Abstract
3q29 duplication syndrome (3q29dup) is a rare genomic disorder caused by a 1.6 Mb duplication (GRCh38 chr3:195,998,000-197,623,000). Case reports indicate the 3q29dup is likely to be pathogenic, but the full range of manifestations is not well understood. We used the 3q29 registry (https://3q29.com) to ascertain 31 individuals with 3q29dup, the largest cohort ever surveyed in a systematic way. For comparison, we ascertained 117 individuals with the reciprocal 3q29 deletion and 64 typically developing controls. We used a custom medical and demographic questionnaire to assess physical and developmental phenotypes, and two standardized instruments, the Social Responsiveness Scale and Child Behavior Checklist/Adult Behavior Checklist, to assess social disability. Participants with 3q29dup report a high rate of problems in the first year of life (80.6%), including feeding problems (55%), failure to gain weight (42%), hypotonia (39%), and respiratory distress (29%). In early childhood, learning problems (71.0%) and seizures (25.8%) are common. Additionally, the rate of self-reported autism spectrum disorder diagnoses (39%) is substantially elevated compared to the general population, suggesting that the 3q29 duplication may be an autism susceptibility locus. This is the most comprehensive description of 3q29dup to date. Our findings can be used to develop evidence-based strategies for early intervention and management of 3q29dup.
Keywords: 3q29 duplication, autism, epilepsy, genomic disorder, intellectual disability
1 ∣. INTRODUCTION
3q29 duplication syndrome (3q29dup) is a genomic disorder caused by duplication of a 1.6 Mb interval on human Chromosome 3 (GRCh38 chr3: 195,998,000–197,623,000). The reciprocal deletion of this interval causes 3q29 deletion syndrome (3q29del). While the breakpoints of the duplication and reciprocal deletion can vary due to the presence of low copy repeats flanking the interval, the canonical interval contains 21 distinct protein-coding genes, three antisense transcripts, three long noncoding RNAs, and one micro-RNA (Ballif et al., 2008; Willatt et al., 2005). 3q29dup has been observed as both an inherited and de novo event (Ballif et al., 2008; Goobie et al., 2009; Vitale et al., 2018). The estimated prevalence of 3q29dup from population-based studies ranges from ~1:75,000 to ~1:8,000 (Kaminsky et al., 2011; Männik et al., 2015; Moreno-De-Luca et al., 2013; Owen et al., 2018; Stefansson et al., 2014). Studies of individuals from clinical cohorts referred for microarray testing indicate a prevalence of ~1:2,000, suggesting that, with larger sample size, the 3q29 duplication may be more common in cohorts ascertained for neurodevelopmental and neuropsychiatric phenotypes than in unselected populations (Ballif et al., 2008; Cooper et al., 2011; Dittwald et al., 2013; Kaminsky et al., 2011; Kushima et al., 2018; Moreno-De-Luca et al., 2013; Sanders et al., 2019). However, the phenotype of 3q29dup is not fully understood; the purpose of the present study is to assess 3q29 duplication-associated phenotypes in a standardized manner.
Case reports of 3q29dup report a range of associated characteristics, including developmental delay, speech delay, intellectual disability (ID), ocular and cardiac anomalies, microcephaly, dental anomalies, obesity, and seizures (Aleixandre Blanquer et al., 2011; Ballif et al., 2008; Fernández-Jaén et al., 2014; Goobie et al., 2009; Kessi et al., 2018; Lesca et al., 2012; Lisi et al., 2008; Schilter et al., 2013; Tassano et al., 2018; Vitale et al., 2018). Additionally, some case reports have described a disruptive behavioral profile (Quintela et al., 2015) and behavioral similarities to autism spectrum disorder (ASD) (Lesca et al., 2012), and one case report identifies an individual with spina bifida (Lawrence, Arreola, Cools, Elton, & Wood, 2017). These case reports are based on extremely small samples, commonly of one individual or of related individuals, highlighting the need for additional phenotypic data on 3q29dup collected from larger patient populations. In the largest case series published to date, which reports on 19 individuals with 3q29dup by Ballif et al. (2008), only five individuals had the canonical 1.6 Mb duplication; the other 14 cases had duplications of sizes varying from 200 kb to 2.4 Mb. Additionally, only seven cases had clinical information (Ballif et al., 2008); based on these factors, it is unclear whether the observed phenotypic heterogeneity is an accurate reflection of heterogeneity in 3q29dup, or if it is largely attributable to the varying duplication sizes within the described cases. For the three subjects with the canonical 1.6 Mb duplication and clinical information, the only common feature was mild/moderate ID (Ballif et al., 2008). Based on the genomic heterogeneity at the 3q29 locus in these cases, there is limited ability to draw conclusions based on this study alone. Taken together, the existing case report literature of 3q29dup is not robust enough to appreciate the full range of syndromic phenotypes associated with the canonical 3q29 duplication.
Because the 3q29 duplication can be inherited from apparently unaffected parents, case reports published to date may reflect the extreme end of the phenotypic distribution associated with 3q29dup. Additionally, carriers of 3q29dup can appear phenotypically normal; these factors combined have resulted in a lack of consensus about the clinical significance of the duplication. For example, in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar), of 19 submission entries for the 1.6 Mb 3q29 duplication, the variant is classified as pathogenic 13 times (68%) with the remaining entries classified as “Uncertain significance” (n = 5) or with “conflicting data from submitters” (n = 1). This means that genetic testing labs identifying the identical variant may classify it differently, with some labs identifying the 3q29 duplication as pathogenic while others fail to do so, which is confusing for families and clinicians alike—especially in cases where a parent transmitting the duplication is seemingly unaffected. To bridge this knowledge gap and better characterize the syndrome, we have created an internet-based registry for individuals with 3q29dup and 3q29del (https://3q29.com) and implemented standardized instruments for systematic ascertainment of self-reported phenotypes. Here, we present results from 31 individuals, the largest cohort of individuals with 3q29dup ever described. We also compare phenotypes to 117 individuals with 3q29del, to identify shared or divergent phenotypes that may be present across the syndromes.
Developing a clearer understanding of the phenotypic spectrum of 3q29dup is crucial for clinicians, caregivers, and the probands themselves, so that evidence-based interventions can be synthesized. Furthermore, these data may provide insight into the potential molecular mechanism and gene dosage effects that have a role in the 3q29 CNVs. Additionally, it will be of clinical utility to determine whether the high rates of neuropsychiatric diagnoses and social disability in 3q29del (Glassford et al., 2016; Pollak et al., 2019) are shared or distinct from 3q29dup phenotypes, as this will provide guidance for developing clinical standards of care for this understudied population.
2 ∣. METHODS
2.1 ∣. Sample
Individuals with 3q29dup were ascertained through the internet-based 3q29 registry (https://3q29.com) as previously reported (Glassford et al., 2016). Briefly, at launch in 2013, information about the registry was emailed to health care providers, medical geneticists, genetic counselors, and support organizations; the registry is currently advertised via Google AdWords, where specific keywords were chosen to target the registry website in internet searches. Participant recruitment, informed consent and assent, and data collection are all performed through the registry website. In April 2019, a data freeze was implemented and existing records were securely downloaded and deidentified for analysis. After data cleaning (removing spam accounts, duplicate records, and individuals with additional significant genetic diagnoses), 31 3q29dup registrants (48.4% male) were included in the present study, ranging in age from 0.3 to 52.2 years (mean = 10.0 ± 10.8 years). Then, 117 individuals with 3q29del (55.6% male) were also obtained through the 3q29 registry, ranging in age from 0.1 to 41.0 years (mean = 9.4 ± 8.0 years). Clinical diagnosis of 3q29dup or 3q29del was confirmed via review of clinical genetics reports and/or medical records. Data from typically developing controls (n = 64, 51.6% male) ranging in age from 1.0 to 41.0 years (mean = 10.5 ± 7.2 years) were obtained as a comparison group (Pollak et al., 2019). Description of the study sample can be found in Table 1. This study was approved by Emory University's Institutional Review Board (IRB00064133).
TABLE 1.
Characteristics of study participants with 3q29dup, 3q29del, and controls
| 3q29dup | 3q29del | Control | |
|---|---|---|---|
| Age, years (mean ± SD) | 10.0 ± 10.8 | 9.4 ± 8.0 | 10.5 ± 7.2 |
| Sex (n, %) | |||
| Male | 15 (48.4%) | 65 (55.6%) | 33 (51.6%) |
| Female | 16 (51.6%) | 52 (44.4%) | 31 (48.4%) |
| Race (n, %) | |||
| White | 27 (87.1%) | 103 (88.0%) | 41 (64.1%) |
| Black/African American | 0 (0%) | 1 (0.9%) | 13 (20.3%) |
| Other | 4 (12.9%) | 11 (9.4%) | 8 (12.5%) |
| Blank | 0 (0%) | 2 (1.7%) | 2 (3.1%) |
Note: Demographic data collected from the custom Medical and Demographic Questionnaire completed by participants upon enrollment in the online 3q29 Registry.
2.2 ∣. Questionnaires
Upon registration, the participant or his/her parent or caregiver completed a custom medical and demographic questionnaire. This questionnaire includes questions on the sex, birthdate, race, and ethnicity of the participant, as well as a detailed medical history covering seven domains of physical and mental development: birth history, development, ear/nose/throat, gastrointestinal, renal, oral/dental, and seizures/psychiatric (Glassford et al., 2016).
In addition to medical phenotypes, two standardized questionnaires were used to assess ASD-related symptomology and general behavioral problems in the participants. The Social Responsiveness Scale (SRS; preschool, school age, and adult forms; n = 15 3q29dup, 67 3q29del, 56 controls) is a 65-item, 4-point Likert-scaled questionnaire designed to assess ASD-related symptoms along a normative continuum (Constantino & Todd, 2012). The Child Behavior Checklist (CBCL) and Adult Behavior Checklist (ABCL) are 100-, 113-, or 126-item (CBCL preschool, CBCL school age, and ABCL, respectively; n = 15 3q29dup, 64 3q29del, 57 controls), 3-point Likert-scaled questionnaires designed to assess behavioral or developmental problems (Achenbach & Rescorla, 2001, 2003). Data from the CBCL and ABCL were pooled for analysis. All standardized questionnaires were adapted for the online 3q29 registry and were completed by the participant or parent/guardian of the participant upon registration. Some participants were not eligible to complete the standardized questionnaires because the proband was too young. Demographic characteristics of the respondents for each questionnaire can be found in Table S1, demonstrating that the average age and sex distribution of participants who completed the medical and demographic questionnaire was not different from the average age and sex distribution of participants who completed each standardized form.
2.3 ∣. Analysis
Diagnoses and health problems from the medical history questionnaire were recoded for analysis as yes/no binary variables; global developmental delay (GDD)/ID diagnosis was recoded as yes (reported diagnosis of GDD and/or ID)/no. Birth weight is coded in 1 lb increments in the online 3q29 registry (https://3q29.com); the midpoint of the interval was assumed as the birth weight of the participant for analysis. Developmental milestones are coded in “bins” of time in the registry; for analysis, it was assumed that the milestone was reached at the midpoint of the selected interval. For milestones marked as “more than 10 years,” it was assumed the participant achieved the milestone at the midpoint between 10 years and their age at registration. For participants who had not yet reached a developmental milestone, their data were treated as censored observations, where time in the study is recorded consistent with age at the time of entry into the registry. To compare birth weight between 3q29dup cases, 3q29del cases, and controls, linear regression and goodness-of-fit analyses were implemented using the stats R package (R Core Team, 2008), controlling for sex, gestational age, and race. To compare reported diagnoses between 3q29dup cases and 3q29del cases, Fisher's exact test and chi-squared tests were implemented using the stats R package (R Core Team, 2008). To compare length of time spent in the hospital between 3q29dup cases and controls, two-sample t test was implemented using the stats R package (R Core Team, 2008). To compare rates of self-reported seizures and psychiatric diagnoses in 3q29dup cases to population prevalence values, one-sample proportion tests with Yates' continuity correction were implemented using the stats R package (R Core Team, 2008). Data from standardized questionnaires were imported into R (R Core Team, 2008) and were recoded and scored according to the publisher's guidelines. To compare standardized questionnaire scores between 3q29dup cases, 3q29del cases, and controls, linear regression was implemented using the stats R package (R Core Team, 2008), controlling for age, race, and sex. To compare scores in participants with 3q29dup to mean values reported for children with idiopathic ASD (Torske, Naerland, Oie, Stenberg, & Andreassen, 2017), one-sample t test was implemented using the stats R package (R Core Team, 2008). Kaplan–Meyer time-to-event analysis for developmental milestones was implemented using the survival R package (Therneau, 2015). Figures were generated using the plotly, ggplot2, and VennDiagram R packages (Chen, 2018; Sievert et al., 2017; Wickham, 2009).
3 ∣. RESULTS
3.1 ∣. Birth weight
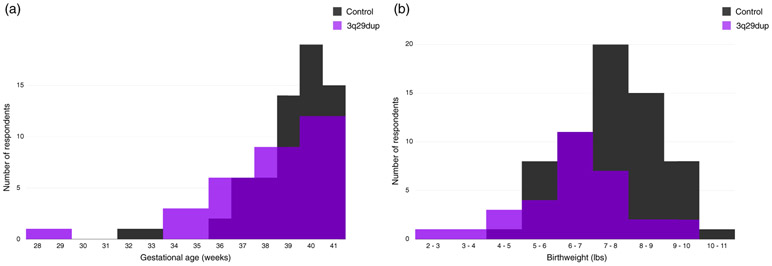
The average birth weight for 31 participants with 3q29dup is 6.50 lbs (2,948.35 g), with an average gestational age of 38.1 weeks, as compared to an average birth weight of 7.6 lbs (3,447.30 g) and average gestational age of 39.2 weeks in our 64 typically developing controls (Figure 1). Gestational age was significantly reduced in 3q29dup cases relative to controls (3q29dup mean = 38.1, control mean = 39.2, p = .04). After adjusting for gestational age, sex, and race, participants with 3q29dup weigh significantly less than controls (p = .005), with an effect size of −0.74, indicating that participants with 3q29dup, on average, weigh 0.74 lbs (11.84 oz, 335.66 g) less than control participants at birth. Additionally, goodness-of-fit analysis shows that including genotype (3q29dup vs. control) in the model fits the data significantly better than only including gestational age, sex, and race (p = .005, Table S2). Because the racial makeup is not matched between participants with 3q29dup and controls, we restricted the analysis to those participants that self-identify as white. We find that the magnitude of the effect size for the 3q29 duplication increases slightly, to −0.83 (p = .001), indicating that within self-identified white registrants, babies with 3q29dup weigh 0.83 lbs (13.28 oz, 376.48 g) less at birth than typically developing controls.
FIGURE 1.
Gestational age and birth weight distributions for 3q29dup and controls. (a) Gestational age distribution for participants with 3q29dup (n = 31) and typically developing controls (n = 64). (b) Birth weight distribution for participants with 3q29dup (n = 31) and typically developing controls (n = 64)
3.2 ∣. Problems in the first year of life
Participants with 3q29dup spent longer in the hospital immediately after birth, with an average stay of 9.8 days (±14.5 days) as compared to an average of 3.8 days (±6.4 days) for typically developing controls (p = .037). Consistent with this longer hospital stay, 80.6% of participants with 3q29dup (n = 25) reported significant health problems in the first year of life, as compared to 39.1% of controls (n = 25). Some of these problems include: feeding problems (54.8%, n = 17); failure to gain weight (41.9%, n = 13); hypotonia (38.7%, n = 12); and respiratory distress (29.0%, n = 9). More data on problems reported in the first year of life can be found in Figure 2 and Table S3.
FIGURE 2.
Reported problems in the first year of life by participants with 3q29dup and controls. Rate of problems in the first year of life reported by participants with 3q29dup (n = 31) and typically developing controls (n = 64), showing that participants with 3q29dup report substantially more problems in the postnatal period. ***, p < .001; **, p < .01; *, p < .05; n.s., not significant
3.3 ∣. Delay of developmental milestones
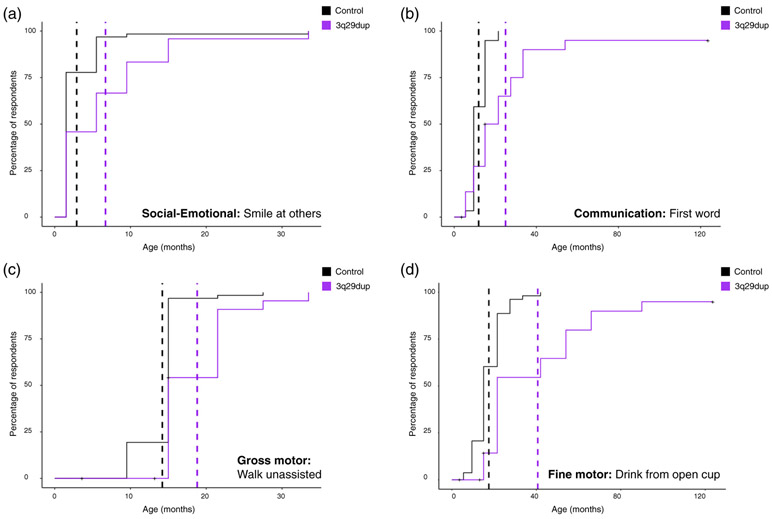
In the 3q29 registry, data are collected on social–emotional, communication, gross motor, and fine motor developmental milestones (Glassford et al., 2016). We used survival analysis to estimate the average time-to-event for developmental milestones for participants with 3q29dup and typically developing controls. One representative milestone was selected for each category; time-to-event curves for participants with 3q29dup and controls are shown in Figure 3. For each milestone shown, participants with 3q29dup achieved that milestone on average 10 to 25 months later than typically developing controls (p < .005); however, the majority of participants do eventually achieve each milestone. A full account of all milestones investigated is available in Table S4. Interestingly, while social–emotional, gross motor, and fine motor milestones on average are delayed by a similar amount (10, 15, and 16 months, respectively), communication milestones are more substantially delayed in participants with 3q29dup, with an average delay of 25 months.
FIGURE 3.
Comparison of developmental milestone achievement between participants with 3q29dup and controls. (a) Kaplan–Meier time-to-event analysis of the representative social–emotional milestone, smile at others, showing that participants with 3q29dup (n = 24) on average achieve this milestone 3.79 months later than typically developing controls (n = 63). (b) Kaplan–Meier time-to-event analysis of the representative communication milestone, first verbal word, showing that participants with 3q29dup (n = 23) on average achieve this milestone 13.4 months later than typically developing controls (n = 59). (c) Kaplan–Meier time-to-event analysis of the representative gross motor milestone, walk unassisted, showing that participants with 3q29dup (n = 26) on average achieve this milestone 4.56 months later than typically developing controls (n = 62). d) Kaplan–Meier time-to-event analysis of the representative fine motor milestone, hold and drink from open cup, showing that participants with 3q29dup (n = 23) on average achieve this milestone 23.29 months later than typically developing controls (n = 53)
3.4 ∣. Learning disabilities
One of the most common phenotypes reported in prior studies of 3q29dup is mild to moderate ID (Aleixandre Blanquer et al., 2011; Ballif et al., 2008; Lisi et al., 2008; Tassano et al., 2018), with one case report of a child with severe ID (Fernández-Jaén et al., 2014). Further, developmental delay, speech delay, and learning disabilities have been reported in individuals with 3q29dup (Goobie et al., 2009; Quintela et al., 2015; Tassano et al., 2018), suggesting that neurodevelopmental and learning disabilities are common to individuals with 3q29dup. Indeed, in our study population 71.0% (n = 22) of participants report at least one diagnosed learning problem, as compared to 4.7% (n = 3) of controls. Commonly reported early learning problems include expressive language delay (54.8%, n = 17), GDD (41.9%, n = 13), and receptive language delay (29.0%, n = 9); common school-age learning problems include learning disability in math (33.3%, n = 7), and learning disability in reading (33.3%, n = 7). A full account of learning disabilities reported by 3q29dup and control participants can be found in Figure 4 and Table S5a,b.
FIGURE 4.
Reported learning problems by participants with 3q29dup and controls. (a) Rate of early learning problems reported by participants with 3q29dup (n = 31) and typically developing controls (n = 64), showing that participants with 3q29dup report substantially more early learning problems. (b) Rate of school-age learning problems reported by participants with 3q29dup (n = 21) and typically developing controls (n = 48) over 5 years of age, showing that participants with 3q29dup report substantially more school-age learning problems. p Values were calculated using Fisher's exact test. ***, p < .001; **, p < .01; n.s., not significant
3.5 ∣. Gastrointestinal phenotypes
While gastrointestinal problems have not been previously reported in individuals with 3q29dup, we find that 54.8% (n = 17) of participants with 3q29dup report at least one gastrointestinal problem (Table S6), including feeding problems beyond the first year of life (38.7%, n = 12) and chronic constipation (35.5%, n = 11).
3.6 ∣. Seizures and neuropsychiatric phenotypes
3.6.1 ∣. Seizures
25.8% (n = 8) of participants with 3q29dup reported seizures, consistent with prior reports of individuals with 3q29dup (Fernández-Jaén et al., 2014; Kessi et al., 2018; Lesca et al., 2012; Tassano et al., 2018) and significantly elevated relative to the general population (general population prevalence = 1.2%, p < 2.20E-16) (Zack & Kobau, 2017).
3.6.2 ∣. Neuropsychiatric diagnosis
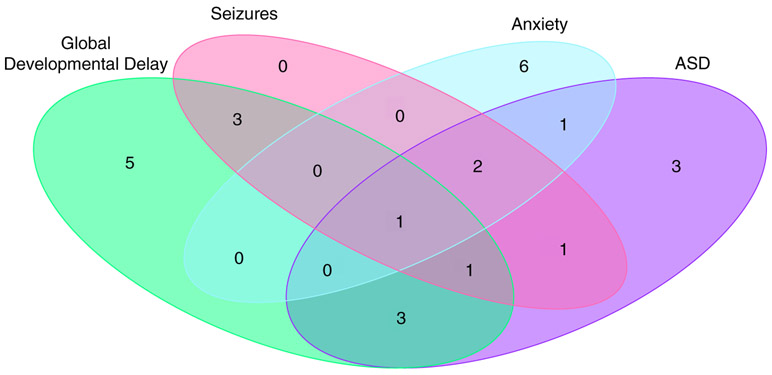
32.3% (n = 10) of participants with 3q29dup reported a diagnosis of anxiety disorder, significantly higher than the general population lifetime prevalence (5.7 vs. 32.3%, p = 1.05E-09). 38.7% (n = 12) of our participants with 3q29dup report a clinical diagnosis of ASD, a rate substantially higher than both that reported for 3q29dup in the literature to date and that reported for the general population, with an estimated 26-fold increased risk for ASD for individuals with 3q29dup. Subsets of participants with 3q29dup also report conduct disorder (6.5%, n = 2), depression (16.1%, n = 5), oppositional defiant disorder (6.5%, n = 2), and panic attacks (6.5%, n = 2) (Table S7). Additionally, there is only partial overlap between participants with 3q29dup reporting GDD, ASD, seizures, and anxiety, indicating that these inflated rates are not due to a subset of severely affected participants, but rather are due to increased risks for these disorders in 3q29dup (Figure 5).
FIGURE 5.
Overlap between reported global developmental delay (GDD), seizures, anxiety, and autism spectrum disorder (ASD) among participants with 3q29dup. Venn diagram showing the overlap between reported GDD, seizures, anxiety, and ASD within our 3q29dup study population, demonstrating that these diagnoses are distributed through the population rather than clustered in a small group of severely affected participants
3.7 ∣. SRS social disability phenotypes and ASD features
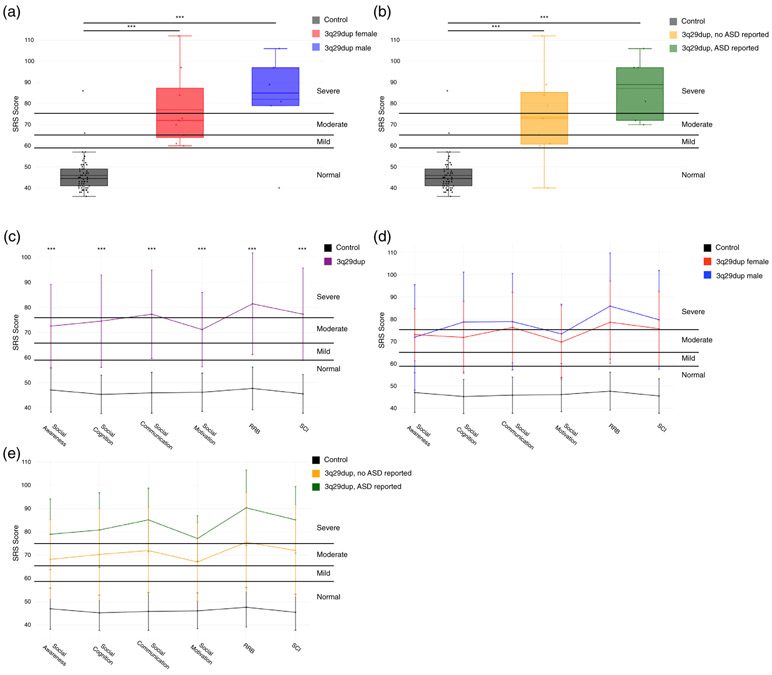
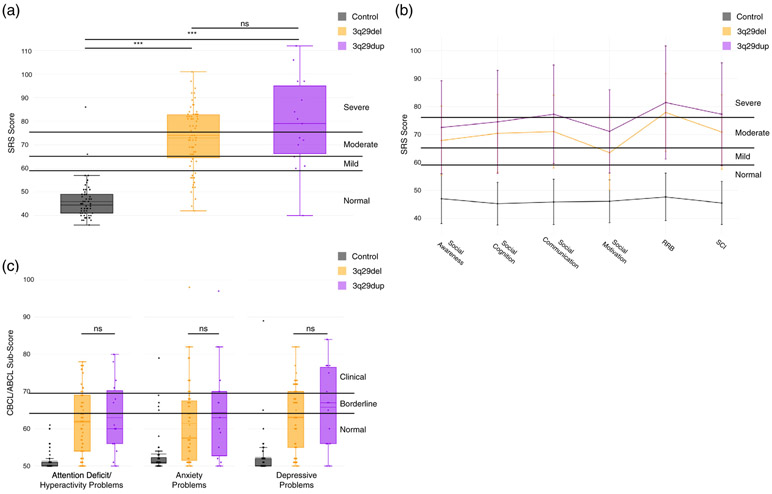
While developmental delay and behavioral similarities to ASD have been identified in 3q29dup (Goobie et al., 2009; Lesca et al., 2012; Tassano et al., 2018), social disability phenotypes have not been quantitatively described. Using standardized self-report tools, we find that participants with 3q29dup have significantly higher total SRS scores than typically developing controls (3q29dup mean T-score = 79.1, control mean T-score = 45.9, p = 1.09E-15), indicating that 3q29dup cases have significantly more social disability than typically developing controls. We observe this increased burden of social disability across sexes and ASD status within our participants with 3q29dup; individuals with 3q29dup score significantly higher than controls irrespective of sex (3q29dup female mean T-score = 77.1, control female mean T-score = 46.0, p = 2.22E-07; 3q29dup male mean T-score = 82.0, control male mean T-score = 45.8, p = 1.83E-07) (Figure 6a) and ASD status (3q29dup with ASD mean T-score = 87.2, control mean T-score = 45.9, p = 3.3E-12; 3q29dup without ASD mean T-score = 73.7, control mean T-score = 45.9, p = 2.9E-09) (Figure 6b) (Table S8).
FIGURE 6.
Comparison of Social Responsiveness Scale (SRS) total scores and sub-scores between 3q29dup and controls. (a) SRS total scores split by controls (n = 56), female with 3q29dup (n = 9), and males with 3q29dup (n = 6), showing that participants with 3q29dup score significantly higher than controls irrespective of sex. For each box plot, the dashed line indicates the mean value, while the solid line indicates the median. (b) SRS total scores split by controls (n = 56), 3q29dup not reporting an autism spectrum disorder (ASD) diagnosis (n = 9), and 3q29dup reporting an ASD diagnosis (n = 6), showing that participants with 3q29dup score significantly higher than typically developing controls irrespective of self-reported autism spectrum disorder (ASD) diagnosis status. For each box plot, the dashed line indicates the mean value, while the solid line indicates the median. (c) Profile of individuals with 3q29dup (n = 15) and controls (n = 56) across SRS subscales, showing moderate to severe impairment of participants with 3q29dup in all domains (Restricted Interests and Repetitive Behaviors [RRB]; Social Communication and Interaction [SCI]). (d) Profile of females with 3q29dup (n = 9), males with 3q29dup (n = 6), and controls (n = 56) across SRS subscales, showing that males and females with 3q29dup both score significantly higher than controls and that the overall shape of the profile is consistent between males and females with 3q29dup. (e) Profile of participants with 3q29dup reporting an ASD diagnosis (n = 6), participants with 3q29dup not reporting an ASD diagnosis (n = 9), and controls (n = 56) across SRS subscales, showing that participants with 3q29dup score significantly higher than controls irrespective of ASD status. ***, p < .001
3.7.1 ∣. SRS subscale profile in 3q29dup
While the SRS total score can give an indication of the overall degree of social impairment for an individual, the SRS subscales can provide more detail about specific domains of social functioning that may be compromised. We analyzed all SRS subscales (Social Awareness, Social Cognition, Social Communication, Social Motivation, Restricted Interests and Repetitive Behaviors, and Social Communication and Interaction) to determine whether the inflated SRS total scores we observed are attributable to substantial deficits in all domains or functioning, or if individuals with 3q29dup show specific impairments in a few domains. Mean scores for Social Communication (T-score = 77.3), Restricted Interests and Repetitive Behaviors (T-score = 81.5), and Social Communication and Interaction (T-score = 77.3) were in the severe range, while mean scores for Social Awareness (T-score = 72.5), Social Cognition (T-score = 74.5), and Social Motivation (T-score = 71.1) were in the moderate range (Figure 6c, Table 2). Notably, participants with 3q29dup have significantly lower Social Motivation scores than those reported in cases of idiopathic ASD (3q29dup Social Motivation T-score = 71.1, idiopathic ASD T-score = 78.4, p = .040) (Torske et al., 2017), indicating a preservation of social motivation relative to other domains assessed by the SRS. Participants with 3q29dup scored significantly higher than typically developing controls on all subscales (p < 4.0E-11) (Table 2).
TABLE 2.
SRS subscore comparison stratified by genotype, sex, and ASD status
| Social awareness |
Social cognition |
Social communication |
Social motivation |
RRB |
SCI |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | p Value | Mean ± SD | p Value | Mean ± SD | p Value | Mean ± SD | p Value | Mean ± SD | p Value | Mean ± SD | p Value | |
| Genotype | ||||||||||||
| Control | 47.04 ± 8.88 | – | 45.27 ± 7.64 | – | 45.88 ± 8.14 | – | 46.13 ± 7.66 | – | 47.66 ± 8.51 | – | 45.50 ± 7.74 | – |
| 3q29dup | 72.53 ± 16.69 | 1.43E-10 | 74.53 ± 18.45 | 4.27E-13 | 77.27 ± 17.65 | 3.37E-14 | 71.13 ± 14.89 | 3.61E-12 | 81.47 ± 20.28 | 1.48E-13 | 77.27 ± 18.43 | 3.24E-14 |
| Sex | ||||||||||||
| Male control | 45.97 ± 9.15 | – | 45.23 ± 7.42 | – | 45.73 ± 6.34 | – | 46.57 ± 6.58 | – | 47.63 ± 5.59 | – | 45.37 ± 6.60 | – |
| Male 3q29dup | 71.83 ± 23.66 | .0003 | 78.67 ± 22.41 | 1.04E-06 | 78.83 ± 21.64 | 3.27E-07 | 73.33 ± 13.35 | 3.72E-07 | 85.83 ± 23.81 | 6.41E-08 | 79.67 ± 22.13 | 3.38E-07 |
| Female control | 48.27 ± 8.56 | – | 45.31 ± 8.03 | – | 46.04 ± 9.95 | – | 45.62 ± 8.85 | – | 47.69 ± 11.08 | – | 45.65 ± 9.01 | – |
| Female 3q29dup | 73.00 ± 11.72 | 1.47E-07 | 71.78 ± 16.15 | 6.24E-07 | 76.22 ± 15.80 | 1.82E-07 | 69.67 ± 16.45 | 6.74E-06 | 78.56 ± 18.47 | 9.13E-07 | 75.67 ± 16.76 | 1.71E-07 |
| ASD status | ||||||||||||
| Control | 47.04 ± 8.88 | – | 45.27 ± 7.64 | – | 45.88 ± 8.14 | – | 46.13 ± 7.66 | – | 47.66 ± 8.51 | – | 45.50 ± 7.74 | – |
| No ASD diagnosis 3q29dup | 68.22 ± 17.09 | 1.74E-06 | 70.33 ± 19.66 | 1.50E-08 | 72.00 ± 18.73 | 3.56E-09 | 67.11 ± 16.83 | 1.00E-07 | 75.56 ±21.37 | 1.29E-08 | 72.00 ± 19.72 | 3.31E-09 |
| ASD diagnosis 3q29dup | 79.00 ± 15.15 | 5.86E-09 | 80.83 ± 15.99 | 1.24E-10 | 85.17 ± 13.64 | 2.85E-12 | 77.17 ± 9.77 | 3.80E-10 | 90.33 ± 16.23 | 7.50E-12 | 85.17 ± 14.27 | 3.15E-12 |
Note: Comparison of mean scores on the SRS subscales between study participants with 3q29dup and controls. Participants with 3q29dup were stratified by sex and ASD status for further analysis. p Values were calculated using simple linear regression, adjusting for age, race, and sex.
Abbreviations: ASD, autism spectrum disorder; RRB, Restricted Interests and Repetitive Behaviors; SCI, Social Communication and Interaction; SRS, Social Responsiveness Scale.
3.7.2 ∣. SRS subscale profile stratified by sex
We find that males and females with 3q29dup do not score significantly differently from each other on any subscale (p > .05); however, both males and females with 3q29dup score significantly higher than controls (p < .0005, Table 2), similar to our previous finding in 3q29del (Pollak et al., 2019). We note that females with 3q29dup score approximately two points higher than males on the Social Awareness subscale, while males with 3q29dup score slightly higher than females on all other subscales (Figures 6d and S1); however, a larger sample size is needed to determine the biological relevance of any sex-specific differences.
3.7.3 ∣. SRS subscale profile stratified by ASD diagnosis
Similar to 3q29del (Pollak et al., 2019), we find that the shape of the SRS sub-score profile is shared between participants with 3q29dup with and without ASD, with participants with 3q29dup reporting an ASD diagnosis scoring on average 10–15 points higher on every subscale than participants with 3q29dup not reporting an ASD diagnosis (Figure 6e). Consistent with participants with 3q29dup having higher total SRS scores than controls irrespective of ASD status, participants with 3q29dup also score in the moderate or severe range, and significantly higher than controls, on all SRS subscales irrespective of ASD status (p < 2.0E-06, Table 2).
3.8 ∣. CBCL/ABCL behavioral phenotypes
3.8.1 ∣. CBCL/ABCL Withdrawn subscale
To further investigate social disability phenotypes in 3q29dup, we used the Withdrawn subscale of the CBCL and ABCL. Previous studies have shown that individuals with idiopathic ASD, on average, score in the borderline range on this subscale, and the majority of individuals score in the borderline or critical range (Mazefsky, Anderson, Conner, & Minshew, 2011; Noterdaeme, Minow, & Amorosa, 1999). Here, we find that participants with 3q29dup score significantly higher than controls (3q29dup mean T-score = 66.2, control mean T-score = 52.4; p = 6.3E-08; Table 3). The average score for participants with 3q29dup overall, and males and females separately, is in the borderline range (Figure 7a,b, Table 3). This supports the SRS data and suggests a previously unidentified social disability phenotype in 3q29dup. Over 50% of participants with 3q29dup score in the borderline or clinical range, with similar proportions observed in participants reporting a diagnosis of ASD and those not reporting a diagnosis of ASD (Figure 7c, Table 3).
TABLE 3.
CBCL/ABCL Withdrawn subscore comparison stratified by genotype, sex, and ASD status
| Mean ± SD | p Value | |
|---|---|---|
| Genotype | ||
| Control | 52.37 ± 5.85 | – |
| 3q29dup | 66.20 ± 12.76 | 6.33E-08 |
| Sex | ||
| Male control | 52.14 ± 3.89 | – |
| Male 3q29dup | 65.00 ± 9.96 | 5.56E-05 |
| Female control | 52.59 ± 7.33 | – |
| Female 3q29dup | 67.00 ± 14.87 | .0004 |
| ASD status | ||
| Control | 52.37 ± 5.85 | – |
| No ASD diagnosis 3q29dup | 64.88 ± 13.91 | 4.42E-05 |
| ASD diagnosis 3q29dup | 67.71 ± 12.20 | 6.06E-06 |
Note: Comparison of mean scores on the CBCL/ABCL Withdrawn subscale between study participants with 3q29dup and controls. Participants with 3q29dup were stratified by sex and ASD status for further analysis. p Values were calculated using simple linear regression, adjusting for age, race, and sex.
Abbreviations: ASD, autism spectrum disorder; CBCL/ABCL, Child Behavior Checklist/Adult Behavior Checklists.
FIGURE 7.
Comparison of Child Behavior Checklist/Adult Behavior Checklists (CBCL/ABCL) Withdrawn and DSM-oriented subscales between 3q29dup and controls. (a) Profile of participants with 3q29dup (n = 15) and controls (n = 57) on the Withdrawn subscale from the CBCL and ABCL, showing a significantly higher score in participants with 3q29dup, with a mean score in the borderline range. For each box plot, the dashed line indicates the mean value, while the solid line indicates the median. (b) Profile of males with 3q29dup (n = 6), females with 3q29dup (n = 9), and controls (n = 57) on the Withdrawn subscale of the CBCL and ABCL, showing that both males and females score significantly higher than typically developing controls. For each box plot, the dashed line indicates the mean value, while the solid line indicates the median. (c) Profile of participants with 3q29dup reporting an autism spectrum disorder (ASD) diagnosis (n = 7) and not reporting an ASD diagnosis (n = 8) and controls (n = 57) on the Withdrawn subscale of the CBCL and ABCL, showing that participants with 3q29dup score significantly higher than typically developing controls irrespective of ASD status. For each box plot, the dashed line indicates the mean value, while the solid line indicates the median. (d) Profile of participants with 3q29dup (n = 15) and controls (n = 57) across three DSM-oriented subscales from the CBCL and ABCL, showing significantly increased pathology in participants with 3q29dup across all domains. For each box plot, the dashed line indicates the mean value, while the solid line indicates the median. ***, p < .001
3.8.2 ∣. CBCL/ABCL DSM-oriented subscales
To assess additional behavioral features of 3q29dup, we examined the DSM-oriented Attention Deficit/Hyperactivity Problems, Anxiety Problems, and Depressive Problems subscales from the CBCL and ABCL. Participants with 3q29dup score significantly higher than controls on every subscale (3q29dup Attention Deficit/Hyperactivity Problems T-score = 63.0, control Attention Deficit/Hyperactivity Problems T-score = 51.3; 3q29dup Anxiety Problems T-score = 64.4, control Anxiety Problems T-score = 53.2; 3q29dup Depressive Problems T-score = 65.8, control Depressive Problems T-score = 52.3; all p < .0005) (Figure 7d). These data suggest additional neuropsychiatric phenotypes are associated with the 3q29 duplication.
3.9 ∣. Other phenotypes
Thirteen (42%) participants reported ear problems (Table S9), 21 (68%) participants reported dental problems (Table S10), two participants (6%) reported heart defects, six participants (19%) reported genitourinary phenotypes, one participant (3%) reported renal phenotypes, and one participant (3%) reported cleft palate (Supplemental Information).
3.10 ∣. Comparison of 3q29dup and 3q29del
3.10.1 ∣. Medical phenotypes
To determine whether there is evidence for divergent phenotypes associated with 3q29dup and 3q29del, we compared overall rates of reported problems in the first year of life, heart defects, learning problems, GDD/ID, ear problems, gastrointestinal problems, genitourinary problems, renal problems, dental problems, seizures, and psychiatric diagnoses between participants with 3q29dup and 3q29del (Table 4). Congenital heart defects are reported at a significantly higher rate by participants with 3q29del as compared to participants with 3q29dup (24.8 vs. 6.5%, p = .047). Although not statistically significant, participants with 3q29dup reported seizures at a rate substantially greater than participants with 3q29del (25.8 vs. 15.4%, p = .276). Rates of all other reported problems were remarkably similar between participants with 3q29dup and 3q29del, suggesting that the reciprocal 3q29 CNVs may have similar effects on organ systems.
TABLE 4.
Comparison of reported medical and neuropsychiatric phenotypes between 3q29dup (n = 31) and 3q29del (n = 117) participants
| Category | 3q29dup (%, n) | 3q29del (%, n) | p Value |
|---|---|---|---|
| Problems in the first year of life | 80.6% (25) | 82.1% (96) | 1.000 |
| Heart defects | 6.5% (2) | 24.8% (29) | .047 |
| Learning problems (excluding GDD/ID) | 71.0% (22) | 78.6% (92) | .500 |
| GDD/ID | 45.2% (14) | 55.6% (65) | .400 |
| Ear problems | 41.9% (13) | 35.9% (42) | .682 |
| Gastrointestinal problems | 54.8% (17) | 67.5% (79) | .270 |
| Genitourinary problems | 19.4% (6) | 17.9% (21) | .800 |
| Renal problems | 3.2% (1) | 6.0% (7) | 1.000 |
| Dental problems | 67.7% (21) | 62.4% (73) | .734 |
| Seizures | 25.8% (8) | 15.4% (18) | .276 |
| Psychiatric diagnoses (including ASD) | 58.1% (18) | 48.7% (57) | .500 |
Note: Comparison of medical and neuropsychiatric diagnosis categories collected from the custom Medical and Demographic Questionnaire completed by participants upon enrollment in the online 3q29 Registry. p Values were calculated using chi-squared test or Fisher's exact test.
Abbreviations: ASD, autism spectrum disorder; GDD, global developmental delay; ID, intellectual disability.
3.10.2 ∣. Psychiatric phenotypes and social disability
ASD and anxiety disorder are present at a similar frequency in both 3q29dup and 3q29del (38.7% in 3q29dup vs. 29.1% in 3q29del, p = .416; 32.3% in 3q29dup vs. 28.2% in 3q29del, p = .826) (Table S7). The 3q29 deletion is established as an ASD-risk variant (Pollak et al., 2019; Sanders et al., 2015) but it is not known whether social disability phenotypes are similarly present in the 3q29 duplication. Using the SRS, we find that participants with 3q29dup score similarly to participants with 3q29del (3q29dup mean T-score = 79.1, 3q29del mean T-score = 72.9, p = .107) (Figure 8a), indicating a similar burden of social disability shared between 3q29dup and 3q29del. The distribution of SRS sub-scores between 3q29dup and 3q29del is qualitatively similar (Figure 8b). However, participants with 3q29dup score significantly higher on Social Motivation than participants with 3q29del (3q29dup Social Motivation T-score = 71.1, 3q29del Social Motivation T-score = 63.5, p = .043), indicating that cases with 3q29dup have an intermediate social motivation phenotype, with significantly more impairment than that observed in 3q29del, but significantly less impairment that that reported in idiopathic ASD (Torske et al., 2017). For the CBCL/ABCL Withdrawn subscale, we have previously reported that individuals with 3q29del have mean scores in the normal range on this subscale, and that over 50% of individuals with 3q29del reporting a diagnosis of ASD score in the borderline or clinical range (Pollak et al., 2019). Here, we find that participants with 3q29dup and 3q29del both score significantly higher than controls (3q29dup mean T-score = 66.2, control mean T-score = 52.4, p = 6.33E-08; 3q29del mean T-score = 63.2, control mean T-score = 52.4, p = 1.6E-09) and that they do not score significantly differently from each other (p = .318). However, the average score for participants with 3q29dup overall, and males and females separately, is in the borderline range (Figure 7a,b, Table 3), whereas the mean score for participants with 3q29del is in the normal range, suggesting a more substantial, and previously unidentified, social disability phenotype in 3q29dup as compared to 3q29del.
FIGURE 8.
Comparison of Social Responsiveness Scale (SRS) and Child Behavior Checklist/Adult Behavior Checklists (CBCL/ABCL) scores between 3q29dup and 3q29del. (a) Total scores on the SRS for participants with 3q29dup (n = 15), participants with 3q29del (n = 67), and controls (n = 56), showing that participants with 3q29dup and 3q29del do not score significantly differently. For each box plot, the dashed line indicates the mean value, while the solid line indicates the median. (b) SRS subscale profile for participants with 3q29dup (n = 15), participants with 3q29del (n = 67), and controls (n = 56), showing that the shape of the SRS subscale profile is conserved between participants with 3q29dup and 3q29del (Restricted Interests and Repetitive Behaviors [RRB]; Social Communication and Interaction [SCI]). (c) Profile of participants with 3q29dup (n = 15), participants with 3q29del (n = 64), and controls (n = 57) across three DSM-oriented subscales from the CBCL and ABCL, showing that participants with 3q29dup and 3q29del have similar levels of pathology in all three domains. For each box plot, the dashed line indicates the mean value, while the solid line indicates the median. ***, p < .001; n.s., not significant
To determine whether 3q29dup shares some behavioral features with 3q29del, we examined the DSM-oriented Attention Deficit/Hyperactivity Problems, Anxiety Problems, and Depressive Problems subscales from the CBCL and ABCL. Both participants with 3q29dup and 3q29del score significantly higher than controls on every subscale (3q29dup Attention Deficit/Hyperactivity Problems T-score = 63.0, 3q29del Attention Deficit/Hyperactivity Problems T-score = 61.7, control Attention Deficit/Hyperactivity Problems T-score = 51.3; 3q29dup Anxiety Problems T-score = 64.4, 3q29del Anxiety Problems T-score = 61.4, control Anxiety Problems T-score = 53.2; 3q29dup Depressive Problems T-score = 65.8, 3q29del Depressive Problems T-score = 63.1, control Depressive Problems T-score = 52.3; all p < .0005), and participants with 3q29dup and 3q29del do not score significantly differently from each other (all p > .282, Figure 8c), suggesting that participants with 3q29dup and 3q29del have shared liability for these neuropsychiatric phenotypes previously associated with 3q29del (Glassford et al., 2016).
4 ∣. DISCUSSION
This study is the first to report on phenotypes associated with 3q29dup using a systematic, standardized approach. We find a high prevalence of problems in the first year of life, including feeding problems, failure to gain weight, hypotonia, and respiratory distress, suggesting that individuals with 3q29 duplication require extra clinical attention during infancy. We also find that seizures, frequently described in case reports of 3q29 duplication syndrome (Fernández-Jaén et al., 2014; Kessi et al., 2018; Lesca et al., 2012; Tassano et al., 2018), are reported in 25% of our study subjects, thus individuals with 3q29 duplication syndrome should be evaluated by a pediatric neurologist. We find feeding problems and chronic constipation are reliably manifest, such that a pediatric gastroenterologist should administer an evaluation. Our data also suggest that ASD and social disability phenotypes are enriched in 3q29 duplication syndrome, and individuals with the 3q29 duplication should therefore be evaluated for ASD using gold-standard clinical measures.
We have found that the 3q29 duplication registry participants report a substantial reduction in birth weight (0.74 lbs, 11.84 oz, 335.66 g), similar to that previously reported for the reciprocal 3q29 deletion (13.9 oz, 394 g) (Glassford et al., 2016). In 3q29del, unpublished data from human subjects assessed by the Emory 3q29 Project (http://genome.emory.edu/3q29/) (Murphy et al., 2018) show that the weight deficit in 3q29del persists into adolescence and data from two independent 3q29 mouse models document diminished weight as a robust feature in these animal models (Baba et al., 2019; Rutkowski et al., in press). However, in an apparent paradox, the 3q29 duplication is associated with obesity in childhood through adulthood (Ballif et al., 2008; Fernández-Jaén et al., 2014; Goobie et al., 2009; Lisi et al., 2008; Vitale et al., 2018), thus 3q29 duplication carriers weigh less at birth but may exhibit accelerated weight gain at an unknown developmental time point. Although the 3q29 registry does not collect data on current weight and height for study participants, these data suggest a compelling future direction for longitudinal data collection on weight and height. These findings also support a complex dose–response relationship between 3q29 interval genes and metabolic phenotypes.
Prior to this study, a significant link between 3q29dup and ASD and related behavioral phenotypes had not been established. One case study reported behavioral similarities to ASD (Lesca et al., 2012); however, 3q29dup cases have not been identified to be significantly enriched in cohort studies of ASD (Sanders et al., 2011; Sanders et al., 2015). It is possible that the population frequency of the 3q29 duplication is low, such that current studies of ASD are underpowered to find association with ASD. As larger cohorts become available, an association between ASD and 3q29dup may become apparent. It is also possible that with existing genomics technologies (such as exome sequencing), duplications are challenging to identify in a research setting and are susceptible to high false-negative rates. Improved analysis methods or new technologies (Mohr et al., 2017) may rectify this problem. It is also possible that our study suffers from ascertainment bias, where individuals with ASD are referred for genetic testing and are coincidentally found to have a 3q29 duplication. However, our own data partially contradict this possibility. In Figures 6 and 7, we show that substantial social disability is present even among individuals without a diagnosis of ASD, as assessed by both the SRS and the CBCL. Our data suggest the 3q29 duplication is a susceptibility locus for ASD, and that gold-standard ASD evaluations should be standard of care for individuals with 3q29dup.
We found that the rate of reported ASD diagnoses in our 3q29dup study population is similar to that reported in 3q29del (38.7 vs. 29.1%, p = .416). Because this is the first study to use standardized, quantitative measures to assess dimensions of social disability in 3q29dup, we are able to evaluate nuances of social behavior in our study sample. Our findings suggest that the degree of social disability in this population has been underappreciated in the literature. Further, we found a profile of ASD features on the SRS that is similar to the profile we had previously identified in 3q29del (Pollak et al., 2019), suggesting that the 3q29 CNVs harbor similar risk for social disability. Furthermore, in both disorders social disability is present irrespective of ASD diagnosis status. Previous work by our group found that individuals with 3q29del have relatively well-preserved social motivation as assessed by the SRS Social Motivation subscale (Pollak et al., 2019); here, we report that individuals with 3q29dup appear to have an intermediate social motivation phenotype, with Social Motivation subscale scores significantly higher (more impaired) than 3q29del, but significantly lower (less impaired) than those reported in a study of idiopathic ASD (Torske et al., 2017). Additionally, we found that participants with 3q29dup, on average, scored in the borderline range for the Withdrawn subscale of the CBCL and ABCL, which further supports a previously unappreciated degree of social disability in the 3q29dup population. Taken together, this indicates that, similar to 3q29del, individuals diagnosed with 3q29dup should receive gold-standard ASD evaluations as a standard of care.
Familial cases of 3q29dup have been reported in the literature (Ballif et al., 2008; Goobie et al., 2009; Vitale et al., 2018); the proportion of 3q29 duplication cases that are inherited is unknown. Anecdotal data from our registry families and prior reports in the literature imply that there are several occurrences where the 3q29 duplication can be inherited from a mildly affected parent. A quantitative estimate of the proportion of 3q29 duplications that are inherited is challenging to obtain because parental genetic testing may not be covered by health insurance, and out of pocket expense for a microarray test may be a significant barrier to testing for many families. Indeed, while most clinical genetics reports for individuals in our study include a recommendation for parental testing and genetic counseling, many of our parents do not follow through in part because they perceive there will be high costs, a high bureaucratic and administrative burden, and hostile interactions with insurance companies for genetic testing to be considered a reimbursable expense. However, knowledge of a parent's carrier status is critical for genetic counseling, and for informing future reproductive choices. In light of our results about the disability that can be associated with the 3q29 duplication, parent genetic testing and genetic counseling should be standard of care for all families of an individual with 3q29dup and should be covered by health insurance to ensure equal access.
While we identified several similarities between 3q29dup and 3q29del, including the effect of the 3q29 CNV on birth weight, SRS scores, and CBCL/ABCL scores, there are also features where 3q29dup and 3q29del diverge. We found that there is a significantly higher rate of congenital heart defects in 3q29del as compared to 3q29dup (24.8 vs. 6.5%, p = .047), which is in line with previously published studies of 3q29del and 3q29dup. We also found a higher rate of participants with 3q29dup reporting seizures as compared to participants with 3q29del (25.8 vs. 15.4%, p = .276); while this difference is not statistically significant, it lends additional support to the association between 3q29dup and seizure phenotypes previously described in case reports (Fernández-Jaén et al., 2014; Kessi et al., 2018; Lesca et al., 2012; Tassano et al., 2018). The registry asks only about the presence or absence of seizures; collecting more data about seizure phenotypes associated with the 3q29 duplication is an important future direction for this work. While the overall rate of individuals reporting at least one psychiatric diagnosis was similar between 3q29dup and 3q29del (58.1 vs. 48.7%, p = .500), the rate of each psychiatric disorder varies between 3q29dup and 3q29del (Table S7). As ongoing efforts to articulate the molecular mechanism and downstream targets impacted by the 3q29 duplication and 3q29 deletion bear fruit, it will be productive to compare convergent and divergent downstream pathways with the concordant and discordant phenotypic spectra of these reciprocal disorders.
Although there are significant strengths to this study, it is not without limitations. While this is the largest cohort of individuals with 3q29dup reported on to date, the small sample size restricts our ability to draw definitive conclusions due to relatively low statistical power. We found substantial differences in some health domains, most notably seizures, that did not reach statistical significance due to our sample size. Studies with larger sample size will be better able to assess the importance of these differences between 3q29dup and 3q29del. Additionally, all of the data used in this study were collected using parent-report measures, which introduce the potential of recall bias due to the data being retrospective. However, a previous study by our group using the same measures as the present study found high concordance between parent-reported diagnoses and direct assessment (Pollak et al., 2019). Finally, there are two potential sources of ascertainment bias in our study. First, our sample of participants with 3q29dup is overwhelmingly white, suggesting that we are not effectively reaching minority populations with our recruitment efforts. Second, we note that parents that register their children and complete time-consuming questionnaires are likely to be highly motivated, potentially because their children are severely affected. If our study sample is taken from the extreme end of 3q29dup phenotypes, scores on the SRS and CBCL/ABCL and reported health problems and diagnoses are likely to be inflated as compared to the true prevalence in the 3q29dup population. Direct assessment of individuals with 3q29dup by the Emory 3q29 Project (http://genome.emory.edu/3q29/) (Murphy et al., 2018) aim to address some of the weakness of this work by performing comprehensive gold-standard evaluations by expert clinicians.
There are significant strengths of this study, most notably that we have reported on the largest cohort of individuals with 3q29dup to date. Further, we have systematically ascertained phenotypes from our study population and from a population of individuals with 3q29del, and we were able to compare phenotypes between the two groups. Although we had a relatively small sample size, we were able to identify significant differences between participants with 3q29dup and 3q29del in the frequency of congenital heart defects, and we were able to find suggestive evidence of other phenotypic divergences between the reciprocal CNVs. We were also able to identify areas of phenotypic concordance between 3q29dup and 3q29del; if these similarities are borne out by studies with larger sample size, it could provide meaningful insight into the molecular mechanisms driving phenotype development, including neuropsychiatric and neurodevelopmental phenotypes. Finally, we found a substantial degree of social disability in our 3q29dup population that has not been previously reported in the literature, which suggests that gold-standard ASD evaluations should be standard of care for individuals with 3q29dup. Taken together, this study serves as a valuable complement to previously published case studies of 3q29dup; by systematically ascertaining phenotypes and comparing them to 3q29del and typically developing controls, we are able to add to the body of knowledge regarding the 3q29dup phenotype and find relationships between 3q29dup and 3q29del, similar to those identified in other reciprocal CNV disorders. These data will assist clinicians, caregivers, and probands in developing comprehensive treatment plans to improve long-term outcomes for individuals with 3q29dup.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge our study population, the 3q29 duplication community, for their participation and commitment to research. The authors also acknowledge the contributions of the members of the Emory 3q29 Project: Hallie Averbach, Emily Black, Gary J. Bassell, T. Lindsey Burrell, Grace Carlock, Tamara Caspary, Joseph F. Cubells, David Cutler, Paul A. Dawson, Michael P. Epstein, Roberto Espana, Michael J. Gambello, Katrina Goines, Henry R. Johnston, Cheryl Klaiman, Sookyong Koh, Elizabeth J. Leslie, Longchuan Li, Bryan Mak, Tamika Malone, Trenell Mosley, Derek Novacek, Ryan Purcell, Timothy Rutkowski, Rossana Sanchez, Celine A. Saulnier, Jason Schroeder, Esra Sefik, Brittney Sholar, Sarah Shultz, Nikisha Sisodiya, Steven Sloan, Elaine F. Walker, Stephen T. Warren, David Weinshenker, and Zhexing Wen.
Funding information
Emory University, Grant/Award Number: Treasure Your Exceptions Project; National Institutes of Health, Grant/Award Numbers: 1R01MH110701-01A1, T32 GM0008490
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Achenbach TM, & Rescorla LA (2001). Manual for the ASEBA school-age forms and profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth and Families. [Google Scholar]
- Achenbach TM, & Rescorla LA (2003). Manual for the ASEBA adult forms and profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth and Families. [Google Scholar]
- Aleixandre Blanquer F, Manchon Trives I, Fornies Arnau MJ, Alcaraz Mas LA, Pico Alfonso N, & Galan Sanchez F (2011). 3q29 microduplication syndrome. Anales de Pediatría (Barcelona, Spain), 75(6), 409–412. 10.1016/j.anpedi.2011.08.002 [DOI] [PubMed] [Google Scholar]
- Baba M, Yokoyama K, Seiriki K, Naka Y, Matsumura K, Kondo M, … Nakazawa T (2019). Psychiatric-disorder-related behavioral phenotypes and cortical hyperactivity in a mouse model of 3q29 deletion syndrome. Neuropsychopharmacology, 44, 2125–2135. 10.1038/s41386-019-0441-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballif BC, Theisen A, Coppinger J, Gowans GC, Hersh JH, Madan-Khetarpal S, … Shaffer LG (2008). Expanding the clinical phenotype of the 3q29 microdeletion syndrome and characterization of the reciprocal microduplication. Molecular Cytogenetics, 1, 8 10.1186/1755-8166-1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H (2018). VennDiagram: Generate high-resolution venn and euler plots. R package version 1.6.20 [Google Scholar]
- Constantino JN, & Todd RD (2012). The social responsiveness scale manual (2nd ed.). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Cooper GM, Coe BP, Girirajan S, Rosenfeld JA, Vu TH, Baker C, …Eichler EE (2011). A copy number variation morbidity map of developmental delay. Nature Genetics, 43(9), 838–846. 10.1038/ng.909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittwald P, Gambin T, Szafranski P, Li J, Amato S, Divon MY, … Stankiewicz P (2013). NAHR-mediated copy-number variants in a clinical population: Mechanistic insights into both genomic disorders and Mendelizing traits. Genome Research, 23(9), 1395–1409. 10.1101/gr.152454.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Jaén A, Castellanos MDC, Fernández-Perrone AL, Fernández-Mayoralas DM, de la Vega AG, Calleja-Pérez B, … Hombre MCS (2014). Cerebral palsy, epilepsy, and severe intellectual disability in a patient with 3q29 microduplication syndrome. American Journal of Medical Genetics. Part A, 164A(8), 2043–2047. 10.1002/ajmg.a.36559 [DOI] [PubMed] [Google Scholar]
- Glassford MR, Rosenfeld JA, Freedman AA, Zwick ME, Mulle JG, & Unique Rare Chromosome Disorder Support Group. (2016). Novel features of 3q29 deletion syndrome: Results from the 3q29 registry. American Journal of Medical Genetics. Part A, 170A(4), 999–1006. 10.1002/ajmg.a.37537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goobie S, Knijnenburg J, Fitzpatrick D, Sharkey FH, Lionel AC, Marshall CR, … Scherer SW (2009). Molecular and clinical characterization of de novo and familial cases with microduplication 3q29: Guidelines for copy number variation case reporting. Cytogenetic and Genome Research, 123(1–4), 65–78. 10.1159/000184693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky EB, Kaul V, Paschall J, Church DM, Bunke B, Kunig D … Martin CL (2011). An evidence-based approach to establish the functional and clinical significance of copy number variants in intellectual and developmental disabilities. Genetics in Medicine, 13(9), 777–784. 10.1097/GIM.0b013e31822c79f9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessi M, Peng J, Yang L, Xiong J, Duan H, Pang N, & Yin F (2018). Genetic etiologies of the electrical status epilepticus during slow wave sleep: Systematic review. BMC Genetics, 19(1), 40 10.1186/s12863-018-0628-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushima I, Aleksic B, Nakatochi M, Shimamura T, Okada T, Uno Y, … Ozaki N (2018). Comparative analyses of copy-number variation in autism spectrum disorder and schizophrenia reveal etiological overlap and biological insights. Cell Reports, 24(11), 2838–2856. 10.1016/j.celrep.2018.08.022 [DOI] [PubMed] [Google Scholar]
- Lawrence MB, Arreola A, Cools M, Elton S, & Wood KS (2017). 3q29 chromosomal duplication in a neonate with associated myelomeningocele and midline cranial defects. Clinical Dysmorphology, 26(4), 221–223. 10.1097/mcd.0000000000000193 [DOI] [PubMed] [Google Scholar]
- Lesca G, Rudolf G, Labalme A, Hirsch E, Arzimanoglou A, Genton P, … Szepetowski P (2012). Epileptic encephalopathies of the Landau-Kleffner and continuous spike and waves during slow-wave sleep types: Genomic dissection makes the link with autism. Epilepsia, 53(9), 1526–1538. 10.1111/j.1528-1167.2012.03559.x [DOI] [PubMed] [Google Scholar]
- Lisi EC, Hamosh A, Doheny KF, Squibb E, Jackson B, Galczynski R, … Batista DA (2008). 3q29 interstitial microduplication: A new syndrome in a three-generation family. American Journal of Medical Genetics. Part A, 146a(5), 601–609. 10.1002/ajmg.a.32190 [DOI] [PubMed] [Google Scholar]
- Männik K, Mägi R, Macá A, Cole B, Guyatt AL, Shihab HA, … Reymond A (2015). Copy number variations and cognitive phenotypes in unselected populations. JAMA, 313(20), 2044–2054. 10.1001/jama.2015.4845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazefsky CA, Anderson R, Conner CM, & Minshew N (2011). Child behavior checklist scores for school-aged children with autism: Preliminary evidence of patterns suggesting the need for referral. Journal of Psychopathology and Behavioral Assessment, 33(1), 31–37. 10.1007/s10862-010-9198-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr D, Naguib A, Weisenfeld N, Kumar V, Shah P, Church D, … Scott A (2017). Improved de novo genome assembly: Linked-read sequencing combined with optical mapping produce a high quality mammalian genome at relatively low cost. BioRxiv, 128348 10.1101/128348 [DOI] [Google Scholar]
- Moreno-De-Luca D, Sanders SJ, Willsey AJ, Mulle JG, Lowe JK, Geschwind DH, … Ledbetter DH (2013). Using large clinical data sets to infer pathogenicity for rare copy number variants in autism cohorts. Molecular Psychiatry, 18(10), 1090–1095. 10.1038/mp.2012.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MM, Lindsey Burrell T, Cubells JF, Espana RA, Gambello MJ, Goines KCB, … Mulle JG (2018). Study protocol for the Emory 3q29 project: Evaluation of neurodevelopmental, psychiatric, and medical symptoms in 3q29 deletion syndrome. BMC Psychiatry, 18(1), 183 10.1186/s12888-018-1760-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noterdaeme M, Minow F, & Amorosa H (1999). Applicability of the child behavior checklist in developmentally delayed children. Zeitschrift für Kinder und Jugendpsychiatrie und Psychotherapie, 27(3), 183–188. 10.1024//1422-4917.27.3.183 [DOI] [PubMed] [Google Scholar]
- Owen D, Bracher-Smith M, Kendall KM, Rees E, Einon M, Escott-Price V, … Kirov G (2018). Effects of pathogenic CNVs on physical traits in participants of the UK Biobank. BMC Genomics, 19(1), 867 10.1186/s12864-018-5292-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak RM, Murphy MM, Epstein MP, Zwick ME, Klaiman C, Saulnier CA, & Mulle JG (2019). Neuropsychiatric phenotypes and a distinct constellation of ASD features in 3q29 deletion syndrome: Results from the 3q29 registry. Molecular Autism, 10(1), 30 10.1186/s13229-019-0281-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintela I, Barros-Angueira F, Pérez-Gay L, Dacruz D, Castro-Gago M, Carracedo A, & Eirís-Puñal J (2015). Molecular characterization and phenotypic description of two patients with reciprocal chromosomal aberrations in the region of the 3q29 microdeletion/microduplication syndromes. RevNeurol, 61(06), 0255–0260. [PubMed] [Google Scholar]
- R Core Team. (2008). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rutkowski TP, Purcell RH, Pollak RM, Grewenow SM, Gafford GM, Malone T, … Mulle JG (in press). Behavioral changes and growth deficits in a CRISPR engineered mouse model of the schizophrenia-associated 3q29 deletion. Molecular Psychiatry. 10.1038/s41380-019-0413-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, … State MW (2011). Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron, 70(5), 863–885. 10.1016/j.neuron.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, He X, Willsey AJ, Ercan-Sencicek AG, Samocha KE, Cicek AE, … State MW (2015). Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron, 87(6), 1215–1233. 10.1016/j.neuron.2015.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, Sahin M, Hostyk J, Thurm A, Jacquemont S, Avillach P, … Bearden CE (2019). A framework for the investigation of rare genetic disorders in neuropsychiatry. Nature Medicine, 25, 1477–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilter KF, Reis LM, Schneider A, Bardakjian TM, Abdul-Rahman O, Kozel BA, … Semina EV (2013). Whole-genome copy number variation analysis in anophthalmia and microphthalmia. Clinical Genetics, 84(5), 473–481. 10.1111/cge.12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievert C, Parmer C, Hocking T, Chamberlain S, Ram K, Corvellec M, & Despouy P (2017). plotly: Create Interactive Web Graphics via ‘plotly.js’. R package version 4.6.0 [Google Scholar]
- Stefansson H, Meyer-Lindenberg A, Steinberg S, Magnusdottir B, Morgen K, Arnarsdottir S, … Stefansson K (2014). CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature, 505(7483), 361–366. 10.1038/nature12818 [DOI] [PubMed] [Google Scholar]
- Tassano E, Uccella S, Giacomini T, Severino M, Siri L, Gherzi M, … Ronchetto P (2018). 3q29 microduplication syndrome: Description of two new cases and delineation of the minimal critical region. European Journal of Medical Genetics, 61(8), 428–433. 10.1016/j.ejmg.2018.02.011 [DOI] [PubMed] [Google Scholar]
- Therneau T (2015). A package for survival analysis in S. Version 2.38. [Google Scholar]
- Torske T, Naerland T, Oie MG, Stenberg N, & Andreassen OA (2017). Metacognitive aspects of executive function are highly associated with social functioning on parent-rated measures in children with autism spectrum disorder. Frontiers in Behavioral Neuroscience, 11, 258 10.3389/fnbeh.2017.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale A, Labruna G, Mancini A, Alfieri A, laffaldano L, Nardelli C, … Lombardo B (2018). 3q29 microduplication in a small family with complex metabolic phenotype from southern Italy. Clinical Chemistry and Laboratory Medicine, 56(7), e167–e170. 10.1515/cclm-2017-1090 [DOI] [PubMed] [Google Scholar]
- Wickham H (2009). ggplot2: Elegant graphics for data analysis. New York, NY: Springer-Verlag. [Google Scholar]
- Willatt L, Cox J, Barber J, Cabanas ED, Collins A, Donnai D, … Raymond FL (2005). 3q29 microdeletion syndrome: Clinical and molecular characterization of a new syndrome. American Journal of Human Genetics, 77(1), 154–160. 10.1086/431653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack MM, & Kobau R (2017). National and state estimates of the numbers of adults and children with active epilepsy - United States, 2015. MMWR Morb Mortal Wkly Rep, 66(31), 821–825. 10.15585/mmwr.mm6631a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.