Nursing Department, Clinical Center, National Institutes of Health, Bethesda, MD, USA
Douglas R. Rosing, Cardiovascular Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA
Pamela L. Wolters, Pediatric Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
Kong Y. Chen, Diabetes, Endocrinology, and Obesity Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA
Jack A. Yanovski, Section on Growth and Obesity, Program in Endocrinology, Metabolism and Genetics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA
Britni R. Belcher, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
References
- 1.Aminian S, Duncan S, White K, Hinckson EA. Using the ActivPAL monitor to quantify time spent sitting, standing and stepping at school: a one-day snapshot. J Sci Res Rep. 2014;3(6):866–73. [Google Scholar]
- 2.Armstrong B, Westen SC, Janicke DM. The role of overweight perception and depressive symptoms in child and adolescent unhealthy weight control behaviors: a mediation model. J Pediatr Psychol. 2013;39(3):340–8. 10.1093/jpepsy/jst091 [DOI] [PubMed] [Google Scholar]
- 3.Bailey DP, Locke CD. Breaking up prolonged sitting with light-intensity walking improves postprandial glycemia, but breaking up sitting with standing does not. J Sci Med Sport. 2015;18(3):294–8. 10.1016/j.jsams.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 4.Basso JC, Suzuki WA. The effects of acute exercise on mood, cognition, neurophysiology, and neurochemical pathways: a review. Brain Plast. 2017;2(2):127–52. 10.3233/BPL-160040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60(6): 2020–7. 10.1152/jappl.1986.60.6.2020 [DOI] [PubMed] [Google Scholar]
- 6.Belcher BR, Berrigan D, Papachristopoulou A, et al. Effects of interrupting children’s sedentary behaviors with activity on metabolic function: a randomized trial. J Clin Endocrinol Metab. 2015;100(10): 3735–43. 10.1210/jc.2015-2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benatti FB, Ried-Larsen M. The effects of breaking up prolonged sitting time: a review of experimental studies. Med Sci Sports Exerc. 2015;47(10):2053–61. 10.1249/MSS.0000000000000654 [DOI] [PubMed] [Google Scholar]
- 8.Bergouignan A, Legget KT, De Jong N, et al. Effect of frequent interruptions of prolonged sitting on self-perceived levels of energy, mood, food cravings and cognitive function. Int J Behav Nutr Phys Act. 2016;13(1):113 10.1186/s12966-016-0437-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broadney MM, Belcher BR, Berrigan DA, et al. Effects of interrupting sedentary behavior with short bouts of moderate physical activity on glucose tolerance in children with overweight and obesity: a randomized, crossover trial. Diabetes Care. 2018;41(10):2220–2. 10.2337/dc18-0774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byun W, Dowda M, Pate RR. Associations between screen-based sedentary behavior and cardiovascular disease risk factors in Korean youth. J Korean Med Sci. 2012;27(4):388–94. 10.3346/jkms.2012.27.4.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carson V, Janssen I. Volume, patterns, and types of sedentary behavior and cardio-metabolic health in children and adolescents: a cross-sectional study. BMC Public Health. 2011;11:274–84. 10.1186/1471-2458-11-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castillo F, Francis L, Wylie-Rosett J, Isasi CR. Depressive symptoms are associated with excess weight and unhealthier lifestyle behaviors in urban adolescents. Child Obes. 2014;10(5):400–7. 10.1089/chi.2014.0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Jong N, Debache I, Pan Z, et al. Breaking up sedentary time in overweight/obese adults on work days and non-work days: results from a feasibility study. Int J Environ Res Public Health. 2018; 15(11):2566 10.3390/ijerph15112566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunstan DW, Kingwell BA, Larsen R, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35(5):976–83. 10.2337/dc11-1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunton GF, Huh J, Leventhal A, et al. Momentary assessment of affect, physical feeling states, and physical activity in children. Health Psychol. 2014;33(3):255–63. 10.1037/a0032640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunton GF, Liao Y, Intille S, Huh J, Leventhal A. Momentary assessment of contextual influences on affective response during physical activity. Health Psychol. 2015;34(12):1145–53. 10.1037/hea0000223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebesutani C, Regan J, Smith A, Reise S, Higa-McMillan C, Chorpita BF. The 10-item positive and negative affect schedule for children, child and parent shortened versions: application of item response theory for more efficient assessment. J Psychopathol Behav Assess. 2012;34(2):191–203. 10.1007/s10862-011-9273-2 [DOI] [Google Scholar]
- 18.Endrighi R, Steptoe A, Hamer M. The effect of experimentally induced sedentariness on mood and psychobiological responses to mental stress. Br J Psychiatr. 2016;208(3):245–51. 10.1192/bjp.bp.114.150755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics. 2001;108(3): 712–8. 10.1542/peds.108.3.712 [DOI] [PubMed] [Google Scholar]
- 20.Gauvin L, Rejeski WJ, Norris JL. A naturalistic study of the impact of acute physical activity on feeling states and affect in women. Health Psychol. 1996;15(5):391–7. 10.1037/0278-6133.15.5.391 [DOI] [PubMed] [Google Scholar]
- 21.Goldfield GS, Murray M, Maras D, et al. Screen time is associated with depressive symptomatology among obese adolescents: a HEARTY study. Eur J Pediatr. 2016;175(7):909–19. 10.1007/s00431-016-2720-z [DOI] [PubMed] [Google Scholar]
- 22.Gunnell KE, Flament MF, Buchholz A, et al. Examining the bidirectional relationship between physical activity, screen time, and symptoms of anxiety and depression over time during adolescence. Prev Med. 2016;88:147–52. 10.1016/j.ypmed.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 23.Jeffery AN, Hyland ME, Hosking J, Wilkin TJ. Mood and its association with metabolic health in adolescents: a longitudinal study, EarlyBird 65. Pediatr Diabetes. 2014;15(8):599–605. 10.1111/pedi.12125 [DOI] [PubMed] [Google Scholar]
- 24.Jeffreys M, Davey Smith G, Martin RM, Frankel S, Gunnell D. Childhood body mass index and later cancer risk: a 50-year follow-up of the Boyd Orr study. Int J Cancer. 2004;112(2):348–51. 10.1002/ijc.20423 [DOI] [PubMed] [Google Scholar]
- 25.Keane E, Li X, Harrington JM, Fitzgerald AP, Perry IJ, Kearney PM. Physical activity, sedentary behavior and the risk of overweight and obesity in school-aged children. Pediatr Exerc Sci. 2017;29(3):408–18. 10.1123/pes.2016-0234 [DOI] [PubMed] [Google Scholar]
- 26.Kim JY, Oh DJ, Yoon TY, Choi JM, Choe BK. The impacts of obesity on psychological well-being: a cross-sectional study about depressive mood and quality of life. J Prev Med Public Health. 2007;40(2):191–5. 10.3961/jpmph.2007.40.2.191 [DOI] [PubMed] [Google Scholar]
- 27.Kristjansson SD, Kircher JC, Webb AK. Multilevel models for repeated measures research designs in psychophysiology: an introduction to growth curve modeling. Psychophysiology. 2007;44(5):728–36. 10.1111/j.1469-8986.2007.00544.x [DOI] [PubMed] [Google Scholar]
- 28.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000(314):1–27. [PubMed] [Google Scholar]
- 29.Larun L, Nordheim LV, Ekeland E, Hagen KB, Heian F. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database Syst Rev. 2006(3):Cd004691. [DOI] [PubMed] [Google Scholar]
- 30.Laurent J, Catanzaro SJ, Joiner TE Jr,et al. A measure of positive and negative affect for children: scale development and preliminary validation. Psycholog Assess. 1999;11(3):326 10.1037/1040-3590.11.3.326 [DOI] [Google Scholar]
- 31.Lo JC, Chandra M, Sinaiko A, et al. Severe obesity in children: prevalence, persistence and relation to hypertension. Int J Pediatr Endocrinol. 2014;2014(1):3 10.1186/1687-9856-2014-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mailey EL, Rosenkranz SK, Ablah E, Swank A, Casey K. Effects of an intervention to reduce sitting at work on arousal, fatigue, and mood among sedentary female employees. J Occup Environ Med. 2017; 59(12):1166–71. 10.1097/JOM.0000000000001131 [DOI] [PubMed] [Google Scholar]
- 33.Markowitz SM, Arent SM. The exercise and affect relationship: evidence for the dual-mode model and a modified opponent process theory. J Sport Exerc Psychol. 2010;32(5):711–30. 10.1123/jsep.32.5.711 [DOI] [PubMed] [Google Scholar]
- 34.Mata J, Hogan CL, Joormann J, Waugh CE, Gotlib IH. Acute exercise attenuates negative affect following repeated sad mood inductions in persons who have recovered from depression. J Abnorm Psychol. 2013;122(1):45–50. 10.1037/a0029881 [DOI] [PubMed] [Google Scholar]
- 35.Maylor BD, Zakrzewski-Fruer JK, Orton CJ, Bailey DP. Beneficial postprandial lipaemic effects of interrupting sedentary time with high-intensity physical activity versus a continuous moderate-intensity physical activity bout: a randomised crossover trial. J Sci Med Sport. 2018;21(12):1250–5. 10.1016/j.jsams.2018.05.022 [DOI] [PubMed] [Google Scholar]
- 36.Miller JC, Krizan Z. Walking facilitates positive affect (even when expecting the opposite). Emotion. 2016;16(5):775–85. 10.1037/a0040270 [DOI] [PubMed] [Google Scholar]
- 37.Morrison EJ, Clark MM, Wieland ML, et al. Relationship between negative mood and health behaviors in an immigrant and refugee population. J Immigr Minor Health. 2016;19(3):655–64. 10.1007/s10903-016-0506-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newman CL, Motta RW. The effects of aerobic exercise on childhood PTSD, anxiety, and depression. Int J Emerg Ment Health. 2007; 9(2):133–58. [PubMed] [Google Scholar]
- 39.Papay JP, Spielberger CD. Assessment of anxiety and achievement in kindergarten and first-and second-grade children. J Abnorm Child Psychol. 1986;14(2):279–86. 10.1007/BF00915446 [DOI] [PubMed] [Google Scholar]
- 40.Pate RR, Mitchell JA, Byun W, Dowda M. Sedentary behaviour in youth. Br J Sports Med. 2011;45(11):906–13. 10.1136/bjsports-2011-090192 [DOI] [PubMed] [Google Scholar]
- 41.Petruzzello SJ, Jones A, Tate A. Affective responses to acute exercise: a test of opponent-process theory. J Sports Med Phys Fitness. 1997;37(3):205–12. [PubMed] [Google Scholar]
- 42.Rankin J, Matthews L, Cobley S, et al. Psychological consequences of childhood obesity: psychiatric comorbidity and prevention. Adolesc Health Med Ther. 2016;7:125–46. 10.2147/AHMT.S101631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reed J, Ones DS. The effect of acute aerobic exercise on positive activated affect: a meta-analysis. Psychol Sport Exerc. 2006;7(5): 477–514. 10.1016/j.psychsport.2005.11.003 [DOI] [Google Scholar]
- 44.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes. 2011;35(7):891 10.1038/ijo.2010.222 [DOI] [PubMed] [Google Scholar]
- 45.Selya AS, Rose JS, Dierker LC, Hedeker D, Mermelstein RJ. A practical guide to calculating Cohen’s f2, a measure of local effect size, from PROC MIXED. Front Psychol. 2012;3:111 10.3389/fpsyg.2012.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheppard KE, Parfitt G. Acute affective responses to prescribed and self-selected exercise intensities in young adolescent boys and girls. Pediatr Exerc Sci. 2008;20(2):129–41. 10.1123/pes.20.2.129 [DOI] [PubMed] [Google Scholar]
- 47.Solomon RL. The opponent-process theory of acquired motivation: the costs of pleasure and the benefits of pain. Am Psychol. 1980; 35(8):691 10.1037/0003-066X.35.8.691 [DOI] [PubMed] [Google Scholar]
- 48.Spielberger CD. State-Trait Anxiety Inventory for Children. Palo Alto, CA: Consulting Psychologists Press; 1973. [Google Scholar]
- 49.Spruijt-Metz D, Belcher B, Anderson D, et al. A high sugar, low fiber meal leads to higher leptin and physical activity levels in overweight Latina females as opposed to a low sugar, high fiber meal. J Am Diet Assoc. 2009;109(6):1058–63. 10.1016/j.jada.2009.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stych K, Parfitt G. Exploring affective responses to different exercise intensities in low-active young adolescents. J Sport Exerc Psychol. 2011;33(4):548–68. 10.1123/jsep.33.4.548 [DOI] [PubMed] [Google Scholar]
- 51.Suchert V, Hanewinkel R, Isensee B. Sedentary behavior and indicators of mental health in school-aged children and adolescents: a systematic review. Prev Med. 2015;76:48–57. 10.1016/j.ypmed.2015.03.026 [DOI] [PubMed] [Google Scholar]
- 52.Sund AM, Larsson B, Wichstrom L. Role of physical and sedentary activities in the development of depressive symptoms in early adolescence. Soc Psychiatry Psychiatr Epidemiol. 2011;46(5):431–41. 10.1007/s00127-010-0208-0 [DOI] [PubMed] [Google Scholar]
- 53.Tevie J, Shaya FT. Anxiety and comorbid obesity and hypertension in United States children. Value Health. 2014;17(3):A193. [Google Scholar]
- 54.Teychenne M, Costigan SA, Parker K. The association between sedentary behaviour and risk of anxiety: a systematic review. BMC Public Health. 2015;15:513 10.1186/s12889-015-1843-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Hees VT, Fang Z, Langford J, et al. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: an evaluation on four continents. J Appl Physiol. 2014;117(7):738–44. 10.1152/japplphysiol.00421.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walter S, Tiemeier H. Variable selection: current practice in epidemiological studies. Eur J Epidemiol. 2009;24(12):733 10.1007/s10654-009-9411-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wen CKF, Liao Y, Maher JP, et al. Relationships among affective states, physical activity, and sedentary behavior in children: moderation by perceived stress. Health Psychol. 2018;37(10):904 10.1037/hea0000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wichers M, Peeters F, Rutten BP, et al. A time-lagged momentary assessment study on daily life physical activity and affect. Health Psychol. 2012;31(2):135–44. 10.1037/a0025688 [DOI] [PubMed] [Google Scholar]
- 59.Zink J, Belcher BR, Kechter A, Stone MD, Leventhal AM. Reciprocal associations between screen time and emotional disorder symptoms during adolescence. Prev Med Rep. 2019;13:281–8. 10.1016/j.pmedr.2019.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.