Abstract
Objective:
The purpose of this study was to explore sleep quality and to determine whether chemotherapy-induced peripheral neurotoxicity is a risk factor for poor sleep quality in breast cancer survivors who receive docetaxel treatment.
Methods:
Secondary data analysis from a cross-sectional study. Sample characteristics were collected using an information sheet. Independent variables included the Hospital Anxiety and Depression Scale (HADS), the Patient Neurotoxicity Questionnaire (PNQ), and the Identification Pain Questionnaire (ID pain). Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI). We performed descriptive analyses and simple logistic regression.
Results:
A total of 98 participants were included. More than 60% of them reported poor sleep quality, with their average PSQI score being 7.54 ± 4.45. Poor subjective sleep quality (1.37 ± 0.88) and short sleep duration (1.37 ± 1.08) were their main problems. In addition, significant risk factors for poor sleep quality were chronic illness (odds ratio [OR] = 2.753, P = 0.041), anxiety (OR = 7.714, P = 0.009), neuropathic pain (OR = 11.261, P = 0.022), sensory neuropathy (OR = 2.529, P = 0.032), motor neuropathy (OR = 3.781, P = 0.002), and undergoing chemotherapy (OR = 2.593, P = 0.027). Targeted therapy that some survivors received served as a protective factor (OR = 0.351, P = 0.015).
Conclusions:
We found a high prevalence of poor sleep quality in breast cancer survivors treated with docetaxel. The results indicated that, in addition to clinical characteristics and psychological discomfort, chemotherapy-induced peripheral neurotoxicity is a significant risk factor for poor sleep quality.
Keywords: Breast cancer, chemotherapy-induced peripheral neurotoxicity, risk factors, sleep quality
Introduction
Poor sleep quality impacts individuals' psychological well-being and cognitive function and diminishes quality of life.[1] Healthy adults with poor sleep quality may be at increased risk for cardiovascular disease, metabolic disease, and cancer.[1] Compared with the general population, cancer survivors have a higher prevalence of sleep problems. Specifically, more than 40% of cancer survivors have been reported in some studies to suffer from sleep problems.[2,3,4,5]
Breast cancer is the most commonly diagnosed cancer among women worldwide,[6] and survivors of the disease often experience poor sleep,[3] with estimates ranging from 14% to 93% of them.[7] Sleep problems have been reported during various phases of survivorship, including during cancer treatment, after the completion of treatment,[8] and during long-term follow-up.[9] In one meta-analyses, breast cancer survivors who were non-Caucasian were found to be more likely to develop sleep problems than those who were Caucasian; additional risk factors for poor sleep included postmenopausal status, pain, emotional distress, fatigue, and hot flashes.[7] A prior systematic review found that breast cancer survivors who received chemotherapy tended to have a poor sleep quality and that sleep problems were more common among breast cancer survivors who did not receive taxane-based chemotherapy than among those who did.[10]
Taxanes, such as docetaxel or paclitaxel, are among the standard antineoplastic agents used in breast cancer chemotherapy. However, a common and challenging adverse effect of these drugs is peripheral neurotoxicity caused by the inhibition of microtubule disassembly.[11] This neurotoxicity, also known as chemotherapy-induced peripheral neuropathy (CIPN), produces such sensory and motor symptoms as tingling, numbness, pain, reduced deep tendon reflex, and muscle cramps and weakness.[11] CIPN occurs in survivors during chemotherapy[12] and continues to present after chemotherapy has been completed.[12,13,14] Cancer survivors with CIPN experience difficulties performing such routine activities as walking, sewing, and opening containers.[15]
Prior studies have also found the severity of CIPN to correlate negatively with the sleep quality of cancer survivors.[16,17,18] However, these studies focused on survivors with colorectal cancer, cervical cancer, and mixed cancer diagnoses and on the severity of CIPN and did not separately report sensory and motor neuropathy. Therefore, the separate effects of sensory neuropathy, motor neuropathy, and neuropathic pain on sleep remain poorly understood. Furthermore, to the best of our knowledge, limited studies have tested whether CIPN is associated with poor sleep quality in breast cancer survivors treated with docetaxel. Accordingly, the aim of the current study was to investigate (1) the sleep quality of breast cancer survivors who have received docetaxel treatment and (2) whether sensory CIPN, motor CIPN, and/or neuropathic pain are potential risk factors for poor sleep quality.
Methods
Design and sample
This study was a secondary analysis of data from an earlier cross-sectional study. The parent study focused on the prevalence and symptoms of CIPN and its effects on function and quality of life among breast cancer survivors receiving chemotherapy; it was conducted in a medical center in Northern Taiwan from April 2015 to June 2016.[19] The study was approved by the Institutional Review Board (IRB) of Taipei Veterans General Hospital (Approval No. 2015-01-003CC). The participants in the parent study who had been diagnosed with breast cancer within the past 5 years, had received as least one cycle of docetaxel, and had never been treated with platinum-based antineoplastic drugs or paclitaxel were included in the present study, a total of 98 individuals.
Measures
The demographic information collected from participants in the parent study included age, education level, employment, income, and levels of smoking, drinking, and regular exercise. The clinical information collected included the presence of chronic illness, menopausal status, cancer stage, recurrence, lymph node involvement, surgical history, any chemotherapy regimen, targeted therapy, radiation therapy, or hormone therapy, anxiety, depression, CIPN, and neuropathic pain.
The Hospital Anxiety and Depression Scale (HADS)[20] was used to screen for anxiety and depression. This scale consists of 14 items, each of which is scored on a scale from 0 to 3. A total score ranging from 0 to 7 is considered normal, a score of 8–11 is considered borderline abnormal, and a score above 11 is considered abnormal.[20] The concurrent validities of anxiety and depression between the Chinese version of the HADS and the mental component summary of the Twelve-Item Short Form Health Survey were −0.53 and −0.52, respectively, in cancer survivors.[21] In the current study, the internal consistency of the HADS depression subscale and anxiety subscale were acceptable to high (Cronbach's α = 0.66 and 0.83, respectively).
The Patient Neurotoxicity Questionnaire (PNQ) includes separate items for self-reporting sensory and motor neuropathy, each of which is ranked from “no symptoms” to “severe symptoms.”[22] The concurrent validities of sensory neuropathy and motor neuropathy between the PNQ and the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity subscale were 0.66 and 0.51, respectively, among breast cancer survivors who received taxane treatment.[23]
The Identification Pain (ID Pain) is a six-item questionnaire used to identify neuropathic pain with total scores ranging from −1 to 5.[24] The Chinese version of the ID Pain had 81% sensitivity and 65% specificity, with a cut-off score of 3; thus, patients with a score of 3 or greater were considered to suffer from neuropathic pain.[25] The internal consistency of the ID Pain in the current study was found to be acceptable (KR reliability = 0.35).
The Pittsburgh Sleep Quality Index (PSQI) was used to assess sleep quality. This 19-item index consists of seven components, including general sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleeping medication, and daytime dysfunction.[26] The index scores ranged from 0 to 21, with a cut-off point of 5, resulting in an 89.6% sensitivity and an 86.5% specificity; thus, a score >5 indicated poor sleep quality.[26] In the current study, the internal consistency of the PSQI was found to be acceptable (Cronbach's α = 0.78).
Statistical analysis
Data were analyzed using IBM SPSS Statistics version 20.0 (IBM Corp, Armonk, New York, USA). We performed descriptive analyses to assess the characteristics of the sample and the distribution of sleep quality. The scores from the HADS, the ID Pain, and the PSQI were further categorized based on the suggested cut-off score. The PNQ was categorized as with and without symptoms based on the responses to the items. We performed a simple logistic regression to test the association between various characteristics of the sample and sleep quality.
Results
Characteristics of the sample
Among the 98 participants who were included in the present study, the mean age was 51.4 years with a standard deviation of 10. Most were under the age of 55 (62.3%), had received at least a high-school education (78.6%), were unemployed (53.1%), earned less than the average income (76.6%), did not drink alcohol (80.7%), did not smoke (99.0%), and engaged in regular exercise (75.5%). Among these individuals, nearly all (99.0%) were newly diagnosed with breast cancer, and most were in the early stages of the disease (i.e., Stages I and II; 70.4%), had lymph nodes involvement (55.1%), were undergoing chemotherapy (61.3%), and had received a docetaxel and CEF regimen (cyclophosphamide, epirubicin, and 5-fluorouracil; 70.4%). Around one-third (30.6%) had one or more chronic illness [Table 1], such as hepatitis B (n = 7), diabetes mellites (n = 4), hypertension (n = 7), heart disease (n = 4), hyperlipidemia (n = 4), or thyroid disease (n = 1). Furthermore, around three-quarters of the individuals in the sample (76.5%) suffered from sensory or motor CIPN to some degree [Table 2].
Table 1.
Distribution of sample characteristics and sleep quality (n=98)
| Characteristics | PSQI ≤5 (n=38), n (%) | PSQI >5 (n=60), n (%) |
|---|---|---|
| Employment | ||
| Unemployed | 20 (20.4) | 32 (32.7) |
| Employed | 18 (18.4) | 28 (28.6) |
| Income | ||
| Below average | 28 (28.6) | 47 (48.0) |
| Above average | 10 (10.2) | 13 (13.3) |
| Regular exercise | ||
| No | 10 (10.2) | 14 (14.3) |
| Yes | 28 (28.6) | 46 (46.9) |
| Chronic illness | ||
| No | 31 (31.6) | 37 (37.8) |
| Yes | 7 (7.1) | 23 (23.5) |
| Menopausal status | ||
| Pre | 26 (26.5) | 37 (37.8) |
| Post | 12 (12.2) | 23 (23.5) |
| Cancer stage | ||
| Early | 24 (24.5) | 45 (45.9) |
| Advanced | 14 (14.3) | 15 (15.3) |
| Surgery | ||
| No | 2 (2.0) | 6 (6.1) |
| Yes | 36 (36.8) | 54 (55.1) |
| Undergoing chemotherapy | ||
| No | 20 (20.4) | 18 (18.4) |
| Yes | 18 (18.4) | 42 (42.9) |
| Targeted therapy | ||
| No | 15 (15.3) | 39 (39.8) |
| Yes | 23 (23.5) | 21 (21.4) |
| Radiation therapy | ||
| No | 23 (23.5) | 45 (45.9) |
| Yes | 15 (15.3) | 15 (15.3) |
| Hormone therapy | ||
| No | 21 (21.4) | 41 (41.8) |
| Yes | 17 (17.3) | 19 (19.4) |
PSQI: Pittsburgh Sleep Quality Index
Table 2.
Distribution of emotional distress, chemotherapyinduced peripheral neurotoxicity and sleep quality (n=98)
| Variable | PSQI ≤5 (n=38), n (%) | PSQI >5 (n=60), n (%) |
|---|---|---|
| Anxiety | ||
| No | 36 (36.7) | 42 (42.9) |
| Borderline/yes | 2 (2.0) | 18 (18.4) |
| Depression | ||
| No | 31 (31.6) | 48 (49.0) |
| Borderline/yes | 7 (7.1) | 12 (12.2) |
| Neuropathic pain | ||
| No | 37 (37.8) | 46 (46.9) |
| Yes | 1 (1.0) | 14 (14.3) |
| Sensory neuropathy | ||
| No | 19 (19.4) | 17 (17.3) |
| Yes | 19 (19.4) | 43 (43.9) |
| Motor neuropathy | ||
| No | 22 (22.4) | 16 (16.3) |
| Yes | 16 (16.3) | 44 (44.9) |
PSQI: Pittsburgh Sleep Quality Index
Distribution of sleep quality
A majority of the individuals included in the sample (61.2%) reported poor sleep quality. The mean ± standard deviation of the PSQI score was 7.54 ± 4.45. Among the seven subscales of the PSQI, poor subjective quality (1.37 ± 0.88), long latency (1.34 ± 1.11), short duration (1.37 ± 1.08), and sleep disturbance (1.34 ± 0.56) were the most severe [Figure 1]. In fact, nearly all (99.0%) reported some form of sleep disturbance, with waking during the night or early in the morning (1.65 ± 1.22; 73.5%) and having to use the bathroom (2.19 ± 1.03; 90.8%) being the most common.
Figure 1.
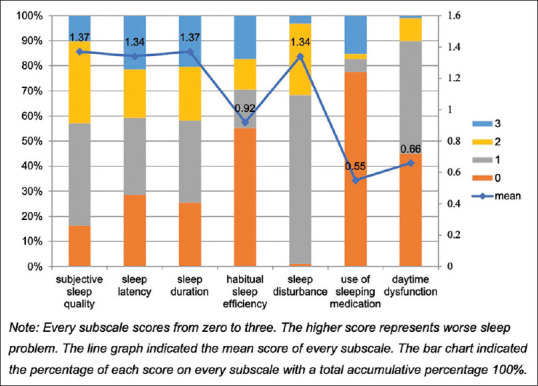
Distribution of sleep quality of participants
Potential risk factors for poor sleep quality
No significant relationships were found between any of the demographic characteristics of the individuals who made up the sample and their sleep quality. However, some of their clinical characteristics were associated with poor sleep quality, including chronic illness, anxiety, neuropathic pain, sensory or motor CIPN, undergoing chemotherapy, and received nontargeted therapy [Table 3].
Table 3.
Relationship between sample characteristics and sleep quality
| Characteristics | OR | 95% confidence interval | P |
|---|---|---|---|
| Chronic illness | |||
| No | 1 | ||
| Yes | 2.75 | 1.04-7.27 | 0.041 |
| Undergoing chemotherapy | |||
| No | 1 | ||
| Yes | 2.59 | 1.12-6.02 | 0.027 |
| Targeted therapy | |||
| No | 1 | ||
| Yes | 0.35 | 0.15-0.81 | 0.015 |
| Anxiety | |||
| No | 1 | ||
| Yes | 7.71 | 1.68-35.53 | 0.009 |
| Neuropathic pain | |||
| No | 1 | ||
| Yes | 11.26 | 1.42-89.63 | 0.022 |
| CIPN-sensory neuropathy | |||
| No | 1 | ||
| Yes | 2.53 | 1.08-5.91 | 0.032 |
| CIPN-motor neuropathy | |||
| No | 1 | ||
| Yes | 3.78 | 1.60-8.95 | 0.002 |
CIPN: Chemotherapy-induced peripheral neuropathy; OR: Odds ratio
Discussion
The present study yielded two main findings. First, more than 60% of the individuals included in the sample reported poor sleep quality, with their main problems relating to latency, duration, and disturbances. Second, CIPN and neuropathic pain were identified as potential risk factors for poor sleep quality, along with chronic illness, undergoing chemotherapy, receiving nontargeted therapy, and anxiety.
The primary aim of this study was to assess the sleep quality of breast cancer survivors who received docetaxel treatment. A prior systematic review found sleep disturbances to be more prevalent among survivors who did not receive taxane chemotherapy than among those who did.[10] Since only survivors who received docetaxel treatment were included in our analysis, we were unable to compare the sleep quality of survivors who received taxane treatment with those who did not. However, the 61.2% of the individuals included in our sample who had sleep problems represented a greater proportion than the average of 40% of breast cancer survivors found to experience such problems by Leysen et al.[7] In addition, general sleep quality, sleep latency, and sleep disturbance were the main problems reported by the survivors among the seven components in the PSQI. A longitudinal study of sleep quality before, during, and after chemotherapy found much the same.[8] Around 60% of the survivors in our sample reported <6 hours of sleep daily. Our findings reinforce the impression that sleep is a significant concern in cancer population, for short sleep duration predicts correlates with increased mortality in advanced cancer survivors.[5]
Our second aim was to determine whether CIPN is a potential risk factor for poor sleep quality. Our findings were consistent with those of Tofthagen et al.[16] and Hong et al.,[17] who reported that CIPN correlated significantly with sleep quality in cancer survivors. However, while we categorized survivors as being either with or without CIPN, the analysis in those studies was based on the severity of CIPN and showed, again, that CIPN was associated not only with poorer sleep quality[16,17] but also with the prevalence of poor sleep quality.[17] We found that motor neuropathy had a greater impact on sleep quality than sensory neuropathy. Since these earlier studies did not separately report sensory and motor neuropathy, we were unable to compare our results with theirs in this respect. Therefore, further study is needed of the separate effects of sensory and motor neuropathy on sleep quality. Pain has been found to be an important risk factor for sleep disturbance in breast cancer survivors.[7] An earlier study also reported that neuropathic pain could diminish sleep quality.[27] Similarly, the survivors in our study who suffered from neuropathic pain were more likely than those who did not to develop poor sleep quality. This result highlights the importance of attention to and management of neuropathic pain in cancer survivors.
We also tested various demographic and clinical characteristics of the individuals included in our sample. Participants with comorbidity or anxiety reported poor sleep quality, a result consistent with Phillips et al.[28] and Desai et al.,[29] respectively. However, unlike Leysen et al.,[7] Palesh et al.,[3] and Phillips et al.,[28] we found no significant relationships between menopausal status, smoking, exercise, radiation therapy or depression and sleep quality. These inconsistencies may at least in part be attributable to differing sleep measures, follow-up time points after diagnosis, and/or cut-off criteria for poor sleep quality.
In terms of treatment, survivors who were currently undergoing chemotherapy tended to have poorer sleep quality than those who were not. These findings are consistent with those of prior studies in which cancer survivors reported the highest prevalence of sleep problems during chemotherapy.[10,30] The systematic review conducted by Costa et al.[10] reported of more sleep disturbances by survivors who received hormone therapy than by those who did not. Similarly, survivors who received radiation therapy reported higher sleep disturbance than those who did not.[10] However, no significant differences in this regard were found in the current study. Moreover, the survivors in our study who received targeted therapy were more likely to report good sleep quality than those who did not. Targeted therapy has been found to improve outcomes for breast cancer survivors with positive human epidermal growth factor receptor 2 (HER-2). Conversely, survivors with negative hormone and HER-2 (those with triple-negative breast cancer) may have relatively poor prognoses; thus, a study of survivors of triple-negative breast cancer found that they experienced significantly greater emotional distress and worry about their cancer than those who were diagnosed with a non-triple-negative breast cancer.[31] This finding, we suggest, may help to explain why, in this study, the survivors who received targeted therapy reported better sleep quality than those who received nontargeted therapy.
Limitations
The sample for this study consisted of breast cancer survivors who had received docetaxel chemotherapy. By assessing sensory CIPN, motor CIPN, and neuropathic pain separately, we were able to distinguish their effects on sleep quality. Our findings shed light on the impact of chemotherapy-induced peripheral neurotoxicity on sleep. This study was subject to some limitations. First, since the data indicated the type of chemotherapy that the survivors received but not whether they were concurrently undergoing hormone therapy, radiation therapy, or targeted therapy, we were unable to capture the exact influence of treatment status on sleep quality. Second, because sleep quality was assessed using a questionnaire that asked the participants to recall their sleep problems over the previous month, there was a possibility of misreporting. For these reasons, future study is needed to use longitudinal study design with a sufficiently large sample size and an objective sleep assessment tool. By doing so, we will be able to clarify the nature of the causal relationship between CIPN and sleep quality.
Conclusions
The findings presented here provide fresh insight into sleep quality and in particular the impact of CIPN on sleep among breast cancer survivors who receive docetaxel treatment. More than 60% of the survivors who made up the sample for this study reported poor sleep quality and sleeping <6 hours per night. Health care providers should, accordingly, be aware of and seek to ameliorate sleep problems in patients treated with docetaxel. In addition, chronic illness, undergoing chemotherapy, treatment with nontargeted therapy, and psychological discomfort were identified as risk factors for poor sleep quality. More specifically, the peripheral neurotoxicity associated with docetaxel treatment can cause the CIPN and neuropathic pain that contribute to poor sleep quality. By remaining attentive to these risk factors, healthcare providers can identify survivors who may suffer from sleep disturbances early in treatment and provide interventions to prevent poor sleep quality from diminishing their quality of life.
Financial support and sponsorship
This study was supported by Yen Tjing Ling Medical Foundation (Grant No. CI-104-29).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151–61. doi: 10.2147/NSS.S134864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoang HT, Molassiotis A, Chan CW, Nguyen TH, Liep Nguyen V. New-onset insomnia among cancer patients undergoing chemotherapy: Prevalence, risk factors, and its correlation with other symptoms. Sleep Breath. 2020;24:241–51. doi: 10.1007/s11325-019-01839-x. [DOI] [PubMed] [Google Scholar]
- 3.Palesh OG, Roscoe JA, Mustian KM, Roth T, Savard J, Ancoli-Israel S, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28:292–8. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akman T, Yavuzsen T, Sevgen Z, Ellidokuz H, Yilmaz AU. Evaluation of sleep disorders in cancer patients based on Pittsburgh Sleep Quality Index. Eur J Cancer Care. 2015;24:553–9. doi: 10.1111/ecc.12296. [DOI] [PubMed] [Google Scholar]
- 5.Collins KP, Geller DA, Antoni M, Donnell DM, Tsung A, Marsh JW, et al. Sleep duration is associated with survival in advanced cancer patients. Sleep Med. 2017;32:208–12. doi: 10.1016/j.sleep.2016.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 7.Leysen L, Lahousse A, Nijs J, Adriaenssens N, Mairesse O, Ivakhnov S, et al. Prevalence and risk factors of sleep disturbances in breast cancer survivors: Systematic review and meta-analyses. Supportive Care Cancer. 2019;27:4401–33. doi: 10.1007/s00520-019-04936-5. [DOI] [PubMed] [Google Scholar]
- 8.Sanford SD, Wagner LI, Beaumont JL, Butt Z, Sweet JJ, Cella D. Longitudinal prospective assessment of sleep quality: Before, during, and after adjuvant chemotherapy for breast cancer. Support Care Cancer. 2013;21:959–67. doi: 10.1007/s00520-012-1612-7. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt ME, Wiskemann J, Steindorf K. Quality of life, problems, and needs of disease-free breast cancer survivors 5 years after diagnosis. Qual Life Res. 2018;27:2077–86. doi: 10.1007/s11136-018-1866-8. [DOI] [PubMed] [Google Scholar]
- 10.Costa AR, Fontes F, Pereira S, Gonçalves M, Azevedo A, Lunet N. Impact of breast cancer treatments on sleep disturbances-A systematic review. Breast. 2014;23:697–709. doi: 10.1016/j.breast.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Velasco R, Bruna J. Taxane-Induced Peripheral Neurotoxicity. Toxics. 2015;3:152–69. doi: 10.3390/toxics3020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ventzel L, Jensen AB, Jensen AR, Jensen TS, Finnerup NB. Chemotherapy-induced pain and neuropathy: A prospective study in patients treated with adjuvant oxaliplatin or docetaxel. Pain. 2016;157:560–8. doi: 10.1097/j.pain.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 13.Bao T, Basal C, Seluzicki C, Li SQ, Seidman AD, Mao JJ. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: Prevalence, risk factors, and fall risk. Breast Cancer Res Treat. 2016;159:327–33. doi: 10.1007/s10549-016-3939-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckhoff L, Knoop A, Jensen MB, Ewertz M. Persistence of docetaxel-induced neuropathy and impact on quality of life among breast cancer survivors. Eur J Cancer. 2015;51:292–300. doi: 10.1016/j.ejca.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Tanay MA, Armes J, Ream E. The experience of chemotherapy-induced peripheral neuropathy in adult cancer patients: A qualitative thematic synthesis. Eur J Cancer Care (Engl) 2017;26:e12443. doi: 10.1111/ecc.12443. [DOI] [PubMed] [Google Scholar]
- 16.Tofthagen C, Donovan KA, Morgan MA, Shibata D, Yeh Y. Oxaliplatin-induced peripheral neuropathy's effects on health-related quality of life of colorectal cancer survivors. Support Care Cancer. 2013;21:3307–13. doi: 10.1007/s00520-013-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong JS, Tian J, Wu LH. The influence of chemotherapy-induced neurotoxicity on psychological distress and sleep disturbance in cancer patients. Curr Oncol. 2014;21:174–80. doi: 10.3747/co.21.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian J, Chen GL, Zhang HR. Sleep status of cervical cancer patients and predictors of poor sleep quality during adjuvant therapy. Support Care Cancer. 2015;23:1401–8. doi: 10.1007/s00520-014-2493-8. [DOI] [PubMed] [Google Scholar]
- 19.Wang YJ. Neurotoxic symptoms predicting quality of life in breast cancer survivors suffering from chemotherapy-induced peripheral neuropathy: A cross-sectional study. Department of Nursing, Da-Yeh University (Unpublished manuscript) [Google Scholar]
- 20.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Lin Y, Hu C, Xu Y, Zhou H, Yang L, et al. The Chinese version of hospital anxiety and depression scale: Psychometric properties in Chinese cancer patients and their family caregivers. Eur J Oncol Nurs. 2016;25:16–23. doi: 10.1016/j.ejon.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. 2006;33:15–49. doi: 10.1053/j.seminoncol.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Shimozuma K, Ohashi Y, Takeuchi A, Aranishi T, Morita S, Kuroi K, et al. Feasibility and validity of the patient neurotoxicity questionnaire during taxane chemotherapy in a phase III randomized trial in patients with breast cancer: N-SAS BC 02. Support Care Cancer. 2009;17:1483–91. doi: 10.1007/s00520-009-0613-7. [DOI] [PubMed] [Google Scholar]
- 24.Portenoy R. Development and testing of a neuropathic pain screening questionnaire: ID Pain. Curr Med Res Opin. 2006;22:1555–65. doi: 10.1185/030079906X115702. [DOI] [PubMed] [Google Scholar]
- 25.Chan A, Wong S, Chen PP, Tsoi TH, Lam J, Ip WY, et al. Validation study of the Chinese Identification Pain Questionnaire for neuropathic pain. Hong Kong Med J. 2011;17:297–300. [PubMed] [Google Scholar]
- 26.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 27.Fontes F, Gonçalves M, Pereira S, Lunet N. Neuropathic pain after breast cancer treatment and its impact on sleep quality one year after cancer diagnosis. Breast. 2017;33:125–31. doi: 10.1016/j.breast.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Phillips KM, Jim HS, Donovan KA, Pinder-Schenck MC, Jacobsen PB. Characteristics and correlates of sleep disturbances in cancer patients. Support Care Cancer. 2012;20:357–65. doi: 10.1007/s00520-011-1106-z. [DOI] [PubMed] [Google Scholar]
- 29.Desai K, Mao JJ, Su I, Demichele A, Li Q, Xie SX, et al. Prevalence and risk factors for insomnia among breast cancer patients on aromatase inhibitors. Support Care Cancer. 2013;21:43–51. doi: 10.1007/s00520-012-1490-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palesh O, Peppone L, Innominato PF, Janelsins M, Jeong M, Sprod L, et al. Prevalence, putative mechanisms, and current management of sleep problems during chemotherapy for cancer. Nat Sci Sleep. 2012;4:151–62. doi: 10.2147/NSS.S18895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vadaparampil ST, Christie J, Donovan KA, Kim J, Augusto B, Kasting ML, et al. Health-related quality of life in Black breast cancer survivors with and without triple-negative breast cancer (TNBC) Breast Cancer Res Treat. 2017;163:331–42. doi: 10.1007/s10549-017-4173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


